Abstract
Congenital heart disease (CHD) is associated with neurodevelopmental and cognitive impairments, but the underlying molecular mechanisms remain unclear. This study investigated cardiac neuronal genomics in CHD using single-nucleus RNA-sequencing data (GSE203274) from 157,273 cardiac nuclei of healthy donors and patients with hypoplastic left heart syndrome (HLHS), Tetralogy of Fallot (TOF), dilated (DCM), and hypertrophic (HCM) cardiomyopathies. The Uniform Manifold Approximation and Projection (UMAP) clustering identified major cardiac cell types, revealing neuron-specific transcriptional programmes. Neuronal populations showed enriched expression of neurodevelopmental disorder-linked genes (NRXN3, CADM2, ZNF536) and synaptic signalling pathways. CHD cardiac neurons exhibited upregulated markers of cognitive dysfunction (APP, SNCA, BDNF) and neurodevelopment regulators (DNMT1, HCFC1) across subtypes. Cardiomyocyte troponin elevation correlated with neuronal exosome receptor expression (TLR2, LRP1), suggesting intercellular communication. Gene ontology analysis highlighted overlaps between cardiovascular disease pathways and neurodevelopmental disorder signatures in CHD neurons. These findings provide the first neuro-genomic map of cardiac neurons in CHD, linking cardiac pathology to neural outcomes through transcriptional dysregulation. Further, the systemic analysis of clinical findings in CHD further supports the risk of neurodevelopmental impacts. In summary, this study identifies transcriptional dysregulation within cardiac neurons in CHD and, together with a systemic analysis of clinical data, provides molecular evidence linking cardiac pathology to neurodevelopmental and cognitive impairments.
1. Introduction
Congenital heart disease (CHD), affecting nearly 1% of live births globally, has transformed from a fatal pediatric condition to a chronic multisystem disorder with growing recognition of neurodevelopmental sequelae [1,2,3]. While surgical advances enable >90% survival to adulthood, 30–40% of survivors exhibit cognitive impairments ranging from executive dysfunction to autism spectrum disorders, with fourfold increased psychiatric morbidity compared to the general population [4]. This neurological burden persists despite optimal cardiac care, suggesting intrinsic biological links between cardiac maldevelopment and neural pathogenesis that extend beyond perioperative factors [3].
Emerging evidence implicates shared genetic underpinnings in CHD and neurodevelopmental disorders (NDDs), with copy number variants at 1q21.1, 16p13.1-11, and 8p23.1 loci disrupting both cardio-genesis and cortical development [2,3]. Critical pathways include Wnt signalling regulators like HCFC1, epigenetic modifiers such as DNMT3A, and synaptic plasticity genes including NRXN3—all implicated in CHD-associated NDDs through population genomics [4]. These findings align with single-cell studies demonstrating that cardiac neuronal populations express neurodevelopmental transcription factors during embryogenesis, suggesting persistent molecular crosstalk [5]. However, the postnatal persistence of such programmes in CHD hearts remains unexplored, creating a critical knowledge gap in understanding cardio-cerebral connections.
Recent advances in single-nucleus RNA sequencing (snRNA-seq) have revolutionized our capacity to decode cellular heterogeneity in cardiovascular tissues [6,7]. Spatial transcriptomics further enable mapping of intercellular communication networks disrupted in disease states. Applying these tools to CHD, preliminary studies reveal cardiomyocyte-specific dysregulation of hypertrophy-related genes and fibroblast activation pathways [6], while developmental analyses identify Hand2-dependent transcriptional networks governing cardiac progenitor fate [7]. Nevertheless, neuronal components within cardiac tissue—long considered limited to autonomic regulation—have not been systematically characterized in CHD despite their potential role in mediating systemic inflammation and neurohumoral signalling.
The cardiomyocyte–neuronal axis may hold particular significance, given emerging links between cardiac troponin elevation and cognitive decline through putative exosome-mediated signalling. Animal models demonstrate that cardiomyocyte-derived exosomes carrying troponin complexes activate neuronal Toll-like receptors, inducing synaptic dysfunction through NF-κB pathways [4]. Human studies further correlate CHD-associated neurodevelopmental impairments with altered cerebral blood flow patterns and neurovascular coupling, suggesting that circulating cardiac factors modulate central nervous system homeostasis [2]. Reports have shown that early developmental delays in infants with CHD predict increased long-term neurodevelopmental risk; however, normal early development does not preclude later deficits, as many children develop impairments by school age despite typical infancy milestones [8,9,10]. However, the molecular mediators of this heart–brain crosstalk remain undefined, particularly within CHD-specific contexts.
In this study, we analyzed integrative single-nucleus RNA sequencing of 157,273 cardiac nuclei from donors and CHD patients, including HLHS and Tetralogy of Fallot (TOF), two common forms of cyanotic CHD lesions, as well as dilated (DCM) and hypertrophic (HCM) cardiomyopathies (Gene Expression Omnibus, GEO accession: GSE203274) [1], to examine whether the CHD hearts harbour aberrant neuronal transcriptional programmes linking cardiac pathology to neural comorbidities through persistent expression of neurodevelopmental regulators, cardiomyocyte–neuronal crosstalk via exosome signalling, and shared genomic architecture between cardiovascular and neurodevelopmental pathways. Subsequently, by leveraging the integration of single-cell genomics with clinical correlation analyses, this work paves the way for targeted therapeutic strategies against CHD-associated neurocognitive impairments.
2. Materials and Methods
2.1. Single-Nucleus RNA-Seq Dataset
The single-nucleus RNA sequencing dataset (GEO Accession: GSE203274) [1] was obtained from the public database accessible at Gene Expression Omnibus (GEO) (accessed on 24 January 2025). This dataset consists of 157,273 nuclei from donors and CHD patients, including HLHS and TOF, two common forms of cyanotic CHD lesions, as well as DCM and HCM.
2.2. Single-Nucleus RNA-Seq Data Analysis
The single-nucleus RNA-seq datasets were analyzed with the statistical programming language R (v. 4.4.2). The following packages were used for loading, saving, and manipulating data, as well as data integration, analysis, and generating plots: Seurat (v 4.3.0), tidyverse (v 1.3.2), dplyr (v 1.1.0), patchwork (v 1.1.2), stringr (v 1.5.0), Harmony (v 0.1.1), org.Hs.eg.db (v 3.16.0), and SingleR (v 2.0.0). The read count matrix was read into R, which subsequently created the Seurat object with the CreateSeuratObject () function. To remove the batch effect, all samples were integrated iteratively. Uniform manifold approximation projections (UMAPs) were then calculated using runUMAP(), respectively, using the first 20 dimensions. The analysis results were visualized using the FeaturePlot(), VlnPlot(), and DotPlot() functions. The single-cell portal (available at https://singlecell.broadinstitute.org/ (accessed on 24 January 2025)) was also utilized.
We performed differential gene expression analysis for each cell type labelled by the original study, comparing patient samples with control samples. The testing method was the Wilcoxon rank sum test. The Benjamin–Hochberg test was used for multiple testing corrections. The adjusted p-value cut-off was 0.05. Enrichment analysis was conducted using the function “enriched ()” in the R package “clusterProfiler” (version 4.16.0).
2.3. Gene Set Enrichment Analysis (GSEA)
GSEA was conducted using ShinyGO v0.82 to examine biological processes, cellular components, and molecular functions in cardiac neurons of CHD. Parameters included FDR q-value ≤ 0.25 and |Normalized Enrichment Score (NES)| ≥ 1.5. Results were visualized through GSEA-based dot plots and network graphs, highlighting enriched pathways across disease conditions. The network graph revealed associations between cardiovascular phenotypes (nodes 1–2) and neurodevelopmental disorders (nodes 3–5), with edges weighted by gene overlap similarity.
2.4. Systemic Analysis
Literature Search Strategy: A comprehensive literature search was conducted to identify studies evaluating the association between congenital heart disease (CHD) and neurodevelopmental disorders. Databases including PubMed, Scopus, and Web of Science were systematically searched using keywords such as “congenital heart disease,” “neurodevelopmental disorders,” “cognitive impairment,” and “odds ratio.” The search included articles published up to June 2025. Additional studies were identified by screening references of relevant reviews.
Study Selection Criteria: Eligible studies were required to meet the following criteria: (1) original research articles involving patients with CHD; (2) qualitative assessment of neurodevelopmental or cognitive outcomes; and (3) publication in peer-reviewed English-language journals. Studies were excluded if they did not provide sufficient qualitative data for synthesis or if they focused exclusively on animal models.
Data Extraction: Relevant information from each included study included author, year of publication, study design, population characteristics, and key qualitative findings related to neurodevelopmental or cognitive outcomes. Any discrepancies in data extraction were resolved through discussion or, when necessary, consultation with a third reviewer.
Qualitative Synthesis: Findings were mapped using a qualitative evidence synthesis approach. Thematic analysis was employed to identify and organize key patterns and themes across studies. A qualitative evidence map was generated to visually summarize the range of neurodevelopmental outcomes reported in CHD patients, highlighting areas of convergence and divergence within the literature.
3. Results
3.1. Single-Nucleus Analysis and Annotations Reveal Major Cardiac Cell Types in Congenital Heart Disease Patients
Despite advancements in genomics, many CHD cases remain unexplained due to genetic heterogeneity and incomplete penetrance. Single-nucleus technologies address this gap by enabling high-resolution profiling of cellular heterogeneity and identifying molecular markers specific to cardiac cell subsets involved in CHD pathology [11,12,13]. Single-nucleus analysis and annotation techniques have proven instrumental in understanding the cellular landscape of CHD. In this study, single-nucleus transcriptomics analysis (GEO accession: GSE203274) [1] was employed to identify and characterize major cardiac cell types in CHD patients, offering new insights into the disease’s molecular and cellular basis. Figure 1A shows a Uniform Manifold Approximation and Projection (UMAP) visualization of heart tissue datasets from CHD patients, revealing distinct cardiac cell populations. Dot plot analysis enabled precise annotation of various cardiac cell types based on their unique gene expression profiles, providing a comprehensive map of the cellular landscape in CHD hearts (Figure 1B). Remarkably, we identified the expression of synaptic genes in cardiac tissues, suggesting the presence of neurocardiac connections in CHD patients. UMAPs revealed cell type-specific expressions of synaptic adhesion proteins and other synapse-associated genes (NLGN4X, NRXN1, CLSTN2, RAPSN, HOMER1, and PCDH10) in cardiac tissues (Figure 1C–H), indicating potential roles in heart development and function. The expression of these synaptic genes in cardiac cells suggests previously unrecognized communication pathways between the nervous system and the heart in CHD.
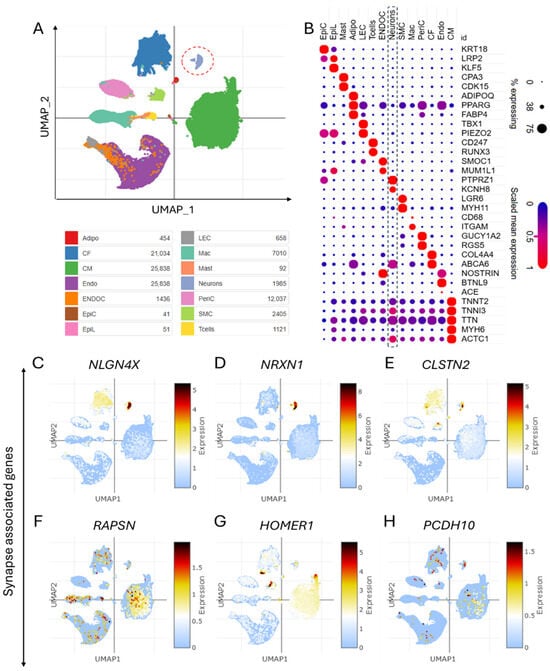
Figure 1.
Single-cell transcriptomics identify diverse cardiac cell populations and neurocardiac synapses in congenital heart disease. (A) UMAP of the dataset showing different cell types in the heart tissues of congenital heart disease (CHD) patients. (B) Dot plot showing the cell type-specific markers-based annotation of different cell types in the heart tissues of CHD patients. (C–H) UMAPs show the cell type-specific expression of synapse-associated genes, including synaptic adhesion proteins encoding the genes NLGN4X, NRXN1, CLSTN2, RAPSN, HOMER1, and PCDH10 in cardiac tissue of CHD.
3.2. Gene Set Enrichment Analysis of Differentially Expressed Genes Enabled the Characterization of Cardiac Neurons in CHD
GSEA of differentially expressed genes could provide crucial insights into the characterization of cardiac neurons in CHD. The dot plot depicts the expression of the top 50 genes predominantly associated with neuronal cell types in the heart (Figure 2A), suggesting their specialized roles in cardiac neuronal function. Notably, UMAPs and violin plots reveal the distribution and predominant expression of key genes such as NRXN3, CADM2, ZNF536, and XKR4 within cardiac neurons (Figure 2B–E), emphasizing their potential regulatory roles in CHD-related neuronal activity. Enrichment plots further elucidate gene ontology-based categorizations, identifying major biological processes, cellular components, and molecular functions linked to cardiac neurons (Figure 2F–H). Importantly, the neurogenesis and nervous system development are found to be the major biological processes associated with differentially expressed genes in cardiac neurons (Figure 2F). In addition, glutamatergic synapse was found to be one of the major cellular components associated with differentially expressed genes in cardiac neurons (Figure 2G). These findings accentuate the importance of understanding neuronal contributions to CHD pathology.
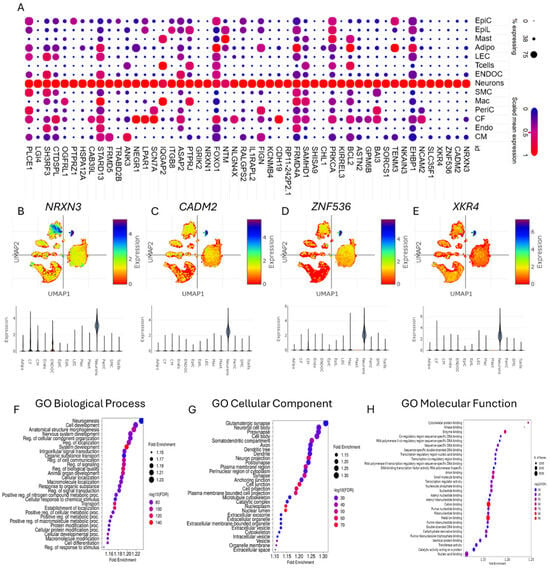
Figure 2.
Characterization of cardiac neurons in congenital heart disease. (A) Dot plot depicting the expression of the top fifty genes predominantly found in neuron cell types in the heart. (B–E) UMAPs and violin plots showing the distribution of (B) NRXN3, (C) CADM2, (D) ZNF536, and (E) XKR4 in different cell types with predominant expression in neuron cell types in the heart. (F–H) Enrichment plots showing the gene ontology (GO)-based major (F) biological processes, (G) cellular components, and (H) molecular functions of the neurons based on differentially expressed genes.
3.3. Genomic Landscape of CHD Features Upregulated Genes Linked to Cognitive Dysfunction and Neurodevelopment Disorder
The genomic landscape of CHD reveals upregulated genes associated with cognitive dysfunction and neurodevelopmental disorders. Investigating these gene expression patterns is essential to uncover the mechanistic interplay between CHD and neurodevelopmental disorders, potentially guiding therapeutic strategies targeting both conditions. The dot plots illustrate the relative expression levels of cognitive dysfunction-associated genes (APP, SNCA, APOE, BDNF, CASS4, DNMTB3A, TOMM40, and PLD3) and neurodevelopmental disorder-associated genes (DNMT1, HCFC1, NR2F1, RANBP17, UPF3B, ACTA1, MYL2, MYL3, and NPPB) across various CHD subtypes, including TOF, HCM, DCM, HF_HLHS, and Neo_HLHS (Figure 3A,B). Supplementary Figure S1A–F depict the cell type-specific distribution of APP, SNCA, APOE, BDNF, CASS4, and DNMTB3A in donor control, TOF DCM, HCM, HF-HLHS, and Neo-HLHS. Further, violin plots further demonstrate age-dependent expression patterns of key genes (APP, SNCA, and DNMT1) in control donors and CHD subtypes (Figure 3C–E). These findings suggest a potential link between CHD pathology and neurological dysfunction, highlighting shared molecular pathways that may influence both cardiac and cognitive development.
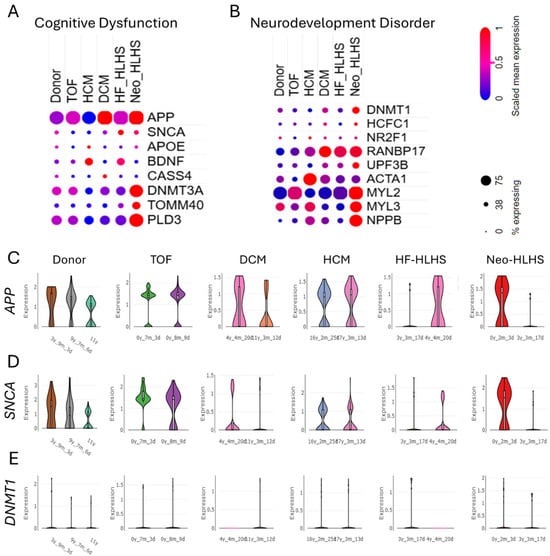
Figure 3.
Genes linked to cognitive dysfunction are upregulated in congenital heart disease. (A,B) Dot plots showing the relative expression level of (A) cognitive dysfunction-associated genes APP, SNCA, APOE, BDNF, CASS4, DNMTB3A, TOMM40, and PLD3 and (B) neurodevelopment disorder-associated genes DNMT1, HCFC1, NR2F1, RANBP17, UPF3B, ACTA1, MYL2, MYL3, NPPB in donor control, TOF, HCM, DCM, HF_HLHS, and Neo_HLHS. (C–E) Violin plots showing the age-dependent expression levels of APP, SNCA, DNMT1 in control donor, TOF, HCM, DCM, HF_HLHS, and Neo_HLHS.
3.4. Cardiac Troponin Elevation in CHD Signals Heart Attack Risk, Revealing Cell-Specific Crosstalk Between Cardiomyocytes and Neurons
Cardiac troponin expression was analyzed across cardiomyocytes in CHD cohorts, including TOF, DCM, and HCM. UMAP visualizations demonstrated elevated expression of troponin genes TNNT2 (Figure 4A–D), TNNI3 (Figure 4E–H), and TTN (Figure 4I–L) in cardiomyocytes of TOF, DCM, HCM, HF-HLHS, and Neo-HLHS compared to the donor control (Supplementary Figure S2A–G). A violin plot revealed upregulated HSP70 expression—a gene linked to exosome biogenesis—in cardiomyocytes of TOF, DCM, HCM, HF_HLHS, and Neo_HLHS versus donor controls (Figure 4M). Neuronal populations exhibited increased expression of exosome uptake receptors, with elevated TLR2 (Figure 4N), LRP1 (Figure 4O), and SCARF (Figure 4P) across all CHD subtypes. These findings suggest enhanced exosome-related signalling pathways in CHD, with cardiomyocytes showing increased troponin and exosome biogenesis markers, while neurons displayed upregulated receptors for exosome internalization. This suggests potential heart attack risk in CHD and exosome-based crosstalk between cardiomyocytes and neurons.
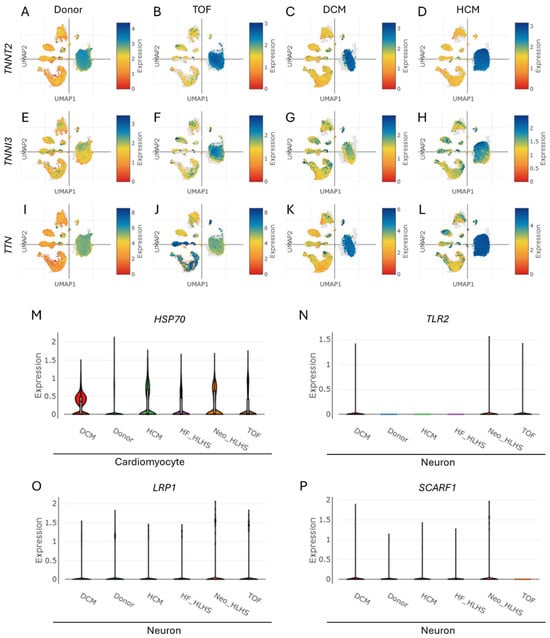
Figure 4.
Cell type-specific expression level of troponin, predominantly enhanced in cardiomyocytes within CHD. (A–L) UMAPs show the enhanced expression levels of (A–D) TNNT2, (E–H) TNNI3, and (I–L) TTN in cardiomyocyte population within various CHDs, namely TOF, DCM, and HCM. (M) A violin plot depicting the enhanced expression level of HSP70 (associated with exosome biogenesis in the cardiomyocytes of TOF, HCM, DCM, HF_HLHS, and Neo_HLHS) compared to the donor control group. (N–P) Violin plots depicting the expression level of genes associated with exosome uptake receptors (N) TLR2, (O) LRP1, and (P) SCARF in the neuron cells of the donor control, TOF, HCM, DCM, HF_HLHS, and Neo_HLHS groups.
3.5. Troponin Levels in CHD Correlate with Markers of Cognitive Dysfunction and Neurodevelopment Disorder
CHD patients face higher risks of cognitive, motor, language, and social–emotional challenges. These neurodevelopmental issues can affect executive functions and overall development [8]. Therefore, we aimed to investigate the relationship between TNNT2 and markers of cognitive dysfunction and neurodevelopment disorder (NDD) in both control and CHD heart cells. The UMAPs show the cell type-specific distribution and expression levels of SNCA, BDNF, DNMT3A, HCFC1, UPF3B, and ACTA1 in the heart for the control and CHD groups. The snRNA-seq analysis revealed significant correlations between TNNT2 expression in CHD and markers linked to cognitive dysfunction and neurodevelopment (Figure 5A–F, Supplementary Figure S3A–D). Positive associations were observed between TNNT2 and SNCA (ρ = 0.007), BDNF (ρ = 0.324), DNMT3A (ρ = 0.202), HCFC1 (ρ = 0.039), UPF3B (ρ = 0.324), and ACTA1 (ρ = 0.202) (Figure 5A–F). These findings align with clinical studies demonstrating troponin’s association with cognitive decline and neurodevelopmental disorder [14,15].
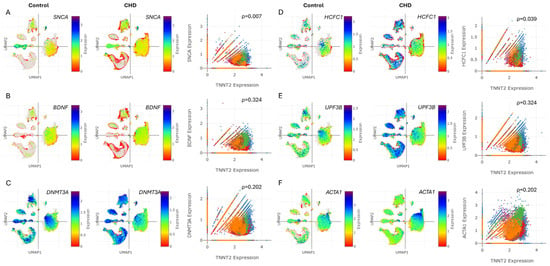
Figure 5.
Cognitive dysfunction and neurodevelopment markers are associated with troponin levels in CHD. (A–F) UMAPs depicting the cell type-specific expression of SNCA, BDNF, DNMT3A, HCFC1, UPF3B, and ACTA1 in the heart for the control and CHD groups. (A–F) Correlation plots showing the correlation for (A) SNCA vs. TNNT2, (B) BDNF vs. TNNT2, (C) DNMT3A vs. TNNT2, (D) HCFC1 vs. TNNT2, (E) UPF3B vs. TNNT2, and (F) ACTA1 vs. TNNT2 in cells from the hearts of the control and CHD patients. ρ depicts the quantitative correlation coefficient.
3.6. Ontology of Differentially Expressed Genes in Cardiac Neurons of CHD Suggests Links Between Cardiovascular Disease Phenotypes and Neurodevelopmental Disorders
We aimed to investigate potential connections between CHD and NDD by analyzing gene expression patterns in cardiac neurons from CHD patients, addressing the increased risk of neurodevelopmental issues in CHD survivors. GSEA of differentially upregulated genes in cardiac neurons from CHD patients revealed significant associations with various cardiovascular phenotypes (including ventricular septal defects, atrial septal defects, Tetralogy of Fallot, and cardiomyopathies) and neurodevelopmental disorders (such as autism spectrum disorders, intellectual disability, developmental delay, and epilepsy) (Figure 6A). The GSEA-based network graph illustrates the complex interplay between cardiovascular phenotypes and neurodevelopmental disorders, with key nodes representing the shared genetic pathways and molecular mechanisms underlying both CHD and NDD (Figure 6B). These findings suggest a potential common genetic etiology between CHD and NDD, with cardiac neuronal gene expression patterns reflecting both cardiovascular and neurodevelopmental abnormalities.
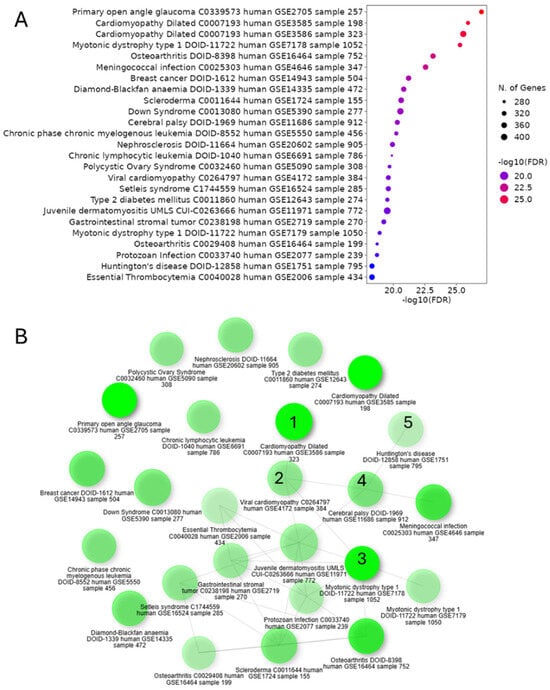
Figure 6.
Cardiac neuronal gene expression in CHD indicates connections between cardiovascular phenotypes and neurodevelopmental disorders. (A) GSEA-based dot plot showing the various disease conditions, as identified through the enrichment of differentially upregulated genes in cardiac neurons of CHD, indicating (B) the association between cardiovascular disease phenotypes (marked as 1 and 2) and neurodevelopment disorders (marked as 3–5), as shown in the GSEA-based network graph.
3.7. System Meta-Analysis Revealed Clinical Association Between CHD and Neurodevelopmental Disorders
To address the need for a comprehensive understanding of the neurodevelopmental risks associated with CHD, as previous individual studies reported varying results (Supplementary Table S1) [16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36], we performed a systematic analysis. Interestingly, the qualitative evidence map presented in Figure 7A,B synthesizes clinical findings related to CHD, focusing on prenatal ultrasound findings and imaging findings associated with neurodevelopmental impairment. The bar graphs quantitatively display the frequency with which specific clinical features are reported across the published studies, offering a visual summary of the evidence landscape, based on a previous studies (Supplementary Tables S1 and S2). Notably, certain prenatal ultrasound markers are more frequently identified in the literature, underscoring their perceived clinical relevance for early CHD detection and risk stratification [37]. The map also highlights imaging findings—such as structural brain abnormalities or altered brain growth patterns—that are recurrently associated with neurodevelopmental impairment in children with CHD [38]. Further, the expression analysis of genes associated with familial Alzheimer’s disease (AD), sporadic AD, and Parkinson’s disease (PD) were found to be relatively upregulated in CHD as compared to the control donor (Supplementary Figure S4), suggesting the common link between the neurodegenerative and CHD genomic landscape. Also, the profile of serum- or cell-free DNA biomarkers of CHD (Supplementary Figure S5) showed the possibility of utilizing biofluids, including serum or amniotic fluid, for potential CHD liquid biopsy-based diagnosis.
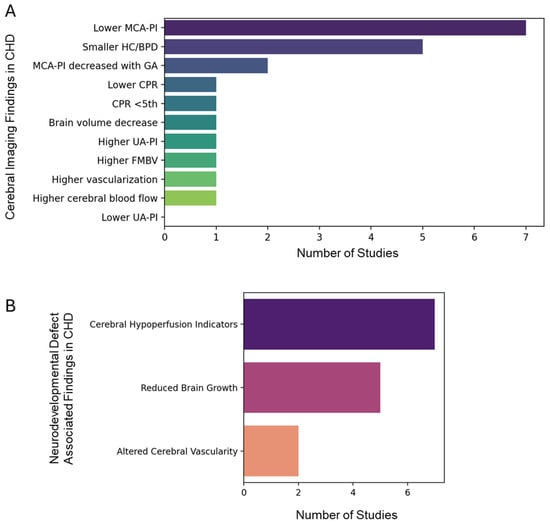
Figure 7.
Qualitative evidence map of clinical findings in congenital heart disease (CHD). Bar graphs illustrate the number of studies reporting (A) prenatal ultrasound findings and (B) imaging findings associated with neurodevelopmental impairment in CHD. Note: MCA-PI: middle cerebral artery pulsatility index; CPR: cerebro-placental ratio; HC/BPD: head circumference or biparietal diameter; UA-PI: umbilical artery PI; FMBV: fractional moving blood volume.
4. Discussion
Single-nucleus transcriptomic analyses have significantly advanced our understanding of cellular heterogeneity in CHD, addressing gaps left by bulk sequencing. Studies using snRNA-seq have identified distinct cardiac cell populations, including dysregulated progenitor subtypes and chamber-specific transcriptional signatures, which correlate with CHD pathology [12,39,40,41]. For instance, Hand2-null models revealed failed outflow tract specification and disrupted retinoic acid signalling in progenitors, while chamber-specific cell cycle dynamics were linked to growth defects [42]. The UMAP visualization and marker-based annotation in the described study (Figure 1A,B) align with established workflows for resolving cardiomyocyte-, endothelium-, and neural crest-derived subsets, as seen in human fetal heart atlases [12,40]. Moreover, the expression of synaptic genes in cardiac tissues of CHD patients (Figure 1C–H) suggests previously unrecognized communication pathways between the nervous system and the heart.
Single-nucleus analyses have revealed significant overlap between cardiac neuronal gene expression and CHD pathology, with GSEA highlighting neurogenesis, nervous system development, and glutamatergic synapse pathways as central to cardiac neuron dysfunction. Genes like NRXN3, CADM2, and HCN1 (implicated in synaptic adhesion and ion channel activity) show enriched expression in CHD-associated cardiac neurons (Figure 2A–E), aligning with prior reports linking neural crest cell migration defects to outflow tract malformations [43,44,45]. These findings mirror pathway enrichments observed in CNV studies of CHD cohorts, where neuronal system genes (ADCY2, NRXN3) correlate with conotruncal defects [43,46]. The glutamatergic synapse signature (Figure 2G) parallels recent snRNA-seq data showing neurotransmitter signalling dysregulation in CHD cardiomyocytes [47].
Recent genomic analyses of CHD have revealed a complex interplay between cardiac malformations and NDDs. The upregulation of genes associated with cognitive dysfunction and NDDs in CHD patients aligns with previous findings linking de novo mutations in heart-expressing genes to both CHD and NDD phenotypes [10]. This genetic overlap supports the “burden of genetic variation” model, where multiple rare variants contribute to both cardiac and neurological outcomes [48]. The expression patterns of genes like APP, SNCA, and DNMT1 across CHD subtypes and age groups (Figure 3A–E) suggest shared molecular pathways influencing cardiac and cognitive development. This corroborates recent studies identifying 60 genes implicated in CHD, with many also contributing to autism and other NDDs [46]. The involvement of chromatin modifiers in both CHD and NDD further emphasizes the genetic link between heart and brain development [49,50].
We found the elevated cardiac troponin expression (TNNT2, TNNI3, TTN) in cardiomyocytes across various CHD subtypes, suggesting increased myocardial stress and potential heart attack risk (Figure 4A–L). This aligns with a previous clinical study showing higher troponin levels in CHD patients, indicating myocardial injury [51]. The upregulation of HSP70 in cardiomyocytes (Figure 4M) further supports this, as HSP70 is associated with cellular stress responses and exosome biogenesis [52]. Although the precise mechanism underlying elevated troponin gene expression in CHD is not fully understood, one possible explanation is that the accumulation of amyloid fibrils—potentially originating from APP—induces myocardial injury, which in turn contributes to troponin elevation [53].
Interestingly, neuronal populations in CHD subtypes exhibited increased expression of exosome uptake receptors (TLR2, LRP1, SCARF) (Figure 4N–P), suggesting enhanced exosome-mediated crosstalk between cardiomyocytes and neurons. This novel finding extends our understanding of cell–cell communication in CHD beyond the established endothelial cell–cardiomyocyte interactions [52,54]. Further, significant correlations were found between TNNT2 expression and markers of cognitive dysfunction (Figure 5A–C, Supplementary Figure S3A,B) and NDDs (Figure 5D–F, Supplementary Figure S3C,D) in CHD patients. This aligns with recent clinical reports showing associations between elevated cardiac troponin levels and cognitive decline, dementia risk, and neurodevelopmental issues in CHD patients [55,56]. The positive correlations between TNNT2 and genes like SNCA, BDNF, and DNMT3A (Figure 5A–C) suggest shared pathways between cardiac dysfunction and neurological processes. This supports the “brain–heart axis” concept, where cardiac biomarkers may serve as early indicators of cognitive vulnerability in CHD [55]. The association with HCFC1 and UPF3B (Figure 5D,E) further emphasizes the link between CHD and NDDs, consistent with previous findings of overlapping genetic factors [57,58].
Interestingly, the GSEA of differentially upregulated genes in cardiac neurons from CHD patients reveals significant associations between cardiovascular phenotypes and NDDs (Figure 6A). This finding aligns with previous studies demonstrating shared genetic contributions to CHD and NDD. For instance, exome sequencing of CHD parent–offspring trios identified an excess of protein-damaging de novo mutations in genes highly expressed in both the developing heart and brain [59]. These mutations accounted for 20% of patients with CHD, NDD, and extra-cardiac congenital anomalies, suggesting pleiotropic effects. Further, the complex interplay between cardiovascular phenotypes and NDDs, illustrated in the GSEA-based network graph (Figure 6A,B), supports the “brain–heart axis” concept. This is consistent with recent findings showing that CHD patients have an increased risk of neurodevelopmental disabilities, with up to 33% of CHD patients with CNVs also having an associated NDD [60]. Our analysis identifies nine major cardiac cell populations, with neuronal clusters showing striking enrichment for NDD-associated genes (NRXN3, CADM2, ZNF536) and synaptic signalling pathways. These neurons co-express cognitive dysfunction markers (APP, SNCA) and chromatin modifiers (DNMT1, HCFC1) across CHD subtypes, suggesting maintained neurodevelopmental potential postnatally. Parallel findings reveal cardiomyocyte-specific troponin upregulation correlated with neuronal exosome receptor expression (TLR2, LRP1), supported by strong SNCA–TNNT2 correlations. Gene ontology analysis demonstrates significant overlap between cardiovascular disease pathways and neurodevelopmental disorder signatures, particularly in Wnt/β-catenin signalling and histone modification modules. In addition, the systemic review and meta-analysis further demonstrated the importance of integrating neurodevelopmental surveillance into routine follow-up for these patients (Figure 7A,B). The evidence map identifies both well-established and underexplored clinical markers, guiding clinicians and researchers toward areas where evidence is robust and highlighting gaps where further investigation is warranted. This approach supports more targeted allocation of resources for diagnosis, intervention, and long-term neurodevelopmental monitoring in CHD populations [37,38]. In conclusion, this study reveals significant links between CHD and NDD through single-nucleus transcriptomics, highlighting shared genetic pathways and potential biomarkers. These findings emphasize the importance of integrated cardiac and neurological care for CHD patients, paving the way for targeted interventions and improved outcomes.
Despite this, this study faces several limitations that impact the interpretation and generalizability of its findings. These include challenges in integrating data across different platforms and limited spatial resolution, which hinder a comprehensive understanding of cell–cell interactions. The reliance on transcriptomic data without functional or electrophysiological validation leaves gaps in understanding the causal relationships between gene expression patterns and the observed phenotypes. The study’s cross-sectional nature precludes establishing causality, while potential confounding factors such as age, medication, comorbidities, hypoxia, and surgical interventions are not fully accounted for. Additionally, the incomplete capture of non-coding variants, epigenetic factors, and somatic mosaicism may overlook important regulatory mechanisms. The analysis also lacks sufficient resolution to fully elucidate spatial interactions between cardiac neurons and other cell types. These limitations pave the way for future studies elucidating various aspects of neurodevelopmental defects in CHD.
5. Conclusions
CHD patients face significant neurodevelopmental risks, including cognitive delays, motor impairments, and motor dysfunction, which persist across the lifespan. Research indicates lower cognitive abilities, particularly in severe CHD subtypes like HLHS, alongside vulnerabilities in attention, memory, and adaptive functioning. These deficits stem from altered cerebral blood flow, genetic factors, and brain immaturity, with white matter injury and stroke further exacerbating outcomes. Emerging evidence highlights shared molecular pathways between CHD and neurodevelopmental disorders, suggesting cardiac–neuronal crosstalk via exosomes. Early monitoring and multidisciplinary interventions are critical to mitigating long-term impacts on education, employment, and independent living.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/life15091400/s1: Figure S1: Identification of cognitive markers’ cell type-specific expression level in control and CHD hearts; Figure S2: Evaluation of cardiac troponin in CHD; Figure S3: Correlation analysis of markers of cognitive dysfunction and neurodevelopment markers with troponin levels in CHD. Figure S4: Genes linked to AD and PD are upregulated in congenital heart disease. Figure S5: Expression analysis of genes encoding serum- or cell-free DNA markers of CHD. Table S1: A list of studies showing key prenatal and preoperative cerebral imaging findings in CHD. Table S2: A list of studies showing neurodevelopment defect-relevant ultrastructure markers in CHD.
Author Contributions
Conceptualization, A.T.; methodology, A.T.; software, A.T.; validation, A.T.; formal analysis, A.T.; investigation, A.T.; resources, R.K.; data curation, A.T.; writing—original draft preparation, A.T.; writing—review and editing, A.T., R.K.; visualization, A.T.; supervision, R.K.; project administration, A.T.; funding acquisition, R.K. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported, in part, by National Institutes of Health grants HL143892, HL134608, HL147841, and HL169405.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in a GEO public database at https://www.ncbi.nlm.nih.gov/geo/ (accessed on 24 January 2025), reference number [GEO accession: GSE203274]. These data were derived from the following resources available in the public domain: https://doi.org/10.1038/s41586-022-04989-3 [1].
Acknowledgments
The graphical abstract was prepared using Biorender.com.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| CHD | Congenital heart disease |
| HLHS | Hypoplastic left heart syndrome |
| TOF | Tetralogy of Fallot |
| DCM | Dilated cardiomyopathy |
| HCM | Hypertrophic cardiomyopathy |
| UMAP | Uniform Manifold Approximation and Projection |
| NRXN3 | Neurexin 3 |
| CADM2 | Cell adhesion molecule 2 |
| ZNF536 | Zinc finger protein 536 |
| APP | Amyloid precursor protein |
| SNCA | Synuclein alpha |
| BDNF | Brain-derived neurotrophic factor |
| DNMT1 | DNA methyltransferase 1 |
| HCFC1 | Host cell factor C1 |
| TLR2 | Toll-like receptor 2 |
| LRP1 | Low-density lipoprotein receptor-related protein 1 |
| NDDs | Neurodevelopmental disorders |
| DNMT3A | DNA methyltransferase 3 alpha |
| GEO | Gene Expression Omnibus |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
References
- Hill, M.C.; Kadow, Z.A.; Long, H.; Morikawa, Y.; Martin, T.J.; Birks, E.J.; Campbell, K.S.; Nerbonne, J.; Lavine, K.; Wadhwa, L.; et al. Integrated multi-omic characterization of congenital heart disease. Nature 2022, 608, 181–191. [Google Scholar] [CrossRef]
- Nattel, S.N.; Adrianzen, L.; Kessler, E.C.; Andelfinger, G.; Dehaes, M.; Côté-Corriveau, G.; Trelles, M.P. Congenital Heart Disease and Neurodevelopment: Clinical Manifestations, Genetics, Mechanisms, and Implications. Can. J. Cardiol. 2017, 33, 1543–1555. [Google Scholar] [CrossRef]
- Morton, P.D.; Ishibashi, N.; Jonas, R.A. Neurodevelopmental Abnormalities and Congenital Heart Disease. Circ. Res. 2017, 120, 960–977. [Google Scholar] [CrossRef] [PubMed]
- Soda, T.; Pasqua, T.; De Sarro, G.; Moccia, F. Cognitive Impairment and Synaptic Dysfunction in Cardiovascular Disorders: The New Frontiers of the Heart–Brain Axis. Biomedicines 2024, 12, 2387. [Google Scholar] [CrossRef]
- Churko, J.M.; Garg, P.; Treutlein, B.; Venkatasubramanian, M.; Wu, H.; Lee, J.; Wessells, Q.N.; Chen, S.-Y.; Chen, W.-Y.; Chetal, K.; et al. Defining human cardiac transcription factor hierarchies using integrated single-cell heterogeneity analysis. Nat. Commun. 2018, 9, 4906. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yin, K.; Chen, L.; Chen, W.; Li, W.; Zhang, T.; Sun, Y.; Yuan, M.; Wang, H.; Song, Y.; et al. Lineage-specific regulatory changes in hypertrophic cardiomyopathy unraveled by single-nucleus RNA-seq and spatial transcriptomics. Cell Discov. 2023, 9, 6. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, Y.; Liu, Y.; Gao, Y.; San, T.; Li, X.; Song, S.; Yan, B.; Zhao, Z. Advances in application of single-cell RNA sequencing in cardiovascular research. Front. Cardiovasc. Med. 2022, 9, 905151. [Google Scholar] [CrossRef]
- Sood, E.; Newburger, J.W.; Anixt, J.S.; Cassidy, A.R.; Jackson, J.L.; Jonas, R.A.; Lisanti, A.J.; Lopez, K.N.; Peyvandi, S.; Marino, B.S. Neurodevelopmental Outcomes for Individuals with Congenital Heart Disease: Updates in Neuroprotection, Risk-Stratification, Evaluation, and Management: A Scientific Statement From the American Heart Association. Circulation 2024, 149, e997–e1022. [Google Scholar] [CrossRef] [PubMed]
- Rollins, C.K.; Newburger, J.W. Neurodevelopmental Outcomes in Congenital Heart Disease. Circulation 2014, 130, e124–e126. [Google Scholar] [CrossRef]
- Rollins, C.K.; Newburger, J.W.; Roberts, A.E. Genetic contribution to neurodevelopmental outcomes in congenital heart disease: Are some patients predetermined to have developmental delay? Curr. Opin. Pediatr. 2017, 29, 529–533. [Google Scholar] [CrossRef]
- Tambi, R.; Zehra, B.; Nandkishore, S.; Sharafat, S.; Kader, F.; Nassir, N.; Mohamed, N.; Ahmed, A.; Abdel Hameid, R.; Alasrawi, S.; et al. Single-cell reconstruction and mutation enrichment analysis identifies dysregulated cardiomyocyte and endothelial cells in congenital heart disease. Physiol. Genom. 2023, 55, 634–646. [Google Scholar] [CrossRef] [PubMed]
- Samad, T.; Wu, S.M. Single cell RNA sequencing approaches to cardiac development and congenital heart disease. Semin. Cell Dev. Biol. 2021, 118, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Ameen, M.; Sundaram, L.; Shen, M.; Banerjee, A.; Kundu, S.; Nair, S.; Shcherbina, A.; Gu, M.; Wilson, K.D.; Varadarajan, A.; et al. Integrative single-cell analysis of cardiogenesis identifies developmental trajectories and non-coding mutations in congenital heart disease. Cell 2022, 185, 4937–4953.e23. [Google Scholar] [CrossRef]
- Jensen, M.; Zeller, T.; Twerenbold, R.; Thomalla, G. Circulating cardiac biomarkers, structural brain changes, and dementia: Emerging insights and perspectives. Alzheimer’s Dement. 2023, 19, 1529–1548. [Google Scholar] [CrossRef]
- Schneider, A.L.C.; Rawlings, A.M.; Sharrett, A.R.; Alonso, A.; Mosley, T.H.; Hoogeveen, R.C.; Ballantyne, C.M.; Gottesman, R.F.; Selvin, E. High-sensitivity cardiac troponin T and cognitive function and dementia risk: The atherosclerosis risk in communities study. Eur. Hear. J. 2014, 35, 1817–1824. [Google Scholar] [CrossRef]
- Ruiz, A.; Cruz-Lemini, M.; Masoller, N.; Sanz-Cortés, M.; Ferrer, Q.; Ribera, I.; Martínez, J.M.; Crispi, F.; Arévalo, S.; Gómez, O.; et al. Longitudinal changes in fetal biometry and cerebroplacental hemodynamics in fetuses with congenital heart disease. Ultrasound Obstet. Gynecol. 2017, 49, 379–386. [Google Scholar] [CrossRef]
- Hahn, E.; Szwast, A.; Cnota, J.; Levine, J.C.; Fifer, C.G.; Jaeggi, E.; Andrews, H.; Williams, I.A. Association between fetal growth, cerebral blood flow and neurodevelopmental outcome in univentricular fetuses. Ultrasound Obstet. Gynecol. 2016, 47, 460–465. [Google Scholar] [CrossRef]
- Zeng, S.; Zhou, Q.C.; Zhou, J.W.; Li, M.; Long, C.; Peng, Q.H. Volume of intracranial structures on three-dimensional ultrasound in fetuses with congenital heart disease. Ultrasound Obstet. Gynecol. 2015, 46, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Zeng, S.; Zhou, J.; Peng, Q.; Tian, L.; Xu, G.; Zhao, Y.; Wang, T.; Zhou, Q. Assessment by three-dimensional power Doppler ultrasound of cerebral blood flow perfusion in fetuses with congenital heart disease. Ultrasound Obstet. Gynecol. 2015, 45, 649–656. [Google Scholar] [CrossRef]
- Masoller, N.; Martínez, J.M.; Gómez, O.; Bennasar, M.; Crispi, F.; Sanz-Cortés, M.; Egaña-Ugrinovic, G.; Bartrons, J.; Puerto, B.; Gratacós, E. Evidence of second-trimester changes in head biometry and brain perfusion in fetuses with congenital heart disease. Ultrasound Obstet. Gynecol. 2014, 44, 182–187. [Google Scholar] [CrossRef]
- Williams, I.A.; Fifer, C.; Jaeggi, E.; Levine, J.C.; Michelfelder, E.C.; Szwast, A.L. The association of fetal cerebrovascular resistance with early neurodevelopment in single ventricle congenital heart disease. Am. Hear. J. 2013, 165, 544–550.e1. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Khoo, N.S.; Brooks, P.A.; Savard, W.; Hirose, A.; Hornberger, L.K. Severe left heart obstruction with retrograde arch flow influences fetal cerebral and placental blood flow. Ultrasound Obstet. Gynecol. 2013, 42, 294–299. [Google Scholar] [CrossRef]
- Szwast, A.; Tian, Z.; McCann, M.; Soffer, D.; Rychik, J. Comparative analysis of cerebrovascular resistance in fetuses with single-ventricle congenital heart disease. Ultrasound Obstet. Gynecol. 2012, 40, 62–67. [Google Scholar] [CrossRef]
- Williams, I.A.; Tarullo, A.R.; Grieve, P.G.; Wilpers, A.; Vignola, E.F.; Myers, M.M.; Fifer, W.P. Fetal cerebrovascular resistance and neonatal EEG predict 18-month neurodevelopmental outcome in infants with congenital heart disease. Ultrasound Obstet. Gynecol. 2012, 40, 304–309. [Google Scholar] [CrossRef]
- Arduini, M.; Rosati, P.; Caforio, L.; Guariglia, L.; Clerici, G.; Di Renzo, G.C.; Scambia, G. Cerebral blood flow autoregulation and congenital heart disease: Possible causes of abnormal prenatal neurologic development. J. Matern. Neonatal Med. 2011, 24, 1208–1211. [Google Scholar] [CrossRef]
- McElhinney, D.B.; Benson, C.B.; Brown, D.W.; Wilkins-Haug, L.E.; Marshall, A.C.; Zaccagnini, L.; Tworetzky, W. Cerebral Blood Flow Characteristics and Biometry in Fetuses Undergoing Prenatal Intervention for Aortic Stenosis with Evolving Hypoplastic Left Heart Syndrome. Ultrasound Med. Biol. 2010, 36, 29–37. [Google Scholar] [CrossRef]
- Berg, C.; Gembruch, O.; Gembruch, U.; Geipel, A. Doppler indices of the middle cerebral artery in fetuses with cardiac defects theoretically associated with impaired cerebral oxygen delivery in utero: Is there a brain-sparing effect? Ultrasound Obstet. Gynecol. 2009, 34, 666–672. [Google Scholar] [CrossRef]
- Brossard-Racine, M.; du Plessis, A.; Vezina, G.; Robertson, R.; Donofrio, M.; Tworetzky, W.; Limperopoulos, C. Brain Injury in Neonates with Complex Congenital Heart Disease: What Is the Predictive Value of MRI in the Fetal Period? Am. J. Neuroradiol. 2016, 37, 1338–1346. [Google Scholar] [CrossRef]
- Brossard-Racine, M.; du Plessis, A.J.; Vezina, G.; Robertson, R.; Bulas, D.; Evangelou, I.E.; Donofrio, M.; Freeman, D.; Limperopoulos, C. Prevalence and Spectrum of in Utero Structural Brain Abnormalities in Fetuses with Complex Congenital Heart Disease. Am. J. Neuroradiol. 2014, 35, 1593–1599. [Google Scholar] [CrossRef]
- Mlczoch, E.; Brugger, P.; Ulm, B.; Novak, A.; Frantal, S.; Prayer, D.; Salzer-Muhar, U. Structural congenital brain disease in congenital heart disease: Results from a fetal MRI program. Eur. J. Paediatr. Neurol. 2013, 17, 153–160. [Google Scholar] [CrossRef]
- Schellen, C.; Ernst, S.; Gruber, G.M.; Mlczoch, E.; Weber, M.; Brugger, P.C.; Ulm, B.; Langs, G.; Salzer-Muhar, U.; Prayer, D.; et al. Fetal MRI detects early alterations of brain development in Tetralogy of Fallot. Am. J. Obstet. Gynecol. 2015, 213, 392.e1–392.e7. [Google Scholar] [CrossRef] [PubMed]
- Al Nafisi, B.; van Amerom, J.F.; Forsey, J.; Jaeggi, E.; Grosse-Wortmann, L.; Yoo, S.-J.; Macgowan, C.K.; Seed, M. Fetal circulation in left-sided congenital heart disease measured by cardiovascular magnetic resonance: A case–control study. J. Cardiovasc. Magn. Reson. 2013, 15, 65. [Google Scholar] [CrossRef]
- Sun, L.; Macgowan, C.K.; Sled, J.G.; Yoo, S.-J.; Manlhiot, C.; Porayette, P.; Grosse-Wortmann, L.; Jaeggi, E.; McCrindle, B.W.; Kingdom, J.; et al. Reduced Fetal Cerebral Oxygen Consumption Is Associated with Smaller Brain Size in Fetuses with Congenital Heart Disease. Circulation 2015, 131, 1313–1323. [Google Scholar] [CrossRef]
- Limperopoulos, C.; Tworetzky, W.; McElhinney, D.B.; Newburger, J.W.; Brown, D.W.; Robertson, R.L.; Guizard, N.; McGrath, E.; Geva, J.; Annese, D.; et al. Brain Volume and Metabolism in Fetuses with Congenital Heart Disease. Circulation 2010, 121, 26–33. [Google Scholar] [CrossRef]
- Masoller, N.; Sanz-Cortés, M.; Crispi, F.; Gómez, O.; Bennasar, M.; Egaña-Ugrinovic, G.; Bargalló, N.; Martínez, J.M.; Gratacós, E. Severity of Fetal Brain Abnormalities in Congenital Heart Disease in Relation to the Main Expected Pattern of in utero Brain Blood Supply. Fetal Diagn. Ther. 2016, 39, 269–278. [Google Scholar] [CrossRef]
- Clouchoux, C.; du Plessis, A.J.; Bouyssi-Kobar, M.; Tworetzky, W.; McElhinney, D.B.; Brown, D.W.; Gholipour, A.; Kudelski, D.; Warfield, S.K.; McCarter, R.J.; et al. Delayed Cortical Development in Fetuses with Complex Congenital Heart Disease. Cereb. Cortex 2013, 23, 2932–2943. [Google Scholar] [CrossRef]
- Patel, S.R.; Michelfelder, E. Prenatal Diagnosis of Congenital Heart Disease: The Crucial Role of Perinatal and Delivery Planning. J. Cardiovasc. Dev. Dis. 2024, 11, 108. [Google Scholar] [CrossRef] [PubMed]
- Abell, B.R.; Eagleson, K.; Auld, B.; Bora, S.; Justo, R.; Parsonage, W.; Sharma, P.; Kularatna, S.; McPhail, S.M. Implementing neurodevelopmental follow-up care for children with congenital heart disease: A scoping review with evidence mapping. Dev. Med. Child Neurol. 2024, 66, 161–175. [Google Scholar] [CrossRef] [PubMed]
- de Soysa, T.Y.; Ranade, S.S.; Okawa, S.; Ravichandran, S.; Huang, Y.; Salunga, H.T.; Schricker, A.; del Sol, A.; Gifford, C.A.; Srivastava, D. Single-cell analysis of cardiogenesis reveals basis for organ-level developmental defects. Nature 2019, 572, 120–124. [Google Scholar] [CrossRef]
- Wei, N.; Lee, C.; Duan, L.; Galdos, F.X.; Samad, T.; Raissadati, A.; Goodyer, W.R.; Wu, S.M. Cardiac Development at a Single-Cell Resolution. Adv. Exp. Med. Biol. 2024, 1441, 253–268. [Google Scholar]
- Thakur, A.; Kishore, R. Neurobiology of the circadian clock and its role in cardiovascular disease: Mechanisms, biomarkers, and chronotherapy. Neurobiol. Sleep Circadian Rhythm. 2025, 19, 100131. [Google Scholar] [CrossRef]
- Li, G.; Tian, L.; Goodyer, W.; Kort, E.J.; Buikema, J.W.; Xu, A.; Wu, J.C.; Jovinge, S.; Wu, S.M. Single cell expression analysis reveals anatomical and cell cycle-dependent transcriptional shifts during heart development. Development 2019, 146, dev173476. [Google Scholar] [CrossRef]
- Landis, B.J.; Helvaty, L.R.; Geddes, G.C.; Lin, J.I.; Yatsenko, S.A.; Lo, C.W.; Border, W.L.; Wechsler, S.B.; Murali, C.N.; Azamian, M.S.; et al. A Multicenter Analysis of Abnormal Chromosomal Microarray Findings in Congenital Heart Disease. J. Am. Hear. Assoc. 2023, 12, e029340. [Google Scholar] [CrossRef]
- Woodward, A.A.; Taylor, D.M.; Goldmuntz, E.; Mitchell, L.E.; Agopian, A.J.; Moore, J.H.; Urbanowicz, R.J. Gene-Interaction-Sensitive enrichment analysis in congenital heart disease. BioData Min. 2022, 15, 4. [Google Scholar] [CrossRef]
- Luo, X.; Liu, L.; Rong, H.; Liu, X.; Yang, L.; Li, N.; Shi, H. ENU-based dominant genetic screen identifies contractile and neuronal gene mutations in congenital heart disease. Genome Med. 2024, 16, 97. [Google Scholar] [CrossRef]
- Sierant, M.C.; Jin, S.C.; Bilguvar, K.; Morton, S.U.; Dong, W.; Jiang, W.; Lu, Z.; Li, B.; López-Giráldez, F.; Tikhonova, I.; et al. Genomic analysis of 11,555 probands identifies 60 dominant congenital heart disease genes. Proc. Natl. Acad. Sci. USA 2025, 122, e2420343122. [Google Scholar] [CrossRef]
- Fear, V.S.; Forbes, C.A.; Shaw, N.C.; Farley, K.O.; Mantegna, J.L.; Htun, J.P.; Syn, G.; Viola, H.; Cserne Szappanos, H.; Hool, L.; et al. Gene editing and cardiac disease modelling for the interpretation of genetic variants of uncertain significance in congenital heart disease. Stem Cell Res. Ther. 2023, 14, 345. [Google Scholar] [CrossRef]
- Blue, G.M.; Kirk, E.P.; Giannoulatou, E.; Sholler, G.F.; Dunwoodie, S.L.; Harvey, R.P.; Winlaw, D.S. Advances in the Genetics of Congenital Heart Disease. J. Am. Coll. Cardiol. 2017, 69, 859–870. [Google Scholar] [CrossRef] [PubMed]
- Ong, L.T. Editor’s Pick: Genetics and Pathophysiology of Co-occurrence of Congenital Heart Disease and Autism Spectrum Disorder. Eur. Med. J. 2024, 9, 73–83. [Google Scholar] [CrossRef]
- Ji, W.; Ferdman, D.; Copel, J.; Scheinost, D.; Shabanova, V.; Brueckner, M.; Khokha, M.K.; Ment, L.R. De novo damaging variants associated with congenital heart diseases contribute to the connectome. Sci. Rep. 2020, 10, 7046. [Google Scholar] [CrossRef]
- Rishika, D.; RN, M.; Anurag, A.; Sumod, K.; Bhawna, M. Highly Sensitive Cardiac Troponin T as a Biomarker of Myocardial Injury in Acyanotic Congenital Heart Disease. Int. J. Pediatr. Res. 2020, 6, 1–5. [Google Scholar] [CrossRef]
- Guo, F.; Zhang, C.-C.; Yin, X.-H.; Li, T.; Fang, C.-H.; He, X.-B. Crosstalk between cardiomyocytes and noncardiomyocytes is essential to prevent cardiomyocyte apoptosis induced by proteasome inhibition. Cell Death Dis. 2020, 11, 783. [Google Scholar] [CrossRef]
- Perfetto, F.; Bergesio, F.; Emdin, M.; Cappelli, F. Troponins in cardiac amyloidosis: Multipurpose markers. Nat. Rev. Cardiol. 2014, 11, 179. [Google Scholar] [CrossRef]
- Colliva, A.; Braga, L.; Giacca, M.; Zacchigna, S. Endothelial cell–cardiomyocyte crosstalk in heart development and disease. J. Physiol. 2020, 598, 2923–2939. [Google Scholar] [CrossRef] [PubMed]
- Hilal, S.; Chai, Y.L.; Ikram, M.K.; Elangovan, S.; Yeow, T.B.; Xin, X.; Chong, J.Y.; Venketasubramanian, N.; Richards, A.M.; Chong, J.P.C.; et al. Markers of Cardiac Dysfunction in Cognitive Impairment and Dementia. Medicine 2015, 94, e297. [Google Scholar] [CrossRef] [PubMed]
- Zonneveld, M.H.; Abbel, D.; Cessie, S.l.; Jukema, J.W.; Noordam, R.; Trompet, S. Cardiac Troponin, Cognitive Function, and Dementia: A Systematic Review. Aging Dis. 2022, 14, 386–397. [Google Scholar] [CrossRef] [PubMed]
- Sterling, L.H.; Liu, A.; Ganni, E.; Therrien, J.; Dancea, A.B.; Guo, L.; Marelli, A.J. Neurocognitive disorders amongst patients with congenital heart disease undergoing procedures in childhood. Int. J. Cardiol. 2021, 336, 47–53. [Google Scholar] [CrossRef]
- Cabrera-Mino, C.; DeVon, H.A.; Aboulhosn, J.; Brecht, M.-L.; Choi, K.R.; Pike, N.A. Neurocognition in adults with congenital heart disease post-cardiac surgery: A systematic review. Hear. Lung 2024, 64, 62–73. [Google Scholar] [CrossRef]
- Homsy, J.; Zaidi, S.; Shen, Y.; Ware, J.S.; Samocha, K.E.; Karczewski, K.J.; DePalma, S.R.; McKean, D.; Wakimoto, H.; Gorham, J.; et al. De novo mutations in congenital heart disease with neurodevelopmental and other congenital anomalies. Science 2015, 350, 1262–1266. [Google Scholar] [CrossRef]
- Dowden, L.; Tucker, D.; Morgan, S.; Uzun, O.; Syed, Y.A. Contribution of Congenital Heart Disorders Associated With Copy Number Variants in Mediating Risk for Brain Developmental Disorders: Evidence From 20-Year Retrospective Cohort Study. Front. Cardiovasc. Med. 2021, 8, 655463. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).