Ultrasound-Guided Interventions for Neuropathic Pain: A Narrative Pictorial Review
Abstract
1. Introduction
2. Literature Search
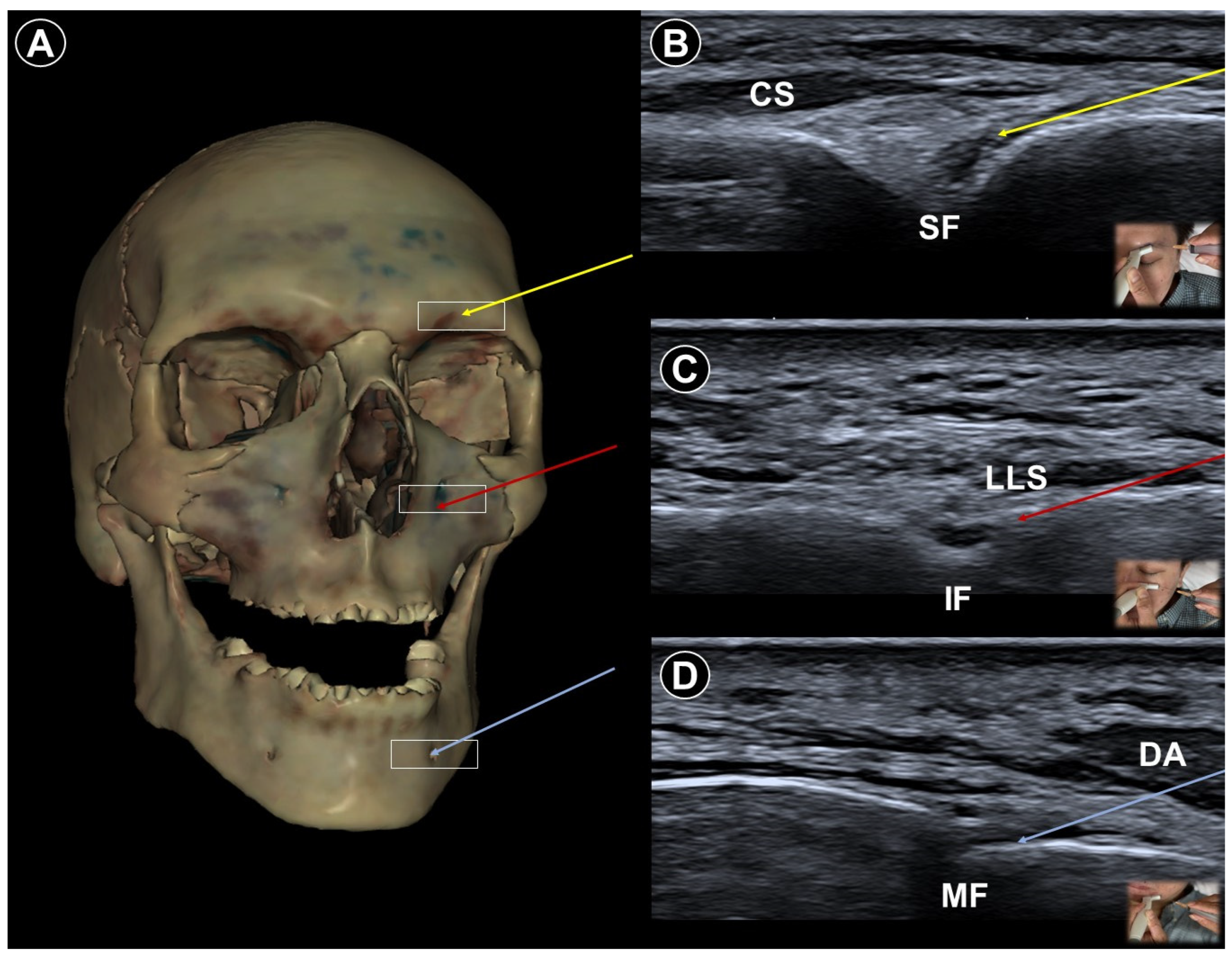
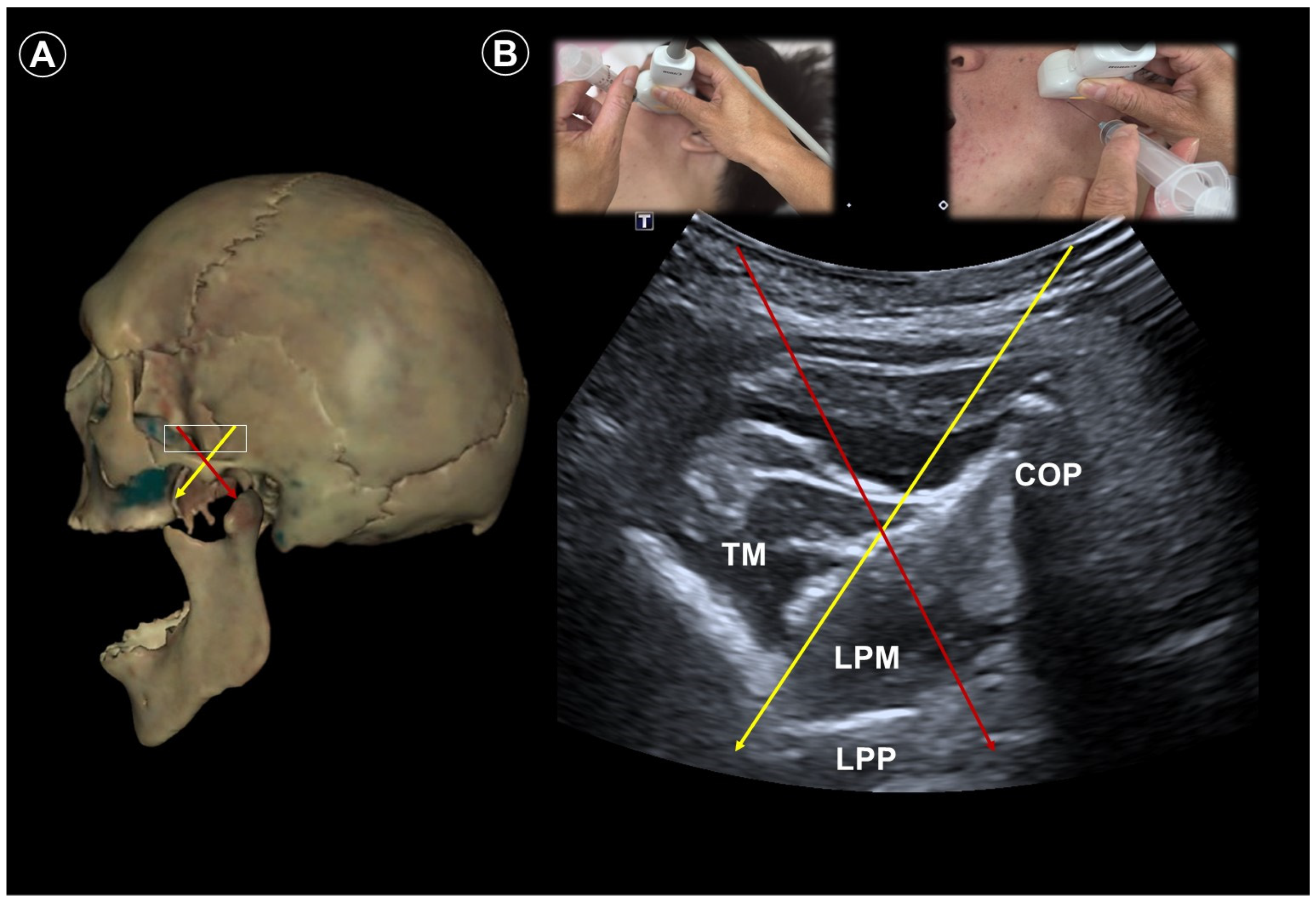
3. Trigeminal Neuralgia
4. Postherpetic Neuralgia
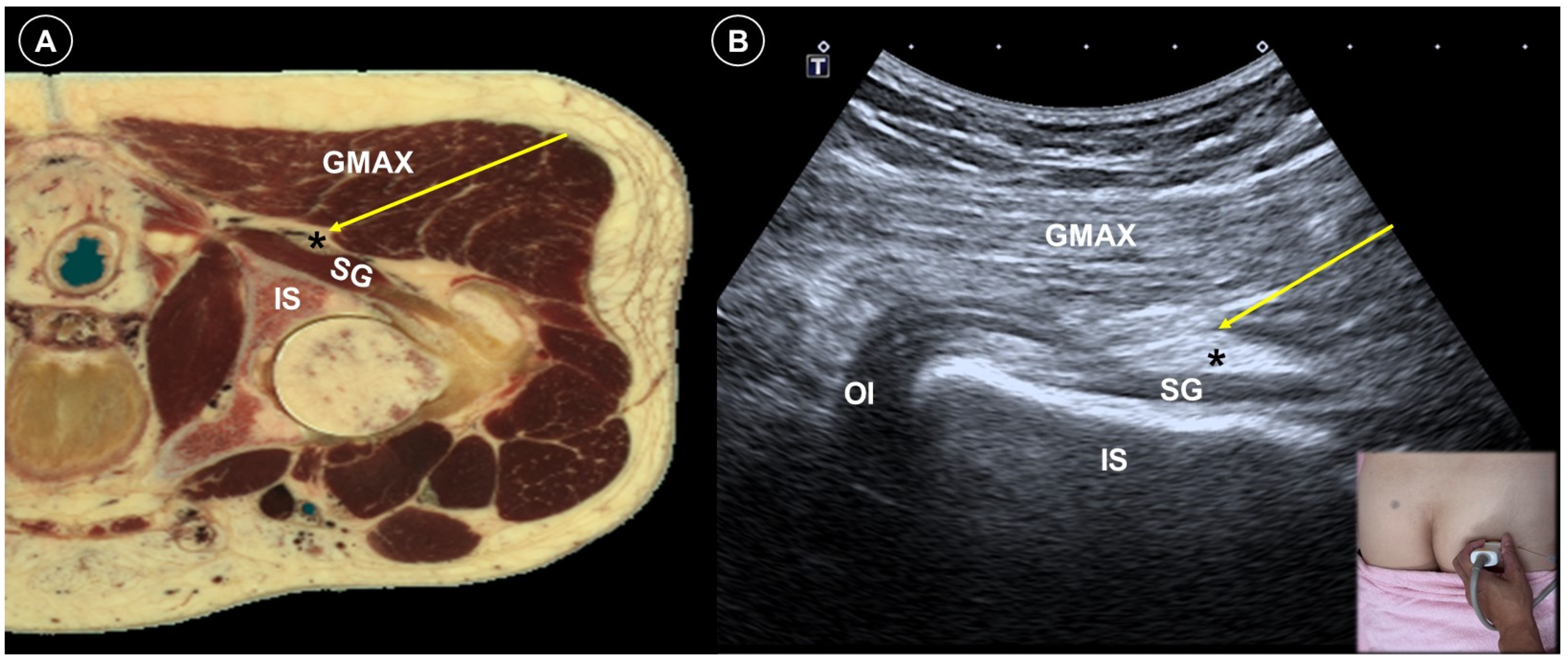
5. Post-Amputation/Mastectomy Pain
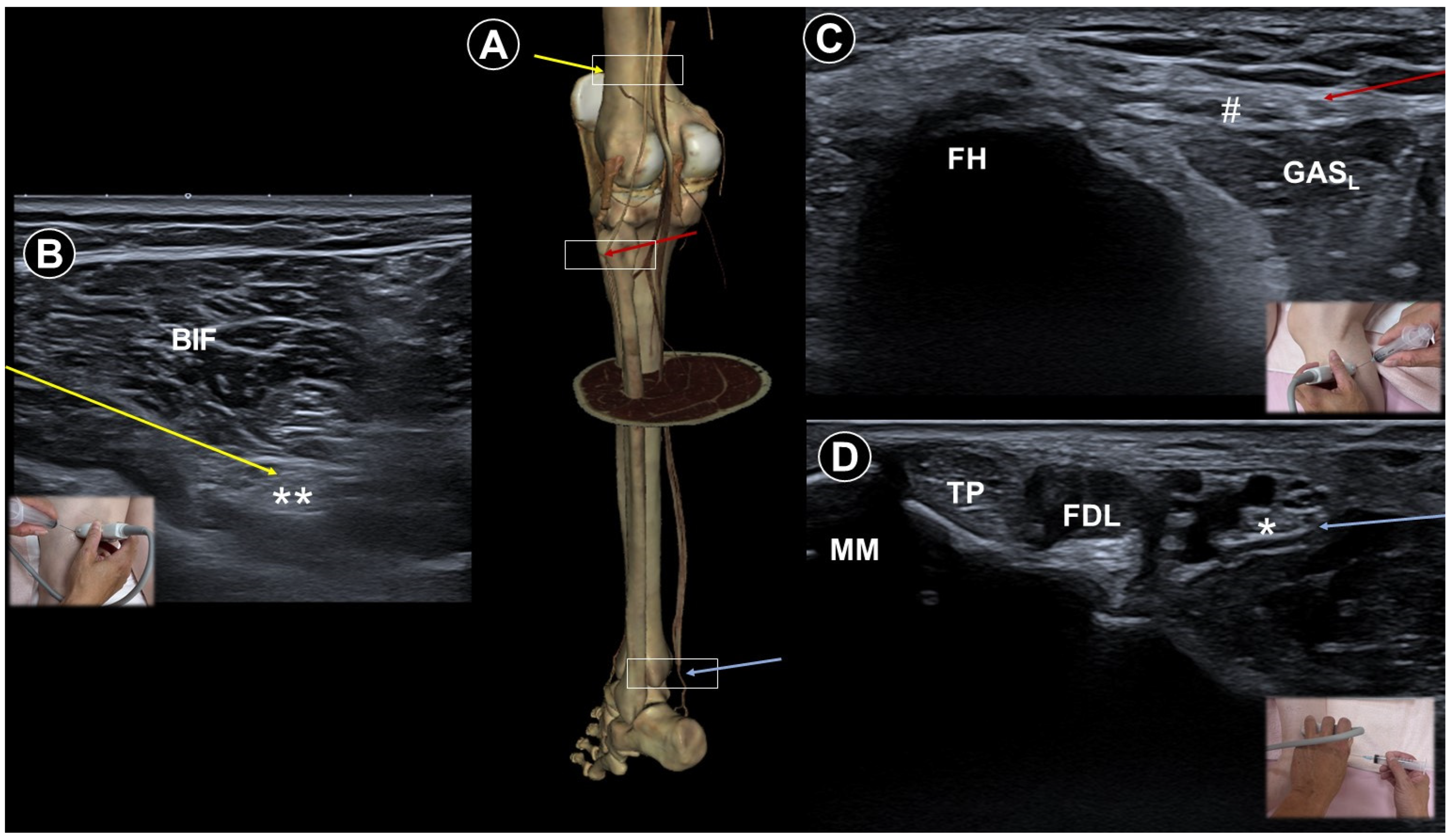
6. Painful Polyneuropathy and Peripheral Nerve Injury Pain
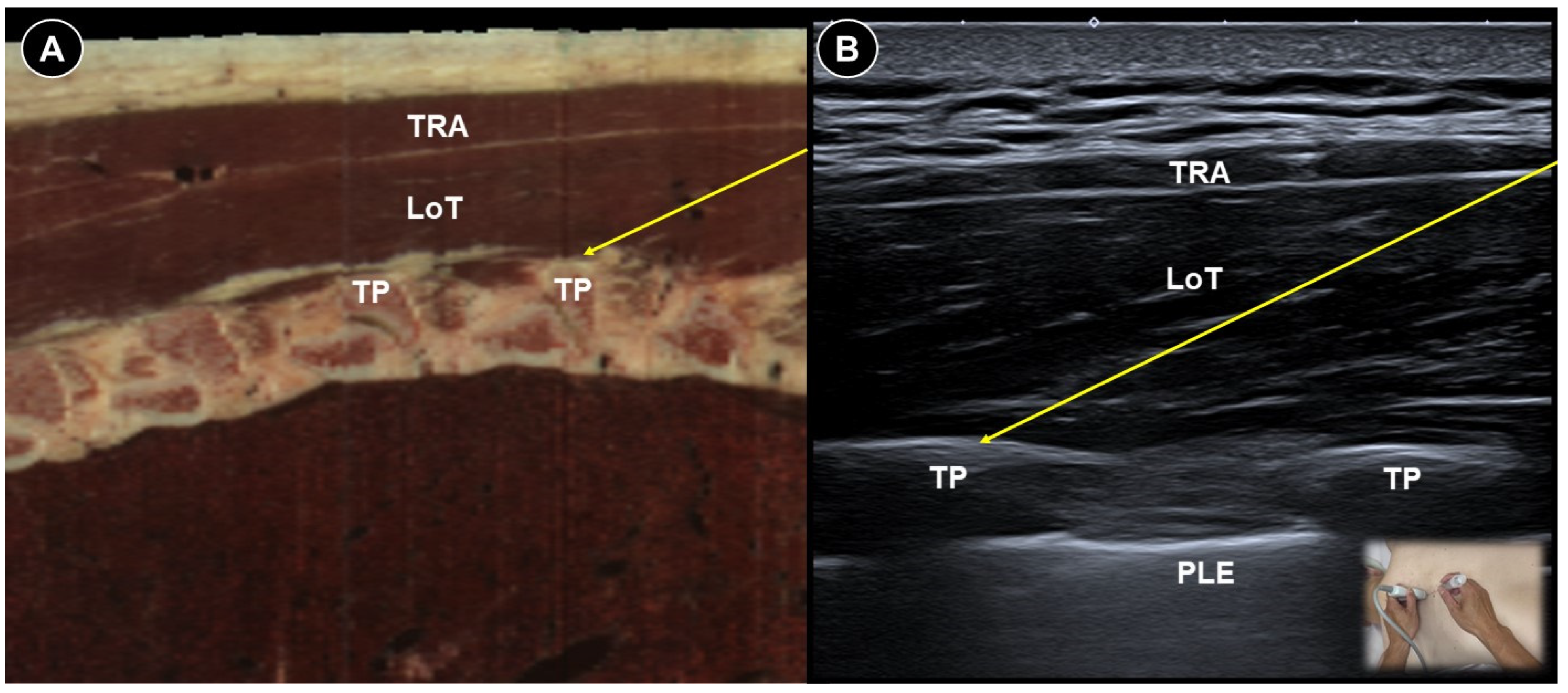
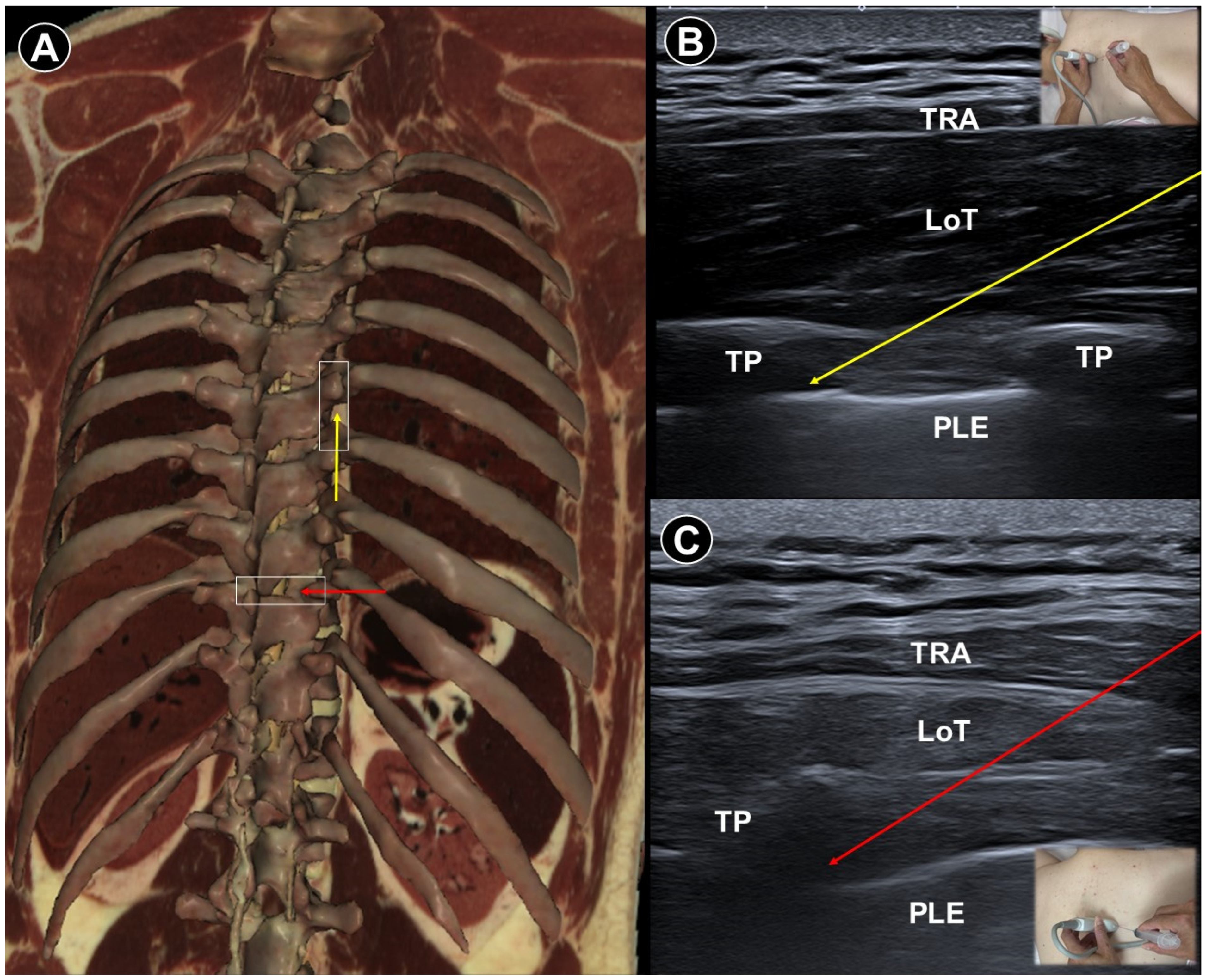
7. Painful Radiculopathy
8. Discussion
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Colloca, L.; Ludman, T.; Bouhassira, D.; Baron, R.; Dickenson, A.H.; Yarnitsky, D.; Freeman, R.; Truini, A.; Attal, N.; Finnerup, N.B. Neuropathic pain. Nat. Rev. Dis. Primers 2017, 3, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Finnerup, N.B.; Kuner, R.; Jensen, T.S. Neuropathic pain: From mechanisms to treatment. Physiol. Rev. 2021, 101, 259–301. [Google Scholar] [CrossRef] [PubMed]
- Gierthmühlen, J.; Baron, R. Neuropathic pain. Semin. Neurol. 2016, 36, 462–468. [Google Scholar]
- Cohen, S.P.; Mao, J. Neuropathic pain: Mechanisms and their clinical implications. BMJ 2014, 348, f7656. [Google Scholar] [CrossRef] [PubMed]
- Meacham, K.; Shepherd, A.; Mohapatra, D.P.; Haroutounian, S. Neuropathic pain: Central vs. peripheral mechanisms. Curr. Pain Headache Rep. 2017, 21, 28. [Google Scholar] [CrossRef]
- Ossipov, M.H.; Dussor, G.O.; Porreca, F. Central modulation of pain. J. Clin. Investig. 2010, 120, 3779–3787. [Google Scholar] [CrossRef]
- Rosner, J.; de Andrade, D.C.; Davis, K.D.; Gustin, S.M.; Kramer, J.L.K.; Seal, R.P.; Finnerup, N.B. Central neuropathic pain. Nat. Rev. Dis. Primers 2023, 9, 73. [Google Scholar] [CrossRef]
- Attal, N.; Bouhassira, D.; Baron, R. Diagnosis and assessment of neuropathic pain through questionnaires. Lancet Neurol. 2018, 17, 456–466. [Google Scholar] [CrossRef]
- Bouhassira, D.; Lantéri-Minet, M.; Attal, N.; Laurent, B.; Touboul, C. Prevalence of chronic pain with neuropathic characteristics in the general population. Pain 2008, 136, 380–387. [Google Scholar] [CrossRef]
- Freynhagen, R.; Baron, R.; Tölle, T.; Stemmler, E.; Gockel, U.; Stevens, M.; Maier, C. Screening of neuropathic pain components in patients with chronic back pain associated with nerve root compression: A prospective observational pilot study (MIPORT). Curr. Med. Res. Opin. 2006, 22, 529–537. [Google Scholar] [CrossRef]
- Attal, N.; Bouhassira, D.; Colvin, L. Advances and challenges in neuropathic pain: A narrative review and future directions. Br. J. Anaesth. 2023, 131, 79–92. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Neri, I.; González-Aguilar, A.; Sandoval, H.; Pineda, C.; Ríos, C. Therapeutic potential of ultrasound neuromodulation in decreasing neuropathic pain: Clinical and experimental evidence. Curr. Neuropharmacol. 2021, 19, 334–348. [Google Scholar] [CrossRef]
- Haffey, P.R.; Bansal, N.; Kaye, E.; Ottestad, E.; Aiyer, R.; Noori, S.; Gulati, A. The regenerative potential of therapeutic ultrasound on neural tissue: A pragmatic review. Pain Med. 2020, 21, 1494–1506. [Google Scholar] [CrossRef]
- Chang, K.-V.; Lin, C.-P.; Lin, C.-S.; Wu, W.-T.; Karmakar, M.K.; Özçakar, L. Sonographic tracking of trunk nerves: Essential for ultrasound-guided pain management and research. J. Pain Res. 2017, 10, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.H.; Chang, K.V.; Özçakar, L.; Hsiao, M.Y.; Hung, C.Y.; Shyu, S.G.; Wang, T.G.; Chen, W.S. Sonographic tracking of the upper limb peripheral nerves: A pictorial essay and video demonstration. Am. J. Phys. Med. Rehabil. 2015, 94, 740–747. [Google Scholar] [CrossRef]
- Hung, C.-Y.; Hsiao, M.-Y.; Özçakar, L.; Chang, K.-V.; Wu, C.-H.; Wang, T.-G.; Chen, W.-S. Sonographic tracking of the lower limb peripheral nerves: A pictorial essay and video demonstration. Am. J. Phys. Med. Rehabil. 2016, 95, 698–708. [Google Scholar] [CrossRef]
- Shen, P.-C.; Lin, T.-Y.; Wu, W.-T.; Özçakar, L.; Chang, K.-V. Comparison of Ultrasound-vs Landmark-Guided Injections For Musculoskeletal Pain: An Umbrella Review. J. Rehabil. Med. 2024, 56, 40769. [Google Scholar] [CrossRef]
- Scholz, J.; Finnerup, N.B.; Attal, N.; Aziz, Q.; Baron, R.; Bennett, M.I.; Benoliel, R.; Cohen, M.; Cruccu, G.; Davis, K.D.; et al. The IASP classification of chronic pain for ICD-11: Chronic neuropathic pain. Pain 2019, 160, 53–59. [Google Scholar] [CrossRef]
- Maarbjerg, S.; Di Stefano, G.; Bendtsen, L.; Cruccu, G. Trigeminal neuralgia—Diagnosis and treatment. Cephalalgia 2017, 37, 648–657. [Google Scholar] [CrossRef]
- Maarbjerg, S.; Wolfram, F.; Gozalov, A.; Olesen, J.; Bendtsen, L. Significance of neurovascular contact in classical trigeminal neuralgia. Brain 2014, 138, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Lambru, G.; Zakrzewska, J.; Matharu, M. Trigeminal neuralgia: A practical guide. Pract. Neurol. 2021, 21, 392–402. [Google Scholar] [CrossRef] [PubMed]
- Nader, A.; Kendall, M.C.; DeOliveria, G.S.; Chen, J.Q.; Vanderby, B.; Rosenow, J.M.; Bendok, B.R. Ultrasound-guided trigeminal nerve block via the pterygopalatine fossa: An effective treatment for trigeminal neuralgia and atypical facial pain. Pain Physician 2013, 16, E537. [Google Scholar] [CrossRef]
- Allam, A.E.; Khalil, A.A.F.; Eltawab, B.A.; Wu, W.T.; Chang, K.V. Ultrasound-Guided Intervention for Treatment of Trigeminal Neuralgia: An Updated Review of Anatomy and Techniques. Pain Res. Manag. 2018, 2018, 5480728. [Google Scholar] [CrossRef]
- Johnson, R.W.; Rice, A.S.C. Postherpetic Neuralgia. N. Engl. J. Med. 2014, 371, 1526–1533. [Google Scholar] [CrossRef]
- Forbes, H.J.; Thomas, S.L.; Smeeth, L.; Clayton, T.; Farmer, R.; Bhaskaran, K.; Langan, S.M. A systematic review and meta-analysis of risk factors for postherpetic neuralgia. Pain 2016, 157, 30–54. [Google Scholar] [CrossRef]
- Kim, H.J.; Ahn, H.S.; Lee, J.Y.; Choi, S.S.; Cheong, Y.S.; Kwon, K.; Yoon, S.H.; Leem, J.G. Effects of applying nerve blocks to prevent postherpetic neuralgia in patients with acute herpes zoster: A systematic review and meta-analysis. Korean J. Pain 2017, 30, 3–17. [Google Scholar] [CrossRef]
- Kikuchi, A.; Kotani, N.; Sato, T.; Takamura, K.; Sakai, I.; Matsuki, A. Comparative therapeutic evaluation of intrathecal versus epidural methylprednisolone for long-term analgesia in patients with intractable postherpetic neuralgia. Reg. Anesth. Pain Med. 1999, 24, 287–293. [Google Scholar] [CrossRef]
- Kotani, N.; Kushikata, T.; Hashimoto, H.; Kimura, F.; Muraoka, M.; Yodono, M.; Asai, M.; Matsuki, A. Intrathecal methylprednisolone for intractable postherpetic neuralgia. N. Engl. J. Med. 2000, 343, 1514–1519. [Google Scholar] [CrossRef]
- Kim, E.D.; Lee, Y.I.; Park, H.J. Comparison of efficacy of continuous epidural block and pulsed radiofrequency to the dorsal root ganglion for management of pain persisting beyond the acute phase of herpes zoster. PLoS ONE 2017, 12, e0183559. [Google Scholar] [CrossRef] [PubMed]
- Chuang, C.M.; Lin, C.R.; Hsieh, Y.L. Nerve Block Efficacy and Safety for Acute Thoracic Herpes Zoster: A Systematic Review and Meta-analysis. Pain Physician 2025, 28, 83–96. [Google Scholar] [CrossRef] [PubMed]
- Deng, A.; Chen, Z.; Lin, S.; Zhou, Y.; He, L. Ultrasound-guided thoracic paravertebral block using paraventricular oblique sagittal (POS) approach for the treatment of acute herpes zoster: A two-blind randomized controlled trial. Pain Ther. 2023, 12, 797–809. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.-V.; Lin, C.-P.; Wu, W.-T.; Özçakar, L. Ultrasound-Guided Selective Cervical Root Injection for Postherpetic Neuralgia. Am. J. Phys. Med. Rehabil. 2017, 96, e189–e190. [Google Scholar] [CrossRef]
- Moon, Y.E.; Choi, J.H.; Park, H.J.; Park, J.H.; Kim, J.H. Ultrasound-Guided Nerve Block with Botulinum Toxin Type A for Intractable Neuropathic Pain. Toxins 2016, 8, 18. [Google Scholar] [CrossRef]
- Zheng, C.; Xie, Y.; Li, H.; Zhang, B.; Chen, S.; Han, W.; Liu, J.; Geng, Y.; Zhang, Y.; Wang, Z. Effectiveness and safety of stellate ganglion block with trioxygen autologous blood retransfusion therapy for facial postherpetic neuralgia in elderly patients. Sci. Rep. 2025, 15, 8025. [Google Scholar] [CrossRef]
- Wang, C.; Yuan, F.; Cai, L.; Lu, H.; Chen, G.; Zhou, J. Ultrasound-guided stellate ganglion block combined with extracorporeal shock wave therapy on postherpetic neuralgia. J. Healthc. Eng. 2022, 2022, 9808994. [Google Scholar] [CrossRef]
- Ma, K.; Fan, Y.; Jin, Y.; Huang, X.; Liu, X.; Cheng, Z.; Huang, C.; Wang, Y. Efficacy of pulsed radiofrequency in the treatment of thoracic postherpetic neuralgia from the angulus costae: A randomized, double-blinded, controlled trial. Pain Physician 2013, 16, 15–25. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, K.; Han, S.; Yu, L. PainVision® Apparatus for Assessment of Efficacy of Pulsed Radiofrequency Combined with Pharmacological Therapy in the Treatment of Postherpetic Neuralgia and Correlations with Measurements. BioMed Res. Int. 2017, 2017, 5670219. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Zhu, T.; Han, Y. Ultrasound-versus computed tomography-guided cervical dorsal root ganglia pulsed radiofrequencies via intervertebral foramen for the treatment of postherpetic neuralgia: A retrospective cohort study. Pain Physician 2023, 26, E171. [Google Scholar]
- Pi, Z.B.; Lin, H.; He, G.D.; Cai, Z.; Xu, X.Z. Randomized and controlled prospective trials of Ultrasound-guided spinal nerve posterior ramus pulsed radiofrequency treatment for lower back post-herpetic neuralgia. Clin. Ter. 2015, 166, e301–e305. [Google Scholar] [CrossRef]
- Lim, S.M.; Park, H.L.; Moon, H.Y.; Kang, K.H.; Kang, H.; Baek, C.H.; Jung, Y.H.; Kim, J.Y.; Koo, G.H.; Shin, H.Y. Ultrasound-guided infraorbital nerve pulsed radiofrequency treatment for intractable postherpetic neuralgia-a case report. Korean J. Pain 2013, 26, 84. [Google Scholar] [CrossRef]
- Li, Y.; Zeng, X.; Zhou, L. Ultrasound-guided peripheral nerve radiofrequency ablation for craniofacial postherpetic neuralgia: Efficacy and safety in a retrospective cohort. J. Clin. Neurosci. 2025, 138, 111408. [Google Scholar] [CrossRef]
- Li, F.; Gong, G.; Zhang, Y.; Ou, C. Efficacy and safety of ultrasound-guided pulsed radiofrequency in the treatment of the ophthalmic branch of postherpetic trigeminal neuralgia. Front. Neurol. 2024, 15, 1398696. [Google Scholar] [CrossRef]
- Lerman, I.R.; Chen, J.L.; Hiller, D.; Souzdalnitski, D.; Sheean, G.; Wallace, M.; Barba, D. Novel high-frequency peripheral nerve stimulator treatment of refractory postherpetic neuralgia: A brief technical note. Neuromodul. Technol. Neural Interface 2015, 18, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Kesikburun, S.; Yaşar, E.; Dede, İ.; Göktepe, S.; Tan, A.K. Ultrasound-guided steroid injection in the treatment of stump neuroma: Pilot study. J. Back Musculoskelet. Rehabil. 2014, 27, 275–279. [Google Scholar] [CrossRef]
- Hung, Y.-H.; Wu, C.-H.; Özçakar, L.; Wang, T.-G. Ultrasound-Guided Steroid Injections for Two Painful Neuromas in the Stump of a Below-Elbow Amputee. Am. J. Phys. Med. Rehabil. 2016, 95, e73–e74. [Google Scholar] [CrossRef] [PubMed]
- Pu, S.; Wu, J.; Han, Q.; Zhang, X.; Lv, Y.; Xu, Y.; Li, C.; Du, D. Ultrasonography-guided radiofrequency ablation for painful stump neuromas to relieve postamputation pain: A pilot study. J. Pain Res. 2020, 13, 3437–3445. [Google Scholar] [CrossRef]
- Guo, S.; Mansour, R.; Slater, D.H. Ultrasound-guided continuous radiofrequency ablation of painful residual limb neuroma in individuals with limb amputation—A retrospective case series. Can. Prosthet. Orthot. J. 2019, 2, 33061. [Google Scholar] [CrossRef]
- Culp, C.J.; Abdi, S. Current understanding of phantom pain and its treatment. Pain Physician 2022, 25, E941. [Google Scholar] [PubMed]
- Birbaumer, N.; Lutzenberger, W.; Montoya, P.; Larbig, W.; Unertl, K.; Töpfner, S.; Grodd, W.; Taub, E.; Flor, H. Effects of regional anesthesia on phantom limb pain are mirrored in changes in cortical reorganization. J. Neurosci. 1997, 17, 5503–5508. [Google Scholar] [CrossRef]
- Ilfeld, B.M.; Smith, C.R.; Turan, A.; Mariano, E.R.; Miller, M.E.; Fisher, R.L.; Trescot, A.M.; Cohen, S.P.; Eisenach, J.C.; Sessler, D.I.; et al. Ultrasound-guided Percutaneous Cryoneurolysis to Treat Chronic Postamputation Phantom Limb Pain: A Multicenter Randomized Controlled Trial. Anesthesiology 2023, 138, 82–97. [Google Scholar] [CrossRef]
- Gilmore, C.; Ilfeld, B.; Rosenow, J.; Li, S.; Desai, M.; Hunter, C.; Rauck, R.; Kapural, L.; Nader, A.; Mak, J.; et al. Percutaneous peripheral nerve stimulation for the treatment of chronic neuropathic postamputation pain: A multicenter, randomized, placebo-controlled trial. Reg. Anesth. Pain Med. 2019, 44, 637–645. [Google Scholar] [CrossRef]
- Chang, P.J.; Asher, A.; Smith, S.R. A targeted approach to post-mastectomy pain and persistent pain following breast cancer treatment. Cancers 2021, 13, 5191. [Google Scholar] [CrossRef] [PubMed]
- Rahimzadeh, P.; Imani, F.; Faiz, S.H.R.; Boroujeni, B.V. Impact of the ultrasound-guided serratus anterior plane block on post-mastectomy pain: A randomised clinical study. Turk. J. Anaesthesiol. Reanim. 2018, 46, 388. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Khanna, A.; Stubblefield, M.D.; Yue, G.H.; Allexandre, D. Ultrasound-guided superficial serratus plane block for persistent post-mastectomy pain: Four case reports. Support. Care Cancer 2022, 30, 2787–2792. [Google Scholar] [CrossRef]
- Abdel Dayem, O.T.; Saeid, M.M.; Ismail, O.M.; El Badrawy, A.M.; Abdel Ghaffar, N.A. Ultrasound guided stellate ganglion block in postmastectomy pain syndrome: A comparison of ketamine versus morphine as adjuvant to bupivacaine. J. Anesthesiol. 2014, 2014, 792569. [Google Scholar] [CrossRef]
- Feldman, E.L.; Callaghan, B.C.; Pop-Busui, R.; Zochodne, D.W.; Wright, D.E.; Bennett, D.L.; Bril, V.; Russell, J.W.; Viswanathan, V. Diabetic neuropathy. Nat. Rev. Dis. Primers 2019, 5, 41. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xu, W.; Wang, Q.; Yang, P.; Kan, Y.; Huang, C.; Lin, F. Efficacy and Safety of Ultrasound-Guided Pulsed Radiofrequency Therapy of Stellate Ganglion on Refractory Painful Diabetic Peripheral Neuropathy. J. Pain Res. 2024, 17, 4521–4531. [Google Scholar] [CrossRef]
- Hu, H.Q.; Huang, H.; Huang, J.; Leng, J.C.; Li, M.; Tang, C.; Li, Y.; Wu, S. Case Report: Successful Outcome for Refractory Diabetic Peripheral Neuropathy in Patients With Ultrasound-Guided Injection Treatment. Front. Endocrinol. 2021, 12, 735132. [Google Scholar] [CrossRef]
- Wang, J.-C.; Chiou, H.-J.; Lu, J.-H.; Hsu, Y.-C.; Chan, R.-C.; Yang, T.-F. Ultrasound-guided perineural steroid injection to treat intractable pain due to sciatic nerve injury. Can. J. Anesth. 2013, 60, 902. [Google Scholar] [CrossRef]
- Kim, J.H.; Shin, S.H.; Lee, Y.R.; Lee, H.S.; Chon, J.Y.; Sung, C.H.; Hong, S.J.; Lee, J.Y.; Moon, H.S. Ultrasound-guided peripheral nerve stimulation for neuropathic pain after brachial plexus injury: Two case reports. J. Anesth. 2017, 31, 453–457. [Google Scholar] [CrossRef]
- Narouze, S.N.; Zakari, A.; Vydyanathan, A. Ultrasound-guided placement of a permanent percutaneous femoral nerve stimulator leads for the treatment of intractable femoral neuropathy. Pain Physician 2009, 12, E305. [Google Scholar] [CrossRef]
- Lin, C.-P.; Chang, K.-V.; Wu, W.-T.; Özçakar, L. Ultrasound-Guided Peripheral Nerve Stimulation for Knee Pain: A Mini-Review of the Neuroanatomy and the Evidence from Clinical Studies. Pain Med. 2020, 21, S56–S63. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-H.; Huang, Y.-K.; Jaw, F.-S. Ultrasound-guided perineural vitamin B12 injection for peripheral neuropathy. J. Med. Ultrasound 2015, 23, 104–106. [Google Scholar] [CrossRef]
- Thoomes, E.J.; Scholten-Peeters, W.; Koes, B.; Falla, D.; Verhagen, A.P. The effectiveness of conservative treatment for patients with cervical radiculopathy: A systematic review. Clin. J. Pain 2013, 29, 1073–1086. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Shi, H.; Zhou, J.; Xu, Y.; Pu, S.; Lv, Y.; Wu, J.; Cheng, Y.; Du, D. The effectiveness of ultrasound-guided cervical transforaminal epidural steroid injections in cervical radiculopathy: A prospective pilot study. J. Pain Res. 2018, 12, 171–177. [Google Scholar] [CrossRef]
- Manchikanti, L.; Knezevic, N.N.; Boswell, M.V.; Kaye, A.D.; Hirsch, J.A. Epidural injections for lumbar radiculopathy and spinal stenosis: A comparative systematic review and meta-analysis. Pain Physician 2016, 19, E365. [Google Scholar] [CrossRef]
- Chou, R.; Hashimoto, R.; Friedly, J.; Fu, R.; Bougatsos, C.; Dana, T.; Sullivan, S.D.; Jarvik, J. Epidural corticosteroid injections for radiculopathy and spinal stenosis: A systematic review and meta-analysis. Ann. Intern. Med. 2015, 163, 373–381. [Google Scholar] [CrossRef]
- Ahmed, M.; Ahmad, A.; Arshad, M.; Naseer, H.; Zamarud, A. Ultrasound-guided versus conventional fluoroscopy-guided epidural injection for radiculopathy. A meta-analysis of randomized controlled trials. World Neurosurg. 2023, 180, 203–212.e204. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Zhang, D.; Zhao, Y.; Song, Y.; He, L.; Zhang, J. An open-label non-inferiority randomized trail comparing the effectiveness and safety of ultrasound-guided selective cervical nerve root block and fluoroscopy-guided cervical transforaminal epidural block for cervical radiculopathy. Ann. Med. 2022, 54, 2669–2679. [Google Scholar] [CrossRef]
- Joo, H.J.; Choi, S.; Kim, B.H.; Kim, M.-S.; Shim, G.Y.; Chung, S.J.; Chon, J.; Yoo, M.C.; Soh, Y. Therapeutic Efficacy of Ultrasound-Guided Selective Nerve Block on Chronic Cervical Radiculopathy. Medicina 2024, 60, 1002. [Google Scholar] [CrossRef]
- Lee, S.H.; Choi, H.H.; Roh, E.Y.; Chang, M.C. Effectiveness of ultrasound-guided pulsed radiofrequency treatment in patients with refractory chronic cervical radicular pain. Pain Physician 2020, 23, E265. [Google Scholar] [CrossRef]
- Wang, B.; Sun, Y.; Zhang, J.; Meng, H.; Zhang, H.; Shan, L. Ultrasound-guided versus fluoroscopy-guided lumbar selective nerve root block: A retrospective comparative study. Sci. Rep. 2024, 14, 3235. [Google Scholar] [CrossRef]
- Kim, Y.H.; Park, H.J.; Moon, D.E. Ultrasound-guided pararadicular injection in the lumbar spine: A comparative study of the paramedian sagittal and paramedian sagittal oblique approaches. Pain Pract. 2015, 15, 693–700. [Google Scholar] [CrossRef]
- Ortega-Romero, A.; Domingo-Rufes, T.; del-Olmo, C.; Ismael, M.-F.; Mayoral, V. Ultrasound-guided interventional procedures for lumbar pain. Tech. Reg. Anesth. Pain Manag. 2013, 17, 96–106. [Google Scholar] [CrossRef]
- Park, Y.; Lee, J.-H.; Park, K.D.; Ahn, J.K.; Park, J.; Jee, H. Ultrasound-guided vs. fluoroscopy-guided caudal epidural steroid injection for the treatment of unilateral lower lumbar radicular pain: A prospective, randomized, single-blind clinical study. Am. J. Phys. Med. Rehabil. 2013, 92, 575–586. [Google Scholar] [CrossRef]
- Hazra, A.K.; Bhattacharya, D.; Mukherjee, S.; Ghosh, S.; Mitra, M.; Mandal, M. Ultrasound versus fluoroscopy-guided caudal epidural steroid injection for the treatment of chronic low back pain with radiculopathy: A randomised, controlled clinical trial. Indian J. Anaesth. 2016, 60, 388–392. [Google Scholar] [CrossRef]
- Elashmawy, M.A.; Shaat, R.M.; Abdelkhalek, A.; El Boghdady, E. Caudal epidural steroid injection ultrasound-guided versus fluoroscopy-guided in treatment of refractory lumbar disc prolapse with radiculopathy. Egypt. J. Radiol. Nucl. Med. 2020, 51, 259. [Google Scholar] [CrossRef]
- Tan, T.; Barry, P.; Reken, S.; Baker, M. Pharmacological management of neuropathic pain in non-specialist settings: Summary of NICE guidance. BMJ 2010, 340, c1079. [Google Scholar] [CrossRef] [PubMed]
- Finnerup, N.B.; Attal, N.; Haroutounian, S.; McNicol, E.; Baron, R.; Dworkin, R.H.; Gilron, I.; Haanpää, M.; Hansson, P.; Jensen, T.S.; et al. Pharmacotherapy for neuropathic pain in adults: A systematic review and meta-analysis. Lancet Neurol. 2015, 14, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Luo, L.; Hu, Y.; Fang, K.; Liu, J. Clinical practice guidelines for the management of neuropathic pain: A systematic review. BMC Anesth. 2016, 16, 12. [Google Scholar] [CrossRef] [PubMed]
- Moisset, X.; Bouhassira, D.; Avez Couturier, J.; Alchaar, H.; Conradi, S.; Delmotte, M.H.; Lanteri-Minet, M.; Lefaucheur, J.P.; Mick, G.; Piano, V.; et al. Pharmacological and non-pharmacological treatments for neuropathic pain: Systematic review and French recommendations. Rev. Neurol. 2020, 176, 325–352. [Google Scholar] [CrossRef] [PubMed]
- Dworkin, R.H.; O’Connor, A.B.; Kent, J.; Mackey, S.C.; Raja, S.N.; Stacey, B.R.; Levy, R.M.; Backonja, M.; Baron, R.; Harke, H.; et al. Interventional management of neuropathic pain: NeuPSIG recommendations. Pain 2013, 154, 2249–2261. [Google Scholar] [CrossRef]
- Wang, X.; Martin, G.; Sadeghirad, B.; Chang, Y.; Florez, I.D.; Couban, R.J.; Mehrabi, F.; Crandon, H.N.; Esfahani, M.A.; Sivananthan, L.; et al. Common interventional procedures for chronic non-cancer spine pain: A systematic review and network meta-analysis of randomised trials. BMJ 2025, 388, e079971. [Google Scholar] [CrossRef]
- Smith, C.C.; McCormick, Z.L.; Mattie, R.; MacVicar, J.; Duszynski, B.; Stojanovic, M.P. The Effectiveness of Lumbar Transforaminal Injection of Steroid for the Treatment of Radicular Pain: A Comprehensive Review of the Published Data. Pain Med. 2019, 21, 472–487. [Google Scholar] [CrossRef] [PubMed]
- Karasek, M.; Bogduk, N. Temporary neurologic deficit after cervical transforaminal injection of local anesthetic. Pain Med. 2004, 5, 202–205. [Google Scholar] [CrossRef]
- Cucinotta, F.A. Radiation risk acceptability and limitations. In Space Radiation Program Element; NASA Publications; NASA Johnson Space Center: Houston, TX, USA, 2010. [Google Scholar]
- Connolly, T.M.; Nadav, D.; Gungor, S. Ultrasound-guided caudal epidural steroid injection for successful treatment of radiculopathy during pregnancy. Pain Manag. 2020, 10, 67–71. [Google Scholar] [CrossRef]
- Wallace, M.A.; Fukui, M.B.; Williams, R.L.; Ku, A.; Baghai, P. Complications of Cervical Selective Nerve Root Blocks Performed with Fluoroscopic Guidance. Am. J. Roentgenol. 2007, 188, 1218–1221. [Google Scholar] [CrossRef]
- Tiso, R.L.; Cutler, T.; Catania, J.A.; Whalen, K. Adverse central nervous system sequelae after selective transforaminal block: The role of corticosteroids. Spine J. 2004, 4, 468–474. [Google Scholar] [CrossRef]
- Wang, D. Image guidance technologies for interventional pain procedures: Ultrasound, fluoroscopy, and CT. Curr. Pain Headache Rep. 2018, 22, 6. [Google Scholar] [CrossRef]
- Viderman, D.; Aubakirova, M.; Aryngazin, A.; Yessimova, D.; Kaldybayev, D.; Tankacheyev, R.; Abdildin, Y.G. Ultrasound-Guided vs. Fluoroscopy-Guided Interventions for Back Pain Management: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Diagnostics 2023, 13, 3474. [Google Scholar] [CrossRef] [PubMed]
- Kimura, R.; Yamamoto, N.; Watanabe, J.; Ono, Y.; Hongo, M.; Miyakoshi, N. Comparative efficacy of ultrasound guidance and fluoroscopy or computed tomography guidance in spinal nerve injections: A systematic review and meta-analysis. Eur. Spine J. 2023, 32, 4101–4110. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.Y.; Park, Y.; Song, J.H.; Ahn, J.; Cho, K.H.; Kim, S. Combined ultrasound and fluoroscopy versus ultrasound versus fluoroscopy-guided caudal epidural steroid injection for the treatment of unilateral lower lumbar radicular pain: A retrospective comparative study. Medicina 2024, 60, 809. [Google Scholar] [CrossRef] [PubMed]
- Martiszus, B.; Hua, S.; Christiansen, S.; Ramsey, K.; Zusmer, E. A Novel Piriformis Injection Technique Utilizing Combined Fluoroscopy and Ultrasound–A Pilot Study. Pain Physician 2022, 25, E365. [Google Scholar]
- Finnerup, N.B.; Haroutounian, S.; Baron, R.; Dworkin, R.H.; Gilron, I.; Haanpaa, M.; Jensen, T.S.; Kamerman, P.R.; McNicol, E.; Moore, A.; et al. Neuropathic pain clinical trials: Factors associated with decreases in estimated drug efficacy. Pain 2018, 159, 2339–2346. [Google Scholar] [CrossRef]
- Lagueny, A.; Burbaud, P. Mechanism of action, clinical indication and results of treatment of botulinum toxin. Neurophysiol. Clin. 1996, 26, 216–226. [Google Scholar] [CrossRef]
- Ranoux, D.; Attal, N.; Morain, F.; Bouhassira, D. Botulinum toxin type A induces direct analgesic effects in chronic neuropathic pain. Ann. Neurol. 2008, 64, 274–283. [Google Scholar] [CrossRef]
- Hong, B.; Yao, L.; Ni, L.; Wang, L.; Hu, X. Antinociceptive effect of botulinum toxin A involves alterations in AMPA receptor expression and glutamate release in spinal dorsal horn neurons. Neuroscience 2017, 357, 197–207. [Google Scholar] [CrossRef]
- Buesing, S.; Costa, M.; Schilling, J.M.; Moeller-Bertram, T. Vitamin B12 as a treatment for pain. Pain Physician 2019, 22, E45. [Google Scholar] [CrossRef]
- Julian, T.; Syeed, R.; Glascow, N.; Angelopoulou, E.; Zis, P. B12 as a Treatment for Peripheral Neuropathic Pain: A Systematic Review. Nutrients 2020, 12, 2221. [Google Scholar] [CrossRef] [PubMed]
- Vallejo, R.; Tilley, D.M.; Williams, J.; Labak, S.; Aliaga, L.; Benyamin, R.M. Pulsed radiofrequency modulates pain regulatory gene expression along the nociceptive pathway. Pain Physician 2013, 16, E601–E613. [Google Scholar] [CrossRef]
- Hagiwara, S.; Iwasaka, H.; Takeshima, N.; Noguchi, T. Mechanisms of analgesic action of pulsed radiofrequency on adjuvant-induced pain in the rat: Roles of descending adrenergic and serotonergic systems. Eur. J. Pain 2009, 13, 249–252. [Google Scholar] [CrossRef]
- Cho, I.T.; Cho, Y.W.; Kwak, S.G.; Chang, M.C. Comparison between ultrasound-guided interfascial pulsed radiofrequency and ultrasound-guided interfascial block with local anesthetic in myofascial pain syndrome of trapezius muscle. Medicine 2017, 96, e6019. [Google Scholar] [CrossRef] [PubMed]
- Sir, E.; Eksert, S. Comparison of block and pulsed radiofrequency of the ganglion impar in coccygodynia. Turk. J. Med. Sci. 2019, 49, 1555–1559. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Fan, Z.; Cheng, F.; Li, Y.; Huo, X.; Cui, J. The efficacy of an ultrasound-guided improved puncture path technique of nerve block/pulsed radiofrequency for pudendal neuralgia: A retrospective study. Brain Sci. 2022, 12, 510. [Google Scholar] [CrossRef]
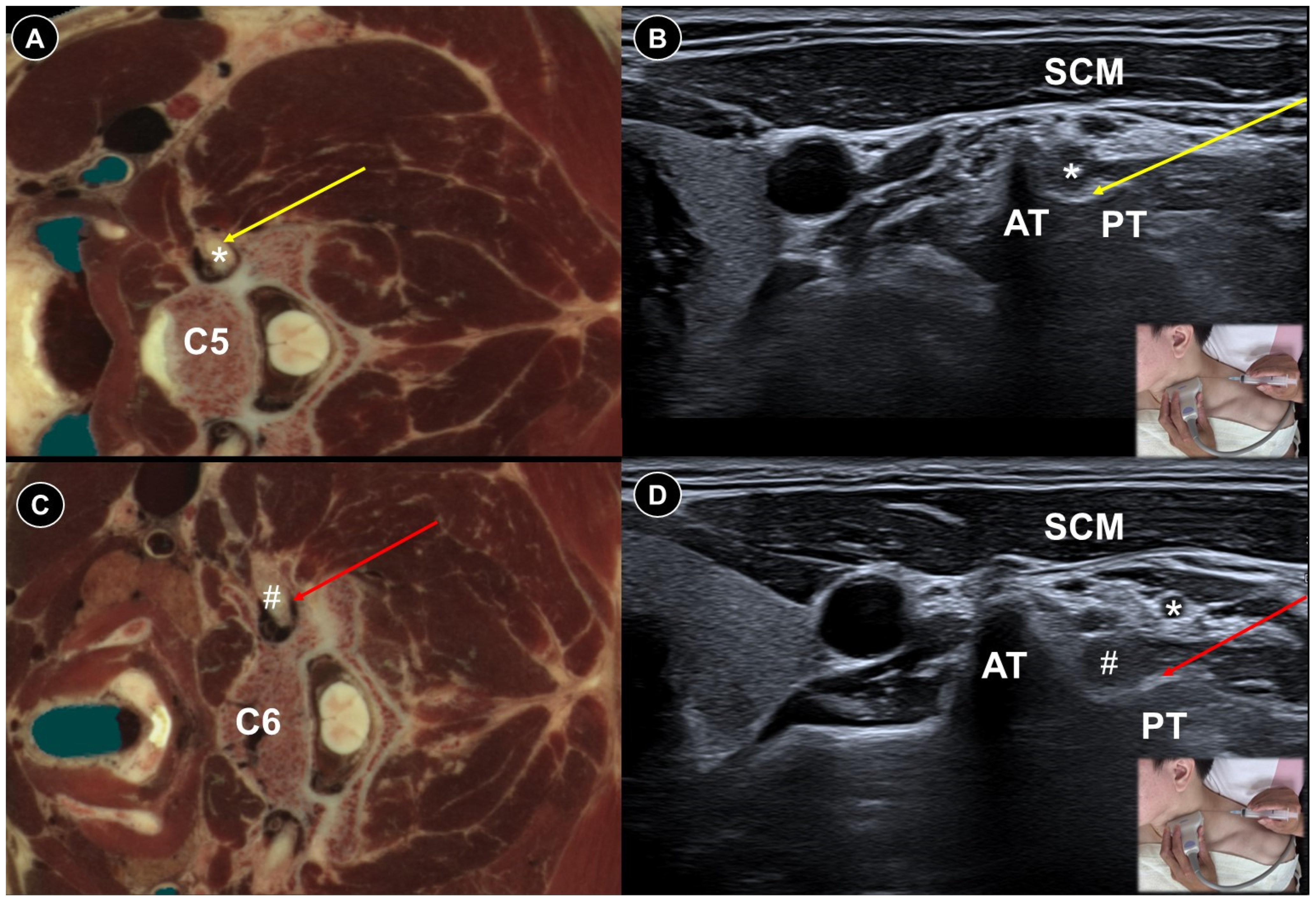
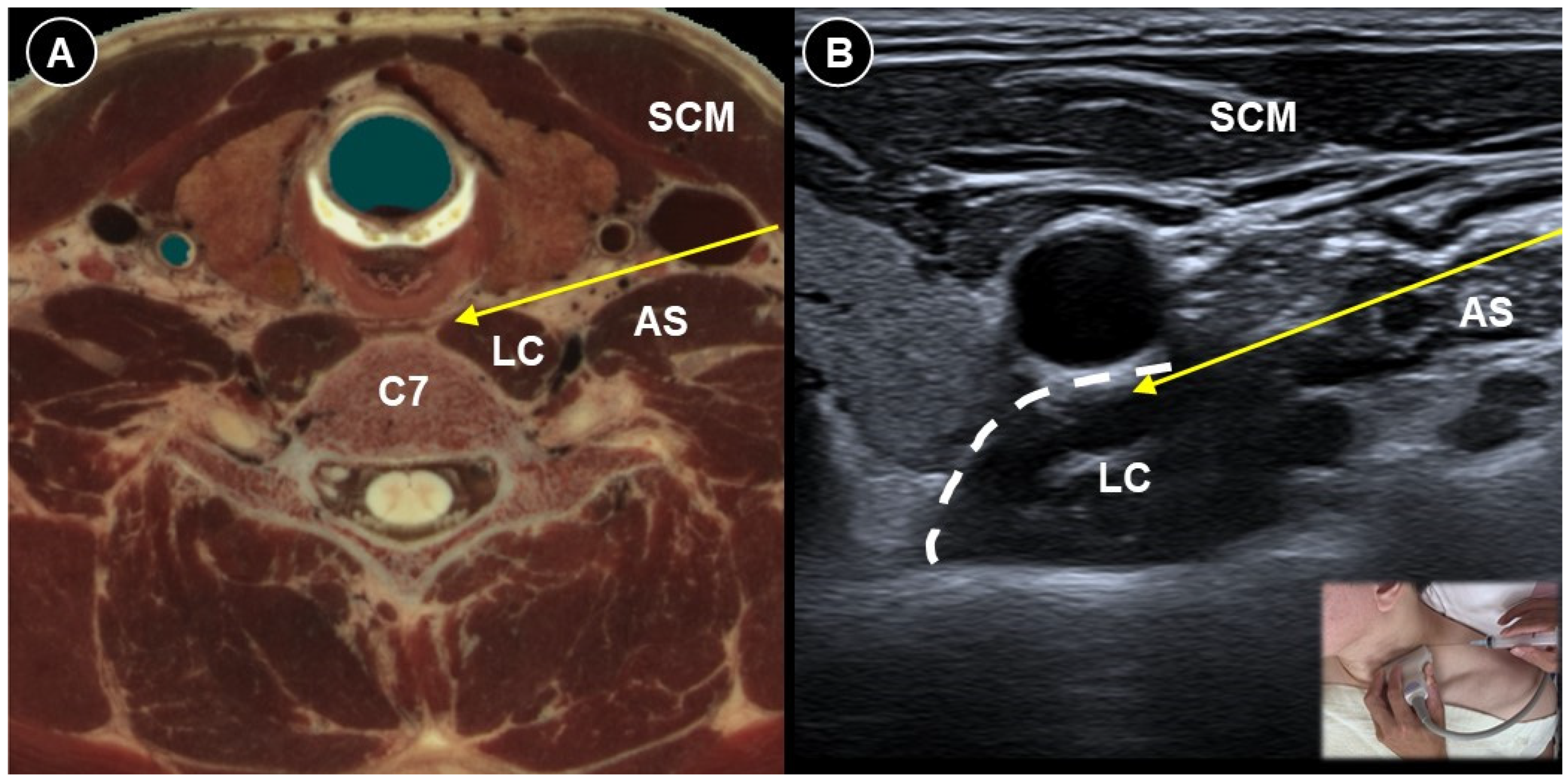
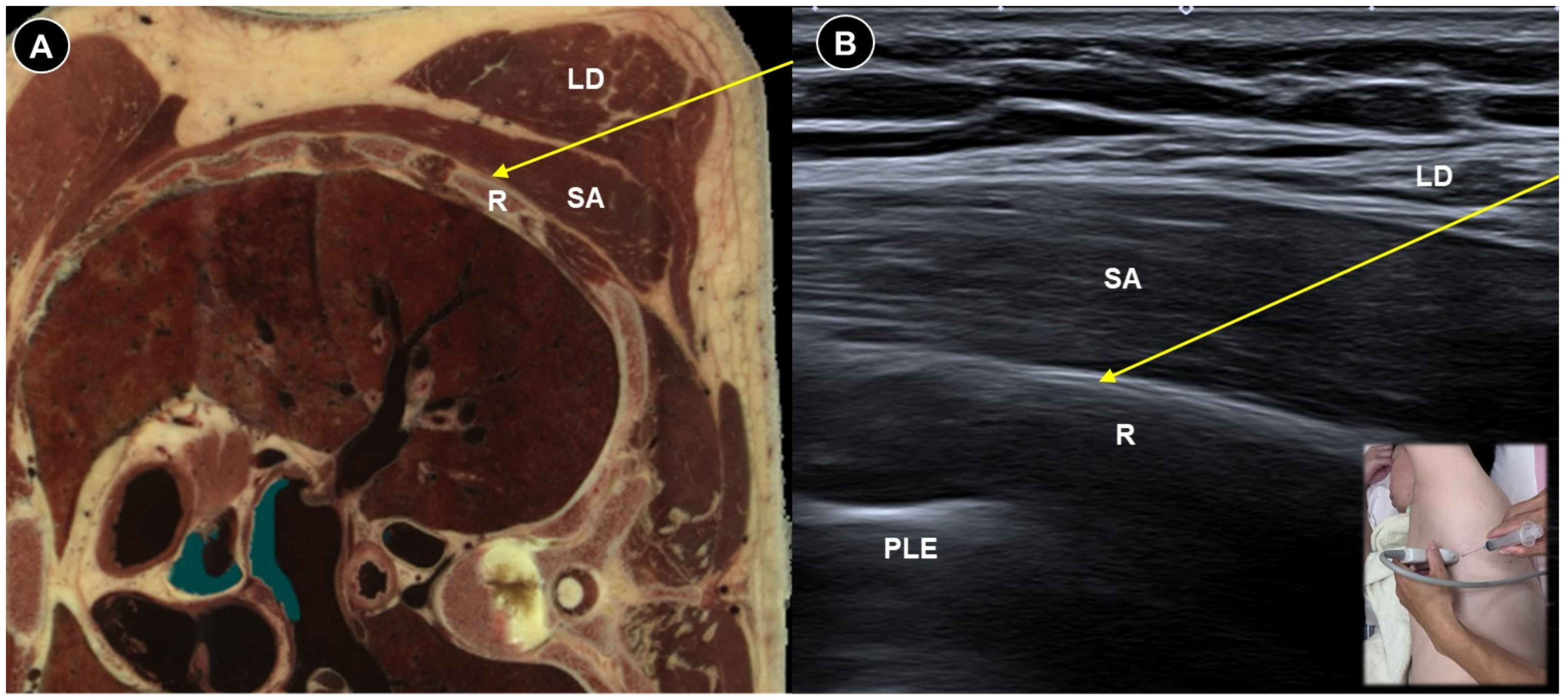
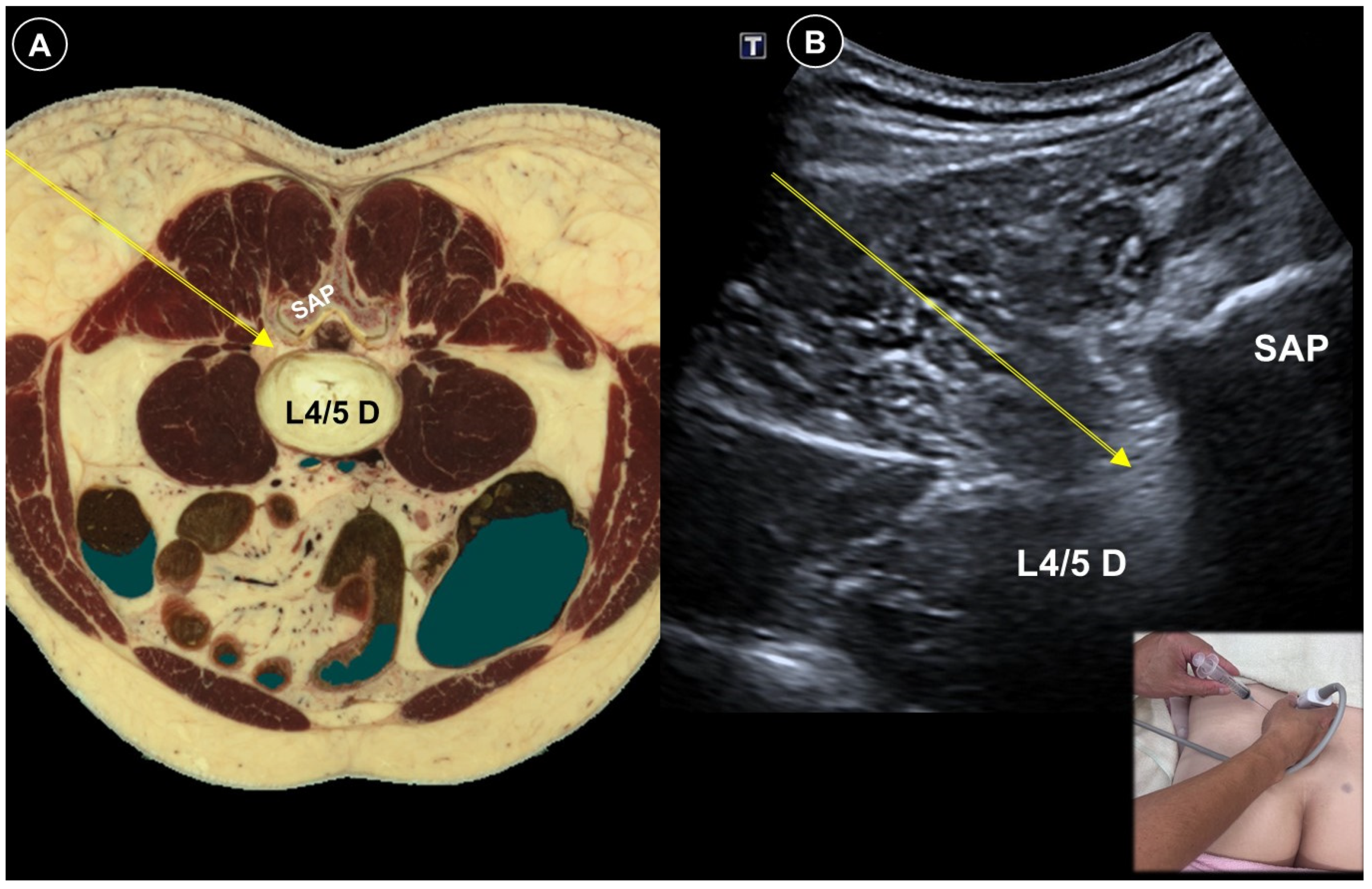

| Pain Characteristic | Cause | Patient Positioning | Ultrasound Probe and Technique | Effectiveness of Ultrasound-Guided Procedures | Additional Notes | |
|---|---|---|---|---|---|---|
| Trigeminal neuralgia | Paroxysmal facial pain in one or more divisions of the trigeminal nerve | Neurovascular compression at root entry zone | Head neutral | Linear probe, in-plane
| Sustained pain relief by nerve block (case study) | Use Doppler imaging to avoid vascular puncture |
| Lateral, with affected side up | Curvilinear probe, out-of-plane
| |||||
| Acute herpes pain | Burning, stabbing, or itching pain in the affected dermatomal distribution | Reactivation of VZV leading to viral nerve damage and inflammation | Cervical: supine, head turned to contralateral side Thoracic: prone | Cervical:
| Reduce pain and lower postherpetic neuralgia incidence by TPVB/ESPB (meta-analysis), PRF/SNRB/SGB (RCT) |
|
| Post-herpetic neuralgia | Persistent pain after rash resolution | Central and peripheral sensitization | Effective pain control by PRF (RCT), TPVB/SNRB/brachial plexus block/SGB/PNS (case study) | |||
| Post-amputation pain | Stump pain and phantom limb pain | Multifactorial, including neuromas, nerve damage, sensitization |
| Significant pain reduction by PNS (RCT), nerve block/PRF (case study) | Avoid direct injection into the nerve | |
| Painful polyneuropathy | Numbness, tingling sensations, dysesthesia, weakness, balance impairment | Metabolic, infections, connective tissue disorders, hereditary, toxins, nerve injuries, etc. |
|
| Ultrasound facilitates targeting small distal nerves | |
| Painful radiculopathy | Pain, numbness, clumsiness and even weakness in the distribution of the affected nerve root | Mechanical compression and inflammatory irritation of the nerve root, often due to disc herniation or spondylosis | Cervical: supine, head turned to contralateral side Lumbar: prone | Cervical: linear, in-plane
| Improvement of pain by ESI (meta-analysis), SNRB/caudal block (RCT) |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, T.-Y.; Chang, K.-V.; Wu, W.-T.; Mezian, K.; Ricci, V.; Özçakar, L. Ultrasound-Guided Interventions for Neuropathic Pain: A Narrative Pictorial Review. Life 2025, 15, 1404. https://doi.org/10.3390/life15091404
Lin T-Y, Chang K-V, Wu W-T, Mezian K, Ricci V, Özçakar L. Ultrasound-Guided Interventions for Neuropathic Pain: A Narrative Pictorial Review. Life. 2025; 15(9):1404. https://doi.org/10.3390/life15091404
Chicago/Turabian StyleLin, Ting-Yu, Ke-Vin Chang, Wei-Ting Wu, Kamal Mezian, Vincenzo Ricci, and Levent Özçakar. 2025. "Ultrasound-Guided Interventions for Neuropathic Pain: A Narrative Pictorial Review" Life 15, no. 9: 1404. https://doi.org/10.3390/life15091404
APA StyleLin, T.-Y., Chang, K.-V., Wu, W.-T., Mezian, K., Ricci, V., & Özçakar, L. (2025). Ultrasound-Guided Interventions for Neuropathic Pain: A Narrative Pictorial Review. Life, 15(9), 1404. https://doi.org/10.3390/life15091404









