Multiorgan Involvement and Particularly Liver Injury in Long COVID: A Narrative Review
Abstract
1. Introduction
2. Materials and Methods
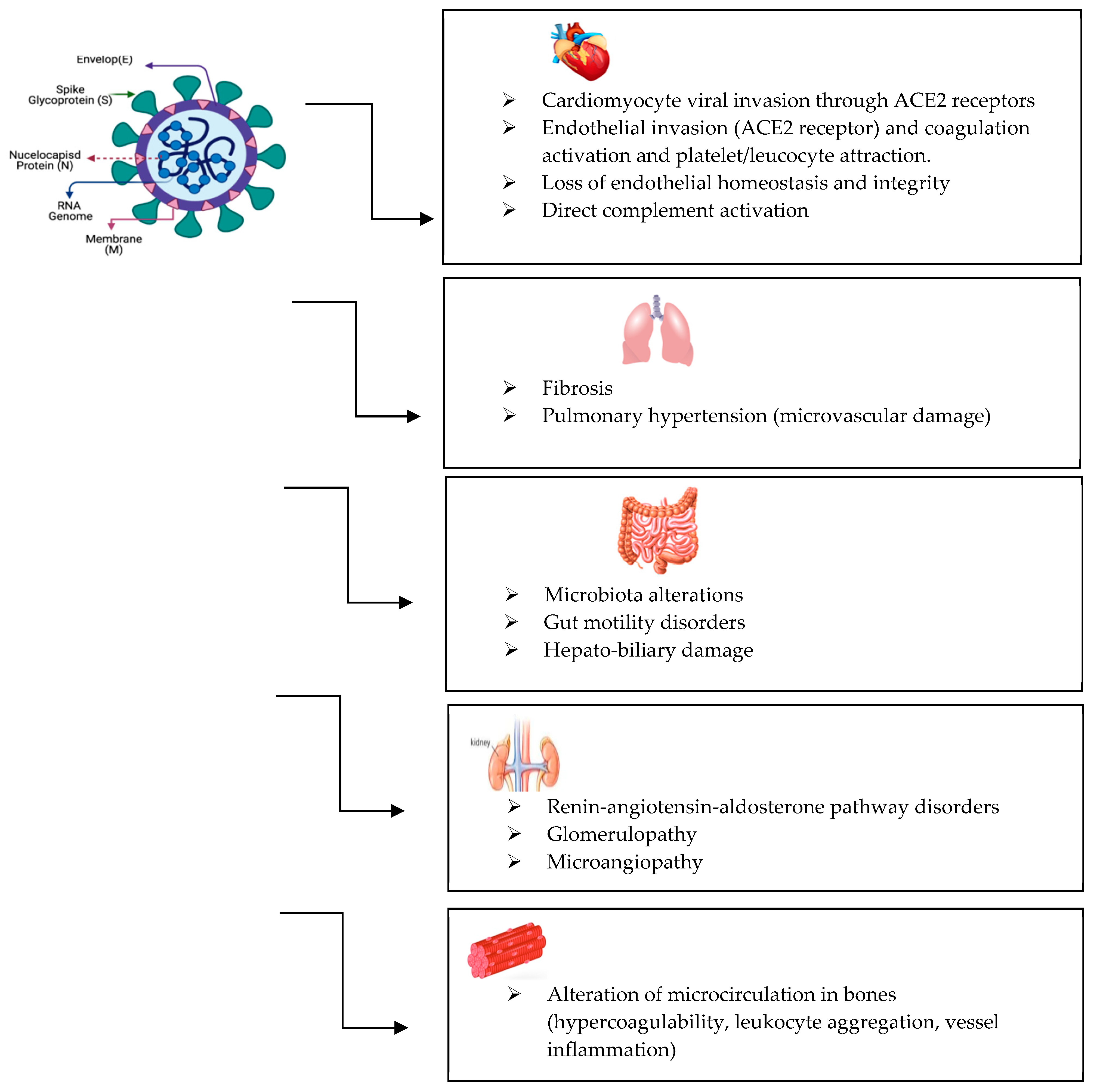
3. SARS-CoV-2 Virus Structure and Mechanism of Infection
- Spike protein—responsible for binding to the host cell receptor (ACE2) and facilitating viral entry. This protein is also the primary target for neutralizing antibodies and vaccine development.
- Envelope protein—involved in virus assembly, budding, and release.
- Membrane protein—the most abundant structural protein, playing a central role in shaping the viral envelope and coordinating virus assembly.
4. Immune-Mediated Severity in COVID-19, Inflammatory Response, and Subsequent Organ Damage
Ferritin as an Inflammatory Marker and Prognostic Indicator
5. Long COVID Symptoms and Affected Organ Systems
6. Liver Involvement in COVID-19 and Long COVID
Pre-Existing Conditions and Other Risk Factors for Liver Injury During/After COVID-19
7. Monitoring, Diagnosis, and Treatment of Long COVID
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Crook, H.; Raza, S.; Nowell, J.; Young, M.; Edison, P. Long covid-mechanisms, risk factors, and management. BMJ 2021, 374, n1944. [Google Scholar] [CrossRef]
- Castanares-Zapatero, D.; Chalon, P.; Kohn, L.; Dauvrin, M.; Detollenaere, J.; Maertens de Noordhout, C.; Primus-de Jong, C.; Cleemput, I.; Van den Heede, K. Pathophysiology and mechanism of long COVID: A comprehensive review. Ann. Med. 2022, 54, 1473–1487. [Google Scholar] [CrossRef]
- Koc, H.C.; Xiao, J.; Liu, W.; Li, Y.; Chen, G. Long COVID and its Management. Int. J. Biol. Sci. 2022, 18, 4768–4780. [Google Scholar] [CrossRef]
- Bar-On, Y.M.; Flamholz, A.; Phillips, R.; Milo, R. SARS-CoV-2 (COVID-19) by the numbers. eLife 2020, 9, e57309. [Google Scholar] [CrossRef]
- Zheng, J. SARS-CoV-2: An Emerging Coronavirus that Causes a Global Threat. Int. J. Biol. Sci. 2020, 16, 1678–1685. [Google Scholar] [CrossRef]
- Chen, B.; Farzan, M.; Choe, H. SARS-CoV-2 spike protein: Structure, viral entry and variants. Nat. Rev. Microbiol. 2025, 23, 455–468. [Google Scholar] [CrossRef] [PubMed]
- Malune, P.; Esposito, F.; Tramontano, E. Unveiling SARS-CoV-2’s heart: Role, structure and inhibition of SARS-CoV-2 RNA-dependent RNA polymerase. Antiviral Res. 2025, 240, 106208. [Google Scholar] [CrossRef]
- Kaku, Y.; Yo, M.S.; Tolentino, J.E.; Uriu, K.; Okumura, K.; Genotype to Phenotype Japan (G2P-Japan) Consortium; Ito, J.; Sato, K. Virological characteristics of the SARS-CoV-2 KP.3, LB.1, and KP.2.3 variants. Lancet Infect. Dis. 2024, 24, e482–e483. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Feng, J.; Fu, Z.; Xu, T.; Liu, J.; Yang, S.; Li, Y.; Deng, J.; Zhang, Y.; Guo, M.; et al. Epitranscriptomic m5C methylation of SARS-CoV-2 RNA regulates viral replication and the virulence of progeny viruses in the new infection. Sci. Adv. 2024, 10, eadn9519. [Google Scholar] [CrossRef]
- Liao, B.; Liu, Z.; Tang, L.; Li, L.; Gan, Q.; Shi, H.; Jiao, Q.; Guan, Y.; Xie, M.; He, X.; et al. Longitudinal clinical and radio- graphic evaluation reveals interleukin-6 as an indicator of persistent pulmonary injury in COVID-19. Int. J. Med. Sci. 2021, 18, 29–41. [Google Scholar] [CrossRef]
- Townsend, L.; Dyer, A.H.; Jones, K.; Dunne, J.; Mooney, A.; Gaffney, F.; O’Connor, L.; Leavy, D.; O’Brien, K.; Dowds, J.; et al. Persistent fatigue following SARS-CoV-2 infection is common and independent of severity of initial infection. PLoS ONE 2020, 15, e0240784. [Google Scholar] [CrossRef]
- Sudre, C.H.; Murray, B.; Varsavsky, T.; Graham, M.S.; Penfold, R.S.; Bowyer, R.C.; Pujol, J.C.; Klaser, K.; Antonelli, M.; Canas, L.S.; et al. Attributes and predictors of long COVID. Nat. Med. 2021, 27, 626–631. [Google Scholar] [CrossRef]
- Coomes, E.A.; Haghbayan, H. Interleukin-6 in Covid-19: A systematic review and meta-analysis. Rev. Med. Virol. 2020, 30, 1–9. [Google Scholar] [CrossRef]
- Moreira, A.C.; Mesquita, G.; Gomes, M.S. Ferritin: An Inflammatory Player Keeping Iron at the Core of Pathogen-Host Interactions. Microorganisms 2020, 8, 589. [Google Scholar] [CrossRef]
- Wang, W.; Knovich, M.A.; Coffman, L.G.; Torti, F.M.; Torti, S.V. Serum ferritin: Past, present and future. Biochim. Biophys. Acta 2010, 1800, 760–769. [Google Scholar] [CrossRef]
- Deng, F.; Zhang, L.; Lyu, L.; Lu, Z.; Gao, D.; Ma, X.; Guo, Y.; Wang, R.; Gong, S.; Jiang, W. Increased levels of ferritin on admission predicts intensive care unit mortality in patients with COVID-19. Med. Clin. 2021, 156, 324–331. [Google Scholar] [CrossRef]
- AbdelFattah, E.B.; Madkour, A.M.; Amer, S.M.I.; Ahmed, N.O. Correlation between the serum level of ferritin and D-dimer and the severity of COVID-19 infection. Egypt. J. Bronchol. 2023, 17, 45. [Google Scholar] [CrossRef]
- Bostanci, A.; Gazi, U.; Tosun, O.; Suer, K.; Unal Evren, E.; Evren, H.; Sanlidag, T. Long-COVID-19 in Asymptomatic, Non-Hospitalized, and Hospitalized Populations: A Cross-Sectional Study. J. Clin. Med. 2023, 12, 2613. [Google Scholar] [CrossRef]
- Pérez-González, A.; Araújo-Ameijeiras, A.; Fernández-Villar, A.; Crespo, M.; Poveda, E. Cohort COVID-19 of the Galicia Sur Health Research Institute. Long COVID in hospitalized and non-hospitalized patients in a large cohort in Northwest Spain, a prospective cohort study. Sci. Rep. 2022, 12, 3369. [Google Scholar] [CrossRef]
- Yong, S.J. Long COVID or post-COVID-19 syndrome: Putative pathophysiology, risk factors, and treatments. Infect. Dis. 2021, 53, 737–754. [Google Scholar] [CrossRef]
- Watanabe, A.; Iwagami, M.; Yasuhara, J.; Takagi, H.; Kuno, T. Protective effect of COVID-19 vaccination against long COVID syndrome: A systematic review and meta-analysis. Vaccine 2023, 41, 1783–1790. [Google Scholar] [CrossRef]
- Aby, E.S.; Moafa, G.; Latt, N.; Sultan, M.T.; Cacioppo, P.A.; Kumar, S.; Chung, R.T.; Bloom, P.P.; Gustafson, J.; Daidone, M.; et al. Long-term clinical outcomes of patients with COVID-19 and chronic liver disease: US multicenter COLD study. Hepatol. Commun. 2023, 7, e8874. [Google Scholar] [CrossRef]
- Scholkmann, F.; May, C.A. COVID-19, post-acute COVID-19 syndrome (PACS, “long COVID”) and post-COVID-19 vaccination syndrome (PCVS, “post-COVIDvac-syndrome”): Similarities and differences. Pathol. Res. Pract. 2023, 246, 154497. [Google Scholar] [CrossRef]
- Herta, T.; Berg, T. COVID-19 and the liver—Lessons learned. Liver Int. 2021, 41, 1–8. [Google Scholar] [CrossRef]
- Zhao, Y.M.; Shang, Y.M.; Song, W.B.; Li, Q.Q.; Xie, H.; Xu, Q.F.; Jia, J.L.; Li, L.M.; Mao, H.L.; Zhou, X.M.; et al. Follow-up study of the pulmonary function and related physiological characteristics of COVID-19 survivors three months after recovery. eClinicalMedicine 2020, 25, 100463. [Google Scholar] [CrossRef] [PubMed]
- Rigo, S.; Urechie, V.; Diedrich, A.; Okamoto, L.E.; Biaggioni, I.; Shibao, C. Impaired parasympathetic function in long-COVID postural orthostatic tachycardia syndrome–a case-control study. Bioelectron. Med. 2023, 9, 19. [Google Scholar] [CrossRef]
- Kruger, A.; Joffe, D.; Lloyd-Jones, G.; Khan, M.A.; Šalamon, Š.; Laubscher, G.J.; Putrino, D.; Kell, D.B.; Pretorius, E. Vascular Pathogenesis in Acute and Long COVID: Current Insights and Therapeutic Outlook. Semin. Thromb. Hemost. 2025, 51, 256–271. [Google Scholar] [CrossRef] [PubMed]
- Greene, C.; Connolly, R.; Brennan, D.; Laffan, A.; O’Keeffe, E.; Zaporojan, L.; O’Callaghan, J.; Thomson, B.; Connolly, E.; Argue, R.; et al. Blood-brain barrier disruption and sustained systemic inflammation in individuals with long COVID-associated cognitive impairment. Nat. Neurosci. 2024, 27, 421–432. [Google Scholar] [CrossRef]
- Marvisi, M.; Ferrozzi, F.; Balzarini, L.; Mancini, C.; Ramponi, S.; Uccelli, M. First report on clinical and radiological features of COVID-19 pneumonitis in a Caucasian population: Factors predicting fibrotic evolution. Int. J. Infect. Dis. 2020, 99, 485–488. [Google Scholar] [CrossRef] [PubMed]
- Boruga, M.; Septimiu-Radu, S.; Nandarge, P.S.; Elagez, A.; Doros, G.; Lazureanu, V.E.; Stoicescu, E.R.; Tanase, E.; Iacob, R.; Dumitrescu, A.; et al. Kidney Function Tests and Continuous eGFR Decrease at Six Months after SARS-CoV-2 Infection in Patients Clinically Diagnosed with Post-COVID Syndrome. Biomedicines 2024, 12, 950. [Google Scholar] [CrossRef]
- Pădureanu, V.; Caragea, D.C.; Florescu, M.M.; Vladu, I.M.; Rădulescu, P.M.; Florescu, D.N.; Rădulescu, D.; Pădureanu, R.; Efrem, I.C. Role of the SARS-COV2 infection in the evolution of acute pancreatitis. Biomed. Rep. 2023, 19, 49. [Google Scholar] [CrossRef] [PubMed]
- Cococcia, S.; Lenti, M.V.; Santacroce, G.; Achilli, G.; Borrelli de Andreis, F.; Di Sabatino, A. Liver-spleen axis dysfunction in COVID-19. World J. Gastroenterol. 2021, 27, 5919–5931. [Google Scholar] [CrossRef]
- Sinagra, E.; Shahini, E.; Crispino, F.; Macaione, I.; Guarnotta, V.; Marasà, M.; Testai, S.; Pallio, S.; Albano, D.; Facciorusso, A.; et al. COVID-19 and the Pancreas: A Narrative Review. Life 2022, 12, 1292. [Google Scholar] [CrossRef]
- Nardo, A.D.; Schneeweiss-Gleixner, M.; Bakail, M.; Dixon, E.D.; Lax, S.F.; Trauner, M. Pathophysiological mechanisms of liver injury in COVID-19. Liver Int. 2021, 41, 20–32. [Google Scholar] [CrossRef]
- Hu, W.S.; Jiang, F.Y.; Shu, W.; Zhao, R.; Cao, J.M.; Wang, D.P. Liver injury in COVID-19: A minireview. World J. Gastroenterol. 2022, 28, 6716–6731. [Google Scholar] [CrossRef]
- Saha, L.; Vij, S.; Rawat, K. Liver injury induced by COVID-19 treatment—what do we know? World J. Gastroenterol. 2022, 28, 6314–6327. [Google Scholar] [CrossRef]
- Kolesova, O.; Vanaga, I.; Laivacuma, S.; Derovs, A.; Kolesovs, A.; Radzina, M.; Platkajis, A.; Eglite, J.; Hagina, E.; Arutjunana, S.; et al. Intriguing findings of liver fibrosis following COVID-19. BMC. Gastroenterol. 2021, 21, 370. [Google Scholar] [CrossRef]
- Caballero-Alvarado, J.; Zavaleta Corvera, C.; Merino Bacilio, B.; Ruiz Caballero, C.; Lozano-Peralta, K. Post-COVID cholangiopathy: A narrative review. Gastroenterol. Hepatol. 2023, 46, 474–482. [Google Scholar] [CrossRef]
- Dissanayake, H. COVID-19 and metabolic syndrome. Best Pract. Res. Clin. Endocrinol. Metab. 2023, 37, 101753. [Google Scholar] [CrossRef] [PubMed]
- Pesti, A.; Danics, K.; Glasz, T.; Várkonyi, T.; Barbai, T.; Reszegi, A.; Kovalszky, I.; Vályi-Nagy, I.; Dobi, D.; Lotz, G.; et al. Liver alterations and detection of SARS-CoV-2 RNA and proteins in COVID-19 autopsies. Geroscience 2023, 45, 1015–1031. [Google Scholar] [CrossRef] [PubMed]
- Lechner-Scott, J.; Levy, M.; Hawkes, C.; Yeh, A.; Giovannoni, G. Long COVID or post COVID-19 syndrome. Mult. Scler. Relat. Disord. 2021, 55, 103268. [Google Scholar] [CrossRef]
- Nalbandian, A.; Sehgal, K.; Gupta, A.; Madhavan, M.V.; McGroder, C.; Stevens, J.S.; Cook, J.R.; Nordvig, A.S.; Shalev, D.; Sehrawat, T.S.; et al. Post-acute COVID-19 syndrome. Nat. Med. 2021, 27, 601–615. [Google Scholar] [CrossRef]
- Afrisham, R.; Jadidi, Y.; Davoudi, M.; Moayedi, K.; Soliemanifar, O.; Eleni Xirouchaki, C.; Ashtary-Larky, D.; Seyyedebrahimi, S.; Alizadeh, S. Gastrointestinal, Liver, Pancreas, Oral and Psychological Long-term Symptoms of COVID-19 After Recovery: A Review. Mini-Rev. Med. Chem. 2023, 23, 852–868. [Google Scholar] [CrossRef] [PubMed]
- Bernasconi, E.; Biagi, M.; Di Agostino, S.; Cursaro, C.; Felicani, C.; Ronconi, E.; Franchi, E.; Costanzo, A.C.; Gabrielli, F.; Cavicchioli, A.; et al. Investigating Acute Hepatitis after SARS-CoV-2 Vaccination or Infection: A Genetic Case Series. Biomedicines 2023, 11, 2848. [Google Scholar] [CrossRef] [PubMed]
- Gupta, T.; Sharma, H. COVID-19 and the liver: Are footprints still there? World J. Gastroenterol. 2023, 29, 656–669. [Google Scholar] [CrossRef] [PubMed]
- Cooper, S.; Tobar, A.; Konen, O.; Orenstein, N.; Kropach Gilad, N.; Landau, Y.E.; Mozer-Glassberg, Y.; Bar-Lev, M.R.; Shaoul, R.; Shamir, R.; et al. Long COVID-19 Liver Manifestation in Children. J. Pediatr. Gastroenterol. Nutr. 2022, 75, 244–251. [Google Scholar] [CrossRef]
- Davis, H.E.; McCorkell, L.; Vogel, J.M.; Topol, E.J. Long COVID: Major findings, mechanisms and recommendations. Nat. Rev. Microbiol. 2023, 21, 133–146. [Google Scholar] [CrossRef]
| Direct cytopathy | Virus invading liver cells => cytopathic effects => liver dysfunction |
| Immune-mediated damage | Virus infection => dysregulated inflammatory response characterized by increased pro-inflammatory cytokines => severe liver dysfunction |
| Hypoxia/ischemia (in severe cases) | Multiple organ dysfunction => hypoxia-related acute respiratory distress syndrome, hypotension, or congestive heart failure => liver dysfunction |
| Microvascular thrombosis | Impaired blood flow to the liver => further exacerbation of liver injury |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Florea, C.-E.; Bălaș-Maftei, B.; Rotaru, A.; Abudanii, P.L.; Vieru, S.T.; Grigoriu, M.; Stoian, A.; Manciuc, C. Multiorgan Involvement and Particularly Liver Injury in Long COVID: A Narrative Review. Life 2025, 15, 1314. https://doi.org/10.3390/life15081314
Florea C-E, Bălaș-Maftei B, Rotaru A, Abudanii PL, Vieru ST, Grigoriu M, Stoian A, Manciuc C. Multiorgan Involvement and Particularly Liver Injury in Long COVID: A Narrative Review. Life. 2025; 15(8):1314. https://doi.org/10.3390/life15081314
Chicago/Turabian StyleFlorea, Carmen-Elena, Bianca Bălaș-Maftei, Alexandra Rotaru, Patricia Lorena Abudanii, Stefana Teodora Vieru, Maria Grigoriu, Adelina Stoian, and Carmen Manciuc. 2025. "Multiorgan Involvement and Particularly Liver Injury in Long COVID: A Narrative Review" Life 15, no. 8: 1314. https://doi.org/10.3390/life15081314
APA StyleFlorea, C.-E., Bălaș-Maftei, B., Rotaru, A., Abudanii, P. L., Vieru, S. T., Grigoriu, M., Stoian, A., & Manciuc, C. (2025). Multiorgan Involvement and Particularly Liver Injury in Long COVID: A Narrative Review. Life, 15(8), 1314. https://doi.org/10.3390/life15081314





