Refractive Outcomes in Keratoconus Patients Following Toric Lens Implantation: A Systematic Review and Single-Group Meta-Analysis
Abstract
1. Introduction
2. Methods
2.1. Search Strategy
2.2. Study Eligibility Criteria
2.3. Screening and Data Extraction
2.4. Quality Assessment
2.5. Outcome Measures and Statistical Analysis
3. Results
3.1. The Literature Search
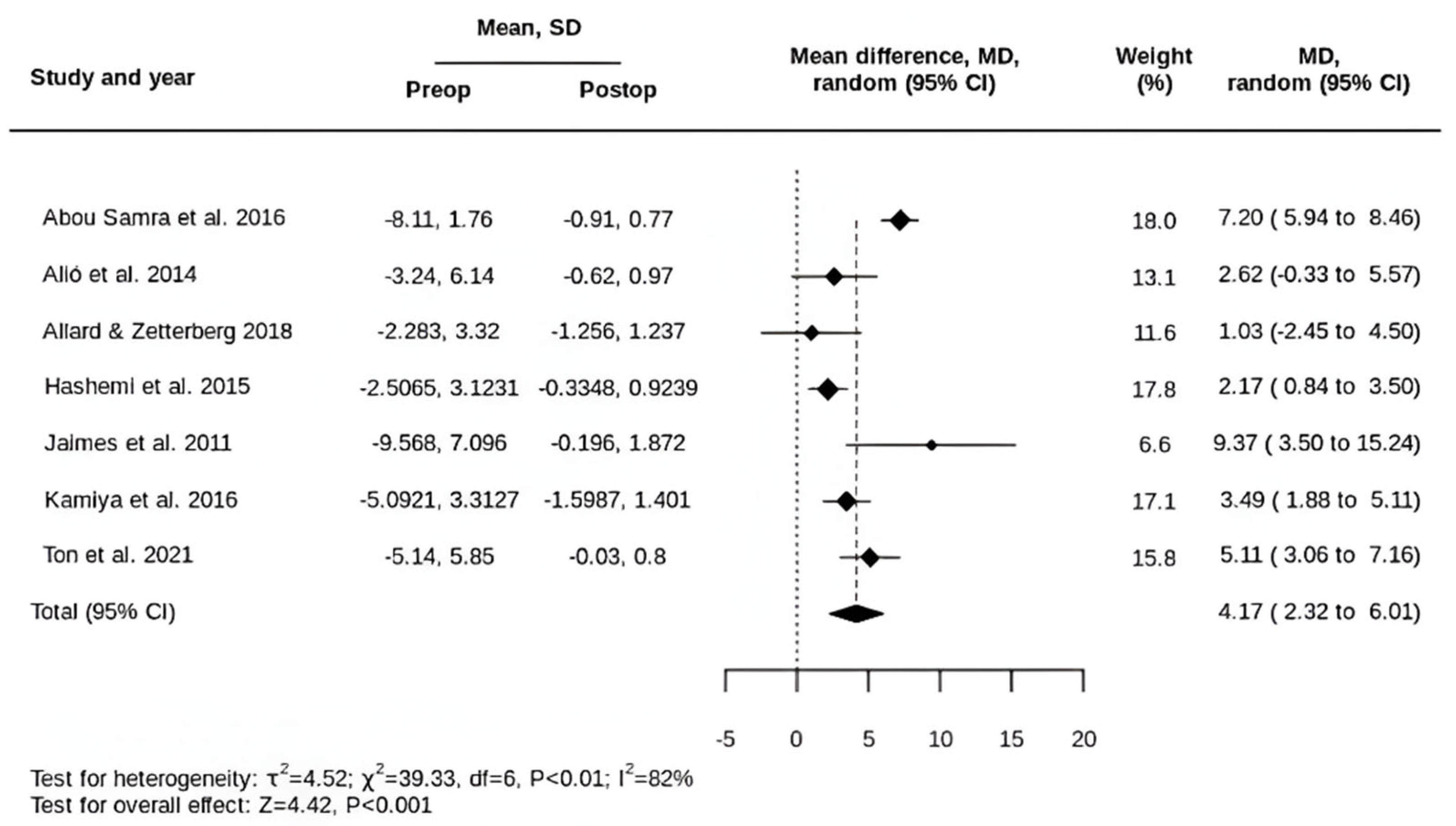
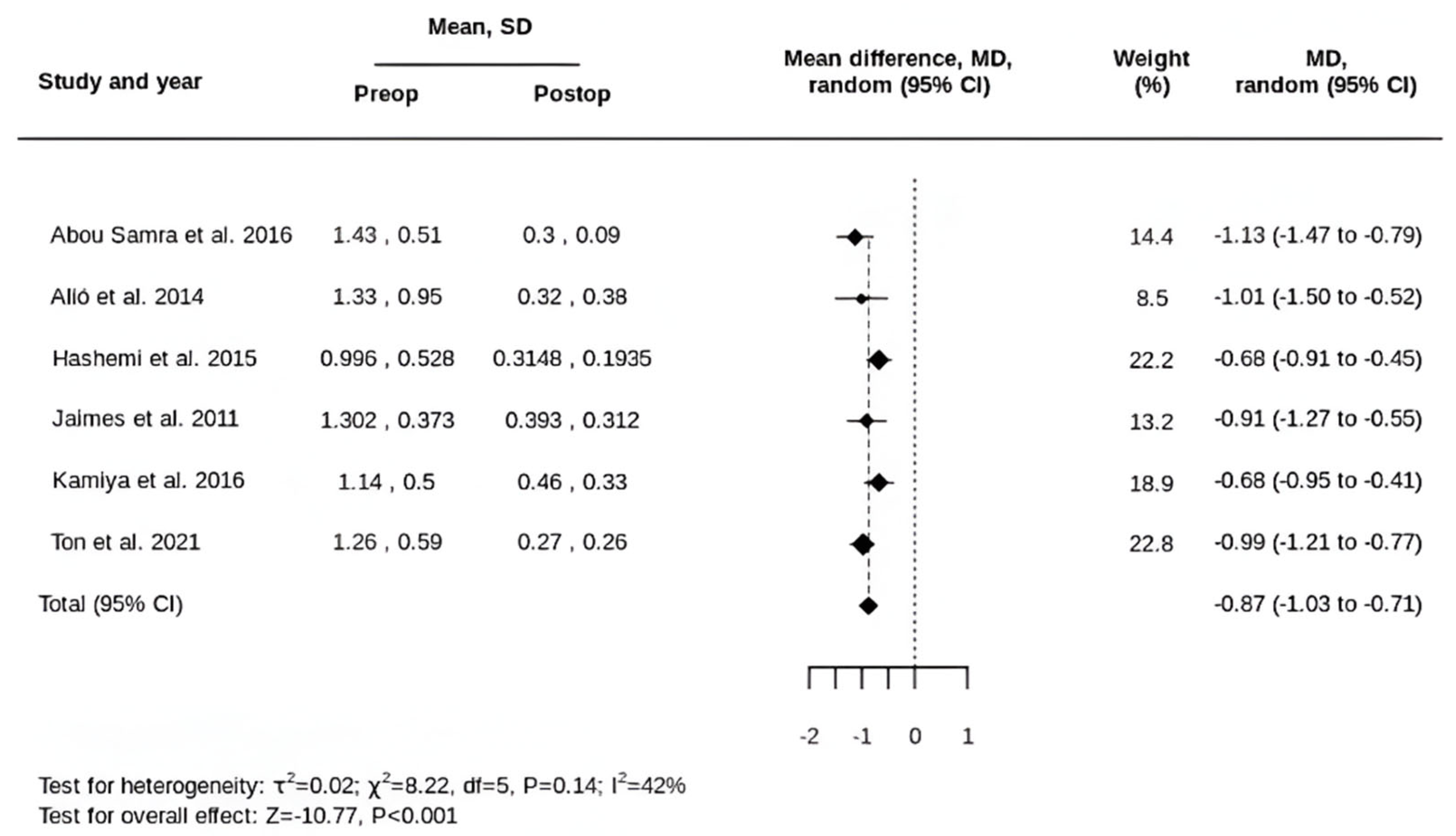
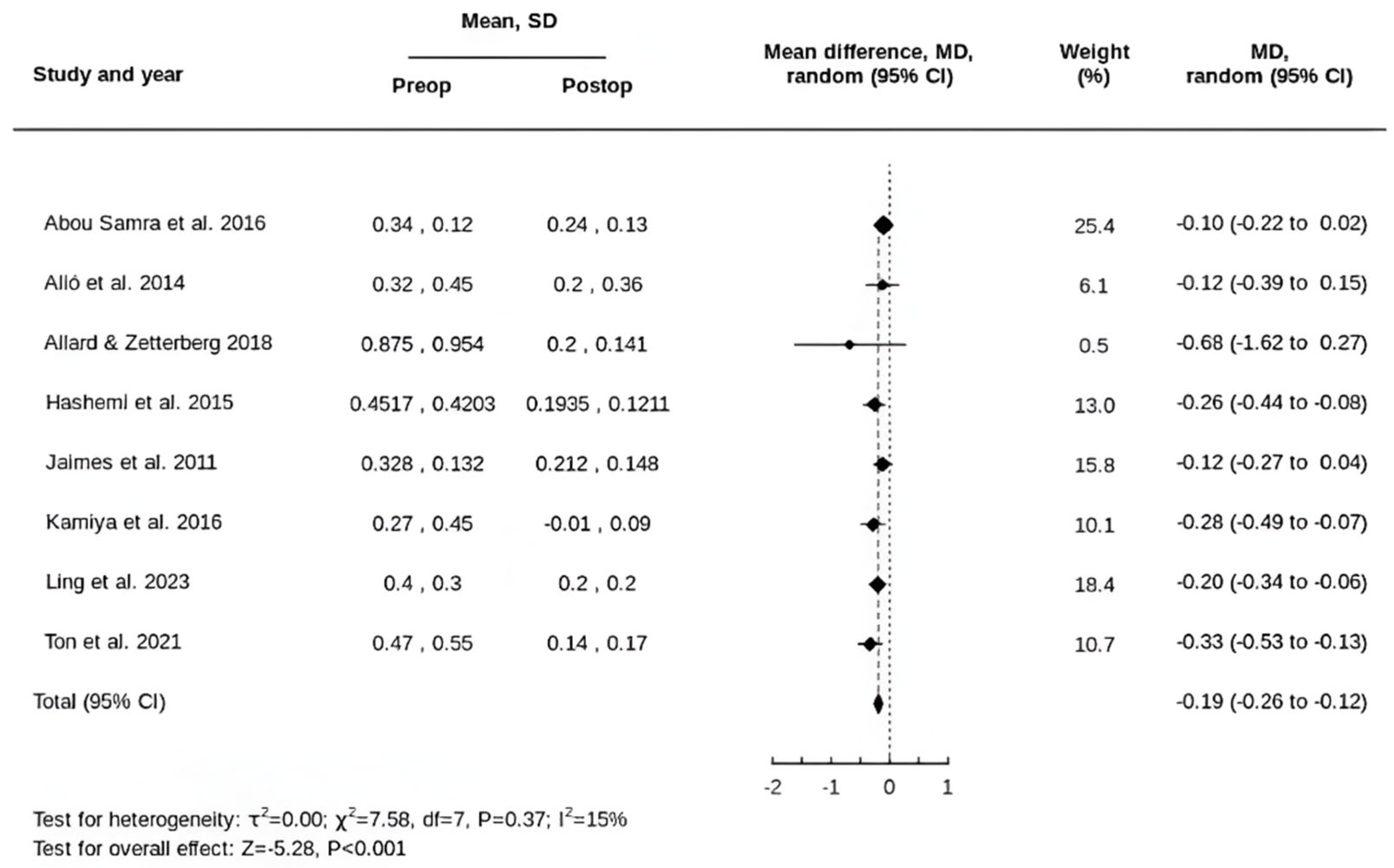
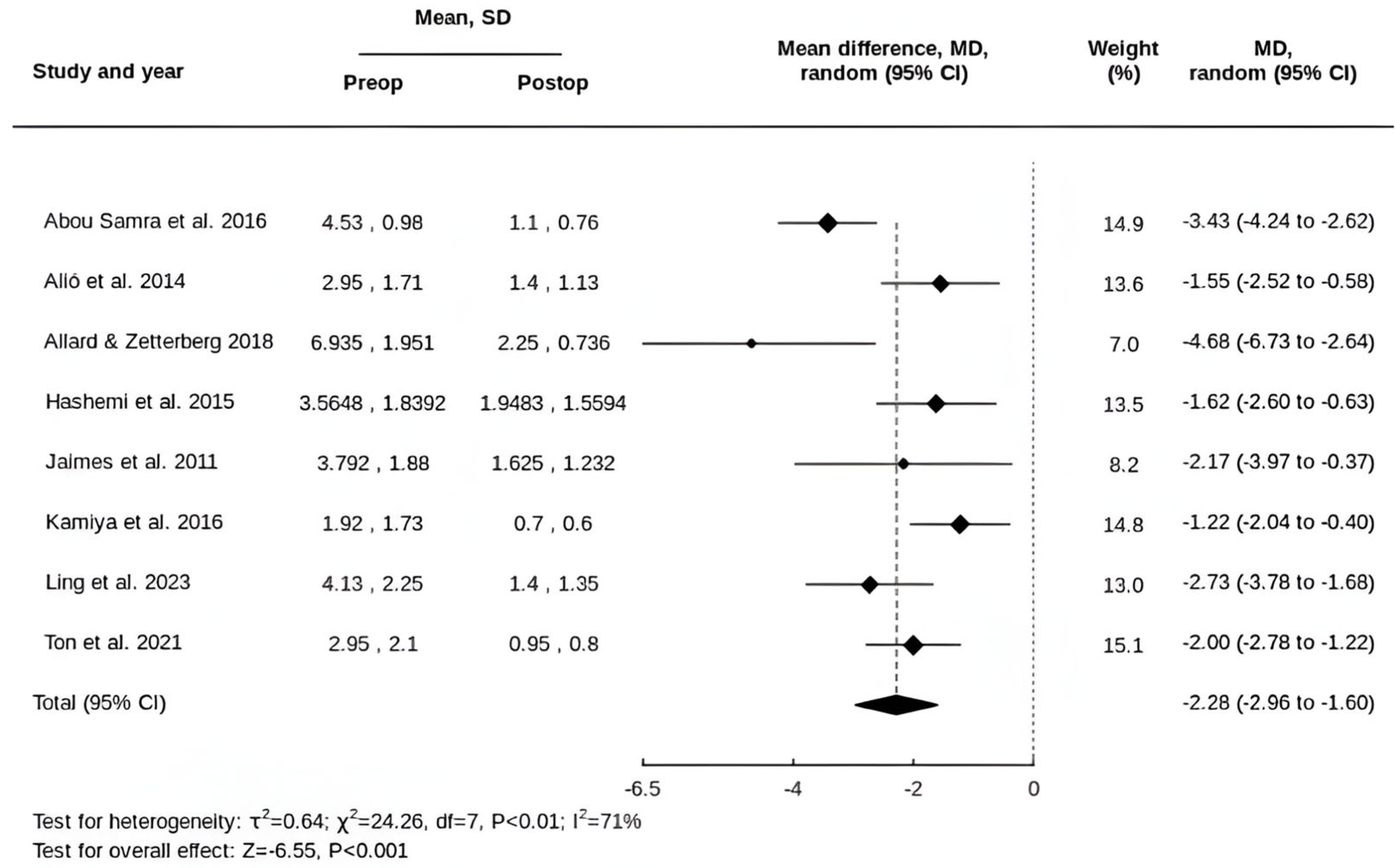
3.2. Primary Outcomes
3.3. Secondary Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| KC | Keratoconus |
| tIOL | Toric intraocular lens |
| SE | Spherical equivalent |
| UDVA | Uncorrected distance vision |
| CDVA | Corrected distance vision |
| REML | Restricted maximum likelihood |
| HOAs | Higher-order aberrations |
| RLE | Refractive lens exchange |
| SD | Standard deviation |
| D | Dioptre |
| MD | Mean difference |
| CMO | Cystoid macular oedema |
| CXL | Corneal cross-linking |
| TIA | Target induced astigmatism |
| SIA | Surgically induced astigmatism |
| DV | Difference vector |
| CI | Correction index |
| DAVD | Double-angle vector diagram |
| OCT | Optical coherence tomography |
| RA | Refractive astigmatism |
| KA | Keratometric astigmatism |
| LA | Lenticular astigmatism |
| PCA | Posterior corneal astigmatism |
| TCA | Total corneal astigmatism |
| IOL | Intraocular lens |
References
- Salmon, J.F. Kanski’s Clinical Ophthalmology: A Systematic Approach, 9th ed.; Elsevier Limited: Amsterdam, The Netherlands, 2000. [Google Scholar]
- Santodomingo-Rubido, J.; Carracedo, G.; Suzaki, A.; Villa-Collar, C.; Vincent, S.J.; Wolffsohn, J.S. Keratoconus: An updated review. Cont. Lens Anterior Eye 2022, 45, 101559. [Google Scholar] [CrossRef]
- Wagner, H.; Barr, J.T.; Zadnik, K. Collaborative Longitudinal Evaluation of Keratoconus (CLEK) Study: Methods and findings to date. Cont. Lens Anterior Eye 2007, 30, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Sideroudi, H.; Labiris, G.; Georgantzoglou, K.; Ntonti, P.; Siganos, C.; Kozobolis, V. Fourier analysis algorithm for the posterior corneal keratometric data: Clinical usefulness in keratoconus. Ophthalmic Physiol. Opt. 2017, 37, 460–466. [Google Scholar] [CrossRef]
- Oshika, T.; Tanabe, T.; Tomidokoro, A.; Amano, S. Progression of keratoconus assessed by fourier analysis of videokeratography data. Ophthalmology 2002, 109, 339–342. [Google Scholar] [CrossRef]
- Hashemi, H.; Khabazkhoob, M.; Jafarzadehpur, E.; Yekta, A.; Emamian, M.H.; Shariati, M.; Fotouhi, A. Higher order aberrations in a normal adult population. J. Curr. Ophthalmol. 2016, 27, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Kessel, L.; Andresen, J.; Tendal, B.; Erngaard, D.; Flesner, P.; Hjortdal, J. Toric Intraocular Lenses in the Correction of Astigmatism During Cataract Surgery: A Systematic Review and Meta-analysis. Ophthalmology 2016, 123, 275–286. [Google Scholar] [CrossRef] [PubMed]
- Güell, J.L.; Vázquez, M.; Malecaze, F.; Manero, F.; Gris, O.; Velasco, F.; Hulin, H.; Pujol, J. Artisan toric phakic intraocular lens for the correction of high astigmatism. Am. J. Ophthalmol. 2003, 136, 442–447. [Google Scholar] [CrossRef]
- Alió, J.L.; Mulet, M.E.; Gutiérrez, R.; Galal, A. Artisan toric phakic intraocular lens for correction of astigmatism. J. Refract. Surg. 2005, 21, 324–331. [Google Scholar] [CrossRef]
- Visser, N.; Beckers, H.J.; Bauer, N.J.; Gast, S.T.; Zijlmans, B.L.; Berenschot, T.T.; Webers, C.A.; Nuijts, R.M. Toric vs aspherical control intraocular lenses in patients with cataract and corneal astigmatism: A randomized clinical trial. JAMA Ophthalmol. 2014, 132, 1462–1468. [Google Scholar] [CrossRef]
- Ruíz-Mesa, R.; Carrasco-Sánchez, D.; Díaz-Alvarez, S.B.; Ruíz-Mateos, M.A.; Ferrer-Blasco, T.; Montés-Micó, R. Refractive lens exchange with foldable toric intraocular lens. Am. J. Ophthalmol. 2009, 147, 990–996.e1. [Google Scholar] [CrossRef]
- McLintock, C.A.; McKelvie, J.; Niyazmand, H.; Apel, A.J. Outcomes of a Toric Monofocal Piggyback Intraocular Lens for Residual Astigmatic Refractive Error in Pseudophakic Eyes. Curr. Eye Res. 2022, 47, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Royo, M.; Jiménez, Á.; Martínez-Alberquilla, I.; Alfonso, J.F. Eight-year follow-up of Artiflex and Artiflex Toric phakic intraocular lens. Eur. J. Ophthalmol. 2022, 32, 2051–2058. [Google Scholar] [CrossRef] [PubMed]
- Sauder, G.; Jonas, J.B. Treatment of Keratoconus by Toric Foldable Intraocular Lenses. Eur. J. Ophthalmol. 2003, 13, 577–579. [Google Scholar] [CrossRef]
- Visser, N.; Gast, S.T.; Bauer, N.J.; Nuijts, R.M. Cataract surgery with toric intraocular lens implantation in keratoconus: A case report. Cornea 2011, 30, 720–723. [Google Scholar] [CrossRef]
- Zvornicanin, J.; Cabric, E.; Jusufovic, V.; Musanovic, Z.; Zvornicanin, E. Use of the toric intraocular lens for keratoconus treatment. Acta Inform. Med. 2014, 22, 139–141. [Google Scholar] [CrossRef] [PubMed]
- Yahalomi, T.; Achiron, A.; Hecht, I.; Arnon, R.; Levinger, E.; Pikkel, J.; Tuuminen, R. Refractive Outcomes of Non-Toric and Toric Intraocular Lenses in Mild, Moderate and Advanced Keratoconus: A Systematic Review and Meta-Analysis. J. Clin. Med. 2022, 11, 2456. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, H.; Yekta, A.; Khabazkhoob, M. Effect of keratoconus grades on repeatability of keratometry readings: Comparison of 5 devices. J. Cataract. Refract. Surg. 2015, 41, 1065–1072. [Google Scholar] [CrossRef]
- Feizi, S.; Einollahi, B.; Raminkhoo, A.; Salehirad, S. Correlation between Corneal Topographic Indices and Higher-Order Aberrations in Keratoconus. J. Ophthalmic Vis. Res. 2013, 8, 113–118. [Google Scholar]
- Maeda, N.; Fujikado, T.; Kuroda, T.; Mihashi, T.; Hirohara, Y.; Nishida, K.; Watanabe, H.; Tano, Y. Wavefront aberrations measured with Hartmann-Shack sensor in patients with keratoconus. Ophthalmology 2002, 109, 1996–2003. [Google Scholar] [CrossRef]
- Pantanelli, S.; MacRae, S.; Jeong, T.M.; Yoon, G. Characterizing the wave aberration in eyes with keratoconus or penetrating keratoplasty using a high-dynamic range wavefront sensor. Ophthalmology 2007, 114, 2013–2021. [Google Scholar] [CrossRef]
- Zhou, W.; Stojanovic, A.; Utheim, T.P. Assessment of refractive astigmatism and simulated therapeutic refractive surgery strategies in coma-like-aberrations-dominant corneal optics. Eye Vis. 2016, 3, 13. [Google Scholar] [CrossRef] [PubMed]
- Wei, R.H.; Lim, L.; Chan, W.K.; Tan, D.T. Higher Order Ocular Aberrations in Eyes With Myopia in a Chinese Population. J. Refract. Surg. 2006, 22, 695–702. [Google Scholar] [CrossRef]
- Zhou, W.; Stojanovic, F.; Reinstein, D.Z.; Archer, T.J.; Chen, X.; Feng, Y.; Stojanovic, A. Coma Influence on Manifest Astigmatism in Coma-Dominant Irregular Corneal Optics. J. Refract. Surg. 2021, 37, 274–282. [Google Scholar] [CrossRef]
- de Gracia, P.; Dorronsoro, C.; Gambra, E.; Marin, G.; Hernández, M.; Marcos, S. Combining coma with astigmatism can improve retinal image over astigmatism alone. Vis. Res. 2010, 50, 2008–2014. [Google Scholar] [CrossRef]
- de Gracia, P.; Dorronsoro, C.; Gambra, E.; Marin, G.; Hernández, M.; Marcos, S. Visual acuity under combined astigmatism and coma: Optical and neural adaptation effects. J. Vis. 2011, 11, 5. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Slim, K.; Nini, E.; Forestier, D.; Kwiatkowski, F.; Panis, Y.; Chipponi, J. Methodological index for non-randomized studies (minors): Development and validation of a new instrument. ANZ J. Surg. 2003, 73, 712–716. [Google Scholar] [CrossRef]
- Munn, Z.; Barker, T.H.; Moola, S.; Tufanaru, C.; Stern, C.; McArthur, A.; Stephenson, M.; Aromataris, E. Methodological quality of case series studies: An introduction to the JBI critical appraisal tool. JBI Evid. Synth. 2020, 18, 2127–2133. [Google Scholar] [CrossRef]
- Viechtbauer, W. Bias and Efficiency of Meta-Analytic Variance Estimators in the Random-Effects Model. J. Educ. Behav. Stat. 2005, 30, 261–293. [Google Scholar] [CrossRef]
- Sterne, J.A.; Sutton, A.J.; Ioannidis, J.P.; Terrin, N.; Jones, D.R.; Lau, J.; Carpenter, J.; Rücker, G.; Harbord, R.M.; Schmid, C.H.; et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ 2011, 343, d4002. [Google Scholar] [CrossRef] [PubMed]
- Abou Samra, W.A.; Awad, E.A.; El Kannishy, A.H. Objective and Subjective Outcome of Clear Lensectomy With Toric IOL Implantation After Corneal Collagen Cross-Linking in Selected Cases of Keratoconus. Eye Contact Lens 2018, 44 (Suppl. 1), S87–S91. [Google Scholar] [CrossRef]
- Alió, J.L.; Peña-García, P.; Abdulla Guliyeva, F.; Soria, F.A.; Zein, G.; Abu-Mustafa, S.K. MICS with toric intraocular lenses in keratoconus: Outcomes and predictability analysis of postoperative refraction. Br. J. Ophthalmol. 2014, 98, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Allard, K.; Zetterberg, M. Implantation of toric intraocular lenses in patients with cataract and keratoconus: A case series. Int. Med. Case Rep. J. 2018, 11, 185–191. [Google Scholar] [CrossRef]
- Hashemi, H.; Heidarian, S.; Seyedian, M.A.; Yekta, A.; Khabazkhoob, M. Evaluation of the Results of Using Toric IOL in the Cataract Surgery of Keratoconus Patients. Eye Contact Lens 2015, 41, 354–358. [Google Scholar] [CrossRef] [PubMed]
- Jaimes, M.; Xacur-García, F.; Alvarez-Melloni, D.; Graue-Hernández, E.O.; Ramirez-Luquín, T.; Navas, A. Refractive lens exchange with toric intraocular lenses in keratoconus. J. Refract. Surg. 2011, 27, 658–664. [Google Scholar] [CrossRef]
- Kamiya, K.; Shimizu, K.; Miyake, T. Changes in astigmatism and corneal higher-order aberrations after phacoemulsification with toric intraocular lens implantation for mild keratoconus with cataract. Jpn. J. Ophthalmol. 2016, 60, 302–308. [Google Scholar] [CrossRef]
- Ling, J.Y.M.; Qiao, G.; Iovieno, A.; Yeung, S.N. Visual Outcomes of Cataract Surgery in Patients With Keratoconus Using Toric and Non-toric Lenses. J. Refract. Surg. 2023, 39, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Ton, Y.; Barrett, G.D.; Kleinmann, G.; Levy, A.; Assia, E.I. Toric intraocular lens power calculation in cataract patients with keratoconus. J. Cataract. Refract. Surg. 2021, 47, 1389–1397. [Google Scholar] [CrossRef]
- Kamiya, K.; Iijima, K.; Nobuyuki, S.; Mori, Y.; Miyata, K.; Yamaguchi, T.; Shimazaki, J.; Watanabe, S.; Maeda, N. Predictability of Intraocular Lens Power Calculation for Cataract with Keratoconus: A Multicenter Study. Sci. Rep. 2018, 8, 1312. [Google Scholar] [CrossRef]
- Savini, G.; Abbate, R.; Hoffer, K.J.; Mularoni, A.; Imburgia, A.; Avoni, L.; D’Eliseo, D.; Schiano-Lomoriello, D. Intraocular lens power calculation in eyes with keratoconus. J. Cataract. Refract. Surg. 2019, 45, 576–581. [Google Scholar] [CrossRef]
- Amsler, M. Le kératôcne fruste au Javal. Ophtalmologica 1938, 96, 77–83. [Google Scholar] [CrossRef]
- Krumeich, J.H.; Daniel, J.; Knülle, A. Live-epikeratophakia for keratoconus. J. Cataract. Refract. Surg. 1998, 24, 456–463. [Google Scholar] [CrossRef] [PubMed]
- Soeters, N.; Muijzer, M.B.; Molenaar, J.; Godefrooij, D.A.; Wisse, R.P.L. Autorefraction Versus Manifest Refraction in Patients with Keratoconus. J. Refract. Surg. 2018, 34, 30–34. [Google Scholar] [CrossRef]
- Nanavaty, M.A.; Lake, D.B.; Daya, S.M. Outcomes of pseudophakic toric intraocular lens implantation in keratoconic eyes with cataract. J. Refract. Surg. 2012, 28, 884–890. [Google Scholar] [CrossRef]
- Schallhorn, S.C.; Hettinger, K.A.; Pelouskova, M.; Teenan, D.; Venter, J.A.; Hannan, S.J.; Schallhorn, J.M. Effect of residual astigmatism on uncorrected visual acuity and patient satisfaction in pseudophakic patients. J. Cataract. Refract. Surg. 2021, 47, 991–998. [Google Scholar] [CrossRef]
- Villegas, E.A.; Alcón, E.; Artal, P. Minimum amount of astigmatism that should be corrected. J. Cataract. Refract. Surg. 2014, 40, 13–19. [Google Scholar] [CrossRef]
- Holladay, J.T.; Wilcox, R.R.; Koch, D.D.; Wang, L. Astigmatism analysis and reporting of surgically induced astigmatism and prediction error. J. Cataract. Refract. Surg. 2022, 48, 799–812. [Google Scholar] [CrossRef] [PubMed]
- Thibos, L.N.; Horner, D. Power vector analysis of the optical outcome of refractive surgery. J. Cataract. Refract. Surg. 2001, 27, 80–85. [Google Scholar] [CrossRef]
- Alpins, N. Astigmatism analysis by the Alpins method. J. Cataract. Refract. Surg. 2001, 27, 31–49. [Google Scholar] [CrossRef] [PubMed]
- Alpins, N. A new method of analyzing vectors for changes in astigmatism. J. Cataract. Refract. Surg. 1993, 19, 524–533. [Google Scholar] [CrossRef] [PubMed]
- Reinstein, D.Z.; Archer, T.J.; Randleman, J.B. JRS standard for reporting astigmatism outcomes of refractive surgery. J Refract Surg. 2014, 30, 654–659. [Google Scholar] [CrossRef]
- Watson, M.P.; Anand, S.; Bhogal, M.; Gore, D.; Moriyama, A.; Pullum, K.; Hau, S.; Tuft, S.J. Cataract surgery outcome in eyes with keratoconus. Br. J. Ophthalmol. 2014, 98, 361–364. [Google Scholar] [CrossRef]
- Kane, J.X.; Connell, B.; Yip, H.; McAlister, J.C.; Beckingsale, P.; Snibson, G.R.; Chan, E. Accuracy of Intraocular Lens Power Formulas Modified for Patients with Keratoconus. Ophthalmology 2020, 127, 1037–1042. [Google Scholar] [CrossRef] [PubMed]
- Dharwadkar, S.; Nayak, B.K. Corneal topography and tomography. J. Clin. Ophthalmol. Res. 2015, 3, 45–62. [Google Scholar] [CrossRef] [PubMed]
- Kanclerz, P.; Khoramnia, R.; Wang, X. Current Developments in Corneal Topography and Tomography. Diagnostics 2021, 11, 1466. [Google Scholar] [CrossRef]
- Jin, A.; Han, X.; Zhang, J.; Qiu, X.; Zhang, Y.; Qu, B.; Tan, X.; Luo, L. Agreement of Total Keratometry and Posterior Keratometry Among IOLMaster 700, CASIA2, and Pentacam. Transl. Vis. Sci. Technol. 2023, 12, 13. [Google Scholar] [CrossRef]
- Crawford, A.Z.; Patel, D.V.; McGhee, C.N. Comparison and repeatability of keratometric and corneal power measurements obtained by Orbscan II, Pentacam, and Galilei corneal tomography systems. Am. J. Ophthalmol. 2013, 156, 53–60. [Google Scholar] [CrossRef]
- Savini, G.; Barboni, P.; Carbonelli, M.; Hoffer, K.J. Repeatability of automatic measurements by a new Scheimpflug camera combined with Placido topography. J. Cataract. Refract. Surg. 2011, 37, 1809–1816. [Google Scholar] [CrossRef] [PubMed]
- Schiano-Lomoriello, D.; Bono, V.; Abicca, I.; Savini, G. Repeatability of anterior segment measurements by optical coherence tomography combined with Placido disk corneal topography in eyes with keratoconus. Sci. Rep. 2020, 10, 1124. [Google Scholar] [CrossRef]
- Javal, É. Mémoires d’Ophtalmométrie: Annotés et Précédés d’une Introduction; G. Masson: Paris, France, 1890; p. 131. [Google Scholar]
- Grosvenor, T.; Quintero, S.; Perrigin, D.M. Predicting refractive astigmatism: A suggested simplification of Javal’s rule. Am. J. Optom. Physiol. Opt. 1988, 65, 292–297. [Google Scholar] [CrossRef]
- Salvador-Roger, R.; Albarrán-Diego, C.; Garzón, N.; García-Montero, M.; Muñoz, G.; Micó, V.; Esteve-Taboada, J.J. Revisiting Javal’s rule: A fresh and improved power vector approach according to age. Graefes Arch. Clin. Exp. Ophthalmol. 2024, 262, 249–260. [Google Scholar] [CrossRef]
- Koch, D.D.; Jenkins, R.B.; Weikert, M.P.; Yeu, E.; Wang, L. Correcting astigmatism with toric intraocular lenses: Effect of posterior corneal astigmatism. J. Cataract. Refract. Surg. 2013, 39, 1803–1809. [Google Scholar] [CrossRef]
- Teus, M.A.; Arruabarrena, C.; Hernández-Verdejo, J.L.; Sales-Sanz, A.; Sales-Sanz, M. Correlation between keratometric and refractive astigmatism in pseudophakic eyes. J. Cataract. Refract. Surg. 2010, 36, 1671–1675. [Google Scholar] [CrossRef]
- Athukorala, S.; Kansara, N.; Lehman, E.; Pantanelli, S.M. Correlation Between Keratometric and Refractive Astigmatism in Pseudophakes. Clin. Ophthalmol. 2021, 15, 3909–3913. [Google Scholar] [CrossRef]
- Koch, D.D.; Ali, S.F.; Weikert, M.P.; Shirayama, M.; Jenkins, R.; Wang, L. Contribution of posterior corneal astigmatism to total corneal astigmatism. J. Cataract. Refract. Surg. 2012, 38, 2080–2087. [Google Scholar] [CrossRef]
- Kim, J.; Whang, W.J.; Kim, H.S. Analysis of total corneal astigmatism with a rotating Scheimpflug camera in keratoconus. BMC Ophthalmol. 2020, 20, 475. [Google Scholar] [CrossRef]
- Thebpatiphat, N.; Hammersmith, K.M.; Rapuano, C.J.; Ayres, B.D.; Cohen, E.J. Cataract surgery in keratoconus. Eye Contact Lens 2007, 33, 244–246. [Google Scholar] [CrossRef] [PubMed]
- Tomidokoro, A.; Oshika, T.; Amano, S.; Higaki, S.; Maeda, N.; Miyata, K. Changes in anterior and posterior corneal curvatures in keratoconus. Ophthalmology 2000, 107, 1328–1332. [Google Scholar] [CrossRef] [PubMed]
- Piñero, D.P.; Alió, J.L.; Alesón, A.; Escaf Vergara, M.; Miranda, M. Corneal volume, pachymetry, and correlation of anterior and posterior corneal shape in subclinical and different stages of clinical keratoconus. J. Cataract. Refract. Surg. 2010, 36, 814–825. [Google Scholar] [CrossRef] [PubMed]
- Kamiya, K.; Shimizu, K.; Igarashi, A.; Miyake, T. Assessment of Anterior, Posterior, and Total Central Corneal Astigmatism in Eyes With Keratoconus. Am. J. Ophthalmol. 2015, 160, 851–857.e1. [Google Scholar] [CrossRef]
- Aslani, F.; Khorrami-Nejad, M.; Aghazadeh Amiri, M.; Hashemian, H.; Askarizadeh, F.; Khosravi, B. Characteristics of Posterior Corneal Astigmatism in Different Stages of Keratoconus. J. Ophthalmic Vis. Res. 2018, 13, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Holladay, T.; Pettit, G. Improving toric intraocular lens calculations using total surgically induced astigmatism for a 2.5 mm temporal incision. J. Cataract. Refract. Surg. 2019, 45, 272–283. [Google Scholar] [CrossRef]
- Kane, J.X. Kane Formula. In Intraocular Lens Calculations. Essentials in Ophthalmology; Aramberri, J., Hoffer, K.J., Olsen, T., Savini, G., Shammas, H.J., Eds.; Springer: Cham, Switzerland, 2024. [Google Scholar] [CrossRef]
- Savini, G.; Næser, K.; Schiano-Lomoriello, D.; Ducoli, P. Optimized keratometry and total corneal astigmatism for toric intraocular lens calculation. J. Cataract. Refract. Surg. 2017, 43, 1140–1148. [Google Scholar] [CrossRef]
- Available online: http://www.ascrs.org/barrett-toric-calculator (accessed on 22 July 2024).
- Abulafia, A.; Koch, D.D.; Wang, L.; Hill, W.E.; Assia, E.I.; Franchina, M.; Barrett, G.D. New regression formula for toric intraocular lens calculations. J. Cataract. Refract. Surg. 2016, 42, 663–671. [Google Scholar] [CrossRef]
- Skrzypecki, J.; Sanghvi Patel, M.; Suh, L.H. Performance of the Barrett Toric Calculator with and without measurements of posterior corneal curvature. Eye 2019, 33, 1762–1767. [Google Scholar] [CrossRef]
- Reitblat, O.; Barnir, M.; Qassoom, A.; Levy, A.; Assia, E.I.; Kleinmann, G. Comparison of the Barrett toric calculator using measured and predicted posterior corneal astigmatism and the Kane and Abulafia-Koch calculators. J. Cataract. Refract. Surg. 2023, 49, 704–710. [Google Scholar] [CrossRef] [PubMed]
- Ianchulev, T.; Salz, J.; Hoffer, K.; Albini, T.; Hsu, H.; Labree, L. Intraoperative optical refractive biometry for intraocular lens power estimation without axial length and keratometry measurements. J. Cataract. Refract. Surg. 2005, 31, 1530–1536. [Google Scholar] [CrossRef] [PubMed]
- Langenbucher, A.; Szentmáry, N.; Cayless, A.; Casaza, M.; Weisensee, J.; Hoffmann, P.; Wendelstein, J. Surgically Induced Astigmatism after Cataract Surgery—A Vector Analysis. Curr. Eye Res. 2022, 47, 1279–1287. [Google Scholar] [CrossRef]
- Abulafia, A.; Koch, D.D.; Holladay, J.T.; Wang, L.; Hill, W. Pursuing perfection in intraocular lens calculations: IV. Rethinking astigmatism analysis for intraocular lens-based surgery: Suggested terminology, analysis, and standards for outcome reports. J. Cataract. Refract. Surg. 2018, 44, 1169–1174. [Google Scholar] [CrossRef]
- Febbraro, J.L.; Wang, L.; Borasio, E.; Richiardi, L.; Khan, H.N.; Saad, A.; Gatinel, D.; Koch, D.D. Astigmatic equivalence of 2.2-mm and 1.8-mm superior clear corneal cataract incision. Graefes. Arch. Clin. Exp. Ophthalmol. 2015, 253, 261–265. [Google Scholar] [CrossRef]
- Yu, Y.B.; Zhu, Y.N.; Wang, W.; Zhang, Y.D.; Yu, Y.H.; Yao, K. A comparable study of clinical and optical outcomes after 1.8, 2.0 mm microcoaxial and 3.0 mm coaxial cataract surgery. Int. J. Ophthalmol. 2016, 9, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Kamiya, K.; Iijima, K.; Ando, W.; Shoji, N. Comparison of Mean and Centroid of Surgically Induced Astigmatism After Standard Cataract Surgery. Front. Med. 2021, 8, 670337. [Google Scholar] [CrossRef]
- Reinstein, D.Z.; Archer, T.J.; Srinivasan, S.; Mamalis, N.; Kohnen, T.; Dupps, W.; Randleman, B.J. Standard for reporting refractive outcomes of intraocular lens–based refractive surgery. J. Cataract. Refract. Surg. 2017, 43, 435–439. [Google Scholar] [CrossRef] [PubMed]
- Sabesan, R.; Yoon, G. Visual performance after correcting higher order aberrations in keratoconic eyes. J. Vis. 2009, 9, 6. [Google Scholar] [CrossRef][Green Version]
- Rocha, K.M.; Nosé, W.; Bottós, K.; Bottós, J.; Morimoto, L.; Soriano, E. Higher-order aberrations of age-related cataract. J. Cataract. Refract. Surg. 2007, 33, 1442–1446. [Google Scholar] [CrossRef]
- Kosaki, R.; Maeda, N.; Bessho, K.; Hori, Y.; Nishida, K.; Suzaki, A.; Hirohara, Y.; Mihashi, T.; Fujikado, T.; Tano, Y. Magnitude and orientation of Zernike terms in patients with keratoconus. Investig. Ophthalmol. Vis. Sci. 2007, 48, 3062–3068. [Google Scholar] [CrossRef]
- van den Berg, R.M.; van den Berg, A.B.; Maia, R.K.; Fetrin de Barros, M.; Dodhia, M.B.S.; Shahid, M.B.A.; Klyce, S.D. Prediction of the small aperture intraocular lens on visual acuity in patients with keratoconus. J. Cataract. Refract. Surg. 2024, 50, 930–935. [Google Scholar] [CrossRef]
- Guirao, A.; Tejedor, J.; Artal, P. Corneal aberrations before and after small-incision cataract surgery. Investig. Ophthalmol. Vis. Sci. 2004, 45, 4312–4319. [Google Scholar] [CrossRef]
- Schröder, S.; Eppig, T.; Liu, W.; Schrecker, J.; Langenbucher, A. Keratoconic eyes with stable corneal tomography could benefit more from custom intraocular lens design than normal eyes. Sci. Rep. 2019, 9, 3479. [Google Scholar] [CrossRef] [PubMed]
- Holland, E.; Lane, S.; Horn, J.D.; Ernest, P.; Arleo, R.; Miller, K.M. The AcrySof Toric intraocular lens in subjects with cataracts and corneal astigmatism: A randomized, subject-masked, parallel-group, 1-year study. Ophthalmology 2010, 117, 2104–2111. [Google Scholar] [CrossRef]
- Tognetto, D.; Perrotta, A.A.; Bauci, F.; Rinaldi, S.; Antonuccio, M.; Pellegrino, F.A.; Fenu, G.; Stamatelatos, G.; Alpins, N. Quality of images with toric intraocular lenses. J. Cataract. Refract. Surg. 2018, 44, 376–381. [Google Scholar] [CrossRef]
- Felipe, A.; Artigas, J.M.; Díez-Ajenjo, A.; García-Domene, C.; Alcocer, P. Residual astigmatism produced by toric intraocular lens rotation. J. Cataract. Refract. Surg. 2011, 37, 1895–1901. [Google Scholar] [CrossRef] [PubMed]
- Ernst, B.J.; Hsu, H.Y. Keratoconus association with axial myopia: A prospective biometric study. Eye Contact Lens 2011, 37, 2–5. [Google Scholar] [CrossRef]
- Zhu, X.; He, W.; Zhang, K.; Lu, Y. Factors influencing 1-year rotational stability of AcrySof Toric intraocular lenses. Br. J. Ophthalmol. 2016, 100, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, H.; Beiranvand, A.; Yekta, A.; Maleki, A.; Yazdani, N.; Khabazkhoob, M. Pentacam top indices for diagnosing subclinical and definite keratoconus. J. Curr. Ophthalmol. 2016, 28, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Gomes, J.A.; Tan, D.; Rapuano, C.J.; Belin, M.W.; Ambrósio, R., Jr.; Guell, J.L.; Malecaze, F.; Nishida, K.; Sangwan, V.S.; Group of Panelists for the Global Delphi Panel of Keratoconus and Ectatic Diseases. Global consensus on keratoconus and ectatic diseases. Cornea 2015, 34, 359–369. [Google Scholar] [CrossRef]
- Henriquez, M.A.; Hadid, M.; Izquierdo, L., Jr. A Systematic Review of Subclinical Keratoconus and Forme Fruste Keratoconus. J. Refract. Surg. 2020, 36, 270–279. [Google Scholar] [CrossRef]
| Study | Study Design | Country | Follow-Up | Preoperative Astigmatism | Sample Size | tIOL Model |
|---|---|---|---|---|---|---|
| Abou Samra WA et al., 2016 [32] | Prospective; CXL then tIOL after 6/12 | Egypt | 12 months | Mean: 4.53 ± 0.98 D | 6 patients (9 eyes) | TECNIS toric IOL |
| Alió JL et al., 2014 [33] | Retrospective | Spain | 3–15 months | Mean: −2.95 ± 1.71 D | 10 patients (17 eyes) | Acrysof IQ toric IOL |
| Allard K et al., 2018 [34] | Prospective case series | Sweden | 1–7 months | Mean: 6.94 ± 1.95 D | 4 patients (4 eyes) | AcrySof IQ toric SN6AT IOL (patient 1 and 2), AT Torbi 709M(P) IOL (patient 3 and 4) |
| Hashemi H et al., 2015 [35] | Case series | Iran | 3 months | Mean: 3.56 ± 1.84 D | 17 patients (23 eyes) | AcrySof toric SN60TT IOL |
| Jaimes M et al., 2011 [36] | Retrospective | Mexico | 3–31 months | Mean: 3.79 ± 1.88 D | 6 patients (7 eyes) | AcrySof toric SN60TT IOL |
| Kamiya K et al., 2015 [37] | Prospective | Japan | 3 months | Mean: 1.92 ± 1.73 D | 19 patients (19 eyes) | AcrySof IQ toric SN6AT IOL |
| Ling JYM et al., 2023 [38] | Case series | Canada | Avg ≥ 2 years | Mean: 4.13 ± 2.25 D | 17 patients (24 eyes) | Multiple models; unspecified |
| Ton Y et al., 2021 [39] | Case series | Israel | 1 month | Mean: 2.95 ± 2.10 D | 23 patients (32 eyes) | AcrySof toric SA6AT IOL, TECNIS toric IOL (ZCT series), Medicontur Bi-Flex toric IOL, Rayner T-flex toric IOL |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, T.G.; O’Kane, K.; Roberts, H.W. Refractive Outcomes in Keratoconus Patients Following Toric Lens Implantation: A Systematic Review and Single-Group Meta-Analysis. Life 2025, 15, 1362. https://doi.org/10.3390/life15091362
Tan TG, O’Kane K, Roberts HW. Refractive Outcomes in Keratoconus Patients Following Toric Lens Implantation: A Systematic Review and Single-Group Meta-Analysis. Life. 2025; 15(9):1362. https://doi.org/10.3390/life15091362
Chicago/Turabian StyleTan, Tun Giap, Kieran O’Kane, and Harry W. Roberts. 2025. "Refractive Outcomes in Keratoconus Patients Following Toric Lens Implantation: A Systematic Review and Single-Group Meta-Analysis" Life 15, no. 9: 1362. https://doi.org/10.3390/life15091362
APA StyleTan, T. G., O’Kane, K., & Roberts, H. W. (2025). Refractive Outcomes in Keratoconus Patients Following Toric Lens Implantation: A Systematic Review and Single-Group Meta-Analysis. Life, 15(9), 1362. https://doi.org/10.3390/life15091362