Ultrasound-Guided Integrated Musculoskeletal and Vascular Landmark Approach for Access to the Facial Nerve Trunk
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval
2.2. Study Design
2.3. Cadaveric Procedure
2.4. Ultrasound Settings and Injection Technique
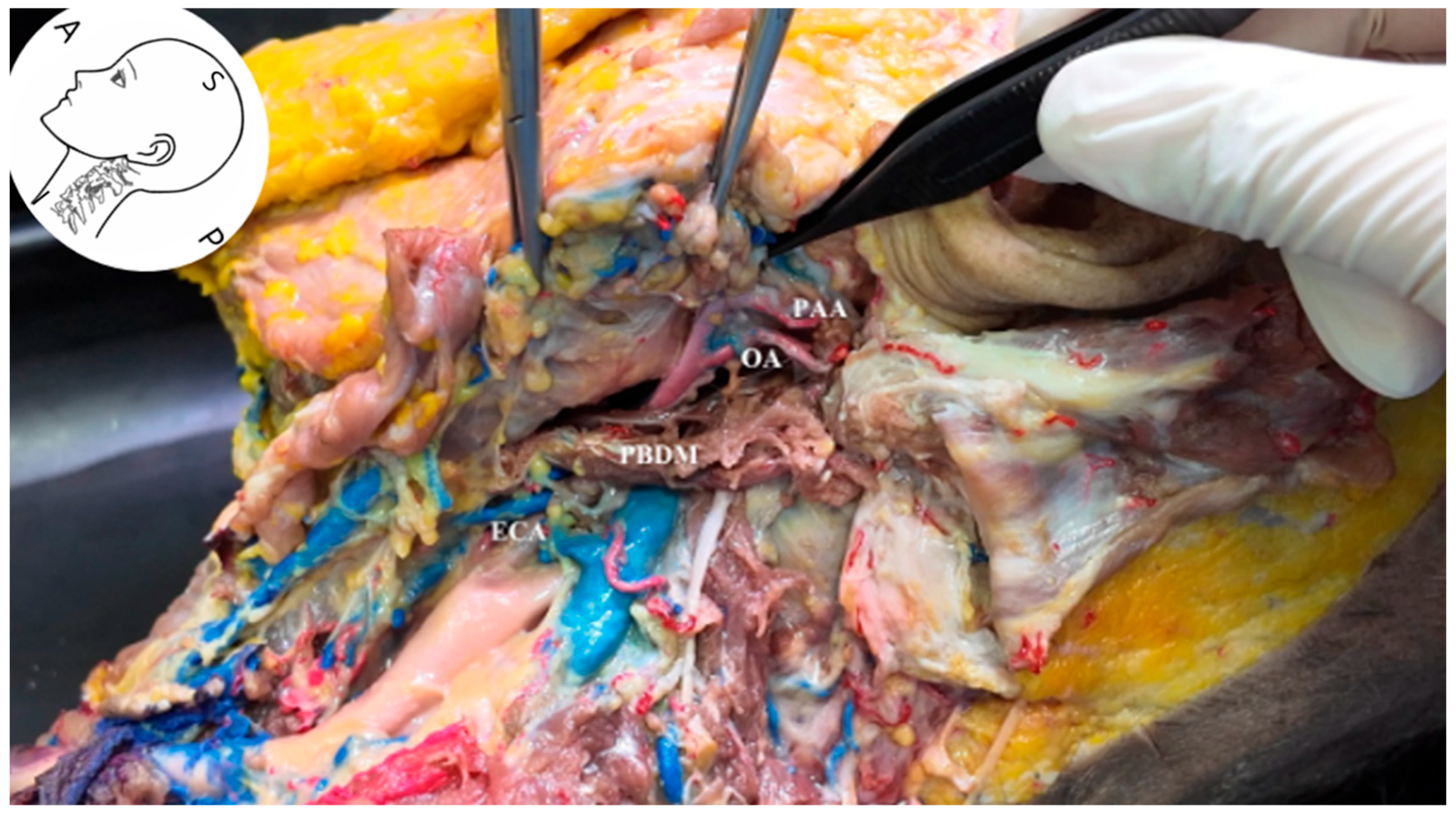
2.5. Anatomical Dissection
2.6. Live Doppler Ultrasound
3. Results
3.1. Overall Findings
3.2. Probe Positioning
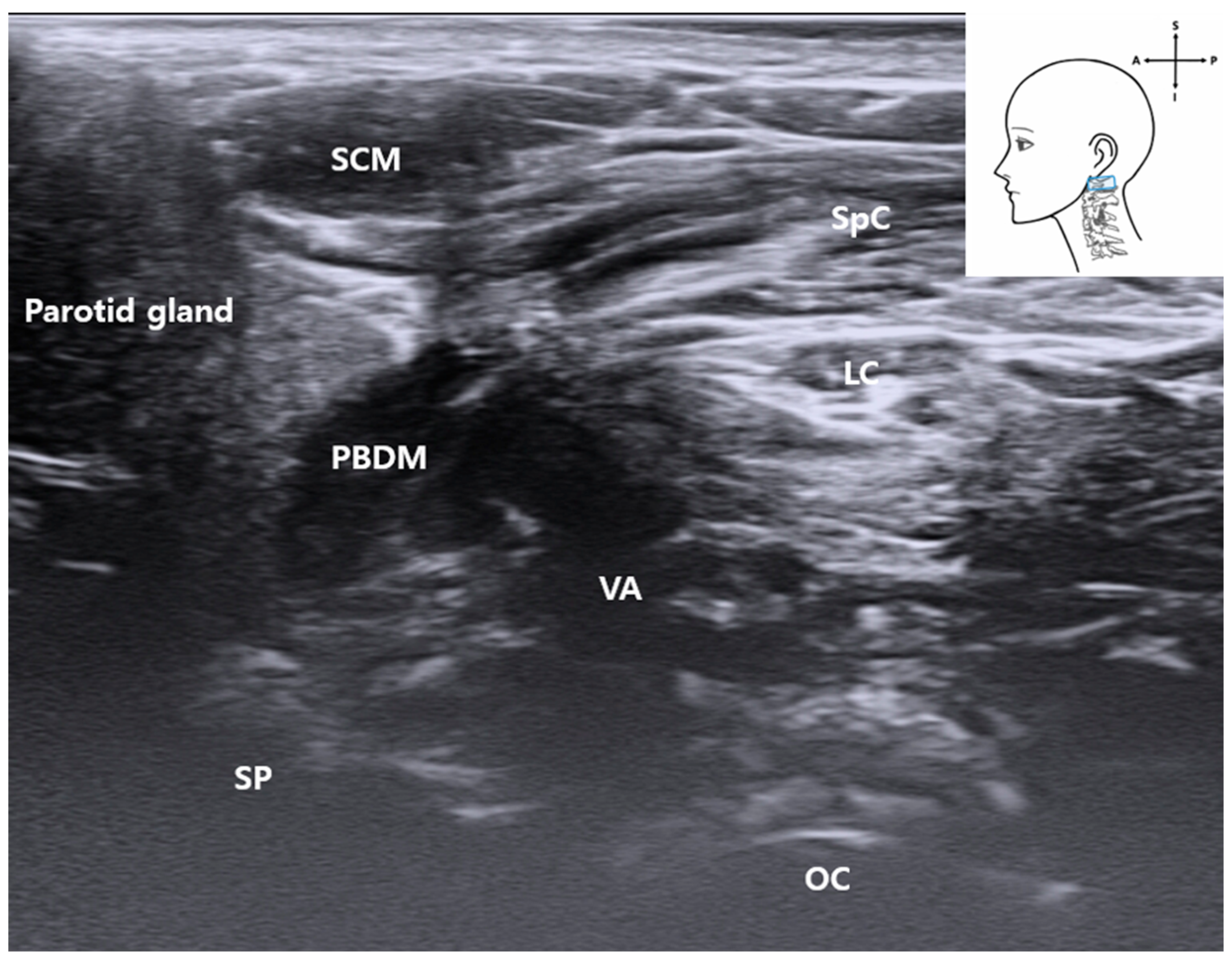
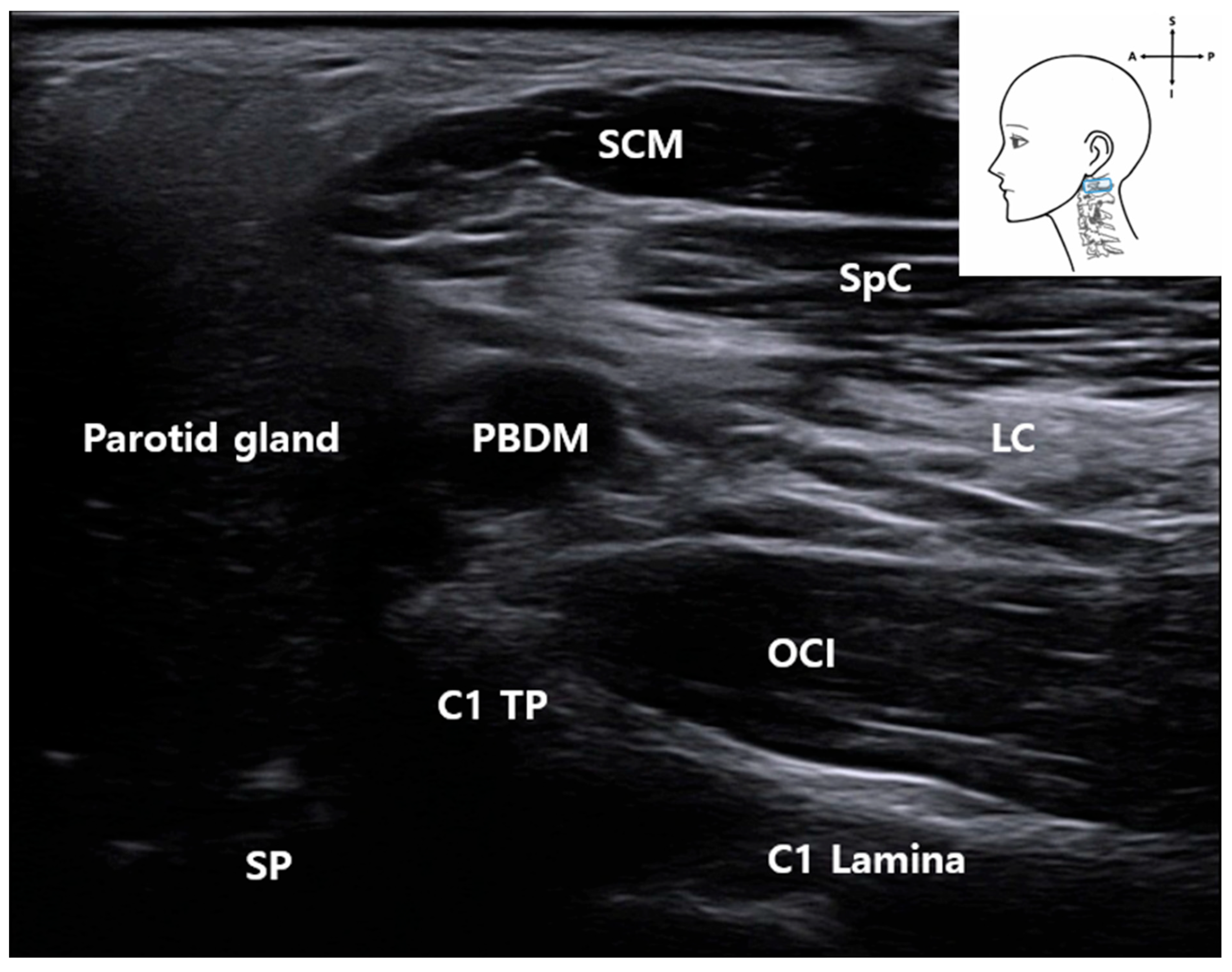
3.3. C1 Detection and Muscles
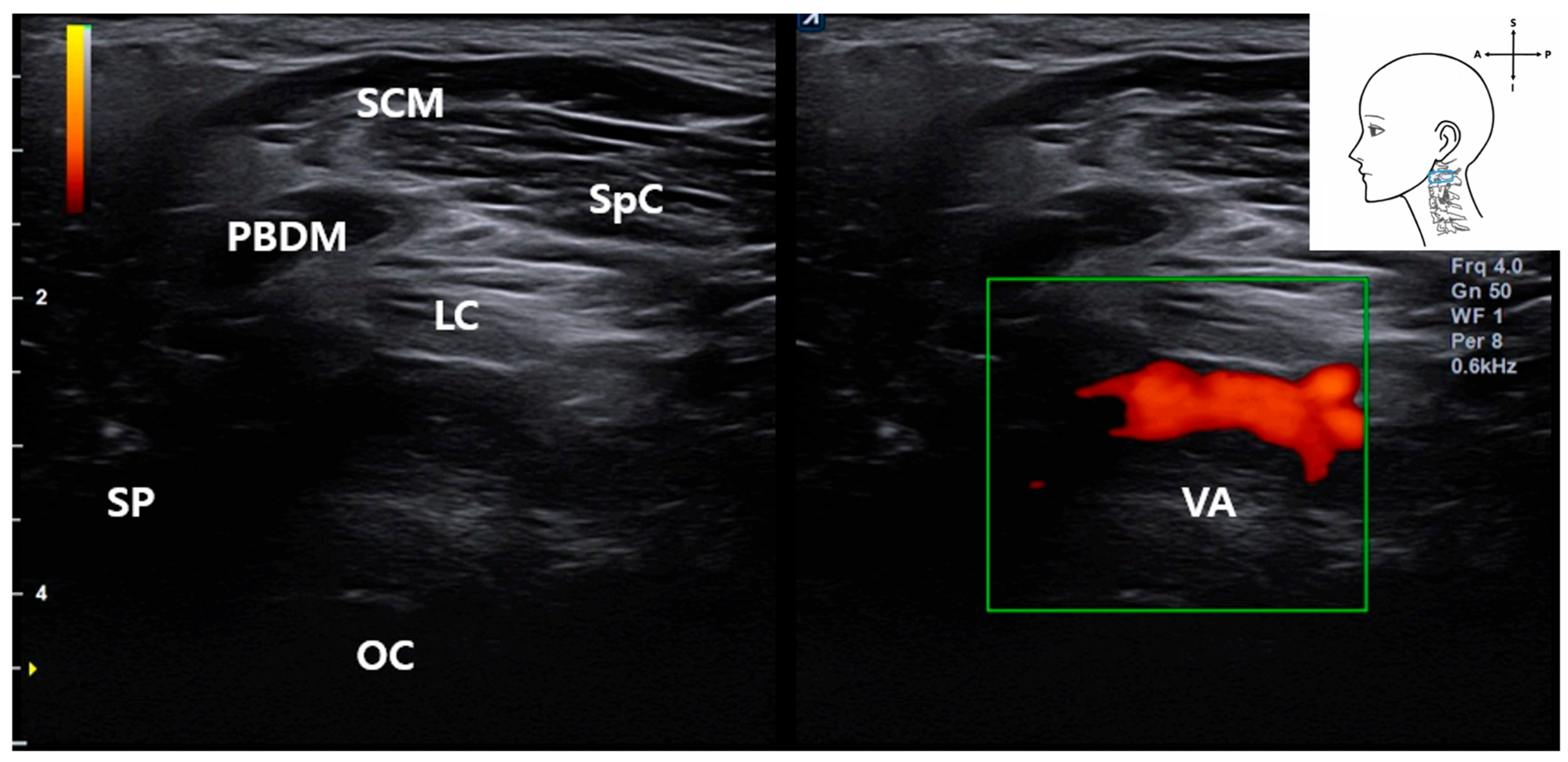
3.4. Identification of the Vertebral Artery (VA)
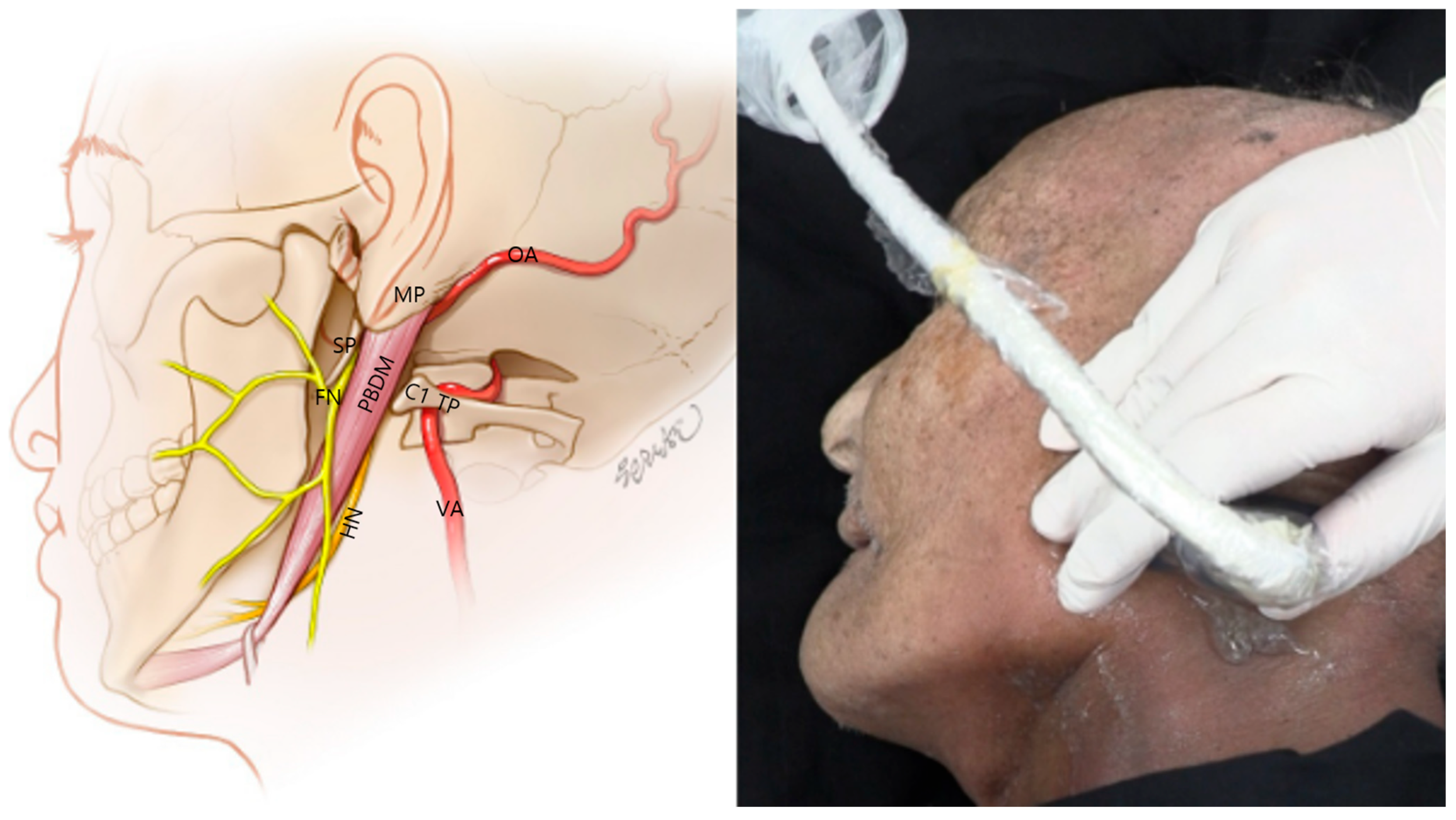
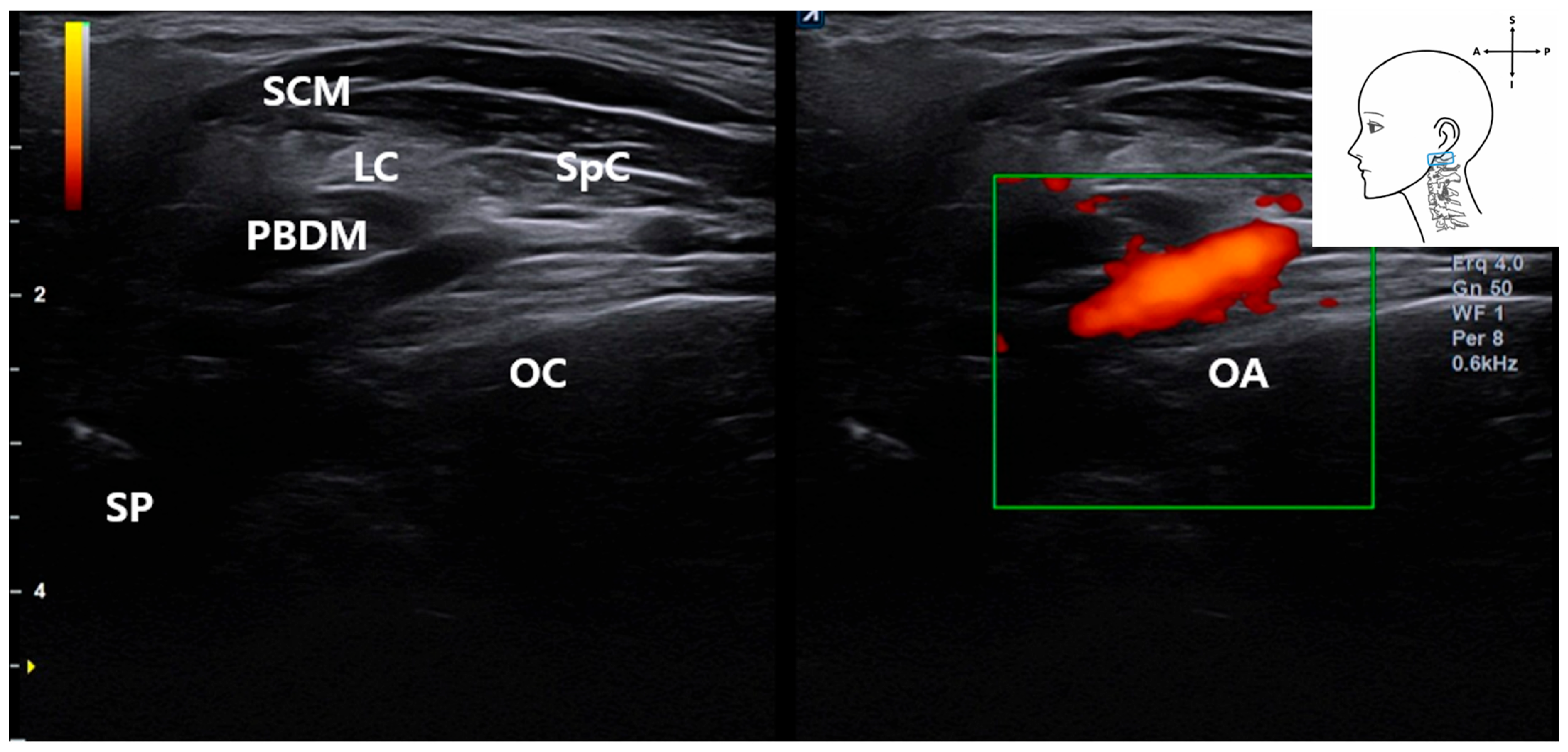
3.5. Identification of the Occipital Artery
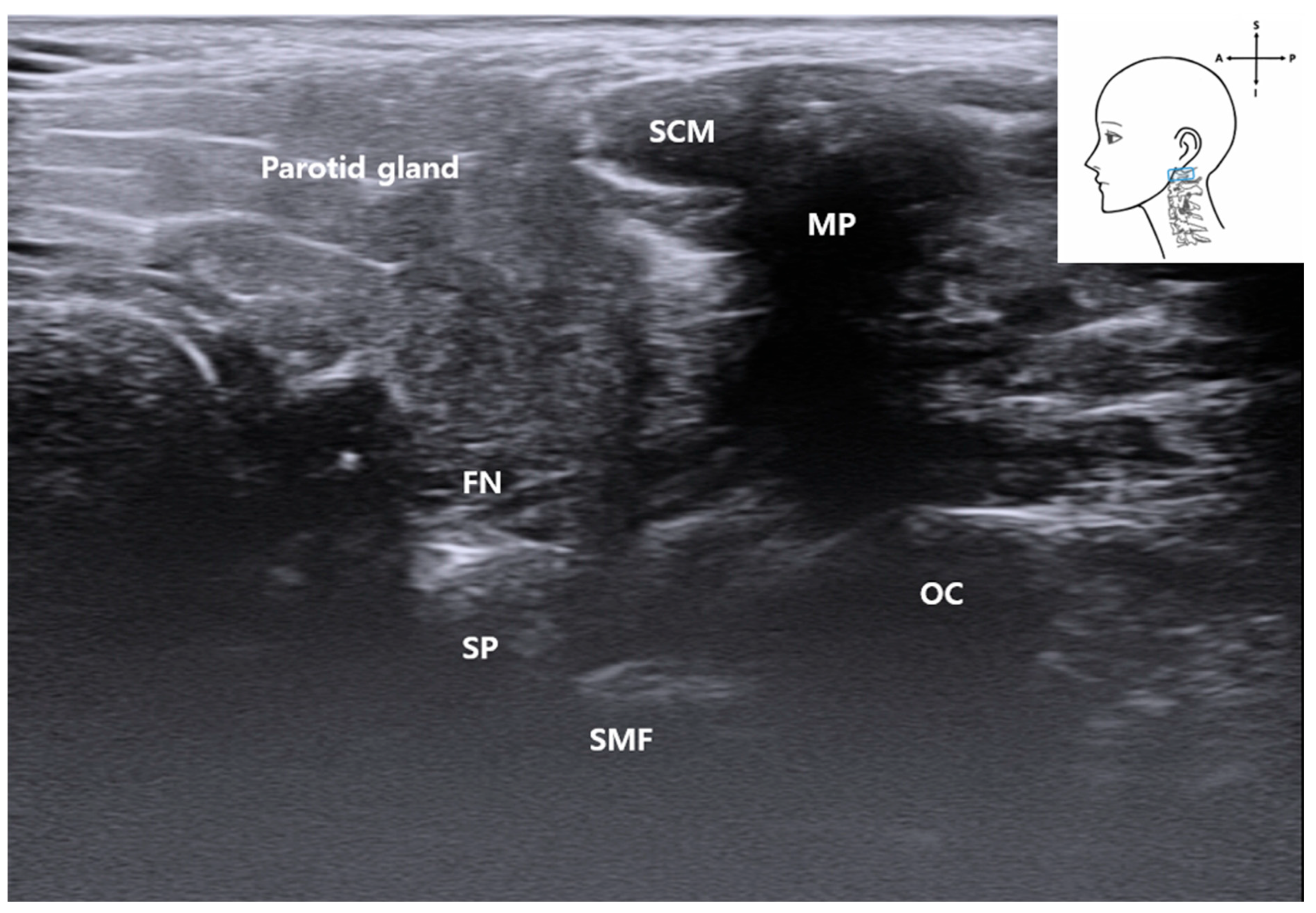
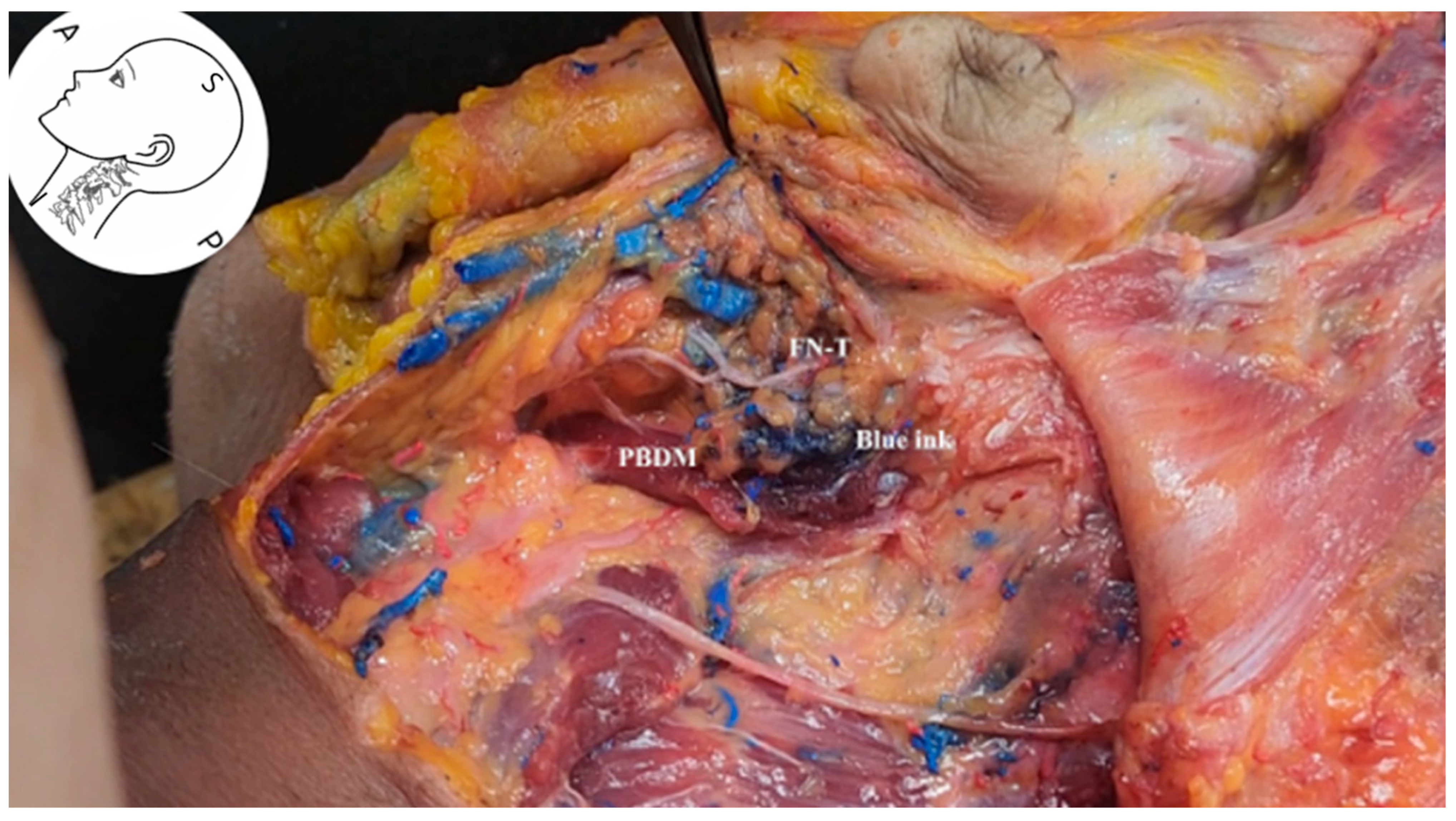
3.6. Visualization of the Stylomastoid Foramen and FN
3.7. Ultrasound-Guided Injection
4. Discussion
4.1. Hydrodissection Potential
4.2. Limitations
4.3. Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Tawfik, E.A.; Walker, F.O.; Cartwright, M.S. Neuromuscular ultrasound of cranial nerves. J. Clin. Neurol. 2015, 11, 109–121. [Google Scholar] [CrossRef] [PubMed]
- Poelaert, J.; Coopman, R.; Ureel, M.; Dhooghe, N.; Genbrugge, E.; Mwewa, T.; Blondeel, P.; Vermeersch, H. Visualization of the Facial Nerve with Ultra-high–Frequency Ultrasound. Plast. Reconstr. Surg. Glob. Open 2023, 11, e5489. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Youn, K.H.; Kim, J.S.; Kim, Y.S.; Ok, S.; Na, H.J. An Anatomic Guideline for Ultrasonographic-Guided Procedures: Ultrasonographic Anatomy of the Face and Neck for Minimally Invasive Procedures; Springer Nature: Singapore, 2021. [Google Scholar]
- Wu, W.T.; Chang, K.V.; Nanka, O.; Chang, H.C.; Ricci, V.; Mezian, K.; Ozcakar, L. Lip Sonoanatomy and Relevance to Aesthetic Filler Injections: A Pictorial Review. J. Cosmet. Dermatol. 2025, 24, e70164. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.T.; Chang, K.V.; Chang, H.C.; Kuan, C.H.; Chen, L.R.; Mezian, K.; Ricci, V.; Ozcakar, L. Ultrasound Imaging of Facial Vascular Neural Structures and Relevance to Aesthetic Injections: A Pictorial Essay. Diagnostics 2022, 12, 1766. [Google Scholar] [CrossRef] [PubMed]
- Rooks, V.J.; Shiels, W.E., 3rd; Murakami, J.W. Soft tissue foreign bodies: A training manual for sonographic diagnosis and guided removal. J. Clin. Ultrasound 2020, 48, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Hooper, N.R.; Sussman, W.I.; Bowers, R.; Williams, C. Ulnar Neuropathy Hydrodissection with Platelet Lysate and Prolotherapy: A Case Series and Review of the Literature. Cureus 2025, 17, e79791. [Google Scholar] [CrossRef] [PubMed]
- Fouda, B.H.; Abdelwahed, W.M.; Negm, E.E. Ultrasound guided hydrodissection versus open surgery in patients with severe carpel tunnel syndrome: A randomized controlled study. Egypt. J. Neurol. Psychiatry Neurosurg. 2025, 61, 23. [Google Scholar] [CrossRef]
- Lam, K.H.S.; Hung, C.Y.; Chiang, Y.P.; Onishi, K.; Su, D.C.J.; Clark, T.B.; Reeves, K.D. Ultrasound-Guided Nerve Hydrodissection for Pain Management: Rationale, Methods, Current Literature, and Theoretical Mechanisms. J. Pain Res. 2020, 13, 1957–1968. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.T.; Chen, Y.P.; Lam, K.H.S.; Reeves, K.D.; Lin, J.A.; Kuo, C.Y. Mechanism of Glucose Water as a Neural Injection: A Perspective on Neuroinflammation. Life 2022, 12, 832. [Google Scholar] [CrossRef] [PubMed]
- Cherng, J.H.; Chang, S.J.; Tsai, H.D.; Chun, C.F.; Fan, G.Y.; Reeves, K.D.; Lam, K.H.S.; Wu, Y.T. The Potential of Glucose Treatment to Reduce Reactive Oxygen Species Production and Apoptosis of Inflamed Neural Cells In Vitro. Biomedicines 2023, 11, 1837. [Google Scholar] [CrossRef] [PubMed]
- Adams, R.B. Ultrasound scanning techniques. Surg. Open Sci. 2022, 10, 182–207. [Google Scholar] [CrossRef] [PubMed]
- Singh, R. Study of Topography of Stylomastoid Foramen with Respect to Nearby Landmarks to Carry Out Facial Nerve Block with Minimum Complications. J. Craniofacl Surg. 2024, 35, 1568–1571. [Google Scholar] [CrossRef] [PubMed]
- Abdulsalam, A.J.; Aksakal, M.F.; Kara, M.; Ozcakar, L. Upscaling Facial Nerve Injections: Ultrasound Guidance to Avoid “Minimal Complications”. J. Craniofacl Surg. 2024, 35, 2200. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhang, J.; Fei, Y.; Cui, X.; Shen, L.; Huang, Y. Proposal of a Route Map for Cervical Spinal Ultrasonography: A Simple and Clear Learning Tool for Beginners. Pain Ther. 2023, 12, 1293–1305. [Google Scholar] [CrossRef] [PubMed]
- Kreutz-Rodrigues, L.; Millesi, E.; Robertson, C.E.; Oishi, T.; Lee, R.; Cook, J.L.; Gibreel, W.; Mardini, S. Unveiling Novel Surgical Treatments for Facial Synkinesis: Myectomy of the Posterior Belly of Digastric and Stylohyoid Muscle. Plast. Reconstr. Surg. Glob. Open 2025, 13, e6677. [Google Scholar] [CrossRef] [PubMed]
- Tewari, S.; Gupta, C.; Palimar, V. A Morphometric Study of Stylomastoid Foramen with Its Clinical Applications. J. Neurol. Surg. B Skull Base 2022, 83, 33–36. [Google Scholar] [CrossRef] [PubMed]
- Meng, S.; Reissig, L.F.; Tzou, C.H.; Meng, K.; Grisold, W.; Weninger, W. Ultrasound of the Hypoglossal Nerve in the Neck: Visualization and Initial Clinical Experience with Patients. Am. J. Neuroradiol. 2016, 37, 354–359. [Google Scholar] [CrossRef] [PubMed]
- Picasso, R.; Zaottini, F.; Pistoia, F.; Carobbio, A.; Ascoli, A.; Barabino, E.; Perez, M.M.; Parrinello, G.; Peretti, G.; Martinoli, C. High-resolution ultrasound of the marginal mandibular branch of the facial nerve: Normal appearance and pathological findings in a postsurgical case series. Head Neck 2021, 43, 2571–2579. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seo, Y.-S.; Yoon, Y.; Lam, K.H.S.; Kim, S.-H.; Kim, I.-B.; Youn, K.-H. Ultrasound-Guided Integrated Musculoskeletal and Vascular Landmark Approach for Access to the Facial Nerve Trunk. Life 2025, 15, 1396. https://doi.org/10.3390/life15091396
Seo Y-S, Yoon Y, Lam KHS, Kim S-H, Kim I-B, Youn K-H. Ultrasound-Guided Integrated Musculoskeletal and Vascular Landmark Approach for Access to the Facial Nerve Trunk. Life. 2025; 15(9):1396. https://doi.org/10.3390/life15091396
Chicago/Turabian StyleSeo, Yeui-Seok, Yonghyun Yoon, King Hei Stanley Lam, Sang-Hyun Kim, In-Beom Kim, and Kwan-Hyun Youn. 2025. "Ultrasound-Guided Integrated Musculoskeletal and Vascular Landmark Approach for Access to the Facial Nerve Trunk" Life 15, no. 9: 1396. https://doi.org/10.3390/life15091396
APA StyleSeo, Y.-S., Yoon, Y., Lam, K. H. S., Kim, S.-H., Kim, I.-B., & Youn, K.-H. (2025). Ultrasound-Guided Integrated Musculoskeletal and Vascular Landmark Approach for Access to the Facial Nerve Trunk. Life, 15(9), 1396. https://doi.org/10.3390/life15091396






