A Novel Small-Molecule GRP94 Modulator Increases PCSK9 Secretion and Promotes LDLR Degradation
Abstract
1. Introduction
2. Materials and Methods
3. Results
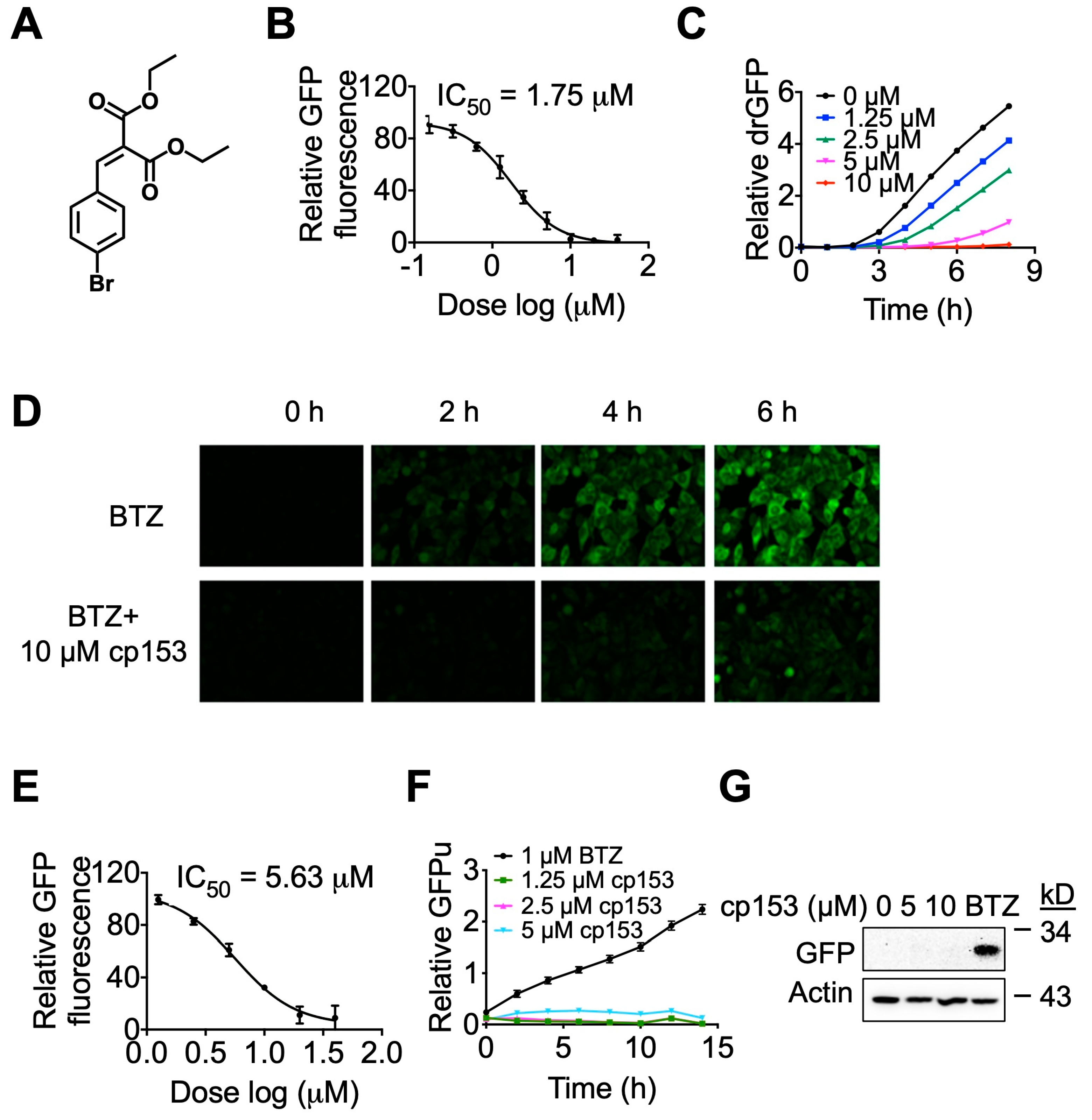
3.1. cp153 Inhibits ER-Associated Protein Dislocation and Substrate Ubiquitination
3.2. cp153 Inhibits Ubiquitination and Stabilizes ERAD Substrates While Inducing ER Stress
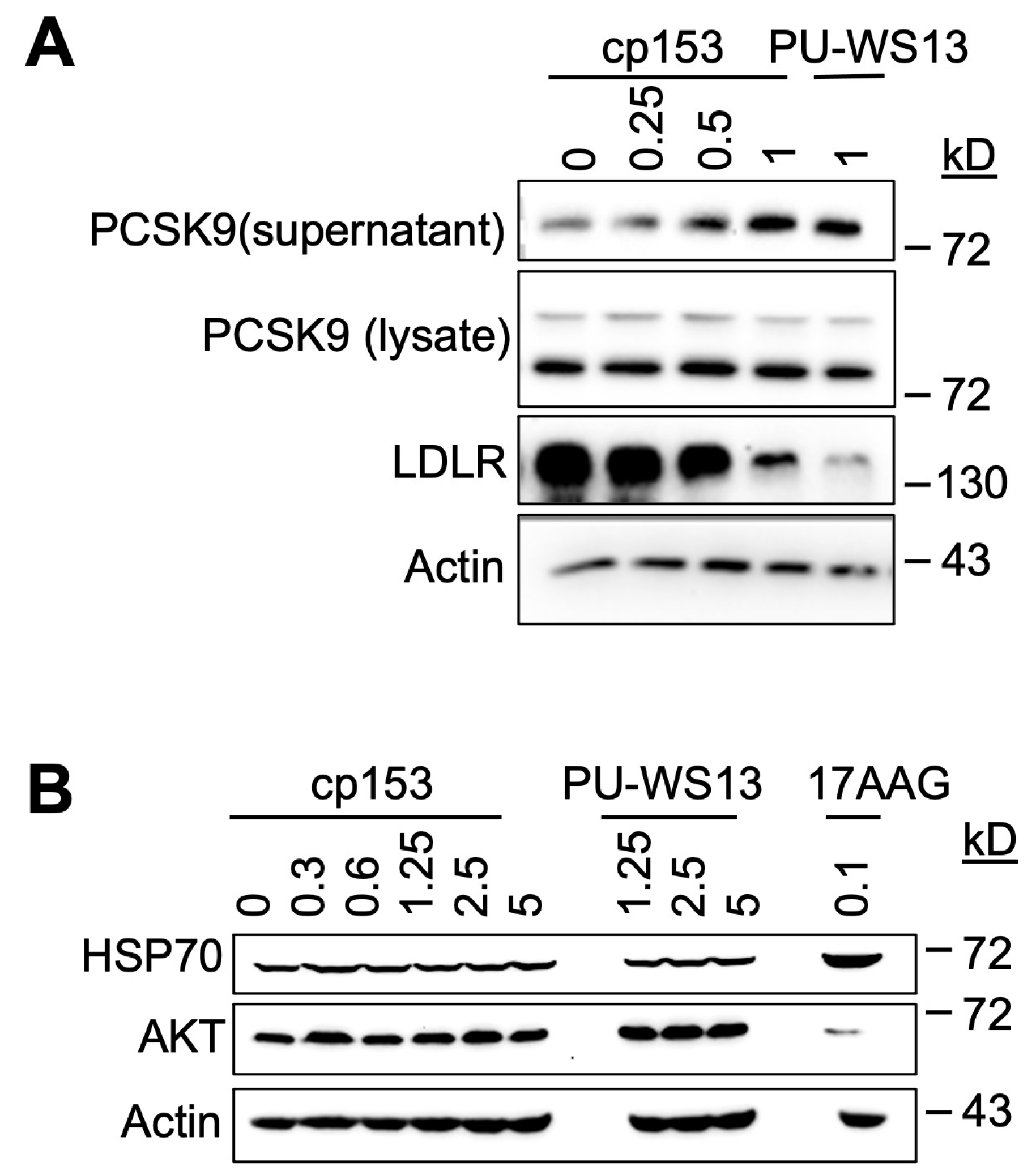
3.3. GRP94 Is a Potential Target of cp153
3.4. cp153 Modulates PCSK9 Secretion and LDLR Degradation Without Inducing Cytosolic HSP90 Responses
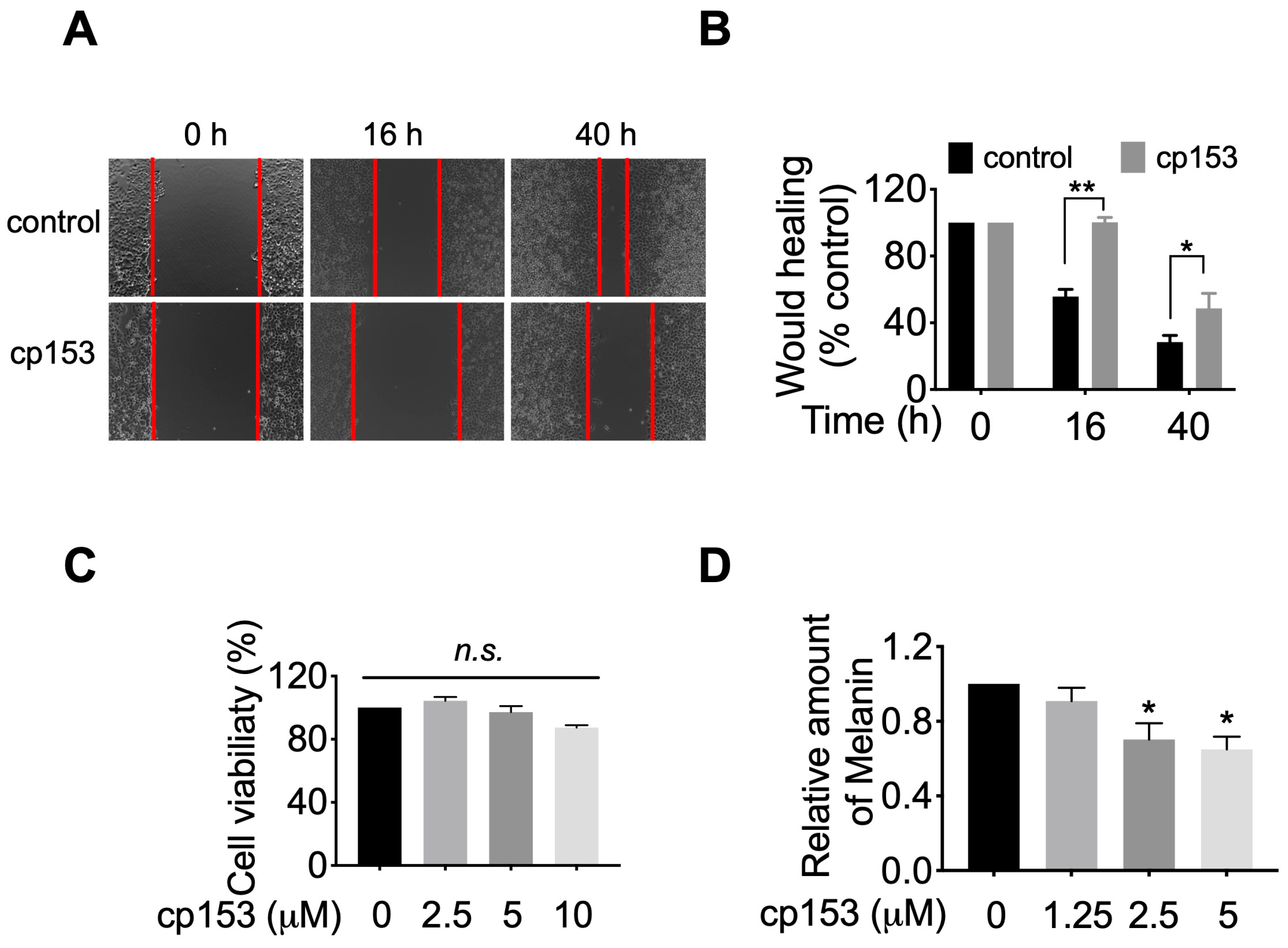
3.5. cp153 Inhibits HeLa Cell Migration and Melanin Production
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ER | Endoplasmic reticulum |
| ERAD | ER-associated degradation |
| CETSA | Cellular Thermal Shift Assay |
References
- Vembar, S.S.; Brodsky, J.L. One step at a time: Endoplasmic reticulum-associated degradation. Nat. Rev. Mol. Cell Biol. 2008, 9, 944–957. [Google Scholar] [CrossRef] [PubMed]
- Christianson, J.C.; Jarosch, E.; Sommer, T. Mechanisms of substrate processing during ER-associated protein degradation. Nat. Rev. Mol. Cell Biol. 2023, 24, 777–796. [Google Scholar] [CrossRef]
- Wu, S.A.; Li, Z.J.; Qi, L. Endoplasmic reticulum (ER) protein degradation by ER-associated degradation and ER-phagy. Trends Cell Biol. 2025, 35, 576–591. [Google Scholar] [CrossRef] [PubMed]
- Hebert, D.N.; Molinari, M. In and out of the ER: Protein folding, quality control, degradation, and related human diseases. Physiol. Rev. 2007, 87, 1377–1408. [Google Scholar] [CrossRef]
- Tsai, B.; Ye, Y.; Rapoport, T.A. Retro-translocation of proteins from the endoplasmic reticulum into the cytosol. Nat. Rev. Mol. Cell Biol. 2002, 3, 246–255. [Google Scholar] [CrossRef]
- Liu, Y.; Ye, Y. Proteostasis regulation at the endoplasmic reticulum: A new perturbation site for targeted cancer therapy. Cell Res. 2011, 21, 867–883. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.X.; Hong, F.; Zhang, Y.; Ansa-Addo, E.; Li, Z. GRP94/gp96 in Cancer: Biology, Structure, Immunology, and Drug Development. Adv. Cancer Res. 2016, 129, 165–190. [Google Scholar]
- Poirier, S.; Mamarbachi, M.; Chen, W.T.; Lee, A.S.; Mayer, G. GRP94 Regulates Circulating Cholesterol Levels Through Blockade of PCSK9-Induced LDLR Degradation. Cell Rep. 2015, 13, 2064–2071. [Google Scholar] [CrossRef]
- Guo, S.; Xia, X.D.; Gu, H.M.; Zhang, D.W. Proprotein Convertase Subtilisin/Kexin-Type 9 and Lipid Metabolism. Adv. Exp. Med. Biol. 2020, 1276, 137–156. [Google Scholar]
- Lebeau, P.F.; Platko, K.; Byun, J.H.; Makda, Y.; Austin, R.C. The Emerging Roles of Intracellular PCSK9 and Their Implications in Endoplasmic Reticulum Stress and Metabolic Diseases. Metabolites 2022, 12, 215. [Google Scholar] [CrossRef]
- Lee, A. Glucose-regulated proteins in cancer: Molecular mechanisms and therapeutic potential. Nat. Rev. Cancer 2014, 14, 263–276. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.F.; Iwanowycz, S.; Ngoi, S.; Hill, M.; Zhao, Q.; Liu, B. Molecular Chaperone GRP94/GP96 in Cancers: Oncogenesis and Therapeutic Target. Front. Oncol. 2021, 11, 629846. [Google Scholar] [CrossRef]
- Duerfeldt, A.S.; Peterson, L.B.; Maynard, J.C.; Ng, C.L.; Eletto, D.; Ostrovsky, O.; Shinogle, H.E.; Moore, D.S.; Argon, Y.; Nicchitta, C.V.; et al. Development of a Grp94 inhibitor. J. Am. Chem. Soc. 2012, 134, 9796–9804. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.D.; Yan, P.; Seidler, P.M.; Patel, H.J.; Sun, W.; Yang, C.; Que, N.S.; Taldone, T.; Finotti, P.; Stephani, R.A.; et al. Paralog-selective Hsp90 inhibitors define tumor-specific regulation of HER2. Nat. Chem. Biol. 2013, 9, 677–684. [Google Scholar] [CrossRef]
- Immormino, R.M.; Metzger, L.E., 4th; Reardon, P.N.; Dollins, D.E.; Blagg, B.S.J.; Gewirth, D.T. Different poses for ligand and chaperone in inhibitor-bound Hsp90 and GRP94: Implications for paralog-specific drug design. J. Mol. Biol. 2009, 388, 1033–1042. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.J.; Ghosh, S.; Stothert, A.R.; Dickey, C.A.; Blagg, B.S.J. Transformation of the Non-Selective Aminocyclohexanol-Based Hsp90 Inhibitor into a Grp94-Seletive Scaffold. ACS Chem. Biol. 2017, 12, 244–253. [Google Scholar] [CrossRef]
- Soldano, K.L.; Jivan, A.; Nicchitta, C.V.; Gewirth, D.T. Structure of the N-terminal domain of GRP94. Basis for ligand specificity and regulation. J. Biol. Chem. 2003, 278, 48330–48338. [Google Scholar] [CrossRef]
- Kim, J.W.; Cho, Y.B.; Lee, S. Cell Surface GRP94 as a Novel Emerging Therapeutic Target for Monoclonal Antibody Cancer Therapy. Cells 2021, 10, 670. [Google Scholar] [CrossRef]
- Pugh, K.W.; Alnaed, M.; Brackett, C.M.; Blagg, B.S.J. The biology and inhibition of glucose-regulated protein 94/gp96. Med. Res. Rev. 2022, 42, 2007–2024. [Google Scholar] [CrossRef]
- Amankwah, Y.S.; Fleifil, Y.; Unruh, E.; Collins, P.; Wang, Y.; Vitou, K.; Bates, A.; Obaseki, I.; Sugoor, M.; Alao, J.P.; et al. Structural transitions modulate the chaperone activities of Grp94. Proc. Natl. Acad. Sci. USA 2024, 121, e2309326121. [Google Scholar] [CrossRef]
- Azam, T.P.; Han, L.; Deans, E.E.; Huang, B.; Hoxie, R.; Friedman, L.J.; Gelles, J.; Street, T.O. Mechanism of client loading from BiP to Grp94 and its disruption by select inhibitors. Nat. Commun. 2025, 16, 3575. [Google Scholar] [CrossRef]
- Que, N.L.S.; Crowley, V.M.; Duerfeldt, A.S.; Zhao, J.; Kent, C.N.; Blagg, B.S.J.; Gewirth, D.T. Structure Based Design of a Grp94-Selective Inhibitor: Exploiting a Key Residue in Grp94 to Optimize Paralog-Selective Binding. J. Med. Chem. 2018, 61, 2793–2805. [Google Scholar] [CrossRef]
- Zhong, Y.W.; Fang, S.Y. Live cell imaging of protein dislocation from the endoplasmic reticulum. J. Biol. Chem. 2012, 287, 28057–28066. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.W.; Shen, H.; Wang, Y.; Yang, Y.; Yang, P.; Fang, S.Y. Identification of ERAD components essential for dislocation of the null Hong Kong variant of α-1-antitrypsin (NHK). Biochem. Biophys. Res. Commun. 2015, 458, 424–428. [Google Scholar] [CrossRef] [PubMed]
- Martinez Molina, D.; Jafari, R.; Ignatushchenko, M.; Seki, T.; Larsson, E.A.; Dan, C.; Sreekumar, L.; Cao, Y.; Nordlund, P. Monitoring drug target engagement in cells and tissues using the cellular thermal shift assay. Science 2013, 341, 84–87. [Google Scholar] [CrossRef]
- Martinez Molina, D.; Nordlund, P. The Cellular Thermal Shift Assay: A Novel Biophysical Assay for In Situ Drug Target Engagement and Mechanistic Biomarker Studies. Annu. Rev. Pharmacol. Toxicol. 2016, 56, 141–161. [Google Scholar] [CrossRef]
- Martinez, N.J.; Asawa, R.R.; Cyr, M.G.; Zakharov, A.; Urban, D.J.; Roth, J.S.; Wallgren, E.; Klumpp-Thomas, C.; Coussens, N.P.; Rai, G.; et al. A widely-applicable high-throughput cellular thermal shift assay (CETSA) using split Nano Luciferase. Sci. Rep. 2018, 8, 9472. [Google Scholar] [CrossRef]
- Reinhard, F.B.; Eberhard, D.; Werner, T.; Franken, H.; Childs, D.; Doce, C.; Savitski, M.F.; Huber, W.; Bantscheff, M.; Savitski, M.M.; et al. Thermal proteome profiling monitors ligand interactions with cellular membrane proteins. Nat. Methods 2015, 12, 1129–1131. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.H.; Jin, S.H.; Kang, H.Y. LPS induces melanogenesis through p38 MAPK activation in human melanocytes. Arch. Dermatol. Res. 2008, 300, 325–329. [Google Scholar] [CrossRef]
- Ruan, J.J.; Rothan, H.A.; Zhong, Y.W.; Yan, W.J.; Henderson, M.J.; Chen, F.H.; Fang, S.Y. A small molecule inhibitor of ER-to-cytosol protein dislocation exhibits anti-dengue and anti-Zika virus activity. Sci. Rep. 2019, 9, 10901. [Google Scholar] [CrossRef]
- Christianson, J.C.; Ye, Y.H. Cleaning up in the endoplasmic reticulum: Ubiquitin in charge. Nat. Struct. Mol. Biol. 2014, 21, 325–335. [Google Scholar] [CrossRef] [PubMed]
- Lebeau, P.; Platko, K.; Al-Hashimi, A.A.; Byun, J.H.; Lhoták, Š.; Holzapfel, N.; Gyulay, G.; Igdoura, S.A.; Cool, D.R.; Trigatti, B.; et al. Loss-of-function PCSK9 mutants evade the unfolded protein response sensor GRP78 and fail to induce endoplasmic reticulum stress when retained. J. Biol. Chem. 2018, 293, 7329–7343. [Google Scholar] [CrossRef]
- Robinson, C.M.; Duggan, A.; Forrester, A. ER exit in physiology and disease. Front Mol. Biosci. 2024, 11, 1352970. [Google Scholar] [CrossRef]
- Gomez-Navarro, N.; Maldutyte, J.; Poljak, K.; Peak-Chew, S.Y.; Orme, J.; Bisnett, B.J.; Lamb, C.H.; Boyce, M.; Gianni, D.; Miller, E.A. Selective inhibition of protein secretion by abrogating receptor-coat interactions during ER export. Proc. Natl. Acad. Sci. USA 2022, 119, e2202080119. [Google Scholar] [CrossRef] [PubMed]
- Bao, X.; Liang, Y.; Chang, H.; Cai, T.; Feng, B.; Gordon, K.; Zhu, Y.; Shi, H.; He, Y.; Xie, L. Targeting proprotein convertase subtilisin/kexin type 9 (PCSK9): From bench to bedside. Signal Transduct. Target. Ther. 2024, 9, 13. [Google Scholar] [CrossRef]
- Liu, M.; Li, M.; Zhou, Y.; Zhou, Q.; Jiang, Y. HSP90 inhibitor 17AAG attenuates sevoflurane-induced neurotoxicity in rats and human neuroglioma cells via induction of HSP70. J. Transl. Med. 2020, 18, 166. [Google Scholar] [CrossRef]
- Trepel, J.; Mollapour, M.; Giaccone, G.; Neckers, L. Targeting the dynamic HSP90 complex in cancer. Nat. Rev. Cancer 2010, 10, 537–549. [Google Scholar] [CrossRef]
- Sato, S.; Fujita, N.; Tsuruo, T. Modulation of Akt kinase activity by binding to Hsp90. Proc. Natl. Acad. Sci. USA 2000, 97, 10832–10837. [Google Scholar] [CrossRef]
- Hong, L.J.; Chen, A.J.; Li, F.Z.; Chen, K.J.; Fang, S. The HSP90 Inhibitor, 17-AAG, Influences the Activation and Proliferation of T Lymphocytes via AKT/GSK3β Signaling in MRL/lpr Mice. Drug Des. Devel. Ther. 2020, 14, 4605–4612. [Google Scholar] [CrossRef]
- Wanderling, S.; Simen, B.B.; Ostrovsky, O.; Ahmed, N.T.; Vogen, S.M.; Gidalevitz, T.; Argon, Y. GRP94 is essential for mesoderm induction and muscle development because it regulates insulin-like growth factor secretion. Mol. Biol. Cell. 2007, 18, 3764–3775. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Velegraki, M.; Wang, Y.; Mandula, J.K.; Chang, Y.; Liu, W.; Song, N.J.; Kwon, H.; Xiao, T.; Bolyard, C.; et al. Spatial and functional targeting of intratumoral Tregs reverses CD8+ T cell exhaustion and promotes cancer immunotherapy. J. Clin. Investig. 2024, 134, e180080. [Google Scholar] [CrossRef]
- Wang, X.Y.; Sun, X.; Chen, X.; Facciponte, J.; Repasky, E.A.; Kane, J.; Subjeck, J.R. Superior antitumor response induced by large stress protein chaperoned protein antigen compared with peptide antigen. J. Immunol. 2010, 184, 6309–6319. [Google Scholar] [CrossRef] [PubMed]
- Bleifuss, E.; Bendz, H.; Sirch, B.; Thompson, S.; Brandl, A.; Milani, V.; Graner, M.W.; Drexler, I.; Kuppner, M.; Katsanis, E.; et al. Differential capacity of chaperone-rich lysates in cross-presenting human endogenous and exogenous melanoma differentiation antigens. Int. J. Hyperth. 2008, 24, 623–637. [Google Scholar] [CrossRef] [PubMed]
- Rothan, H.A.; Zhong, Y.W.; Sanborn, M.A.; Teoh, T.C.; Ruan, J.J.; Yusof, R.; Hang, J.; Henderson, M.J.; Fang, S.Y. Small molecule grp94 inhibitors block dengue and Zika virus replication. Antivir. Res. 2019, 171, 104590. [Google Scholar] [CrossRef] [PubMed]
- Ruan, J.J.; Liang, D.D.; Yan, W.J.; Zhong, Y.W.; Talley, D.C.; Rai, G.; Tao, D.Y.; LeClair, C.A.; Simeonov, A.; Zhang, Y.H.; et al. A small-molecule inhibitor and degrader of the RNF5 ubiquitin ligase. Mol. Biol. Cell. 2022, 33, ar120. [Google Scholar] [CrossRef]
- Yan, W.J.; Zhong, Y.W.; Hu, X.; Xu, T.; Zhang, Y.H.; Kales, S.; Qu, Y.; Talley, D.C.; Baljinnyam, B.; LeClair, C.A.; et al. Auranofin targets UBA1 and enhances UBA1 activity by facilitating ubiquitin trans-thioesterification to E2 ubiquitin-conjugating enzymes. Nat. Commun. 2023, 14, 4798. [Google Scholar] [CrossRef]
- Chauhan, J.S.; Hölzel, M.; Lambert, J.P.; Buffa, F.M.; Goding, C.R. The MITF regulatory network in melanoma. Pigment Cell Melanoma Res. 2022, 35, 517–533. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, W.; Zhong, Y.; Fang, S. A Novel Small-Molecule GRP94 Modulator Increases PCSK9 Secretion and Promotes LDLR Degradation. Life 2025, 15, 1321. https://doi.org/10.3390/life15081321
Yan W, Zhong Y, Fang S. A Novel Small-Molecule GRP94 Modulator Increases PCSK9 Secretion and Promotes LDLR Degradation. Life. 2025; 15(8):1321. https://doi.org/10.3390/life15081321
Chicago/Turabian StyleYan, Wenjing, Yongwang Zhong, and Shengyun Fang. 2025. "A Novel Small-Molecule GRP94 Modulator Increases PCSK9 Secretion and Promotes LDLR Degradation" Life 15, no. 8: 1321. https://doi.org/10.3390/life15081321
APA StyleYan, W., Zhong, Y., & Fang, S. (2025). A Novel Small-Molecule GRP94 Modulator Increases PCSK9 Secretion and Promotes LDLR Degradation. Life, 15(8), 1321. https://doi.org/10.3390/life15081321






