Neuro-Genomic Mapping of Cardiac Neurons with Systemic Analysis Reveals Cognitive and Neurodevelopmental Impacts in Congenital Heart Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Single-Nucleus RNA-Seq Dataset
2.2. Single-Nucleus RNA-Seq Data Analysis
2.3. Gene Set Enrichment Analysis (GSEA)
2.4. Systemic Analysis
3. Results
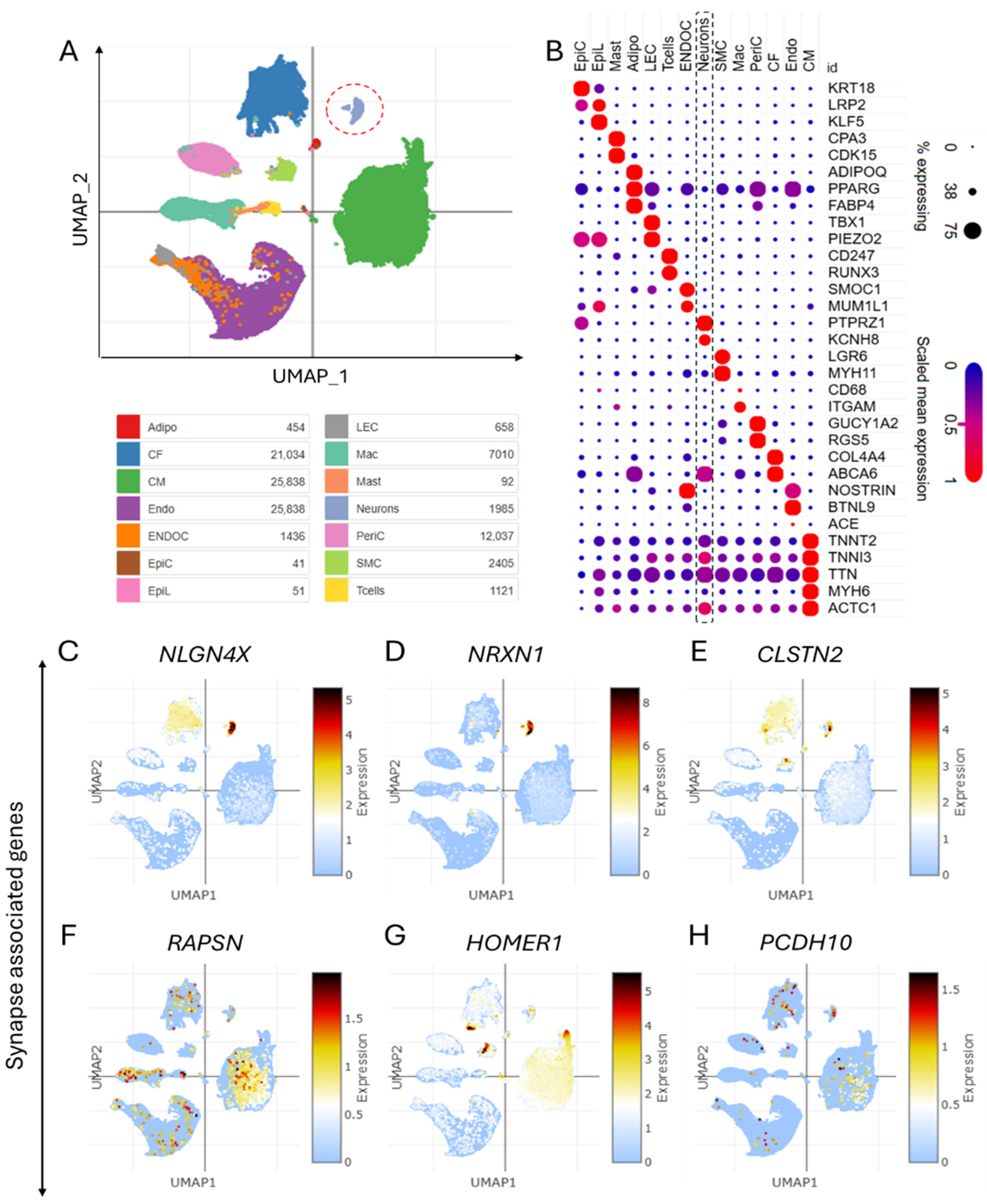
3.1. Single-Nucleus Analysis and Annotations Reveal Major Cardiac Cell Types in Congenital Heart Disease Patients
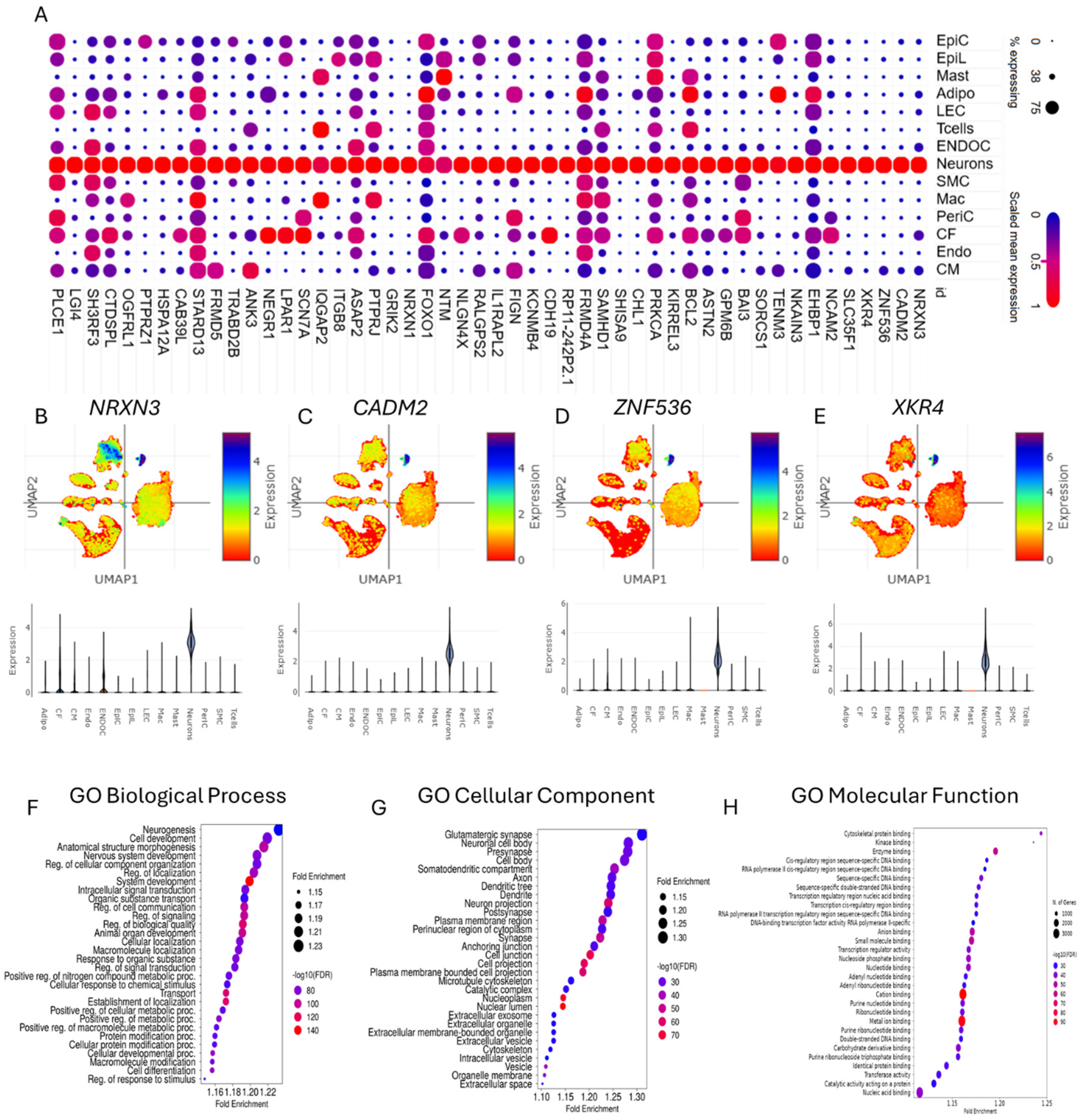
3.2. Gene Set Enrichment Analysis of Differentially Expressed Genes Enabled the Characterization of Cardiac Neurons in CHD
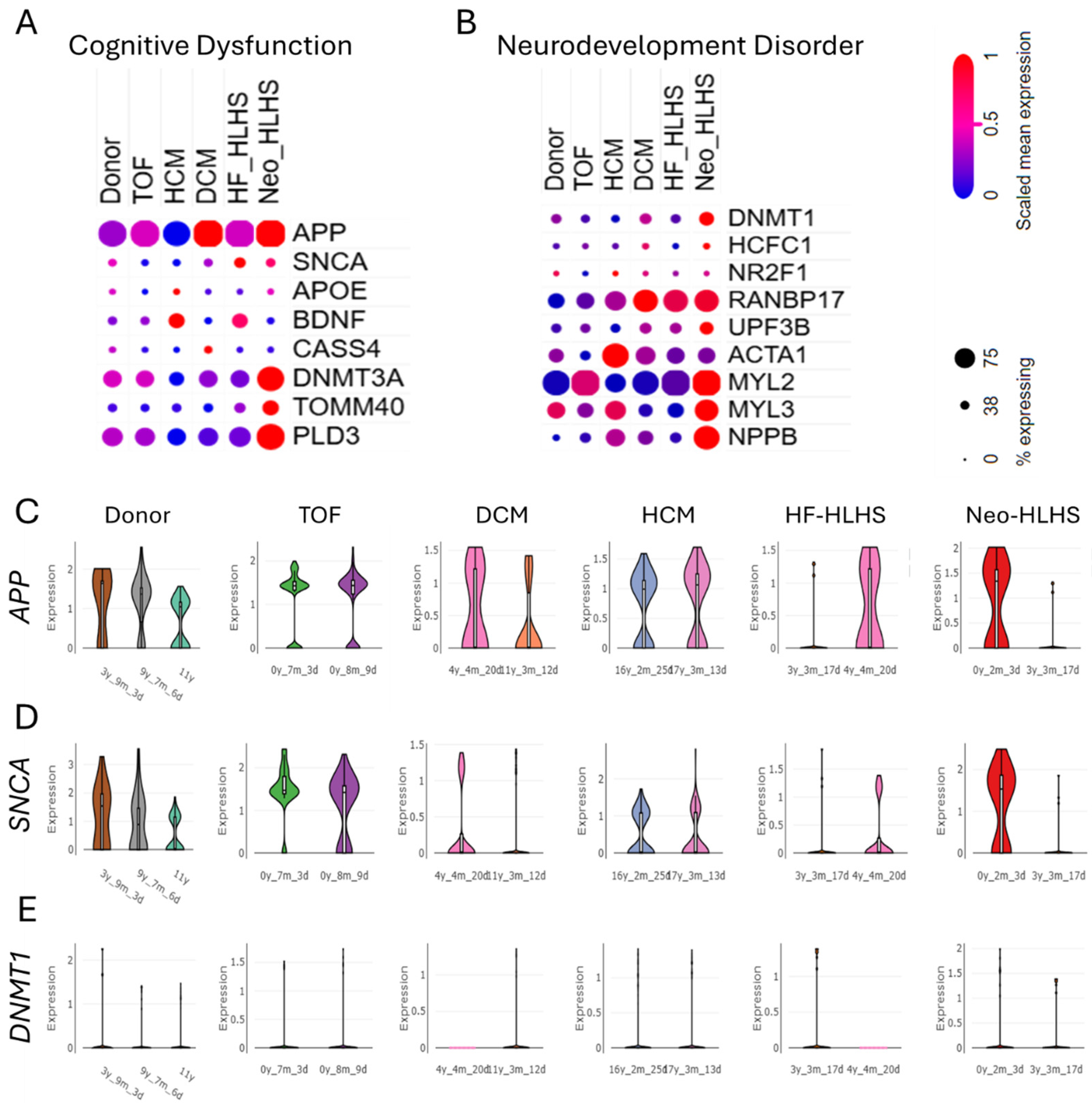
3.3. Genomic Landscape of CHD Features Upregulated Genes Linked to Cognitive Dysfunction and Neurodevelopment Disorder
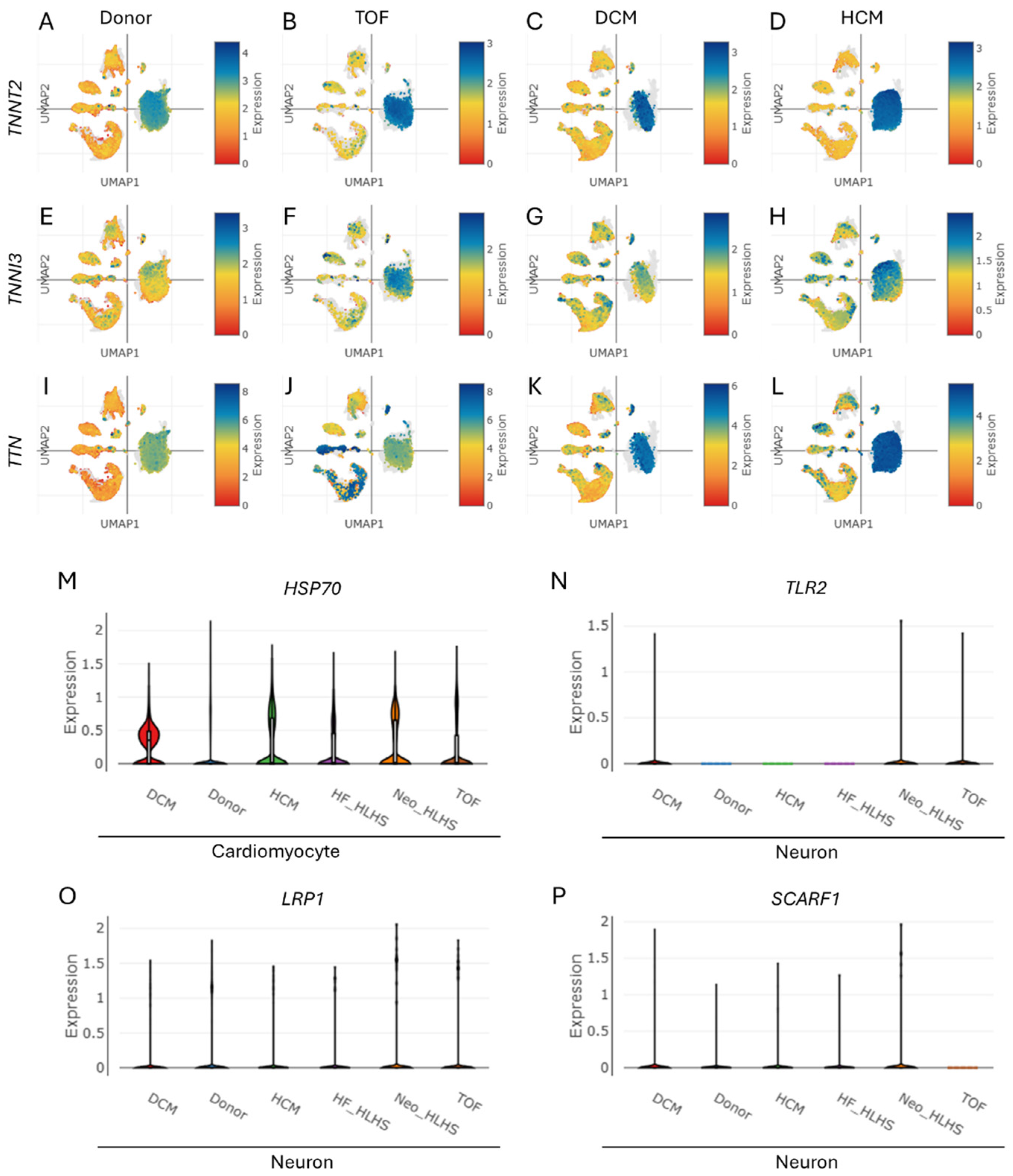
3.4. Cardiac Troponin Elevation in CHD Signals Heart Attack Risk, Revealing Cell-Specific Crosstalk Between Cardiomyocytes and Neurons
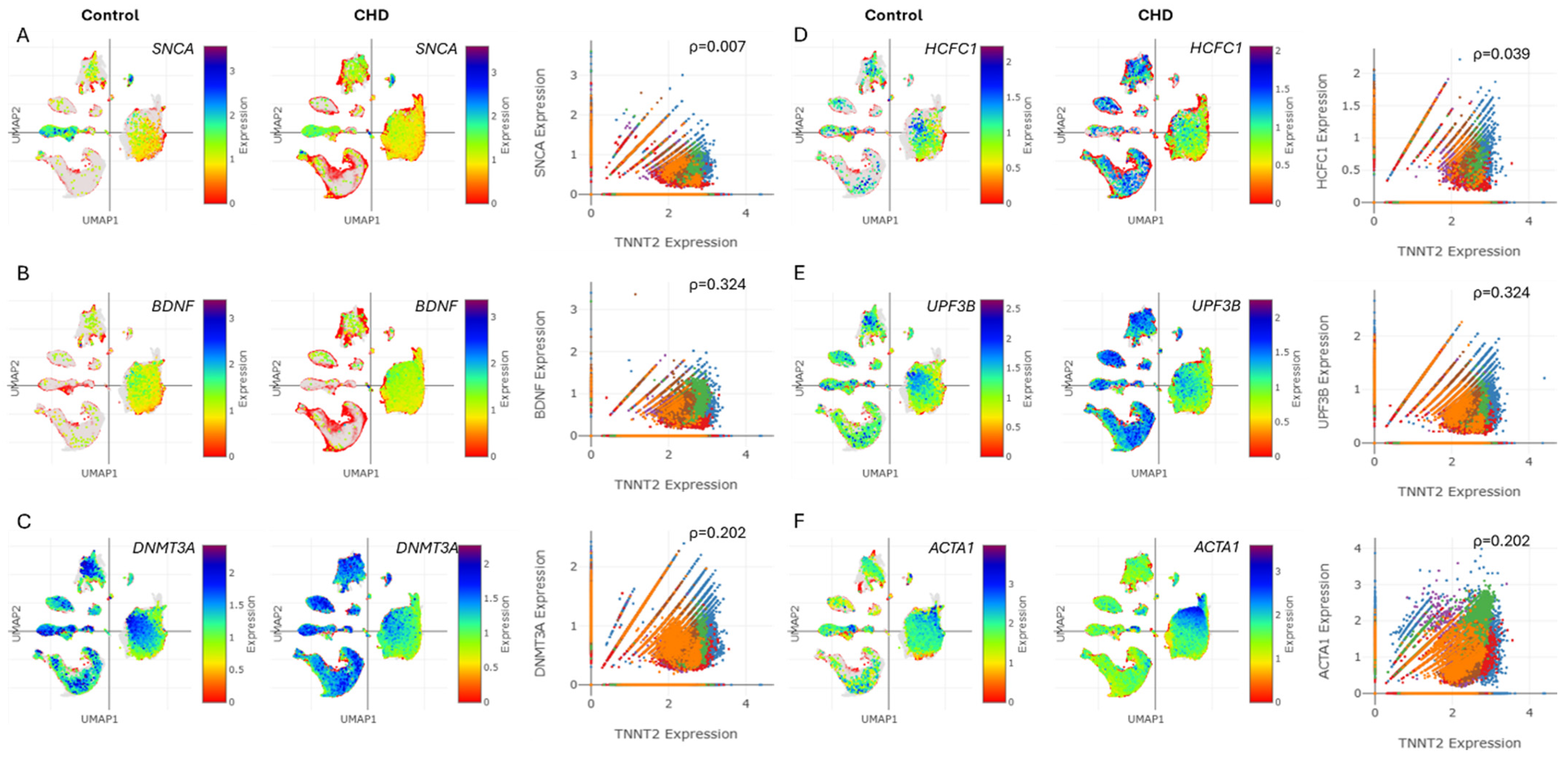
3.5. Troponin Levels in CHD Correlate with Markers of Cognitive Dysfunction and Neurodevelopment Disorder
3.6. Ontology of Differentially Expressed Genes in Cardiac Neurons of CHD Suggests Links Between Cardiovascular Disease Phenotypes and Neurodevelopmental Disorders
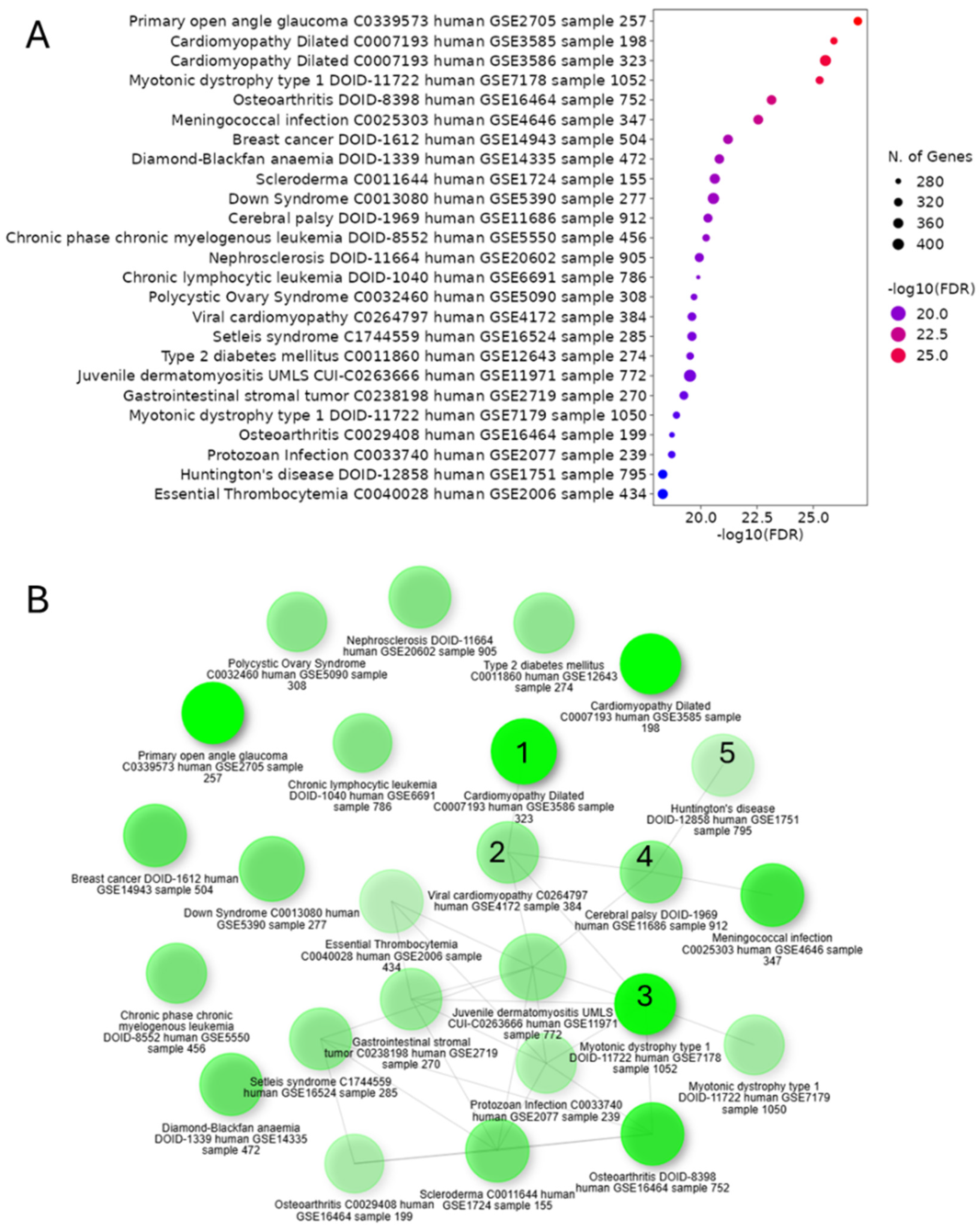
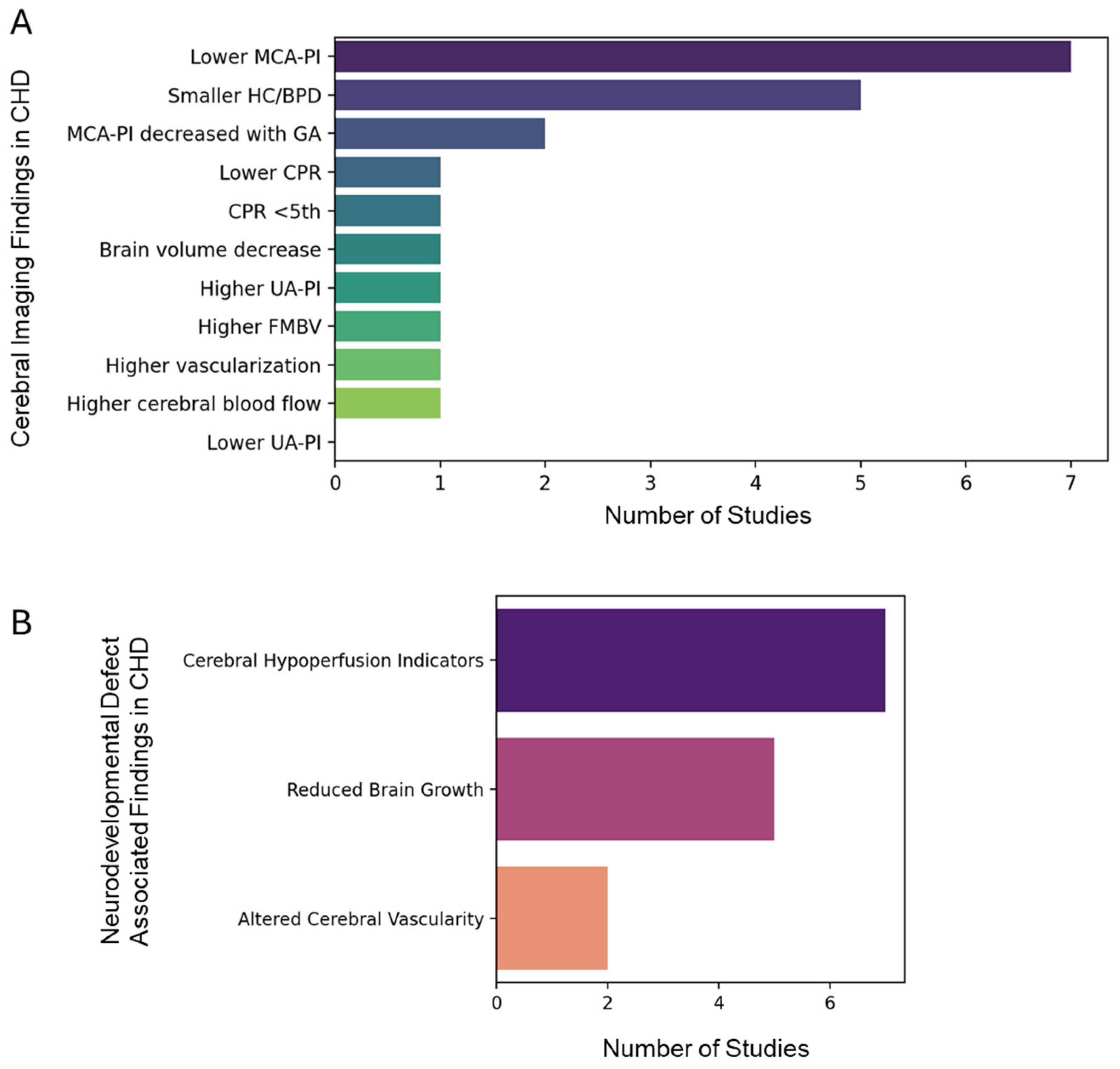
3.7. System Meta-Analysis Revealed Clinical Association Between CHD and Neurodevelopmental Disorders
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CHD | Congenital heart disease |
| HLHS | Hypoplastic left heart syndrome |
| TOF | Tetralogy of Fallot |
| DCM | Dilated cardiomyopathy |
| HCM | Hypertrophic cardiomyopathy |
| UMAP | Uniform Manifold Approximation and Projection |
| NRXN3 | Neurexin 3 |
| CADM2 | Cell adhesion molecule 2 |
| ZNF536 | Zinc finger protein 536 |
| APP | Amyloid precursor protein |
| SNCA | Synuclein alpha |
| BDNF | Brain-derived neurotrophic factor |
| DNMT1 | DNA methyltransferase 1 |
| HCFC1 | Host cell factor C1 |
| TLR2 | Toll-like receptor 2 |
| LRP1 | Low-density lipoprotein receptor-related protein 1 |
| NDDs | Neurodevelopmental disorders |
| DNMT3A | DNA methyltransferase 3 alpha |
| GEO | Gene Expression Omnibus |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
References
- Hill, M.C.; Kadow, Z.A.; Long, H.; Morikawa, Y.; Martin, T.J.; Birks, E.J.; Campbell, K.S.; Nerbonne, J.; Lavine, K.; Wadhwa, L.; et al. Integrated multi-omic characterization of congenital heart disease. Nature 2022, 608, 181–191. [Google Scholar] [CrossRef]
- Nattel, S.N.; Adrianzen, L.; Kessler, E.C.; Andelfinger, G.; Dehaes, M.; Côté-Corriveau, G.; Trelles, M.P. Congenital Heart Disease and Neurodevelopment: Clinical Manifestations, Genetics, Mechanisms, and Implications. Can. J. Cardiol. 2017, 33, 1543–1555. [Google Scholar] [CrossRef]
- Morton, P.D.; Ishibashi, N.; Jonas, R.A. Neurodevelopmental Abnormalities and Congenital Heart Disease. Circ. Res. 2017, 120, 960–977. [Google Scholar] [CrossRef] [PubMed]
- Soda, T.; Pasqua, T.; De Sarro, G.; Moccia, F. Cognitive Impairment and Synaptic Dysfunction in Cardiovascular Disorders: The New Frontiers of the Heart–Brain Axis. Biomedicines 2024, 12, 2387. [Google Scholar] [CrossRef]
- Churko, J.M.; Garg, P.; Treutlein, B.; Venkatasubramanian, M.; Wu, H.; Lee, J.; Wessells, Q.N.; Chen, S.-Y.; Chen, W.-Y.; Chetal, K.; et al. Defining human cardiac transcription factor hierarchies using integrated single-cell heterogeneity analysis. Nat. Commun. 2018, 9, 4906. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yin, K.; Chen, L.; Chen, W.; Li, W.; Zhang, T.; Sun, Y.; Yuan, M.; Wang, H.; Song, Y.; et al. Lineage-specific regulatory changes in hypertrophic cardiomyopathy unraveled by single-nucleus RNA-seq and spatial transcriptomics. Cell Discov. 2023, 9, 6. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, Y.; Liu, Y.; Gao, Y.; San, T.; Li, X.; Song, S.; Yan, B.; Zhao, Z. Advances in application of single-cell RNA sequencing in cardiovascular research. Front. Cardiovasc. Med. 2022, 9, 905151. [Google Scholar] [CrossRef]
- Sood, E.; Newburger, J.W.; Anixt, J.S.; Cassidy, A.R.; Jackson, J.L.; Jonas, R.A.; Lisanti, A.J.; Lopez, K.N.; Peyvandi, S.; Marino, B.S. Neurodevelopmental Outcomes for Individuals with Congenital Heart Disease: Updates in Neuroprotection, Risk-Stratification, Evaluation, and Management: A Scientific Statement From the American Heart Association. Circulation 2024, 149, e997–e1022. [Google Scholar] [CrossRef] [PubMed]
- Rollins, C.K.; Newburger, J.W. Neurodevelopmental Outcomes in Congenital Heart Disease. Circulation 2014, 130, e124–e126. [Google Scholar] [CrossRef]
- Rollins, C.K.; Newburger, J.W.; Roberts, A.E. Genetic contribution to neurodevelopmental outcomes in congenital heart disease: Are some patients predetermined to have developmental delay? Curr. Opin. Pediatr. 2017, 29, 529–533. [Google Scholar] [CrossRef]
- Tambi, R.; Zehra, B.; Nandkishore, S.; Sharafat, S.; Kader, F.; Nassir, N.; Mohamed, N.; Ahmed, A.; Abdel Hameid, R.; Alasrawi, S.; et al. Single-cell reconstruction and mutation enrichment analysis identifies dysregulated cardiomyocyte and endothelial cells in congenital heart disease. Physiol. Genom. 2023, 55, 634–646. [Google Scholar] [CrossRef] [PubMed]
- Samad, T.; Wu, S.M. Single cell RNA sequencing approaches to cardiac development and congenital heart disease. Semin. Cell Dev. Biol. 2021, 118, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Ameen, M.; Sundaram, L.; Shen, M.; Banerjee, A.; Kundu, S.; Nair, S.; Shcherbina, A.; Gu, M.; Wilson, K.D.; Varadarajan, A.; et al. Integrative single-cell analysis of cardiogenesis identifies developmental trajectories and non-coding mutations in congenital heart disease. Cell 2022, 185, 4937–4953.e23. [Google Scholar] [CrossRef]
- Jensen, M.; Zeller, T.; Twerenbold, R.; Thomalla, G. Circulating cardiac biomarkers, structural brain changes, and dementia: Emerging insights and perspectives. Alzheimer’s Dement. 2023, 19, 1529–1548. [Google Scholar] [CrossRef]
- Schneider, A.L.C.; Rawlings, A.M.; Sharrett, A.R.; Alonso, A.; Mosley, T.H.; Hoogeveen, R.C.; Ballantyne, C.M.; Gottesman, R.F.; Selvin, E. High-sensitivity cardiac troponin T and cognitive function and dementia risk: The atherosclerosis risk in communities study. Eur. Hear. J. 2014, 35, 1817–1824. [Google Scholar] [CrossRef]
- Ruiz, A.; Cruz-Lemini, M.; Masoller, N.; Sanz-Cortés, M.; Ferrer, Q.; Ribera, I.; Martínez, J.M.; Crispi, F.; Arévalo, S.; Gómez, O.; et al. Longitudinal changes in fetal biometry and cerebroplacental hemodynamics in fetuses with congenital heart disease. Ultrasound Obstet. Gynecol. 2017, 49, 379–386. [Google Scholar] [CrossRef]
- Hahn, E.; Szwast, A.; Cnota, J.; Levine, J.C.; Fifer, C.G.; Jaeggi, E.; Andrews, H.; Williams, I.A. Association between fetal growth, cerebral blood flow and neurodevelopmental outcome in univentricular fetuses. Ultrasound Obstet. Gynecol. 2016, 47, 460–465. [Google Scholar] [CrossRef]
- Zeng, S.; Zhou, Q.C.; Zhou, J.W.; Li, M.; Long, C.; Peng, Q.H. Volume of intracranial structures on three-dimensional ultrasound in fetuses with congenital heart disease. Ultrasound Obstet. Gynecol. 2015, 46, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Zeng, S.; Zhou, J.; Peng, Q.; Tian, L.; Xu, G.; Zhao, Y.; Wang, T.; Zhou, Q. Assessment by three-dimensional power Doppler ultrasound of cerebral blood flow perfusion in fetuses with congenital heart disease. Ultrasound Obstet. Gynecol. 2015, 45, 649–656. [Google Scholar] [CrossRef]
- Masoller, N.; Martínez, J.M.; Gómez, O.; Bennasar, M.; Crispi, F.; Sanz-Cortés, M.; Egaña-Ugrinovic, G.; Bartrons, J.; Puerto, B.; Gratacós, E. Evidence of second-trimester changes in head biometry and brain perfusion in fetuses with congenital heart disease. Ultrasound Obstet. Gynecol. 2014, 44, 182–187. [Google Scholar] [CrossRef]
- Williams, I.A.; Fifer, C.; Jaeggi, E.; Levine, J.C.; Michelfelder, E.C.; Szwast, A.L. The association of fetal cerebrovascular resistance with early neurodevelopment in single ventricle congenital heart disease. Am. Hear. J. 2013, 165, 544–550.e1. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Khoo, N.S.; Brooks, P.A.; Savard, W.; Hirose, A.; Hornberger, L.K. Severe left heart obstruction with retrograde arch flow influences fetal cerebral and placental blood flow. Ultrasound Obstet. Gynecol. 2013, 42, 294–299. [Google Scholar] [CrossRef]
- Szwast, A.; Tian, Z.; McCann, M.; Soffer, D.; Rychik, J. Comparative analysis of cerebrovascular resistance in fetuses with single-ventricle congenital heart disease. Ultrasound Obstet. Gynecol. 2012, 40, 62–67. [Google Scholar] [CrossRef]
- Williams, I.A.; Tarullo, A.R.; Grieve, P.G.; Wilpers, A.; Vignola, E.F.; Myers, M.M.; Fifer, W.P. Fetal cerebrovascular resistance and neonatal EEG predict 18-month neurodevelopmental outcome in infants with congenital heart disease. Ultrasound Obstet. Gynecol. 2012, 40, 304–309. [Google Scholar] [CrossRef]
- Arduini, M.; Rosati, P.; Caforio, L.; Guariglia, L.; Clerici, G.; Di Renzo, G.C.; Scambia, G. Cerebral blood flow autoregulation and congenital heart disease: Possible causes of abnormal prenatal neurologic development. J. Matern. Neonatal Med. 2011, 24, 1208–1211. [Google Scholar] [CrossRef]
- McElhinney, D.B.; Benson, C.B.; Brown, D.W.; Wilkins-Haug, L.E.; Marshall, A.C.; Zaccagnini, L.; Tworetzky, W. Cerebral Blood Flow Characteristics and Biometry in Fetuses Undergoing Prenatal Intervention for Aortic Stenosis with Evolving Hypoplastic Left Heart Syndrome. Ultrasound Med. Biol. 2010, 36, 29–37. [Google Scholar] [CrossRef]
- Berg, C.; Gembruch, O.; Gembruch, U.; Geipel, A. Doppler indices of the middle cerebral artery in fetuses with cardiac defects theoretically associated with impaired cerebral oxygen delivery in utero: Is there a brain-sparing effect? Ultrasound Obstet. Gynecol. 2009, 34, 666–672. [Google Scholar] [CrossRef]
- Brossard-Racine, M.; du Plessis, A.; Vezina, G.; Robertson, R.; Donofrio, M.; Tworetzky, W.; Limperopoulos, C. Brain Injury in Neonates with Complex Congenital Heart Disease: What Is the Predictive Value of MRI in the Fetal Period? Am. J. Neuroradiol. 2016, 37, 1338–1346. [Google Scholar] [CrossRef]
- Brossard-Racine, M.; du Plessis, A.J.; Vezina, G.; Robertson, R.; Bulas, D.; Evangelou, I.E.; Donofrio, M.; Freeman, D.; Limperopoulos, C. Prevalence and Spectrum of in Utero Structural Brain Abnormalities in Fetuses with Complex Congenital Heart Disease. Am. J. Neuroradiol. 2014, 35, 1593–1599. [Google Scholar] [CrossRef]
- Mlczoch, E.; Brugger, P.; Ulm, B.; Novak, A.; Frantal, S.; Prayer, D.; Salzer-Muhar, U. Structural congenital brain disease in congenital heart disease: Results from a fetal MRI program. Eur. J. Paediatr. Neurol. 2013, 17, 153–160. [Google Scholar] [CrossRef]
- Schellen, C.; Ernst, S.; Gruber, G.M.; Mlczoch, E.; Weber, M.; Brugger, P.C.; Ulm, B.; Langs, G.; Salzer-Muhar, U.; Prayer, D.; et al. Fetal MRI detects early alterations of brain development in Tetralogy of Fallot. Am. J. Obstet. Gynecol. 2015, 213, 392.e1–392.e7. [Google Scholar] [CrossRef] [PubMed]
- Al Nafisi, B.; van Amerom, J.F.; Forsey, J.; Jaeggi, E.; Grosse-Wortmann, L.; Yoo, S.-J.; Macgowan, C.K.; Seed, M. Fetal circulation in left-sided congenital heart disease measured by cardiovascular magnetic resonance: A case–control study. J. Cardiovasc. Magn. Reson. 2013, 15, 65. [Google Scholar] [CrossRef]
- Sun, L.; Macgowan, C.K.; Sled, J.G.; Yoo, S.-J.; Manlhiot, C.; Porayette, P.; Grosse-Wortmann, L.; Jaeggi, E.; McCrindle, B.W.; Kingdom, J.; et al. Reduced Fetal Cerebral Oxygen Consumption Is Associated with Smaller Brain Size in Fetuses with Congenital Heart Disease. Circulation 2015, 131, 1313–1323. [Google Scholar] [CrossRef]
- Limperopoulos, C.; Tworetzky, W.; McElhinney, D.B.; Newburger, J.W.; Brown, D.W.; Robertson, R.L.; Guizard, N.; McGrath, E.; Geva, J.; Annese, D.; et al. Brain Volume and Metabolism in Fetuses with Congenital Heart Disease. Circulation 2010, 121, 26–33. [Google Scholar] [CrossRef]
- Masoller, N.; Sanz-Cortés, M.; Crispi, F.; Gómez, O.; Bennasar, M.; Egaña-Ugrinovic, G.; Bargalló, N.; Martínez, J.M.; Gratacós, E. Severity of Fetal Brain Abnormalities in Congenital Heart Disease in Relation to the Main Expected Pattern of in utero Brain Blood Supply. Fetal Diagn. Ther. 2016, 39, 269–278. [Google Scholar] [CrossRef]
- Clouchoux, C.; du Plessis, A.J.; Bouyssi-Kobar, M.; Tworetzky, W.; McElhinney, D.B.; Brown, D.W.; Gholipour, A.; Kudelski, D.; Warfield, S.K.; McCarter, R.J.; et al. Delayed Cortical Development in Fetuses with Complex Congenital Heart Disease. Cereb. Cortex 2013, 23, 2932–2943. [Google Scholar] [CrossRef]
- Patel, S.R.; Michelfelder, E. Prenatal Diagnosis of Congenital Heart Disease: The Crucial Role of Perinatal and Delivery Planning. J. Cardiovasc. Dev. Dis. 2024, 11, 108. [Google Scholar] [CrossRef] [PubMed]
- Abell, B.R.; Eagleson, K.; Auld, B.; Bora, S.; Justo, R.; Parsonage, W.; Sharma, P.; Kularatna, S.; McPhail, S.M. Implementing neurodevelopmental follow-up care for children with congenital heart disease: A scoping review with evidence mapping. Dev. Med. Child Neurol. 2024, 66, 161–175. [Google Scholar] [CrossRef] [PubMed]
- de Soysa, T.Y.; Ranade, S.S.; Okawa, S.; Ravichandran, S.; Huang, Y.; Salunga, H.T.; Schricker, A.; del Sol, A.; Gifford, C.A.; Srivastava, D. Single-cell analysis of cardiogenesis reveals basis for organ-level developmental defects. Nature 2019, 572, 120–124. [Google Scholar] [CrossRef]
- Wei, N.; Lee, C.; Duan, L.; Galdos, F.X.; Samad, T.; Raissadati, A.; Goodyer, W.R.; Wu, S.M. Cardiac Development at a Single-Cell Resolution. Adv. Exp. Med. Biol. 2024, 1441, 253–268. [Google Scholar]
- Thakur, A.; Kishore, R. Neurobiology of the circadian clock and its role in cardiovascular disease: Mechanisms, biomarkers, and chronotherapy. Neurobiol. Sleep Circadian Rhythm. 2025, 19, 100131. [Google Scholar] [CrossRef]
- Li, G.; Tian, L.; Goodyer, W.; Kort, E.J.; Buikema, J.W.; Xu, A.; Wu, J.C.; Jovinge, S.; Wu, S.M. Single cell expression analysis reveals anatomical and cell cycle-dependent transcriptional shifts during heart development. Development 2019, 146, dev173476. [Google Scholar] [CrossRef]
- Landis, B.J.; Helvaty, L.R.; Geddes, G.C.; Lin, J.I.; Yatsenko, S.A.; Lo, C.W.; Border, W.L.; Wechsler, S.B.; Murali, C.N.; Azamian, M.S.; et al. A Multicenter Analysis of Abnormal Chromosomal Microarray Findings in Congenital Heart Disease. J. Am. Hear. Assoc. 2023, 12, e029340. [Google Scholar] [CrossRef]
- Woodward, A.A.; Taylor, D.M.; Goldmuntz, E.; Mitchell, L.E.; Agopian, A.J.; Moore, J.H.; Urbanowicz, R.J. Gene-Interaction-Sensitive enrichment analysis in congenital heart disease. BioData Min. 2022, 15, 4. [Google Scholar] [CrossRef]
- Luo, X.; Liu, L.; Rong, H.; Liu, X.; Yang, L.; Li, N.; Shi, H. ENU-based dominant genetic screen identifies contractile and neuronal gene mutations in congenital heart disease. Genome Med. 2024, 16, 97. [Google Scholar] [CrossRef]
- Sierant, M.C.; Jin, S.C.; Bilguvar, K.; Morton, S.U.; Dong, W.; Jiang, W.; Lu, Z.; Li, B.; López-Giráldez, F.; Tikhonova, I.; et al. Genomic analysis of 11,555 probands identifies 60 dominant congenital heart disease genes. Proc. Natl. Acad. Sci. USA 2025, 122, e2420343122. [Google Scholar] [CrossRef]
- Fear, V.S.; Forbes, C.A.; Shaw, N.C.; Farley, K.O.; Mantegna, J.L.; Htun, J.P.; Syn, G.; Viola, H.; Cserne Szappanos, H.; Hool, L.; et al. Gene editing and cardiac disease modelling for the interpretation of genetic variants of uncertain significance in congenital heart disease. Stem Cell Res. Ther. 2023, 14, 345. [Google Scholar] [CrossRef]
- Blue, G.M.; Kirk, E.P.; Giannoulatou, E.; Sholler, G.F.; Dunwoodie, S.L.; Harvey, R.P.; Winlaw, D.S. Advances in the Genetics of Congenital Heart Disease. J. Am. Coll. Cardiol. 2017, 69, 859–870. [Google Scholar] [CrossRef] [PubMed]
- Ong, L.T. Editor’s Pick: Genetics and Pathophysiology of Co-occurrence of Congenital Heart Disease and Autism Spectrum Disorder. Eur. Med. J. 2024, 9, 73–83. [Google Scholar] [CrossRef]
- Ji, W.; Ferdman, D.; Copel, J.; Scheinost, D.; Shabanova, V.; Brueckner, M.; Khokha, M.K.; Ment, L.R. De novo damaging variants associated with congenital heart diseases contribute to the connectome. Sci. Rep. 2020, 10, 7046. [Google Scholar] [CrossRef]
- Rishika, D.; RN, M.; Anurag, A.; Sumod, K.; Bhawna, M. Highly Sensitive Cardiac Troponin T as a Biomarker of Myocardial Injury in Acyanotic Congenital Heart Disease. Int. J. Pediatr. Res. 2020, 6, 1–5. [Google Scholar] [CrossRef]
- Guo, F.; Zhang, C.-C.; Yin, X.-H.; Li, T.; Fang, C.-H.; He, X.-B. Crosstalk between cardiomyocytes and noncardiomyocytes is essential to prevent cardiomyocyte apoptosis induced by proteasome inhibition. Cell Death Dis. 2020, 11, 783. [Google Scholar] [CrossRef]
- Perfetto, F.; Bergesio, F.; Emdin, M.; Cappelli, F. Troponins in cardiac amyloidosis: Multipurpose markers. Nat. Rev. Cardiol. 2014, 11, 179. [Google Scholar] [CrossRef]
- Colliva, A.; Braga, L.; Giacca, M.; Zacchigna, S. Endothelial cell–cardiomyocyte crosstalk in heart development and disease. J. Physiol. 2020, 598, 2923–2939. [Google Scholar] [CrossRef] [PubMed]
- Hilal, S.; Chai, Y.L.; Ikram, M.K.; Elangovan, S.; Yeow, T.B.; Xin, X.; Chong, J.Y.; Venketasubramanian, N.; Richards, A.M.; Chong, J.P.C.; et al. Markers of Cardiac Dysfunction in Cognitive Impairment and Dementia. Medicine 2015, 94, e297. [Google Scholar] [CrossRef] [PubMed]
- Zonneveld, M.H.; Abbel, D.; Cessie, S.l.; Jukema, J.W.; Noordam, R.; Trompet, S. Cardiac Troponin, Cognitive Function, and Dementia: A Systematic Review. Aging Dis. 2022, 14, 386–397. [Google Scholar] [CrossRef] [PubMed]
- Sterling, L.H.; Liu, A.; Ganni, E.; Therrien, J.; Dancea, A.B.; Guo, L.; Marelli, A.J. Neurocognitive disorders amongst patients with congenital heart disease undergoing procedures in childhood. Int. J. Cardiol. 2021, 336, 47–53. [Google Scholar] [CrossRef]
- Cabrera-Mino, C.; DeVon, H.A.; Aboulhosn, J.; Brecht, M.-L.; Choi, K.R.; Pike, N.A. Neurocognition in adults with congenital heart disease post-cardiac surgery: A systematic review. Hear. Lung 2024, 64, 62–73. [Google Scholar] [CrossRef]
- Homsy, J.; Zaidi, S.; Shen, Y.; Ware, J.S.; Samocha, K.E.; Karczewski, K.J.; DePalma, S.R.; McKean, D.; Wakimoto, H.; Gorham, J.; et al. De novo mutations in congenital heart disease with neurodevelopmental and other congenital anomalies. Science 2015, 350, 1262–1266. [Google Scholar] [CrossRef]
- Dowden, L.; Tucker, D.; Morgan, S.; Uzun, O.; Syed, Y.A. Contribution of Congenital Heart Disorders Associated With Copy Number Variants in Mediating Risk for Brain Developmental Disorders: Evidence From 20-Year Retrospective Cohort Study. Front. Cardiovasc. Med. 2021, 8, 655463. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thakur, A.; Kishore, R. Neuro-Genomic Mapping of Cardiac Neurons with Systemic Analysis Reveals Cognitive and Neurodevelopmental Impacts in Congenital Heart Disease. Life 2025, 15, 1400. https://doi.org/10.3390/life15091400
Thakur A, Kishore R. Neuro-Genomic Mapping of Cardiac Neurons with Systemic Analysis Reveals Cognitive and Neurodevelopmental Impacts in Congenital Heart Disease. Life. 2025; 15(9):1400. https://doi.org/10.3390/life15091400
Chicago/Turabian StyleThakur, Abhimanyu, and Raj Kishore. 2025. "Neuro-Genomic Mapping of Cardiac Neurons with Systemic Analysis Reveals Cognitive and Neurodevelopmental Impacts in Congenital Heart Disease" Life 15, no. 9: 1400. https://doi.org/10.3390/life15091400
APA StyleThakur, A., & Kishore, R. (2025). Neuro-Genomic Mapping of Cardiac Neurons with Systemic Analysis Reveals Cognitive and Neurodevelopmental Impacts in Congenital Heart Disease. Life, 15(9), 1400. https://doi.org/10.3390/life15091400






