CFTR Modulators Counteract F508del CFTR Functional Defects in a Pancreatic Epithelial Model of Cystic Fibrosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Cell Culture
2.3. Pharmacological Treatments and Inflammatory Stimulation
2.4. Transepithelial Electrical Resistance (TEER) Measurements
2.5. Measurement of Fluid Absorption (Jw) and Total Protein Content of the Apical Surface Fluid (ASF)
2.6. pH Measurement in Apical Surface Fluid (ASF)
2.7. Western Blot and Dot Blot Analyses
2.8. Multiple Particle Tracking (MPT) Analysis
2.9. Statistics
3. Results
3.1. CFTR Modulators Enhance Transepithelial Conductance (TEC) in CFPAC-1 Epithelial Preparations
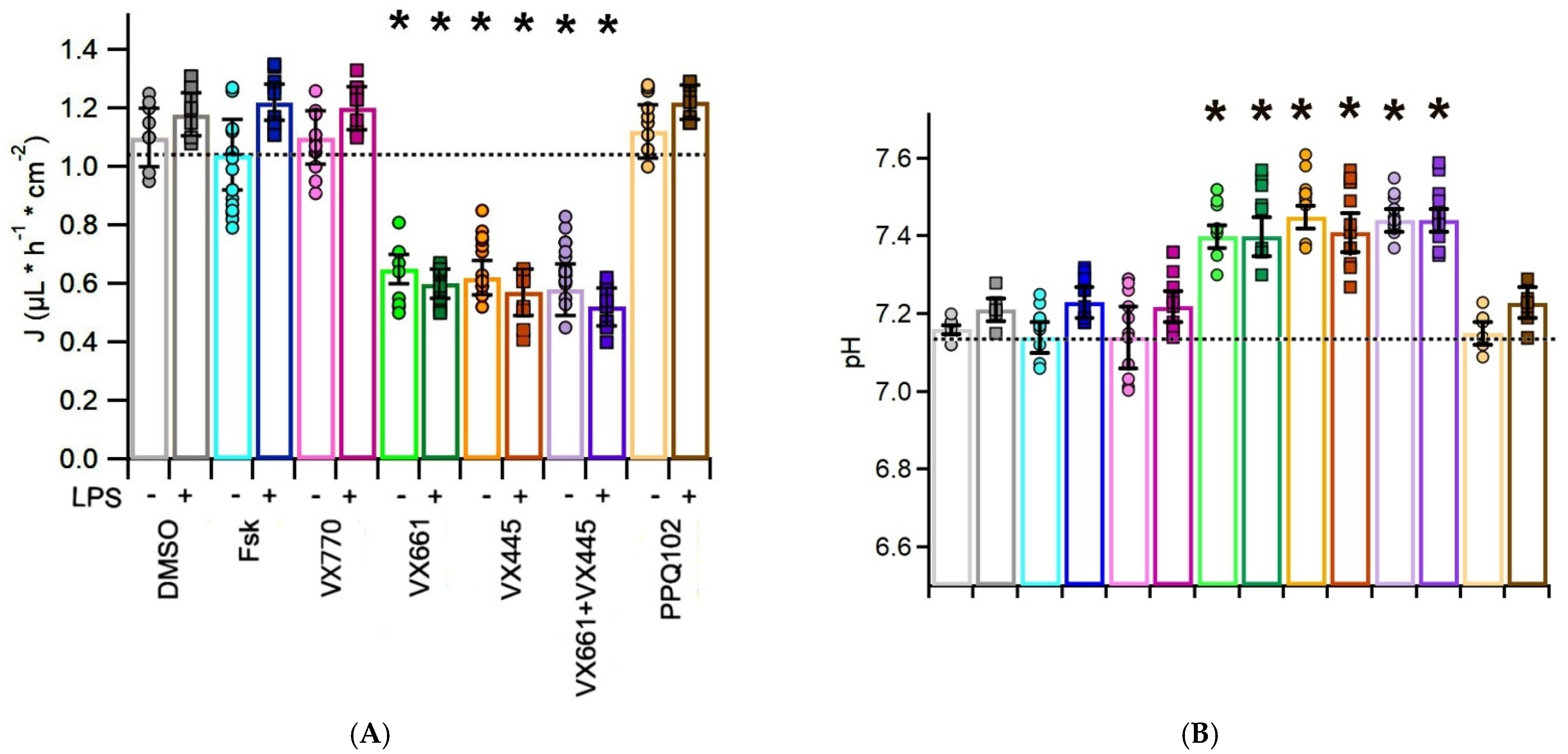
3.2. CFTR Modulators Reduce Apical Surface Fluid Reabsorption in CFPAC-1 Monolayers
3.3. CFTR Modulators Increase the pH of the Apical Surface Fluid in CFPAC-1 Cells, with Additive Effects in the Presence of LPS
3.4. CFTR Modulators Do Not Affect Total Protein Concentration in the Apical Surface Fluid (ASF) of CF-PAC1 Epithelial Layers
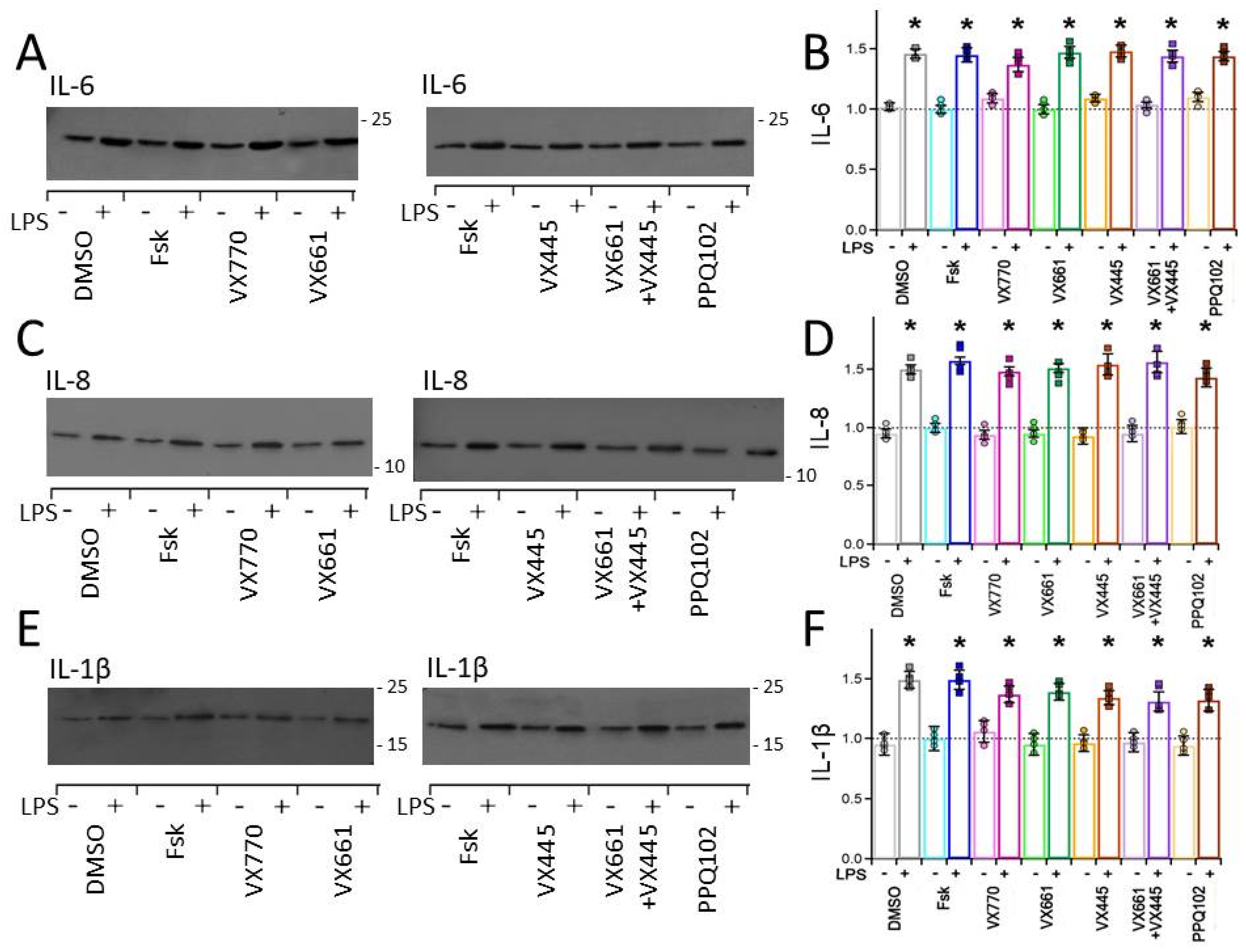
3.5. CFTR Modulators Enhance Endogenous F508del CFTR Levels in CFPAC-1 Cells but Do Not Reduce Pro−Inflammatory Cytokine Expression
3.6. CFTR Modulators Promote Mucin Secretion in the Apical Surface Fluid (ASF) of CFPAC-1 Epithelial Preparations
3.7. CFTR Modulators Decrease the Viscosity of the Apical Surface Fluid (ASF) in CFPAC-1 Epithelial Layers
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bobadilla, J.L.; Macek, M., Jr.; Fine, J.P.; Farrell, P.M. Cystic fibrosis: A worldwide analysis of CFTR mutations--correlation with incidence data and application to screening. Hum. Mutat. 2002, 19, 575–606. [Google Scholar] [CrossRef] [PubMed]
- Riordan, J.R.; Rommens, J.M.; Kerem, B.-S.; Alon, N.; Rozmahel, R.; Grzelczak, Z.; Zielenski, J.; Lok, S.; Plavsic, N.; Chou, J.-L.; et al. Identification of the cystic fibrosis gene: Cloning and characterization of complementary DNA. Science 1989, 245, 1066–1073, Erratum in Science 1989, 245, 1437. [Google Scholar] [CrossRef] [PubMed]
- Sosnay, P.R.; Siklosi, K.R.; Van Goor, F.; Kaniecki, K.; Yu, H.; Sharma, N.; Ramalho, A.S.; Amaral, M.D.; Dorfman, R.; Zielenski, J.; et al. Defining the disease liability of variants in the cystic fibrosis transmembrane conductance regulator gene. Nat. Genet. 2013, 45, 1160–1167. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Savant, A.; Lyman, B.; Bojanowski, C.; Upadia, J. Cystic Fibrosis, 26 Mar 2001 [updated 2024 Aug 8]. In GeneReviews®; Adam, M.P., Feldman, J., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 1993–2025. [Google Scholar] [PubMed]
- Ratjen, F.; Döring, G. Cystic fibrosis. Lancet 2003, 361, 681–689. [Google Scholar] [CrossRef] [PubMed]
- Kristidis, P.; Bozon, D.; Corey, M.; Markiewicz, D.; Rommens, J.; Tsui, L.C.; Durie, P. Genetic determination of exocrine pancreatic function in cystic fibrosis. Am. J. Hum. Genet. 1992, 50, 1178–1184. [Google Scholar] [PubMed] [PubMed Central]
- Gibson-Corley, K.N.; Meyerholz, D.K.; Engelhardt, J.F. Pancreatic pathophysiology in cystic fibrosis. J. Pathol. 2015, 238, 311–320. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lukacs, G.L.; Verkman, A. CFTR: Folding, misfolding and correcting the ΔF508 conformational defect. Trends Mol. Med. 2012, 18, 81–91. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Van Goor, F.; Hadida, S.; Grootenhuis, P.D.J.; Burton, B.; Stack, J.H.; Straley, K.S.; Decker, C.J.; Miller, M.; McCartney, J.; Olson, E.R.; et al. Correction of the F508del-CFTR protein processing defect in vitro by the investigational drug VX-809. Proc. Natl. Acad. Sci. USA 2011, 108, 18843–18848. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Baroni, D. Unraveling the Mechanism of Action, Binding Sites, and Therapeutic Advances of CFTR Modulators: A Narrative Review. Curr. Issues Mol. Biol. 2025, 47, 119. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Middleton, P.G.; Mall, M.A.; Dřevínek, P.; Lands, L.C.; McKone, E.F.; Polineni, D.; Ramsey, B.W.; Taylor-Cousar, J.L.; Tullis, E.; Vermeulen, F.; et al. Elexacaftor-Tezacaftor-Ivacaftor for Cystic Fibrosis with a Single Phe508del Allele. N. Engl. J. Med. 2019, 381, 1809–1819. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nichols, D.P.; Paynter, A.C.; Heltshe, S.L.; Donaldson, S.H.; Frederick, C.A.; Freedman, S.D.; Gelfond, D.; Hoffman, L.R.; Kelly, A.; Narkewicz, M.R.; et al. Clinical Effectiveness of Elexacaftor/Tezacaftor/Ivacaftor in People with Cystic Fibrosis: A Clinical Trial. Am. J. Respir. Crit. Care Med. 2022, 205, 529–539. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Reid, G.J.; Hyde, K.; Ho, S.B.; Harris, A.; Weatherall, D. Cystic fibrosis of the pancreas: Involvement of MUC6 mucin in obstruction of pancreatic ducts. Mol. Med. 1997, 3, 403–411. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wilschanski, M.; Novak, I. The cystic fibrosis of exocrine pancreas. Cold Spring Harb. Perspect. Med. 2013, 3, a009746. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kim, Y.; Jun, I.; Shin, D.H.; Yoon, J.; Piao, H.; Jung, J.; Park, H.; Cheng, M.H.; Bahar, I.; Whitcomb, D.C.; et al. Regulation of CFTR Bicarbonate Channel Activity by WNK1: Implications for Pancreatitis and CFTR-Related Disorders. Cell. Mol. Gastroenterol. Hepatol. 2020, 9, 79–103. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Park, H.W.; Lee, M.G. Transepithelial bicarbonate secretion: Lessons from the pancreas. Cold Spring Harb. Perspect. Med. 2012, 2, a009571. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Durie, P.R.; Forstner, G.G. Pathophysiology of the exocrine pancreas in cystic fibrosis. J. R. Soc. Med. 1989, 82, 2–10. [Google Scholar] [PubMed] [PubMed Central]
- Quinton, P.M. The neglected ion: HCO3-. Nat. Med. 2001, 7, 292–293. [Google Scholar] [CrossRef] [PubMed]
- Quinton, P.M. Role of epithelial HCO3- transport in mucin secretion: Lessons from cystic fibrosis. Am. J. Physiol. Cell Physiol. 2010, 299, C1222–C1233. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kreda, S.M.; Davis, C.W.; Rose, M.C. CFTR, Mucins, and Mucus Obstruction in Cystic Fibrosis. Cold Spring Harb. Perspect. Med. 2012, 2, a009589. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gustafsson, J.K.; Ermund, A.; Ambort, D.; Johansson, M.E.; Nilsson, H.E.; Thorell, K.; Hebert, H.; Sjövall, H.; Hansson, G.C. Bicarbonate and functional CFTR channel are required for proper mucin secretion and link cystic fibrosis with its mucus phenotype. J. Exp. Med. 2012, 209, 1263–1272. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Morrison, C.B.; Markovetz, M.R.; Ehre, C. Mucus, mucins, and cystic fibrosis. Pediatr. Pulmonol. 2019, 54 (Suppl. S3), S84–S96. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Angyal, D.; Bijvelds, M.J.C.; Bruno, M.J.; Peppelenbosch, M.P.; de Jonge, H.R. Bicarbonate Transport in Cystic Fibrosis and Pancreatitis. Cells 2021, 11, 54. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Madácsy, T.; Pallagi, P.; Maleth, J. Fibrosis of the Pancreas: The Role of CFTR Channel in the Regulation of Intracellular Ca2+ Signaling and Mitochondrial Function in the Exocrine Pancreas. Front. Physiol. 2018, 9, 1585. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hegyi, P.; Seidler, U.; Kunzelmann, K. CFTR-beyond the airways: Recent findings on the role of the CFTR channel in the pancreas, the intestine and the kidneys. J. Cyst. Fibros. 2023, 22 (Suppl. S1), S17–S22. [Google Scholar] [CrossRef] [PubMed]
- McKay, I.R.; Ooi, C.Y. The Exocrine Pancreas in Cystic Fibrosis in the Era of CFTR Modulation: A Mini Review. Front. Pediatr. 2022, 10, 914790. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mattke, J.; Darden, C.M.; Lawrence, M.C.; Kuncha, J.; Shah, Y.A.; Kane, R.R.; Naziruddin, B. Toll-like receptor 4 in pancreatic damage and immune infiltration in acute pancreatitis. Front. Immunol. 2024, 15, 1362727. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pooran, N.; Indaram, A.; Singh, P.; Bank, S. Cytokines (IL-6, IL-8, TNF): Early and reliable predictors of severe acute pancreatitis. J. Clin. Gastroenterol. 2003, 37, 263–266. [Google Scholar] [CrossRef] [PubMed]
- Angyal, D.; Groeneweg, T.A.; Leung, A.; Desain, M.; Dulla, K.; de Jonge, H.R.; Bijvelds, M.J.C. Pro-inflammatory cytokines stimulate CFTR-dependent anion secretion in pancreatic ductal epithelium. Cell. Mol. Biol. Lett. 2024, 29, 18. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, X.; Tian, X.; Wang, Y.; Yan, Y.; Wang, Y.; Su, M.; Lv, H.; Li, K.; Hao, X.; Xing, X.; et al. Application of lipopolysaccharide in establishing inflammatory models. Int. J. Biol. Macromol. 2024, 279 Pt 4, 135371. [Google Scholar] [CrossRef] [PubMed]
- Sivam, H.G.P.; Chin, B.Y.; Gan, S.Y.; Ng, J.H.; Gwenhure, A.; Chan, E.W.L. Lipopolysaccharide (LPS) stimulation of Pancreatic Ductal Adenocarcinoma (PDAC) and macrophages activates the NLRP3 inflammasome that influences the levels of pro-inflammatory cytokines in a co-culture model. Cancer Biol. Ther. 2023, 24, 2284857. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Constantinescu, A.A.; Gleizes, C.; Alhosin, M.; Yala, E.; Zobairi, F.; Leclercq, A.; Stoian, G.; Mitrea, I.L.; Prévost, G.; Toti, F.; et al. Exocrine cell-derived microparticles in response to lipopolysaccharide promote endocrine dysfunction in cystic fibrosis. J. Cyst. Fibros. 2014, 13, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Schoumacher, R.A.; Ram, J.; Iannuzzi, M.C.; Bradbury, N.A.; Wallace, R.W.; Hon, C.T.; Kelly, D.R.; Schmid, S.M.; Gelder, F.B.; A Rado, T. A cystic fibrosis pancreatic adenocarcinoma cell line. Proc. Natl. Acad. Sci. USA 1990, 87, 4012–4016. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kersting, U.; Kersting, D.; Spring, K.R. Ketoconazole activates Cl- conductance and blocks Cl- and fluid absorption by cultured cystic fibrosis (CFPAC-1) cells. Proc. Natl. Acad. Sci. USA 1993, 90, 4047–4051. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Carew, M.A.; Yang, X.; Schultz, C.; Shears, S.B. myo-inositol 3,4,5,6-tetrakisphosphate inhibits an apical calcium-activated chloride conductance in polarized monolayers of a cystic fibrosis cell line. J. Biol. Chem. 2000, 275, 26906–26913. [Google Scholar] [CrossRef] [PubMed]
- Rakonczay, Z., Jr.; Fearn, A.; Hegyi, P.; Boros, I.; Gray, M.A.; Argent, B.E. Characterization of H+ and HCO3- transporters in CFPAC-1 human pancreatic duct cells. World J. Gastroenterol. 2006, 12, 885–895. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chan, H.C.; Law, S.H.; Leung, P.S.; Fu, L.X.; Wong, P.Y. Angiotensin II receptor type I-regulated anion secretion in cystic fibrosis pancreatic duct cells. J. Membr. Biol. 1997, 156, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Achoute, L.; Margolskee, R.F.; Jiang, P.; Wang, H. Lipopolysaccharide-Induced Inflammatory Cytokine Expression in Taste Organoids. Chem Senses. 2020, 45, 187–194. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lin, T.Y.; Fan, C.W.; Maa, M.C.; Leu, T.H. Lipopolysaccharide-promoted proliferation of Caco-2 cells is mediated by c-Src induction and ERK activation. BioMedicine 2015, 5, 5. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, B.; Cao, J. Effects of transfection on inflammatory factor production in LPS-induced HBE cells. Histol. Histopathol. 2025, 40, 541–545. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Liu, C.; Bao, C.; Hu, J.; Li, Z.; Ma, X.; Zhu, Y.; Xu, S. Inhibition of the ATP synthase c subunit ameliorates HDM/LPS-induced inflammatory responses in asthmatic bronchial epithelial cells by blocking the mPTP-mtDNA-cGAS-STING axis. Respir. Res. 2025, 26, 219. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nova, Z.; Skovierova, H.; Strnadel, J.; Halasova, E.; Calkovska, A. Short-Term versus Long-Term Culture of A549 Cells for Evaluating the Effects of Lipopolysaccharide on Oxidative Stress, Surfactant Proteins and Cathelicidin LL-37. Int. J. Mol. Sci. 2020, 21, 1148. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhao, W.; Shen, T.; Zhao, B.; Li, M.; Deng, Z.; Huo, Y.; Aernouts, B.; Loor, J.J.; Psifidi, A.; Xu, C. Epigallocatechin-3-gallate protects bovine ruminal epithelial cells against lipopolysaccharide-induced inflammatory damage by activating autophagy. J. Anim. Sci. Biotechnol. 2024, 15, 109. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, W.; Xu, R.-L.; He, P.; Chen, R. MAR1 suppresses inflammatory response in LPS-induced RAW 264.7 macrophages and human primary peripheral blood mononuclear cells via the SIRT1/PGC-1α/PPAR-γ pathway. J. Inflamm. 2021, 18, 8. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gianotti, A.; Capurro, V.; Scudieri, P.; Galietta, L.J.; Moran, O.; Zegarra-Moran, O. Pharmacological rescue of mutant CFTR protein improves the viscoelastic properties of CF mucus. J. Cyst. Fibros. 2016, 15, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Gianotti, A.; Capurro, V.; Delpiano, L.; Mielczarek, M.; García-Valverde, M.; Carreira-Barral, I.; Ludovico, A.; Fiore, M.; Baroni, D.; Moran, O.; et al. Small Molecule Anion Carriers Correct Abnormal Airway Surface Liquid Properties in Cystic Fibrosis Airway Epithelia. Int. J. Mol. Sci. 2020, 21, 1488. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ludovico, A.; Moran, O.; Baroni, D. Modulator Combination Improves In Vitro the Microrheological Properties of the Airway Surface Liquid of Cystic Fibrosis Airway Epithelia. Int. J. Mol. Sci. 2022, 23, 11396. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods. 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Qian, H.; Sheetz, M.P.; Elson, E.L. Single particle tracking. Analysis of diffusion and flow in two-dimensional systems. Biophys. J. 1991, 60, 910–921. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wirtz, D. Particle-tracking microrheology of living cells: Principles and applications. Annu. Rev. Biophys. 2009, 38, 301–326. [Google Scholar] [CrossRef] [PubMed]
- Wigmore, S.J.; Fearon, K.C.; Sangster, K.; Maingay, J.P.; Garden, O.J.; Ross, J.A. Cytokine regulation of constitutive production of interleukin-8 and -6 by human pancreatic cancer cell lines and serum cytokine concentrations in patients with pancreatic cancer. Int. J. Oncol. 2002, 21, 881–886. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Shi, M.; Yu, G.Z.; Qin, X.R.; Jin, G.; Chen, P.; Zhu, M.H. Interleukin-8, a promising predictor for prognosis of pancreatic cancer. World J. Gastroenterol. 2012, 18, 1123–1129. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mendis, E.; Kim, M.-M.; Rajapakse, N.; Kim, S.-K. Suppression of cytokine production in lipopolysaccharide-stimulated mouse macrophages by novel cationic glucosamine derivative involves down-regulation of NF-kappaB and MAPK expressions. Bioorganic Med. Chem. 2008, 16, 8390–8396. [Google Scholar] [CrossRef] [PubMed]
- Duan, W.; Wang, H.; Fan, Q.; Chen, L.; Huang, H.; Ran, H. Cystatin F involvement in adenosine A2A receptor-mediated neuroinflammation in BV2 microglial cells. Sci. Rep. 2018, 8, 6820. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, X.; Cheng, Y.; Xiong, Y.; Ye, C.; Zheng, H.; Sun, H.; Zhao, H.; Ren, Z.; Xu, J.; Bose, S. Enterohemorrhagic Escherichia coli specific enterohemolysin induced IL-1β in human macrophages and EHEC-induced IL-1β required activation of NLRP3 inflammasome. PLoS ONE 2012, 7, e50288. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Montserrat, C.; Merten, M.; Figarella, C. Defective ATP-dependent mucin secretion by cystic fibrosis pancreatic epithelial cells. FEBS Lett. 1996, 393, 264–268. [Google Scholar] [CrossRef] [PubMed]
- Bose, M.; Grover, P.; Sanders, A.J.; Zhou, R.; Ahmad, M.; Shwartz, S.; Lala, P.; Nath, S.; Yazdanifar, M.; Brouwer, C.; et al. Overexpression of MUC1 Induces Non-Canonical TGF-β Signaling in Pancreatic Ductal Adenocarcinoma. Front. Cell Dev. Biol. 2022, 10, 821875. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Qu, C.F.; Li, Y.; Song, Y.J.; Rizvi, S.M.A.; Raja, C.; Zhang, D.; Samra, J.; Smith, R.; Perkins, A.C.; Apostolidis, C.; et al. MUC1 expression in primary and metastatic pancreatic cancer cells for in vitro treatment by (213)Bi-C595 radioimmunoconjugate. Br. J. Cancer 2004, 91, 2086–2093. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Montserrat, C.; Hollande, E.; Guy-Crotte, O.; Figarella, C. Direct double antibody sandwich immunoassay of mucin M1 epitopes in human mucus secreting pancreatic cell lines. Clin. Chim. Acta 1995, 243, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Dahiya, R.; Kwak, K.S.; Ho, S.B.; Yoon, W.H.; Kim, Y.S. Cystic fibrosis and pancreatic cancer cells synthesize and secrete MUC1 type mucin gene product. Biochem. Mol. Biol. Int. 1995, 35, 351–362. [Google Scholar] [PubMed]
- Zhu, Y.; Chidekel, A.; Shaffer, T.H. Cultured human airway epithelial cells (calu-3): A model of human respiratory function, structure, and inflammatory responses. Crit. Care Res. Pract. 2010, 2010, 394578. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Haghi, M.; Young, P.M.; Traini, D.; Jaiswal, R.; Gong, J.; Bebawy, M. Time- and passage-dependent characteristics of a Calu-3 respiratory epithelial cell model. Drug Dev. Ind. Pharm. 2010, 36, 1207–1214. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.F.; Lethem, M.I.; Lansley, A.B. A comparison of three mucus-secreting airway cell lines (Calu-3, SPOC1 and UNCN3T) for use as biopharmaceutical models of the nose and lung. Eur. J. Pharm. Biopharm. 2021, 167, 159–174. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kreda, S.M.; Okada, S.F.; Van Heusden, C.A.; O'NEal, W.; Gabriel, S.; Abdullah, L.; Davis, C.W.; Boucher, R.C.; Lazarowski, E.R. Coordinated release of nucleotides and mucin from human airway epithelial Calu-3 cells. J. Physiol. 2007, 584 Pt 1, 245–259. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kim, D.; Maharjan, S.; Kim, J.; Park, S.; Park, J.-A.; Park, B.K.; Lee, Y.; Kwon, H.-J. MUC1-C influences cell survival in lung adenocarcinoma Calu-3 cells after SARS-CoV-2 infection. BMB Rep. 2021, 54, 425–430. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Okuda, K.; Chen, G.; Subramani, D.B.; Wolf, M.; Gilmore, R.C.; Kato, T.; Radicioni, G.; Kesimer, M.; Chua, M.; Dang, H.; et al. Localization of Secretory Mucins MUC5AC and MUC5B in Normal/Healthy Human Airways. Am. J. Respir. Crit. Care Med. 2019, 199, 715–727. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- McCague, A.F.; Raraigh, K.S.; Pellicore, M.J.; Davis-Marcisak, E.F.; Evans, T.A.; Han, S.T.; Lu, Z.; Joynt, A.T.; Sharma, N.; Castellani, C.; et al. Correlating Cystic Fibrosis Transmembrane Conductance Regulator Function with Clinical Features to Inform Precision Treatment of Cystic Fibrosis. Am. J. Respir. Crit. Care Med. 2019, 199, 1116–1126. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Keating, D.; Marigowda, G.; Burr, L.D.; Daines, C.; Mall, M.A.; McKone, E.F.; Ramsey, B.W.; Rowe, S.M.; Sass, L.A.; Tullis, E.; et al. VX-445-Tezacaftor-Ivacaftor in Patients with Cystic Fibrosis and One or Two Phe508del Alleles. N. Engl. J. Med. 2018, 379, 1612–1620. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hisert, K.B.; Birket, S.E.; Clancy, J.P.; Downey, D.G.; Engelhardt, J.F.; Fajac, I.; Gray, R.D.; E Lachowicz-Scroggins, M.; Mayer-Hamblett, N.; Thibodeau, P.; et al. Understanding and addressing the needs of people with cystic fibrosis in the era of CFTR modulator therapy. Lancet Respir. Med. 2023, 11, 916–931. [Google Scholar] [CrossRef] [PubMed]
- Becq, F.; Mirval, S.; Carrez, T.; Lévêque, M.; Billet, A.; Coraux, C.; Sage, E.; Cantereau, A. The rescue of F508del-CFTR by elexacaftor/tezacaftor/ivacaftor (Trikafta) in human airway epithelial cells is underestimated due to the presence of ivacaftor. Eur. Respir. J. 2022, 59, 2100671. [Google Scholar] [CrossRef] [PubMed]
- Kleinfelder, K.; Lotti, V.; Eramo, A.; Amato, F.; Cicero, S.L.; Castelli, G.; Spadaro, F.; Farinazzo, A.; Dell’oRco, D.; Preato, S.; et al. In silico analysis and theratyping of an ultra-rare CFTR genotype (W57G/A234D) in primary human rectal and nasal epithelial cells. iScience 2023, 26, 108180. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ahmadi, S.; Bozoky, Z.; Di Paola, M.; Xia, S.; Li, C.; Wong, A.P.; Wellhauser, L.; Molinski, S.V.; Ip, W.; Ouyang, H.; et al. Phenotypic profiling of CFTR modulators in patient-derived respiratory epithelia. NPJ Genom. Med. 2017, 2, 12. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Veit, G.; Roldan, A.; Hancock, M.A.; Da Fonte, D.F.; Xu, H.; Hussein, M.; Frenkiel, S.; Matouk, E.; Velkov, T.; Lukacs, G.L. Allosteric folding correction of F508del and rare CFTR mutants by elexacaftor-tezacaftor-ivacaftor (Trikafta) combination. JCI Insight 2020, 5, e139983. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yeh, H.I.; Hwang, T.C. In vitro assessment of triple combination therapy for the most common disease-associated mutation in cystic fibrosis. Eur. Respir. J. 2022, 59, 2102380. [Google Scholar] [CrossRef] [PubMed]
- Awatade, N.T.; Wong, S.L.; Hewson, C.K.; Fawcett, L.K.; Kicic, A.; Jaffe, A.; Waters, S.A. Human Primary Epithelial Cell Models: Promising Tools in the Era of Cystic Fibrosis Personalized Medicine. Front. Pharmacol. 2018, 9, 1429. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Balázs, A.; Mall, M.A. Mucus obstruction and inflammation in early cystic fibrosis lung disease: Emerging role of the IL-1 signaling pathway. Pediatr. Pulmonol. 2019, 54 (Suppl. S3), S5–S12. [Google Scholar] [CrossRef] [PubMed]
- Wainwright, C.E. Airway inflammation and lung function in cystic fibrosis. Respirology 2023, 28, 509–510. [Google Scholar] [CrossRef] [PubMed]
- Stoltz, D.A.; Meyerholz, D.K.; Pezzulo, A.A.; Ramachandran, S.; Rogan, M.P.; Davis, G.J.; Hanfland, R.A.; Wohlford-Lenane, C.; Dohrn, C.L.; Bartlett, J.A.; et al. Cystic fibrosis pigs develop lung disease and exhibit defective bacterial eradication at birth. Sci. Transl. Med. 2010, 2, 29ra31. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- John, G.; Yildirim, A.Ö.; Rubin, B.K.; Gruenert, D.C.; Henke, M.O. TLR-4-mediated innate immunity is reduced in cystic fibrosis airway cells. Am. J. Respir. Cell Mol. Biol. 2010, 42, 424–431. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kulka, M.; Dery, R.; Nahirney, D.; Duszyk, M.; Befus, A.D. Differential regulation of cystic fibrosis transmembrane conductance regulator by interferon gamma in mast cells and epithelial cells. J. Pharmacol. Exp. Ther. 2005, 315, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Bitam, S.; Pranke, I.; Hollenhorst, M.; Servel, N.; Moquereau, C.; Tondelier, D.; Hatton, A.; Urbach, V.; Sermet-Gaudelus, I.; Hinzpeter, A.; et al. An unexpected effect of TNF-α on F508del-CFTR maturation and function. F1000Research 2015, 4, 218. [Google Scholar] [CrossRef] [PubMed] [PubMed Central][Green Version]
- McAllister, F.; Henry, A.; Kreindler, J.L.; Dubin, P.J.; Ulrich, L.; Steele, C.; Finder, J.D.; Pilewski, J.M.; Carreno, B.M.; Goldman, S.J.; et al. Role of IL-17A, IL-17F, and the IL-17 receptor in regulating growth-related oncogene-alpha and granulocyte colony-stimulating factor in bronchial epithelium: Implications for airway inflammation in cystic fibrosis. J. Immunol. 2005, 175, 404–412. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hsu, D.; Taylor, P.; Fletcher, D.; van Heeckeren, R.; Eastman, J.; van Heeckeren, A.; Davis, P.; Chmiel, J.F.; Pearlman, E.; Bonfield, T.L.; et al. Interleukin-17 Pathophysiology and Therapeutic Intervention in Cystic Fibrosis Lung Infection and Inflammation. Infect. Immun. 2016, 84, 2410–2421. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gentzsch, M.; Cholon, D.M.; Quinney, N.L.; Martino, M.E.B.; Minges, J.T.; Boyles, S.E.; Lee, T.N.G.; Esther, C.R.; Ribeiro, C.M.P. Airway Epithelial Inflammation In Vitro Augments the Rescue of Mutant CFTR by Current CFTR Modulator Therapies. Front. Pharmacol. 2021, 12, 628722. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ribeiro, C.M.P.; Gentzsch, M. Impact of Airway Inflammation on the Efficacy of CFTR Modulators. Cells 2021, 10, 3260. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Koehler, D.R.; Downey, G.P.; Sweezey, N.B.; Tanswell, A.K.; Hu, J. Lung inflammation as a therapeutic target in cystic fibrosis. Am. J. Respir. Cell Mol. Biol. 2004, 31, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Smyth, R.L.; Croft, N.M.; O’Hea, U.; Marshall, T.G.; Ferguson, A. Intestinal inflammation in cystic fibrosis. Arch. Dis. Child. 2000, 82, 394–399. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rosenstein, B.J.; Zeitlin, P.L. Cystic fibrosis. Lancet 1998, 351, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Ooi, C.Y.; Dorfman, R.; Cipolli, M.; Gonska, T.; Castellani, C.; Keenan, K.; Freedman, S.D.; Zielenski, J.; Berthiaume, Y.; Corey, M.; et al. Type of CFTR mutation determines risk of pancreatitis in patients with cystic fibrosis. Gastroenterology 2011, 140, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Wen, E.; Xin, G.; Su, W.; Li, S.; Zhang, Y.; Dong, Y.; Yang, X.; Wan, C.; Chen, Z.; Yu, X.; et al. Activation of TLR4 induces severe acute pancreatitis-associated spleen injury via ROS-disrupted mitophagy pathway. Mol. Immunol. 2022, 142, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Yu, L.; Wang, L.; He, J.; Sun, J.; Wang, X.; Wang, H.; Bai, Z.; Feng, H.; Pei, H. Inflammatory stimuli promote oxidative stress in pancreatic acinar cells via Toll-like receptor 4/nuclear factor-κB pathway. Int. J. Mol. Med. 2018, 42, 3582–3590. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Lu, J.; Antony, S.; Juhasz, A.; Liu, H.; Jiang, G.; Meitzler, J.L.; Hollingshead, M.; Haines, D.C.; Butcher, D.; et al. Activation of TLR4 is required for the synergistic induction of dual oxidase 2 and dual oxidase A2 by IFN-γ and lipopolysaccharide in human pancreatic cancer cell lines. J. Immunol. 2013, 190, 1859–1872. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Courtney, J.; Ennis, M.; Elborn, J. Cytokines and inflammatory mediators in cystic fibrosis. J. Cyst. Fibros. 2004, 3, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Bonfield, T.L.; Panuska, J.R.; Konstan, M.W.; Hilliard, K.A.; Hilliard, J.B.; Ghnaim, H.; Berger, M. Inflammatory cytokines in cystic fibrosis lungs. Am. J. Respir. Crit. Care Med. 1995, 152, 2111–2118, Erratum in Am. J. Respir. Crit. Care Med. 1996, 154 Pt 1, 1217. [Google Scholar] [CrossRef] [PubMed]
- Rehman, T.; Pezzulo, A.A.; Thurman, A.L.; Zemans, R.L.; Welsh, M.J. Epithelial responses to CFTR modulators are improved by inflammatory cytokines and impaired by antiinflammatory drugs. JCI Insight 2024, 9, e181836. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bacalhau, M.; Camargo, M.; Magalhães-Ghiotto, G.A.V.; Drumond, S.; Castelletti, C.H.M.; Lopes-Pacheco, M. Elexacaftor-Tezacaftor-Ivacaftor: A Life-Changing Triple Combination of CFTR Modulator Drugs for Cystic Fibrosis. Pharmaceuticals 2023, 16, 410. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bongiorno, R.; Ludovico, A.; Moran, O.; Baroni, D. Elexacaftor Mediates the Rescue of F508del CFTR Functional Expression Interacting with MSD2. Int. J. Mol. Sci. 2023, 24, 12838. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Amico, G.; Brandas, C.; Moran, O.; Baroni, D. Unravelling the Regions of Mutant F508del-CFTR More Susceptible to the Action of Four Cystic Fibrosis Correctors. Int. J. Mol. Sci. 2019, 20, 5463. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ribeiro, C.M.P.; Boucher, R.C. Role of endoplasmic reticulum stress in cystic fibrosis-related airway inflammatory responses. Proc. Am. Thorac. Soc. 2010, 7, 387–394. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gentzsch, M.; Cholon, D.M.; Quinney, N.L.; Boyles, S.E.; Martino, M.E.B.; Ribeiro, C.M.P. The cystic fibrosis airway milieu enhances rescue of F508del in a pre-clinical model. Eur. Respir. J. 2018, 52, 1801133. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hisert, K.B.; Heltshe, S.L.; Pope, C.; Jorth, P.; Wu, X.; Edwards, R.M.; Radey, M.; Accurso, F.J.; Wolter, D.J.; Cooke, G.; et al. Restoring Cystic Fibrosis Transmembrane Conductance Regulator Function Reduces Airway Bacteria and Inflammation in People with Cystic Fibrosis and Chronic Lung Infections. Am. J. Respir. Crit. Care Med. 2017, 195, 1617–1628. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Saluzzo, F.; Riberi, L.; Messore, B.; Loré, N.I.; Esposito, I.; Bignamini, E.; De Rose, V. CFTR Modulator Therapies: Potential Impact on Airway Infections in Cystic Fibrosis. Cells 2022, 11, 1243. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jarosz-Griffiths, H.H.; Scambler, T.; Wong, C.H.; Lara-Reyna, S.; Holbrook, J.; Martinon, F.; Savic, S.; Whitaker, P.; Etherington, C.; Spoletini, G.; et al. Different CFTR modulator combinations downregulate inflammation differently in cystic fibrosis. eLife 2020, 9, e54556. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Corfield, A.P. Mucins: A biologically relevant glycan barrier in mucosal protection. Biochim. Biophys. Acta 2015, 1850, 236–252. [Google Scholar] [CrossRef] [PubMed]


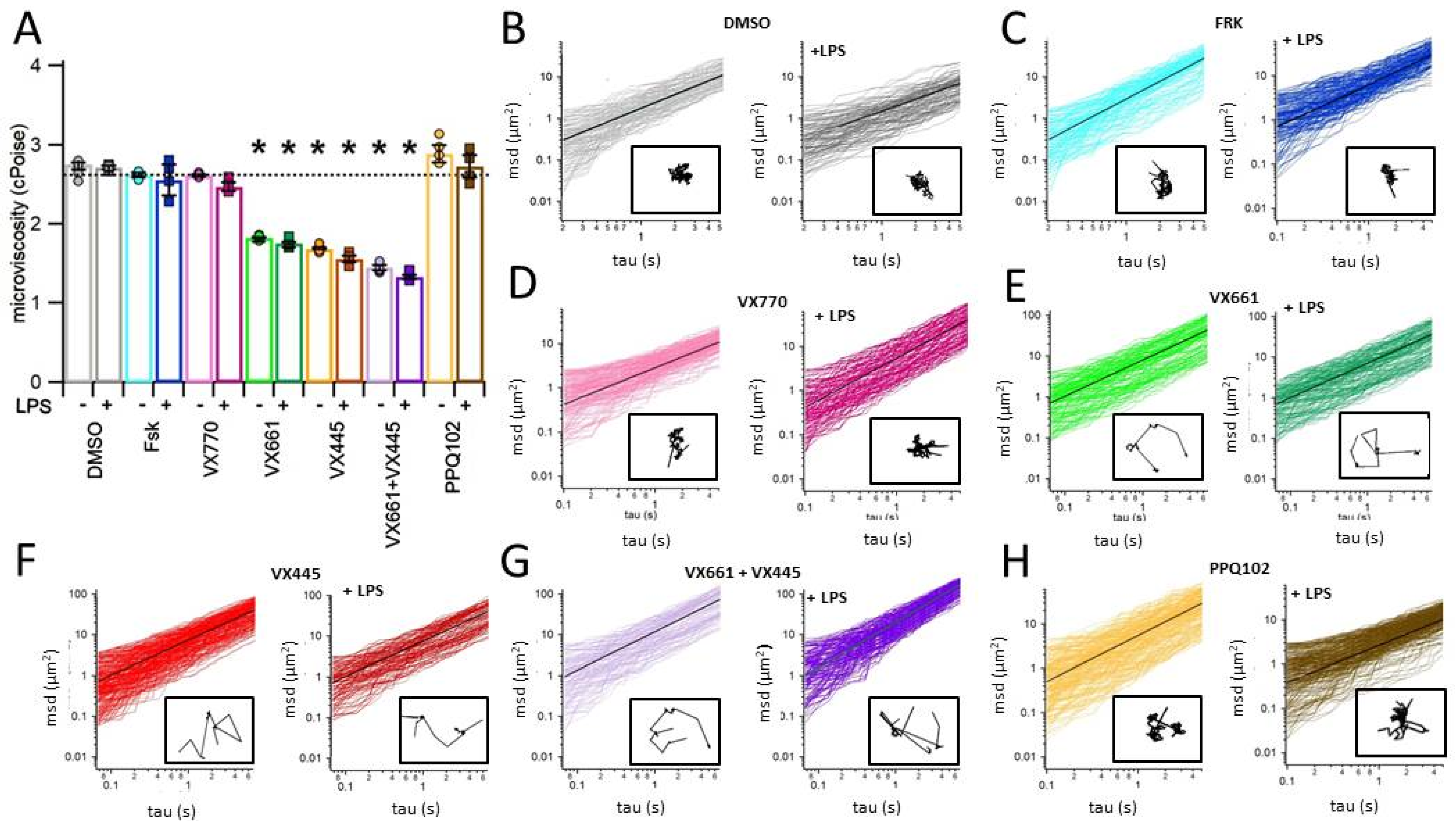
| DMSO | Fsk | VX770 | VX661 | VX445 | VX661 + VX445 | PPQ102 | |
|---|---|---|---|---|---|---|---|
| −LPS | 1.29 | 1.36 | 1.40 | 1.28 | 1.28 | 1.44 | 1.31 |
| ± | ± | ± | ± | ± | ± | ± | |
| 29.79 | 20.21 | 42.26 | 37.38 | 39.63 | 29.50 | 39.65 | |
| (5) | (5) | (5) | (5) | (4) | (5) | (5) | |
| +LPS | 1.78 | 1.68 | 1.83 | 1.78 | 1.97 | 1.87 | 1.72 |
| ± | ± | ± | ± | ± | ± | ± | |
| 59.96 | 22.53 | 44.23 | 31.22 | 40.29 | 19.60 | 27.83 | |
| (4) | (6) | (4) | (4) | (4) | (5) | (4) | |
| * | * | * | * | * | * | * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ludovico, A.; Baroni, D. CFTR Modulators Counteract F508del CFTR Functional Defects in a Pancreatic Epithelial Model of Cystic Fibrosis. Life 2025, 15, 1315. https://doi.org/10.3390/life15081315
Ludovico A, Baroni D. CFTR Modulators Counteract F508del CFTR Functional Defects in a Pancreatic Epithelial Model of Cystic Fibrosis. Life. 2025; 15(8):1315. https://doi.org/10.3390/life15081315
Chicago/Turabian StyleLudovico, Alessandra, and Debora Baroni. 2025. "CFTR Modulators Counteract F508del CFTR Functional Defects in a Pancreatic Epithelial Model of Cystic Fibrosis" Life 15, no. 8: 1315. https://doi.org/10.3390/life15081315
APA StyleLudovico, A., & Baroni, D. (2025). CFTR Modulators Counteract F508del CFTR Functional Defects in a Pancreatic Epithelial Model of Cystic Fibrosis. Life, 15(8), 1315. https://doi.org/10.3390/life15081315






