Comparative Effectiveness and Safety of Fractional Laser and Fractional Radiofrequency for Atrophic Acne Scars: A Retrospective Propensity Score Analysis
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Baseline Characteristics and Propensity Score Stratification
3.2. Efficacy Outcomes
3.3. Safety and Tolerability
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AFL | Ablative Fractional Laser |
| CI | Confidence Interval |
| EBD | Energy-Based Device |
| Er:YAG | Erbium-Doped Yttrium Aluminum Garnet |
| FL | Fractional Laser |
| FRF | Fractional Radiofrequency |
| IQR | Interquartile Range |
| PIH | Post-Inflammatory Hyperpigmentation |
| PS | Propensity Score |
| RF | Radiofrequency |
| SD | Standard Deviation |
| STD | Standardized Difference |
| TOST | Two One-Sided Test |
References
- Layton, A.; Henderson, C.; Cunliffe, W. A clinical evaluation of acne scarring and its incidence. Clin. Exp. Dermatol. 1994, 19, 303–308. [Google Scholar] [CrossRef]
- Holland, D.; Jeremy, A.; Roberts, S.; Seukeran, D.; Layton, A.; Cunliffe, W. Inflammation in acne scarring: A comparison of the responses in lesions from patients prone and not prone to scar. Br. J. Dermatol. 2004, 150, 72–81. [Google Scholar] [CrossRef]
- Lee, W.; Jung, H.; Lim, H.; Jang, Y.; Lee, S.J.; Kim, D. Serial sections of atrophic acne scars help in the interpretation of microscopic findings and the selection of good therapeutic modalities. J. Eur. Acad. Dermatol. Venereol. 2013, 27, 643–646. [Google Scholar] [CrossRef]
- Jacob, C.I.; Dover, J.S.; Kaminer, M.S. Acne scarring: A classification system and review of treatment options. J. Am. Acad. Dermatol. 2001, 45, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Dréno, B.; Tan, J.; Kang, S.; Rueda, M.-J.; Torres Lozada, V.; Bettoli, V.; Layton, A.M. How people with facial acne scars are perceived in society: An online survey. Dermatol. Ther. 2016, 6, 207–218. [Google Scholar] [CrossRef]
- Bhargava, S.; Cunha, P.R.; Lee, J.; Kroumpouzos, G. Acne scarring management: Systematic review and evaluation of the evidence. Am. J. Clin. Dermatol. 2018, 19, 459–477. [Google Scholar] [CrossRef] [PubMed]
- Kroepfl, L.; Emer, J.J. Combination Therapy for Acne Scarring: Personal Experience and Clinical Suggestions. J. Drugs Dermatol. 2016, 15, 1413–1419. [Google Scholar]
- Salameh, F.; Shumaker, P.R.; Goodman, G.J.; Spring, L.K.; Seago, M.; Alam, M.; Al-Niaimi, F.; Cassuto, D.; Chan, H.H.; Dierickx, C. Energy-based devices for the treatment of Acne Scars: 2022 International consensus recommendations. Lasers Surg. Med. 2022, 54, 10–26. [Google Scholar] [CrossRef] [PubMed]
- Sadick, N.S.; Cardona, A. Laser treatment for facial acne scars: A review. J. Cosmet. Laser Ther. 2018, 20, 424–435. [Google Scholar] [CrossRef]
- Manuskiatti, W.; Iamphonrat, T.; Wanitphakdeedecha, R.; Eimpunth, S. Comparison of fractional erbium-doped yttrium aluminum garnet and carbon dioxide lasers in resurfacing of atrophic acne scars in Asians. Dermatol. Surg. Off. Publ. Am. Soc. Dermatol. Surg. 2013, 39, 111–120. [Google Scholar] [CrossRef]
- Kwon, H.H.; Park, H.Y.; Choi, S.C.; Bae, Y.; Kang, C.; Jung, J.Y.; Park, G.H. Combined Fractional Treatment of Acne Scars Involving Non-ablative 1550-nm Erbium-glass Laser and Micro-needling Radiofrequency: A 16-week Prospective, Randomized Split-face Study. Acta Derm.-Venereol. 2017, 97, 947–951. [Google Scholar] [CrossRef]
- Abdel Hay, R.; Shalaby, K.; Zaher, H.; Hafez, V.; Chi, C.C.; Dimitri, S.; Nabhan, A.F.; Layton, A.M. Interventions for acne scars. Cochrane Database Syst. Rev. 2016, 4, Cd011946. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.G.; Jo, C.E.; Chapas, A.; Khetarpal, S.; Dover, J.S. Radiofrequency Microneedling: A Comprehensive and Critical Review. Dermatol. Surg. Off. Publ. Am. Soc. Dermatol. Surg. 2021, 47, 755–761. [Google Scholar] [CrossRef]
- Hruza, G.; Taub, A.F.; Collier, S.L.; Mulholland, S.R. Skin rejuvenation and wrinkle reduction using a fractional radiofrequency system. J. Drugs Dermatol. 2009, 8, 259–265. [Google Scholar]
- Tan, J.; Thiboutot, D.; Gollnick, H.; Kang, S.; Layton, A.; Leyden, J.J.; Torres, V.; Guillemot, J.; Dréno, B. Development of an atrophic acne scar risk assessment tool. J. Eur. Acad. Dermatol. Venereol. JEADV 2017, 31, 1547–1554. [Google Scholar] [CrossRef]
- Alajlan, A.M.; Alsuwaidan, S.N. Acne scars in ethnic skin treated with both non-ablative fractional 1550 nm and ablative fractional CO2 lasers: Comparative retrospective analysis with recommended guidelines. Lasers Surg. Med. 2011, 43, 787–791. [Google Scholar] [CrossRef] [PubMed]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. BMJ 2007, 335, 806–808. [Google Scholar] [CrossRef]
- Zhou, Y.; Hamblin, M.R.; Wen, X. An update on fractional picosecond laser treatment: Histology and clinical applications. Lasers Med. Sci. 2023, 38, 45. [Google Scholar] [CrossRef] [PubMed]
- Lyu, J.-J.; Liu, S.-X. Radiofrequency in facial rejuvenation. Int. J. Dermatol. Venereol. 2022, 5, 94–100. [Google Scholar] [CrossRef]
- Dai, R.; Cao, Y.; Su, Y.; Cai, S. Comparison of 1064-nm Nd:YAG picosecond laser using fractional micro-lens array vs. ablative fractional 2940-nm Er:YAG laser for the treatment of atrophic acne scar in Asians: A 20-week prospective, randomized, split-face, controlled pilot study. Front. Med. 2023, 10, 1248831. [Google Scholar] [CrossRef]
- Li, J.; Duan, F.; Kuang, J. Fractional picosecond laser for atrophic acne scars: A meta-analysis. J. Cosmet. Dermatol. 2023, 22, 2205–2217. [Google Scholar] [CrossRef]
- Zhang, M.; Fang, J.; Wu, Q.; Lin, T. A comparison of efficacy and safety of picosecond alexandrite laser versus ultrapulsed fractional CO2 laser for the treatment of facial atrophic acne scars. Chin. J. Dermatol. 2020, 53, 602–606. [Google Scholar] [CrossRef]
- Li, J.; Duan, F.; Kuang, J.; Liu, X.; Pan, J.; Wei, J.; Zhao, J. Fractional picosecond laser treatment of non-acne atrophic scars and scar erythema in Chinese patients. Skin Res. Technol. 2024, 30, e13856. [Google Scholar] [CrossRef]
- Hendel, K.; Karmisholt, K.; Hedelund, L.; Haedersdal, M. Fractional CO2 -laser versus microneedle radiofrequency for acne scars: A randomized, single treatment, split-face trial. Lasers Surg. Med. 2023, 55, 335–343. [Google Scholar] [CrossRef]
- Rajput, C.D.; Gore, S.B.; Ansari, M.K.; Shah, S.M. A Prospective, Nonrandomized, Open-label Study, Comparing the Efficacy, Safety, and Tolerability of Fractional CO2 Laser versus Fractional Microneedling Radio Frequency in Acne Scars. J. Cutan. Aesthetic Surg. 2021, 14, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Sriram, R.; Chandrashekar, B.S.; Madura, C.; Gowda, H.H. Comparative study in treatment of acne scars fractional carbon dioxide laser versus micro needling fractional radio frequency—A retrospective study. J. Cutan. Aesthetic Surg. 2024, 17, 214–218. [Google Scholar] [CrossRef] [PubMed]
- Nobari, N.N.; Tabavar, A.; Sadeghi, S.; Dehghani, A.; Kalantari, Y.; Ghassemi, M.; Atefi, N.; Goodarzi, A. A systematic review of the comparison between needling (RF-needling, meso-needling, and micro-needling) and ablative fractional lasers (CO2, erbium YAG) in the treatment of atrophic and hypertrophic scars. Lasers Med. Sci. 2023, 38, 67. [Google Scholar] [CrossRef] [PubMed]
- Li, M.Y.; Huang, Y.L.; Chen, J.H.; Kang, Y.N.; Chen, C. Effectiveness and Safety of Energy-Based Devices for Acne Scars: A Network Meta-Analysis of Randomized Controlled Trials. Facial Plast. Surg. Aesthetic Med. 2023, 25, 521–527. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, Z.; Cai, L.; Zhang, F. Comparative efficacy and safety of six photoelectric therapies for the atrophic acne scars: A network meta-analysis. Indian J. Dermatol. Venereol. Leprol. 2023, 89, 353–362. [Google Scholar] [CrossRef]
- Cohen, S.R.; Goodacre, A.; Lim, S.; Johnston, J.; Henssler, C.; Jeffers, B.; Saad, A.; Leong, T. Clinical Outcomes and Complications Associated with Fractional Lasers: A Review of 730 Patients. Aesthetic Plast. Surg. 2017, 41, 171–178. [Google Scholar] [CrossRef]
- Hantash, B.M.; Bedi, V.P.; Kapadia, B.; Rahman, Z.; Jiang, K.; Tanner, H.; Chan, K.F.; Zachary, C.B. In vivo histological evaluation of a novel ablative fractional resurfacing device. Lasers Surg. Med. 2007, 39, 96–107. [Google Scholar] [CrossRef] [PubMed]
- Juhasz, M.L.W.; Cohen, J.L. Microneedling for the Treatment of Scars: An Update for Clinicians. Clin. Cosmet. Investig. Dermatol. 2020, 13, 997–1003. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Fractional Laser Treatment (n = 254) | Fractional Radiofrequency Treatment (n = 143) | Standardized Difference (STD) Before PS Stratification | Standardized Difference (STD) After PS Stratification |
|---|---|---|---|---|
| N (%) or Mean ± SD | N (%) or Mean ± SD | |||
| Demographic factors | ||||
| Age (years) | 33.17 ± 7.41 | 34.29 ± 10.67 | 0.216 | 0.051 |
| Gender | −0.079 | −0.080 | ||
| Male | 96 (37.80) | 42 (29.37) | ||
| Female | 158 (62.20) | 101 (70.63) | ||
| Skin phototypes | ||||
| III | 56 (22.05) | 37 (25.87) | 0.110 | −0.017 |
| IV | 166 (65.35) | 90 (62.94) | −0.115 | 0.005 |
| V | 32 (12.60) | 16 (11.19) | 0.026 | 0.016 |
| Clinical factors | ||||
| Scar age (years) | 15.30 ± 7.07 | 8.47 ± 3.96 | −1.040 | −0.045 |
| Severity of acne scar | 0.092 | −0.020 | ||
| Almost clear—Mild | 27 (10.63) | 7 (4.90) | ||
| Moderate—very severe | 227 (89.37) | 136 (95.10) | ||
| Type of acne scar | −0.139 | 0.051 | ||
| 1 | 54 (21.26) | 44 (30.77) | ||
| >1 | 200 (78.74) | 99 (69.23) | ||
| Outcome | Fractional Laser Treatment (95% CI) | Fractional Radiofrequency Treatment (95% CI) | Mean Difference (95% CI) | p Value |
|---|---|---|---|---|
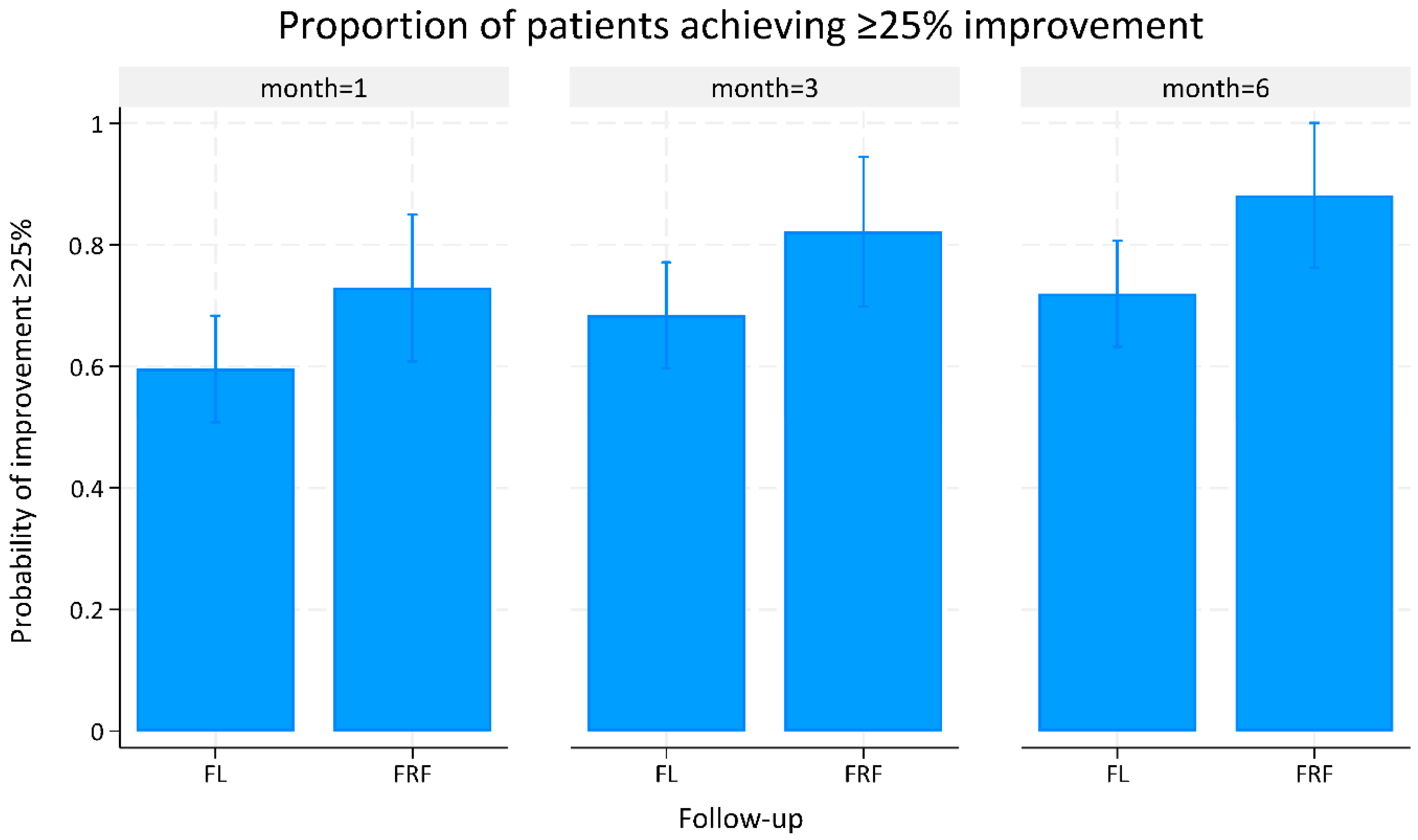
| Proportion of ≥25% clinical improvement | ||||
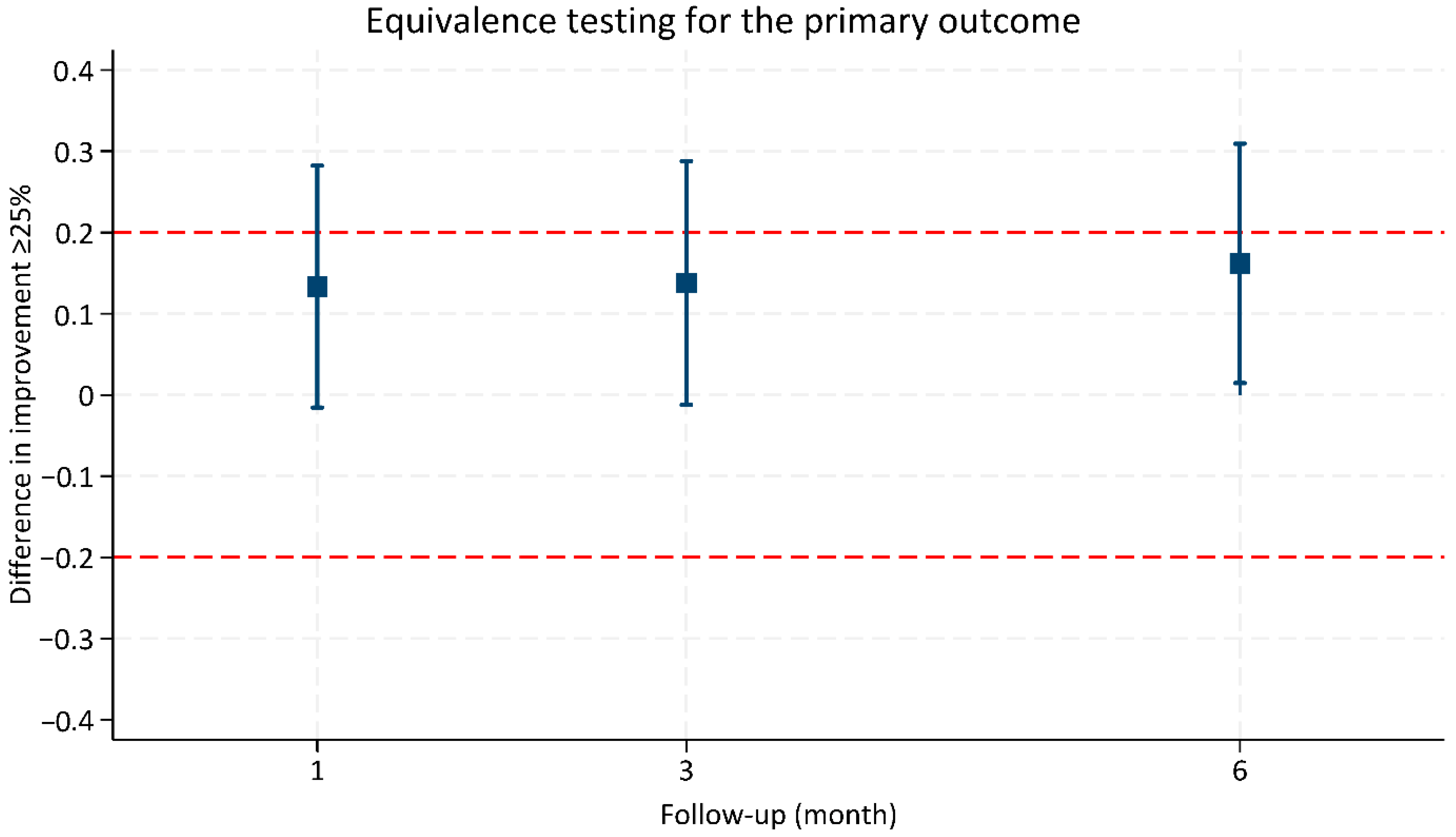
| 1-month follow-up | 0.60 (0.51 to 0.68) | 0.73 (0.61 to 0.85) | 0.13 (−0.02 to 0.28) | 0.080 |
| 3-month follow-up | 0.69 (0.60 to 0.77) | 0.82 (0.70 to 0.94) | 0.13 (−0.01 to 0.29) | 0.072 |
| 6-month follow-up | 0.72 (0.63 to 0.81) | 0.88 (0.76 to 1.00) | 0.16 (0.01 to 0.31) | 0.031 * |
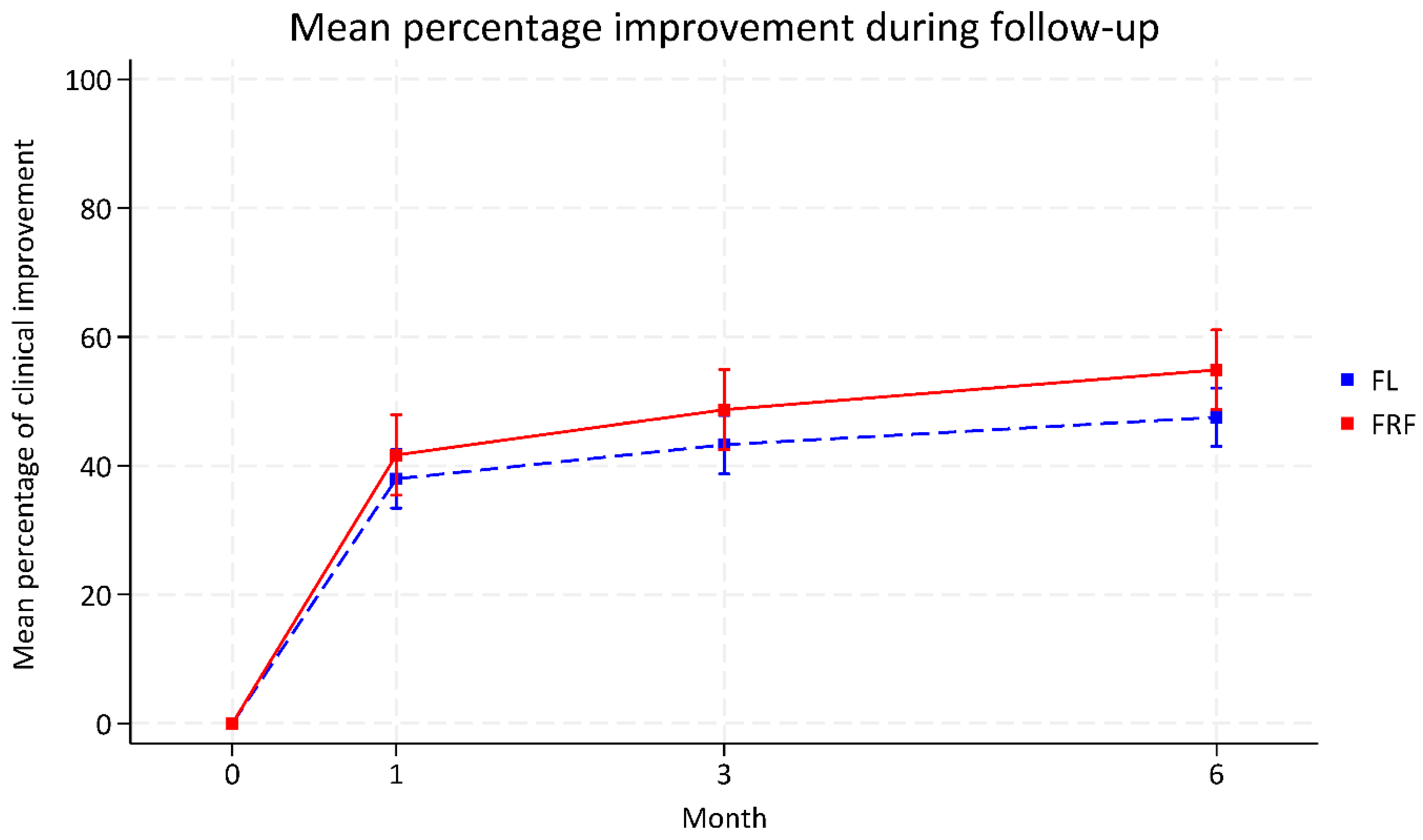
| Percentage of clinical improvement | ||||
| 1-month follow-up | 37.98 (33.44 to 42.52) | 41.68 (35.44 to 47.92) | 3.70 (−4.01 to 11.41) | 0.347 |
| 3-month follow-up | 43.27 (38.73 to 47.81) | 48.71 (42.47 to 54.94) | 5.43 −2.28 to 13.15 | 0.167 |
| 6-month follow-up | 47.53 (43.00 to 52.05) | 54.89 (48.70 to 61.07) | 7.35 −0.29 to 15.00) | 0.059 |
| Side Effects/Adverse Events | Fractional Laser Treatment (n = 254) | Fractional Radiofrequency Treatment (n = 143) | p-Value | Standardized Difference (STD) |
|---|---|---|---|---|
| Average Pain score (n = 203) | 4.14 ± 1.83 | 5.65 ± 1.74 | <0.001 | −0.846 |
| Post-inflammatory hyperpigmentation (n = 325) | 13 (5.42) | 4 (4.71) | 1.000 | 0.032 |
| Acneiform eruption (n = 325) | 16 (6.67) | 3 (3.53) | 0.421 | 0.143 |
| Infection (n = 325) | 0 (0) | 0 (0) | - | - |
| Post-treatment scar (n = 372) | 2 (0.83) | 4 (3.03) | 0.191 | −0.160 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, C.; Phinyo, P.; Yogya, Y.; Chuamanochan, M.; Wanitphakdeedecha, R. Comparative Effectiveness and Safety of Fractional Laser and Fractional Radiofrequency for Atrophic Acne Scars: A Retrospective Propensity Score Analysis. Life 2025, 15, 1379. https://doi.org/10.3390/life15091379
Yan C, Phinyo P, Yogya Y, Chuamanochan M, Wanitphakdeedecha R. Comparative Effectiveness and Safety of Fractional Laser and Fractional Radiofrequency for Atrophic Acne Scars: A Retrospective Propensity Score Analysis. Life. 2025; 15(9):1379. https://doi.org/10.3390/life15091379
Chicago/Turabian StyleYan, Chadakan, Phichayut Phinyo, Yuri Yogya, Mati Chuamanochan, and Rungsima Wanitphakdeedecha. 2025. "Comparative Effectiveness and Safety of Fractional Laser and Fractional Radiofrequency for Atrophic Acne Scars: A Retrospective Propensity Score Analysis" Life 15, no. 9: 1379. https://doi.org/10.3390/life15091379
APA StyleYan, C., Phinyo, P., Yogya, Y., Chuamanochan, M., & Wanitphakdeedecha, R. (2025). Comparative Effectiveness and Safety of Fractional Laser and Fractional Radiofrequency for Atrophic Acne Scars: A Retrospective Propensity Score Analysis. Life, 15(9), 1379. https://doi.org/10.3390/life15091379







