Differential Performance of Children and Adults in a Vision-Deprived Maze Spatial Navigation Task and Exploration of the Impact of tDCS over the Right Posterior Parietal Cortex on Performance in Adults
Abstract
1. Introduction
2. Study 1
2.1. Materials and Methods
2.1.1. Participants
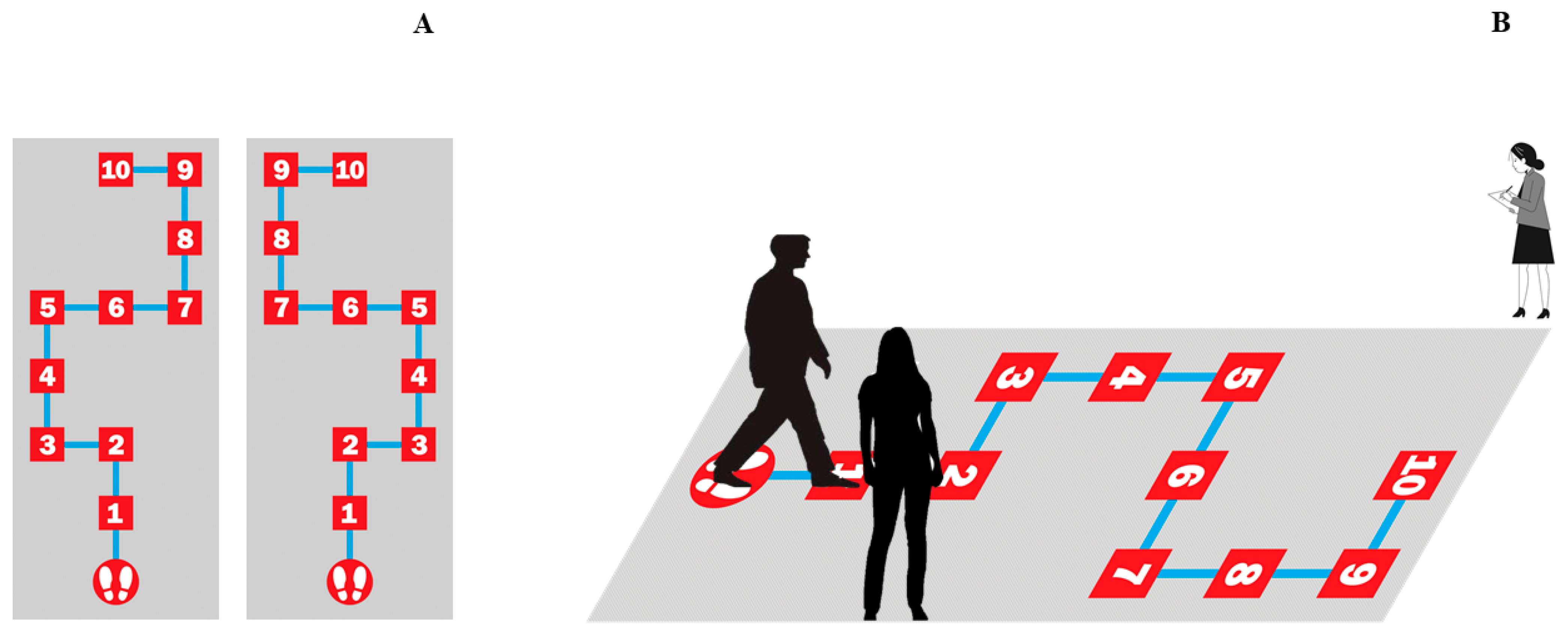
2.1.2. Maze Spatial Navigation Task
2.1.3. Data Analysis
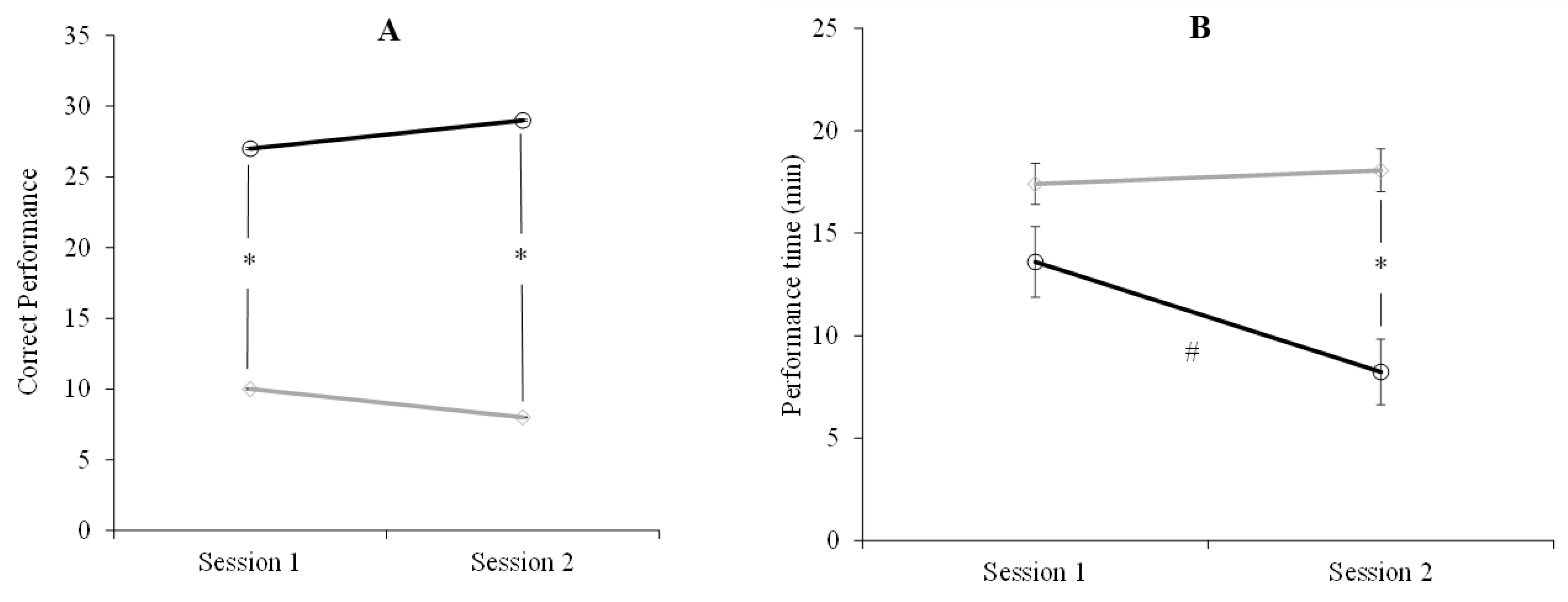
2.2. Results
3. Study 2
3.1. Material and Methods
3.1.1. Participants
3.1.2. Maze Spatial Navigation Task
3.1.3. Transcranial Direct Current Stimulation (tDCS)
3.1.4. Procedure
3.1.5. Data Analysis
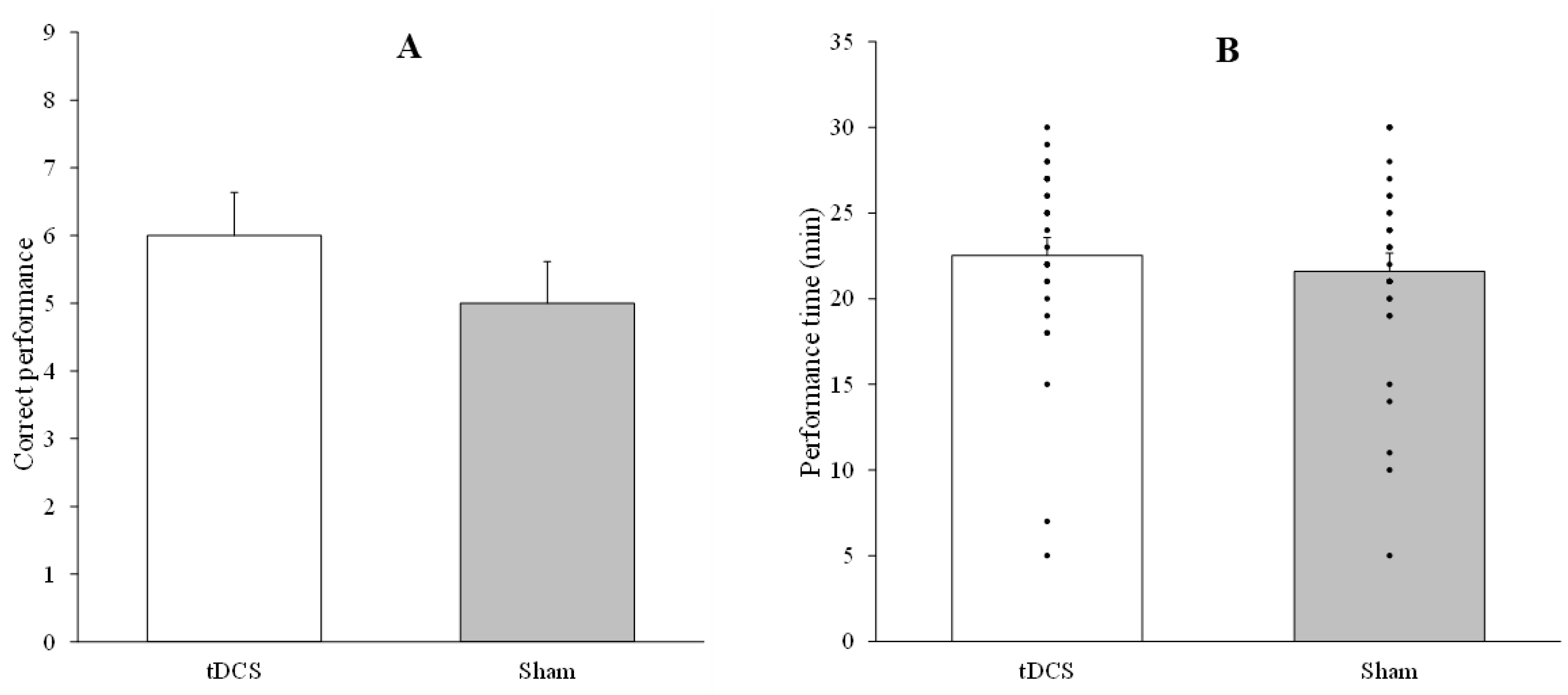
3.2. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Castillo Escamilla, J.; León Estrada, I.; Alcaraz-Iborra, M.; Cimadevilla Redondo, J.M. Aging: Working Memory Capacity and Spatial Strategies in a Virtual Orientation Task. GeroScience 2023, 45, 159–175. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Baizan, C.; Arias, J.L.; Mendez, M. Spatial Memory in Young Adults: Gender Differences in Egocentric and Allocentric Performance. Behav. Brain Res. 2019, 359, 694–700. [Google Scholar] [CrossRef]
- Moffat, S.D.; Resnick, S.M. Effects of Age on Virtual Environment Place Navigation and Allocentric Cognitive Mapping. Behav. Neurosci. 2002, 116, 851–859. [Google Scholar] [CrossRef]
- Richmond, L.L.; Sargent, J.Q.; Flores, S.; Zacks, J.M. Age Differences in Spatial Memory for Mediated Environments. Psychol. Aging 2018, 33, 892–903. [Google Scholar] [CrossRef] [PubMed]
- Rieser, J.J.; Narasimham, G.; Erdemir, A. Spatial Orientation. In Encyclopedia of Human Behavior; Elsevier: Amsterdam, The Netherlands, 2012; pp. 519–524. ISBN 978-0-08-096180-4. [Google Scholar]
- Forster, P.-P.; Fiehler, K.; Karimpur, H. Egocentric Cues Influence the Allocentric Spatial Memory of Object Configurations for Memory-Guided Actions. J. Neurophysiol. 2023, 130, 1142–1149. [Google Scholar] [CrossRef] [PubMed]
- Jin, W.; Qin, H.; Zhang, K.; Chen, X. Spatial Navigation. In Neural Circuits of Innate Behaviors; Wang, H., Ed.; Advances in Experimental Medicine and Biology; Springer: Singapore, 2020; Volume 1284, pp. 63–90. ISBN 9789811570858. [Google Scholar]
- Tomás, D.J.; Nascimento Alves, P.; Vânia Silva-Nunes, M. Spatial Orientation: A Relationship with Inferential Memory. Brain Cogn. 2023, 170, 106059. [Google Scholar] [CrossRef]
- Zorzo, C.; Arias, J.L.; Méndez, M. Functional Neuroanatomy of Allocentric Remote Spatial Memory in Rodents. Neurosci. Biobehav. Rev. 2022, 136, 104609. [Google Scholar] [CrossRef]
- Hayne, H.; Rovee-Collier, C.; Borza, M.A. Infant Memory for Place Information. Mem. Cognit. 1991, 19, 378–386. [Google Scholar] [CrossRef]
- Murias, K.; Slone, E.; Tariq, S.; Iaria, G. Development of Spatial Orientation Skills: An fMRI Study. Brain Imaging Behav. 2019, 13, 1590–1601. [Google Scholar] [CrossRef]
- Bleau, M.; Paré, S.; Chebat, D.-R.; Kupers, R.; Nemargut, J.P.; Ptito, M. Neural Substrates of Spatial Processing and Navigation in Blindness: An Activation Likelihood Estimation Meta-Analysis. Front. Neurosci. 2022, 16, 1010354. [Google Scholar] [CrossRef]
- Newcombe, N.S. Navigation and the Developing Brain. J. Exp. Biol. 2019, 222, jeb186460. [Google Scholar] [CrossRef] [PubMed]
- Toga, A.W.; Thompson, P.M.; Sowell, E.R. Mapping Brain Maturation. Trends Neurosci. 2006, 29, 148–159. [Google Scholar] [CrossRef] [PubMed]
- Moraresku, S.; Hammer, J.; Janca, R.; Jezdik, P.; Kalina, A.; Marusic, P.; Vlcek, K. Timing of Allocentric and Egocentric Spatial Processing in Human Intracranial EEG. Brain Topogr. 2023, 36, 870–889. [Google Scholar] [CrossRef]
- Bécu, M.; Sheynikhovich, D.; Ramanoël, S.; Tatur, G.; Ozier-Lafontaine, A.; Authié, C.N.; Sahel, J.-A.; Arleo, A. Landmark-Based Spatial Navigation across the Human Lifespan. eLife 2023, 12, e81318. [Google Scholar] [CrossRef]
- Stahn, A.C.; Riemer, M.; Wolbers, T.; Werner, A.; Brauns, K.; Besnard, S.; Denise, P.; Kühn, S.; Gunga, H.-C. Spatial Updating Depends on Gravity. Front. Neural Circuits 2020, 14, 20. [Google Scholar] [CrossRef] [PubMed]
- Chebat, D.-R.; Schneider, F.C.; Ptito, M. Neural Networks Mediating Perceptual Learning in Congenital Blindness. Sci. Rep. 2020, 10, 495. [Google Scholar] [CrossRef]
- Chebat, D.-R.; Schneider, F.C.; Kupers, R.; Ptito, M. Navigation with a Sensory Substitution Device in Congenitally Blind Individuals. NeuroReport 2011, 22, 342–347. [Google Scholar] [CrossRef]
- Schinazi, V.R.; Thrash, T.; Chebat, D. Spatial Navigation by Congenitally Blind Individuals. WIREs Cogn. Sci. 2016, 7, 37–58. [Google Scholar] [CrossRef]
- Tinti, C.; Adenzato, M.; Tamietto, M.; Cornoldi, C. Visual Experience Is Not Necessary for Efficient Survey Spatial Cognition: Evidence from Blindness. Q. J. Exp. Psychol. 2006, 59, 1306–1328. [Google Scholar] [CrossRef]
- O’Keefe, J.; Dostrovsky, J. The Hippocampus as a Spatial Map. Preliminary Evidence from Unit Activity in the Freely-Moving Rat. Brain Res. 1971, 34, 171–175. [Google Scholar] [CrossRef]
- Robinson, N.T.M.; Descamps, L.A.L.; Russell, L.E.; Buchholz, M.O.; Bicknell, B.A.; Antonov, G.K.; Lau, J.Y.N.; Nutbrown, R.; Schmidt-Hieber, C.; Häusser, M. Targeted Activation of Hippocampal Place Cells Drives Memory-Guided Spatial Behavior. Cell 2020, 183, 1586–1599.e10. [Google Scholar] [CrossRef]
- Whitlock, J.R.; Sutherland, R.J.; Witter, M.P.; Moser, M.-B.; Moser, E.I. Navigating from Hippocampus to Parietal Cortex. Proc. Natl. Acad. Sci. USA 2008, 105, 14755–14762. [Google Scholar] [CrossRef]
- Whitlock, J.R. Posterior Parietal Cortex. Curr. Biol. 2017, 27, R691–R695. [Google Scholar] [CrossRef]
- Nitz, D. Parietal Cortex, Navigation, and the Construction of Arbitrary Reference Frames for Spatial Information. Neurobiol. Learn. Mem. 2009, 91, 179–185. [Google Scholar] [CrossRef]
- Nitsche, M.A.; Paulus, W. Excitability Changes Induced in the Human Motor Cortex by Weak Transcranial Direct Current Stimulation. J. Physiol. 2000, 527, 633–639. [Google Scholar] [CrossRef] [PubMed]
- Buch, E.R.; Santarnecchi, E.; Antal, A.; Born, J.; Celnik, P.A.; Classen, J.; Gerloff, C.; Hallett, M.; Hummel, F.C.; Nitsche, M.A.; et al. Effects of tDCS on Motor Learning and Memory Formation: A Consensus and Critical Position Paper. Clin. Neurophysiol. 2017, 128, 589–603. [Google Scholar] [CrossRef]
- Wertheim, J.; Colzato, L.S.; Nitsche, M.A.; Ragni, M. Enhancing Spatial Reasoning by Anodal Transcranial Direct Current Stimulation over the Right Posterior Parietal Cortex. Exp. Brain Res. 2020, 238, 181–192. [Google Scholar] [CrossRef]
- Hampstead, B.M.; Brown, G.S.; Hartley, J.F. Transcranial Direct Current Stimulation Modulates Activation and Effective Connectivity During Spatial Navigation. Brain Stimulat. 2014, 7, 314–324. [Google Scholar] [CrossRef] [PubMed]
- Krishnamurthy, V.; Gopinath, K.; Brown, G.S.; Hampstead, B.M. Resting-State fMRI Reveals Enhanced Functional Connectivity in Spatial Navigation Networks after Transcranial Direct Current Stimulation. Neurosci. Lett. 2015, 604, 80–85. [Google Scholar] [CrossRef]
- Cho, I.-F.; Chao, C.-C.; Lin, T.-T.; Yang, Y.; Tang, P.-F. Effects of Posterior Parietal Cortex Anodal Transcranial Direct Current Stimulation on Ankle Tracking Visuomotor Control in Healthy Young Adults. Hum. Mov. Sci. 2025, 101, 103351. [Google Scholar] [CrossRef] [PubMed]
- Hsu, G.; Shereen, A.D.; Cohen, L.G.; Parra, L.C. Robust Enhancement of Motor Sequence Learning with 4 mA Transcranial Electric Stimulation. Brain Stimulat. 2023, 16, 56–67. [Google Scholar] [CrossRef]
- Rivera-Urbina, G.N.; Molero-Chamizo, A.; Nitsche, M.A. Discernible Effects of tDCS over the Primary Motor and Posterior Parietal Cortex on Different Stages of Motor Learning. Brain Struct. Funct. 2022, 227, 1115–1131. [Google Scholar] [CrossRef]
- Philippen, S.; Hanert, A.; Schönfeld, R.; Granert, O.; Yilmaz, R.; Jensen-Kondering, U.; Splittgerber, M.; Moliadze, V.; Siniatchkin, M.; Berg, D.; et al. Transcranial Direct Current Stimulation of the Right Temporoparietal Junction Facilitates Hippocampal Spatial Learning in Alzheimer’s Disease and Mild Cognitive Impairment. Clin. Neurophysiol. 2024, 157, 48–60. [Google Scholar] [CrossRef]
- Willacker, L.; Dowsett, J.; Dieterich, M.; Taylor, P.C.J. Egocentric Processing in the Roll Plane and Dorsal Parietal Cortex: A TMS-ERP Study of the Subjective Visual Vertical. Neuropsychologia 2019, 127, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Kang, H. Sample Size Determination and Power Analysis Using the G*Power Software. J. Educ. Eval. Health Prof. 2021, 18, 17. [Google Scholar] [CrossRef]
- Antal, A.; Alekseichuk, I.; Bikson, M.; Brockmöller, J.; Brunoni, A.R.; Chen, R.; Cohen, L.G.; Dowthwaite, G.; Ellrich, J.; Flöel, A.; et al. Low Intensity Transcranial Electric Stimulation: Safety, Ethical, Legal Regulatory and Application Guidelines. Clin. Neurophysiol. 2017, 128, 1774–1809. [Google Scholar] [CrossRef]
- Wexler, A. A Pragmatic Analysis of the Regulation of Consumer Transcranial Direct Current Stimulation (TDCS) Devices in the United States. J. Law Biosci. 2015, 2, 669–696. [Google Scholar] [CrossRef] [PubMed]
- Niemann, F.; Shababaie, A.; Paßmann, S.; Riemann, S.; Malinowski, R.; Kocataş, H.; Caisachana Guevara, L.M.; Abdelmotaleb, M.; Antonenko, D.; Blankenburg, F.; et al. Neuronavigated Focalized Transcranial Direct Current Stimulation Administered During Functional Magnetic Resonance Imaging. J. Vis. Exp. 2024, 213, 67155. [Google Scholar] [CrossRef] [PubMed]
- Bikson, M.; Datta, A.; Rahman, A.; Scaturro, J. Electrode Montages for tDCS and Weak Transcranial Electrical Stimulation: Role of “Return” Electrode’s Position and Size. Clin. Neurophysiol. 2010, 121, 1976–1978. [Google Scholar] [CrossRef]
- Nitsche, M.A.; Doemkes, S.; Karaköse, T.; Antal, A.; Liebetanz, D.; Lang, N.; Tergau, F.; Paulus, W. Shaping the Effects of Transcranial Direct Current Stimulation of the Human Motor Cortex. J. Neurophysiol. 2007, 97, 3109–3117. [Google Scholar] [CrossRef]
- Bikson, M.; Grossman, P.; Thomas, C.; Zannou, A.L.; Jiang, J.; Adnan, T.; Mourdoukoutas, A.P.; Kronberg, G.; Truong, D.; Boggio, P.; et al. Safety of Transcranial Direct Current Stimulation: Evidence Based Update 2016. Brain Stimulat. 2016, 9, 641–661. [Google Scholar] [CrossRef]
- Chhatbar, P.Y.; Chen, R.; Deardorff, R.; Dellenbach, B.; Kautz, S.A.; George, M.S.; Feng, W. Safety and Tolerability of Transcranial Direct Current Stimulation to Stroke Patients—A Phase I Current Escalation Study. Brain Stimulat. 2017, 10, 553–559. [Google Scholar] [CrossRef]
- Gillick, B.T.; Kirton, A.; Carmel, J.B.; Minhas, P.; Bikson, M. Pediatric Stroke and Transcranial Direct Current Stimulation: Methods for Rational Individualized Dose Optimization. Front. Hum. Neurosci. 2014, 8, 739. [Google Scholar] [CrossRef]
- Nitsche, M.A.; Paulus, W. Sustained Excitability Elevations Induced by Transcranial DC Motor Cortex Stimulation in Humans. Neurology 2001, 57, 1899–1901. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Urbina, G.N.; Batsikadze, G.; Molero-Chamizo, A.; Paulus, W.; Kuo, M.; Nitsche, M.A. Parietal Transcranial Direct Current Stimulation Modulates Primary Motor Cortex Excitability. Eur. J. Neurosci. 2015, 41, 845–855. [Google Scholar] [CrossRef] [PubMed]
- Saturnino, G.B.; Siebner, H.R.; Thielscher, A.; Madsen, K.H. Accessibility of Cortical Regions to Focal TES: Dependence on Spatial Position, Safety, and Practical Constraints. NeuroImage 2019, 203, 116183. [Google Scholar] [CrossRef] [PubMed]
- Saturnino, G.B.; Antunes, A.; Thielscher, A. On the Importance of Electrode Parameters for Shaping Electric Field Patterns Generated by tDCS. NeuroImage 2015, 120, 25–35. [Google Scholar] [CrossRef]
- Liu, A.; Vöröslakos, M.; Kronberg, G.; Henin, S.; Krause, M.R.; Huang, Y.; Opitz, A.; Mehta, A.; Pack, C.C.; Krekelberg, B.; et al. Immediate Neurophysiological Effects of Transcranial Electrical Stimulation. Nat. Commun. 2018, 9, 5092. [Google Scholar] [CrossRef]
- Molero-Chamizo, A.; Nitsche, M.A.; Gutiérrez Lérida, C.; Salas Sánchez, Á.; Martín Riquel, R.; Andújar Barroso, R.T.; Alameda Bailén, J.R.; García Palomeque, J.C.; Rivera-Urbina, G.N. Standard Non-Personalized Electric Field Modeling of Twenty Typical tDCS Electrode Configurations via the Computational Finite Element Method: Contributions and Limitations of Two Different Approaches. Biology 2021, 10, 1230. [Google Scholar] [CrossRef]
- Kostakos, K.; Pliakopanou, A.; Meimaridis, V.; Galanou, O.-N.O.; Anagnostou, A.A.; Sertidou, D.; Katis, P.; Anastasiou, P.; Katsoulidis, K.; Lykogiorgos, Y.; et al. Development of Spatial Memory: A Behavioral Study. NeuroSci 2024, 5, 713–728. [Google Scholar] [CrossRef]
- Deen, B.; Saxe, R.; Bedny, M. Occipital Cortex of Blind Individuals Is Functionally Coupled with Executive Control Areas of Frontal Cortex. J. Cogn. Neurosci. 2015, 27, 1633–1647. [Google Scholar] [CrossRef] [PubMed]
- Bostelmann, M.; Lavenex, P.; Banta Lavenex, P. Children Five-to-Nine Years Old Can Use Path Integration to Build a Cognitive Map without Vision. Cognit. Psychol. 2020, 121, 101307. [Google Scholar] [CrossRef]
- Moffat, S.D.; Elkins, W.; Resnick, S.M. Age Differences in the Neural Systems Supporting Human Allocentric Spatial Navigation. Neurobiol. Aging 2006, 27, 965–972. [Google Scholar] [CrossRef]
- Aggius-Vella, E.; Chebat, D.-R.; Maidenbaum, S.; Amedi, A. Activation of Human Visual Area V6 during Egocentric Navigation with and without Visual Experience. Curr. Biol. 2023, 33, 1211–1219.e5. [Google Scholar] [CrossRef]
- Chebat, D.-R.; Maidenbaum, S.; Amedi, A. Navigation Using Sensory Substitution in Real and Virtual Mazes. PLoS ONE 2015, 10, e0126307. [Google Scholar] [CrossRef]
- Oyama, F.; Ishibashi, K.; Iwanaga, K. Cathodal Transcranial Direct-Current Stimulation over Right Posterior Parietal Cortex Enhances Human Temporal Discrimination Ability. J. Physiol. Anthropol. 2017, 36, 41. [Google Scholar] [CrossRef]
- Evans, C.; Zich, C.; Lee, J.S.A.; Ward, N.; Bestmann, S. Inter-Individual Variability in Current Direction for Common tDCS Montages. NeuroImage 2022, 260, 119501. [Google Scholar] [CrossRef] [PubMed]
- Li, L.M.; Uehara, K.; Hanakawa, T. The Contribution of Interindividual Factors to Variability of Response in Transcranial Direct Current Stimulation Studies. Front. Cell. Neurosci. 2015, 9, 181. [Google Scholar] [CrossRef]
- Mikkonen, M.; Laakso, I.; Tanaka, S.; Hirata, A. Cost of Focality in TDCS: Interindividual Variability in Electric Fields. Brain Stimulat. 2020, 13, 117–124. [Google Scholar] [CrossRef]
- Strube, W.; Bunse, T.; Malchow, B.; Hasan, A. Corrigendum to “Efficacy and Interindividual Variability in Motor-Cortex Plasticity Following Anodal tDCS and Paired-Associative Stimulation”. Neural Plast. 2015, 2015, 903265. [Google Scholar] [CrossRef] [PubMed]
- Vergallito, A.; Feroldi, S.; Pisoni, A.; Romero Lauro, L.J. Inter-Individual Variability in tDCS Effects: A Narrative Review on the Contribution of Stable, Variable, and Contextual Factors. Brain Sci. 2022, 12, 522. [Google Scholar] [CrossRef] [PubMed]
- Iannone, A.; Santiago, I.; Ajao, S.T.; Brasil-Neto, J.; Rothwell, J.C.; Spampinato, D.A. Comparing the Effects of Focal and Conventional tDCS on Motor Skill Learning: A Proof of Principle Study. Neurosci. Res. 2022, 178, 83–86. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.; Truong, D.Q.; Khadka, N.; Bikson, M. Spatial and Polarity Precision of Concentric High-Definition Transcranial Direct Current Stimulation (HD-tDCS). Phys. Med. Biol. 2016, 61, 4506–4521. [Google Scholar] [CrossRef]
- He, W.; Fong, P.-Y.; Leung, T.W.H.; Huang, Y.-Z. Protocols of Non-Invasive Brain Stimulation for Neuroplasticity Induction. Neurosci. Lett. 2020, 719, 133437. [Google Scholar] [CrossRef]
- Molero-Chamizo, A.; Alameda Bailén, J.R.; Garrido Béjar, T.; García López, M.; Jaén Rodríguez, I.; Gutiérrez Lérida, C.; Pérez Panal, S.; González Ángel, G.; Lemus Corchero, L.; Ruiz Vega, M.J.; et al. Poststimulation Time Interval-Dependent Effects of Motor Cortex Anodal tDCS on Reaction-Time Task Performance. Cogn. Affect. Behav. Neurosci. 2018, 18, 167–175. [Google Scholar] [CrossRef] [PubMed]

| Study 1 | |
| Children group | 30 |
| Age | 10.97 ± 0.55 |
| Sex | 15 girls |
| Education | 5 years |
| Adults group | 30 |
| Age | 21.16 ± 1.76 |
| Sex | 15 women |
| Education | 13 years |
| Study 2 | |
| Adults | 30 |
| Age | 21.23 ± 2.01 |
| Sex | 15 women |
| Education | 13 years |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rivera-Urbina, G.N.; Kemp, N.M.; Nitsche, M.A.; Molero-Chamizo, A. Differential Performance of Children and Adults in a Vision-Deprived Maze Spatial Navigation Task and Exploration of the Impact of tDCS over the Right Posterior Parietal Cortex on Performance in Adults. Life 2025, 15, 1323. https://doi.org/10.3390/life15081323
Rivera-Urbina GN, Kemp NM, Nitsche MA, Molero-Chamizo A. Differential Performance of Children and Adults in a Vision-Deprived Maze Spatial Navigation Task and Exploration of the Impact of tDCS over the Right Posterior Parietal Cortex on Performance in Adults. Life. 2025; 15(8):1323. https://doi.org/10.3390/life15081323
Chicago/Turabian StyleRivera-Urbina, G. Nathzidy, Noah M. Kemp, Michael A. Nitsche, and Andrés Molero-Chamizo. 2025. "Differential Performance of Children and Adults in a Vision-Deprived Maze Spatial Navigation Task and Exploration of the Impact of tDCS over the Right Posterior Parietal Cortex on Performance in Adults" Life 15, no. 8: 1323. https://doi.org/10.3390/life15081323
APA StyleRivera-Urbina, G. N., Kemp, N. M., Nitsche, M. A., & Molero-Chamizo, A. (2025). Differential Performance of Children and Adults in a Vision-Deprived Maze Spatial Navigation Task and Exploration of the Impact of tDCS over the Right Posterior Parietal Cortex on Performance in Adults. Life, 15(8), 1323. https://doi.org/10.3390/life15081323









