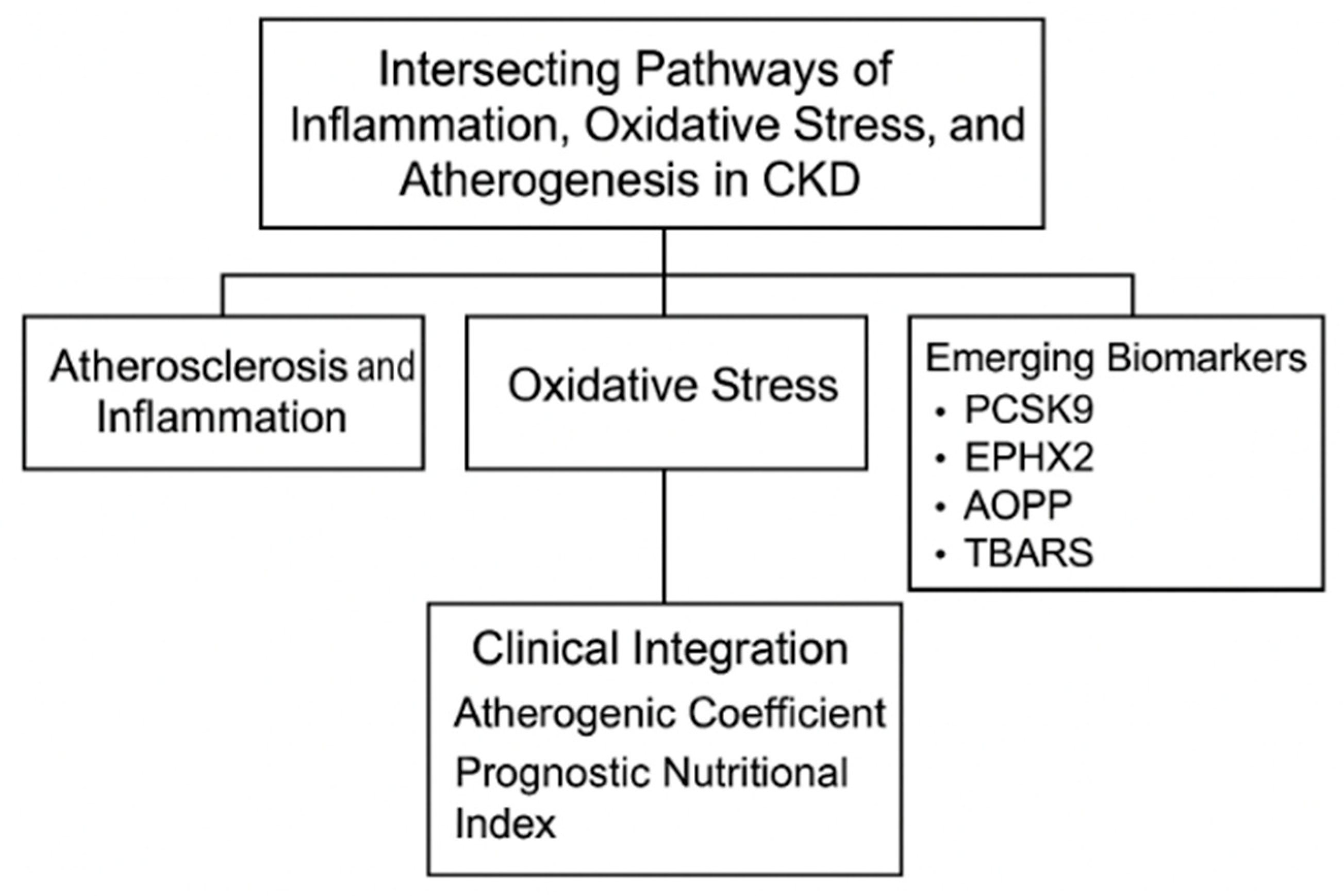
Intersecting Pathways of Inflammation, Oxidative Stress, and Atherogenesis in the Evaluation of CKD: Emerging Biomarkers PCSK9, EPHX2, AOPPs, and TBARSs
Abstract
1. Introduction
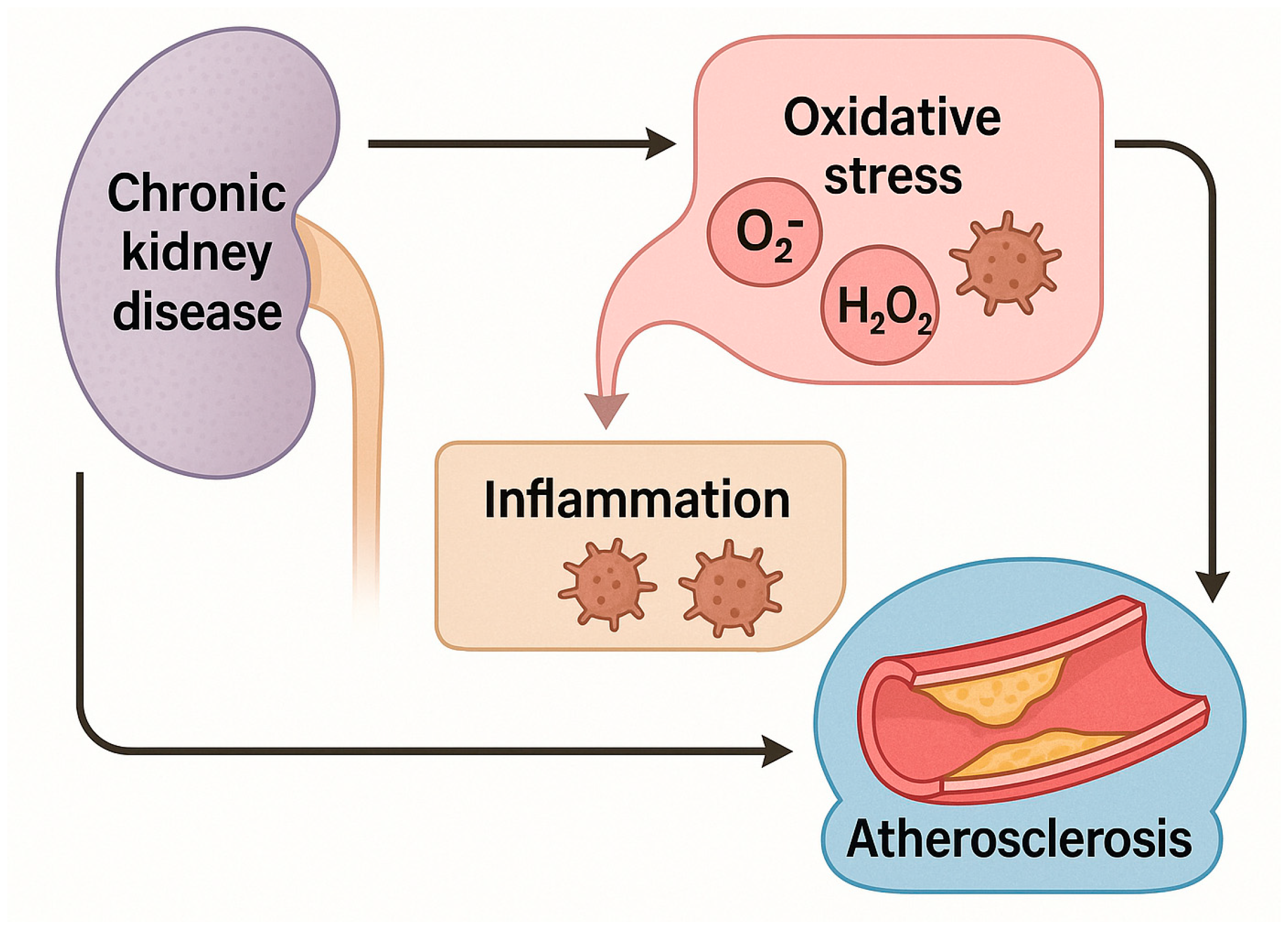
2. Atherosclerosis and Inflammation in Chronic Kidney Disease
2.1. Overview: A Vicious Cycle
2.2. Inflammatory Mediators in CKD-Associated Atherosclerosis
2.3. Role of Dyslipidemia
2.4. Endothelial Dysfunction and Vascular Calcification
2.5. Inflammation as a Prognostic Indicator: PNI and Beyond
3. Role of Oxidative Stress in Chronic Kidney Disease
3.1. Mechanisms of Oxidative Stress in CKD
- Uremic toxin accumulation (e.g., indoxyl sulfate, p-cresyl sulfate), which induces mitochondrial dysfunction and NADPH oxidase activation (NOX);
- Inflammatory cytokines (e.g., IL-6, TNF-α), which amplify ROS production via activation of immune cells and endothelial cells;
- Impaired antioxidant systems, including reduced levels of glutathione, superoxide dismutase (SOD), catalase, and selenium-dependent enzymes.
3.2. Lipid Peroxidation and TBARSs
- A reduced glomerular filtration rate (GFR);
- An increase in cardiovascular events;
- Endothelial dysfunction and arterial stiffness.
3.3. Protein Oxidation and AOPPs
- Inflammation (correlating with CRP and IL-8);
- Malnutrition (inverse relationship with albumin);
- Endothelial dysfunction and arterial stiffness.
3.4. Other Key Redox Pathways: Nrf2/Keap1 Signaling, Mitochondrial ROS Regulation, MitoQ, Bardoxolone Methyl
3.5. Interactions with Traditional Risk Factors and Comorbidities
- Diabetes mellitus: hyperglycemia induces ROS via AGEs and polyol pathway flux;
- Hypertension: angiotensin II stimulates ROS generation through activation of NOX and impairs endothelial nitric oxide synthesis;
- Dyslipidemia: oxidized LDL further promotes ROS production and immune activation.
3.6. Potential Therapeutic Targets and Antioxidant Strategies
- Vitamin E and C supplementation has shown modest effects in reducing TBARS and improving endothelial function, particularly in early-stage CKD;
- N-acetylcysteine (NAC) replenishes glutathione stores and has been shown to reduce proteinuria and improve oxidative markers in some trials;
- Bardoxolone methyl is an Nrf2 activator that enhances endogenous antioxidant defenses; while promising in phase II trials, it showed adverse cardiovascular outcomes in later-stage CKD.
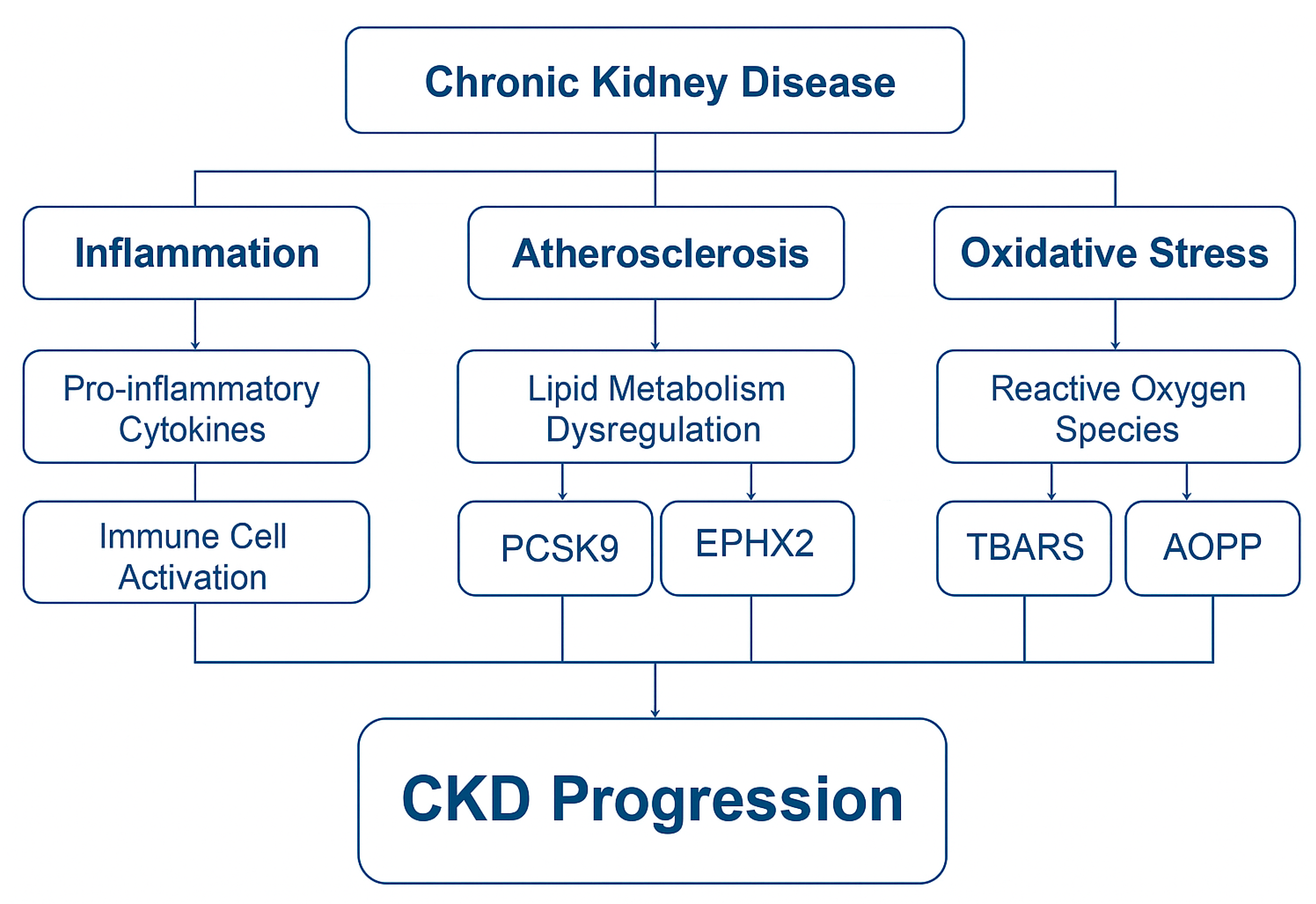
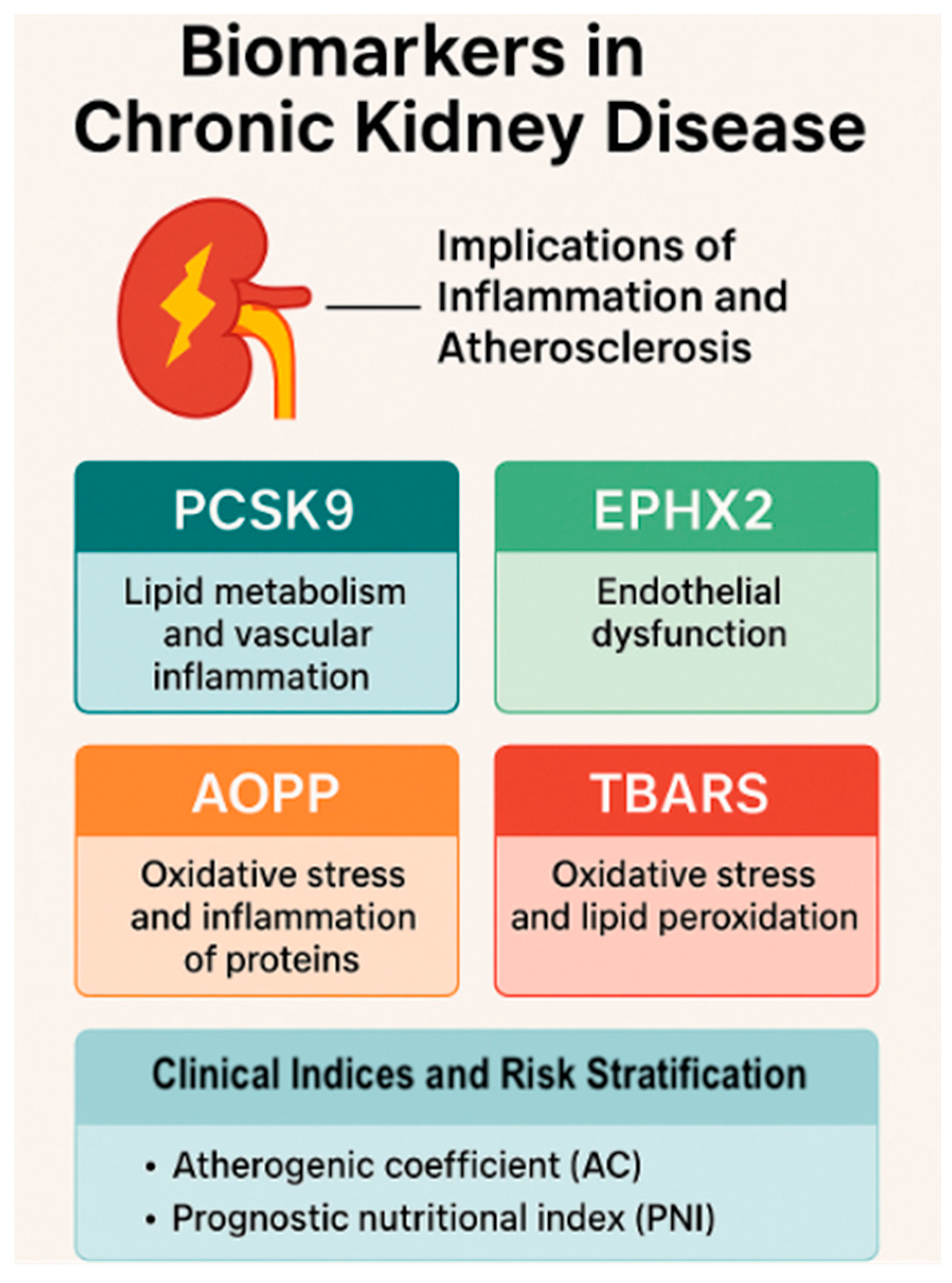
4. Integrated Roles of PCSK9, EPHX2, AOPPs, and TBARSs in CKD Pathophysiology
4.1. PCSK9: A Link Between Dyslipidemia, Inflammation, and CKD Progression
4.1.1. Overview
4.1.2. Mechanisms of PCSK9 in Lipid and Inflammatory Pathways
4.1.3. PCSK9 in CKD and Diabetic Nephropathy
4.1.4. Association with Cardiovascular Risk in CKD
4.1.5. PCSK9 Inhibitors: Therapeutic Relevance in CKD
- Monoclonal antibodies (e.g., evolocumab, alirocumab), which bind circulating PCSK9, preventing it from degrading LDL receptors;
- siRNA-based therapies (e.g., inclisiran) which silence PCSK9 mRNA in hepatocytes, offering sustained inhibition with infrequent dosing.
4.2. EPHX2: A Mediator of Vascular Inflammation and Renal Injury
4.2.1. Overview
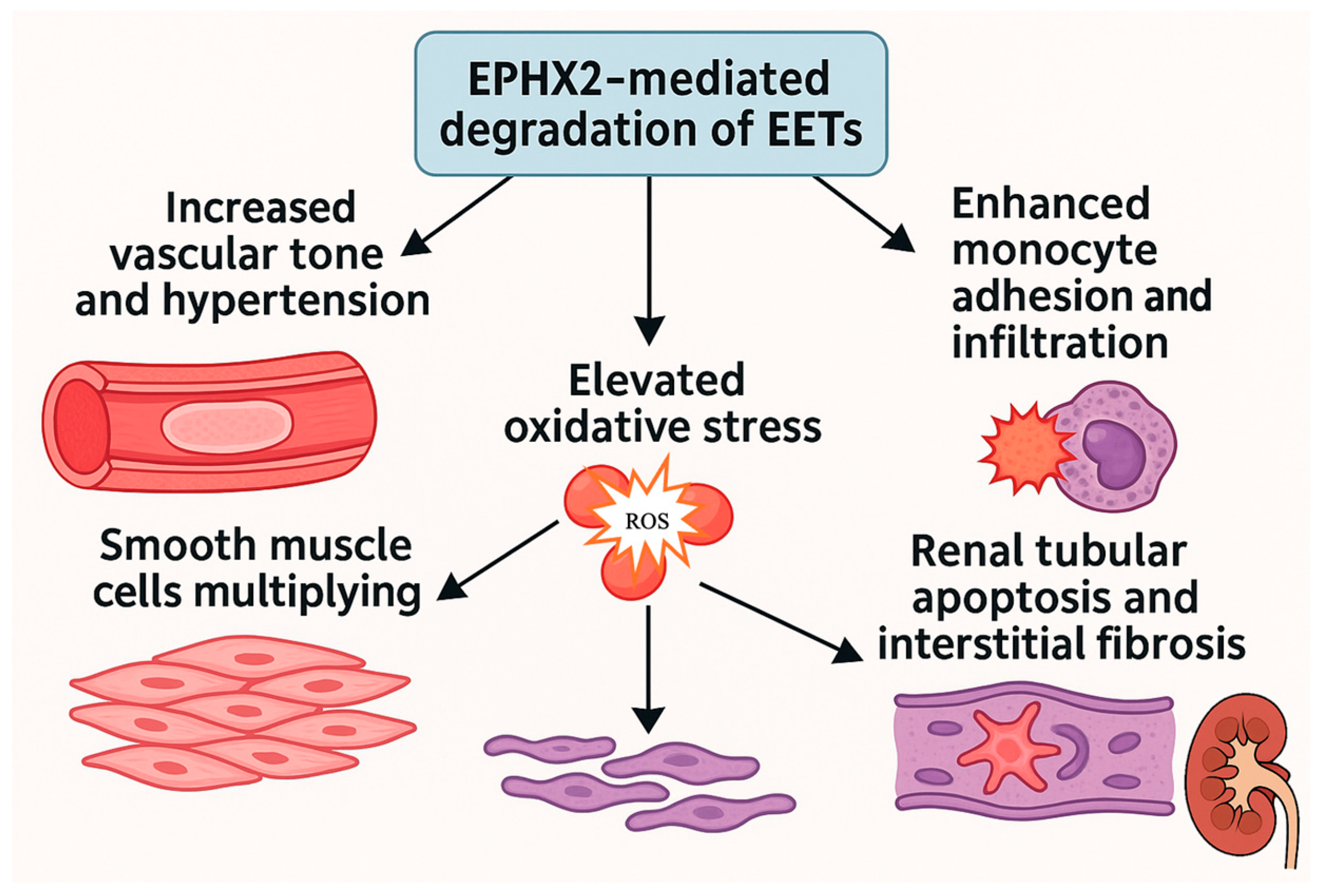
4.2.2. Mechanistic Role of EPHX2 in Vascular and Renal Pathophysiology
- Increased vascular tone and hypertension;
- Enhanced monocyte adhesion and infiltration;
- Elevated oxidative stress;
- Proliferation of vascular smooth muscle cells;
- Renal tubular apoptosis and interstitial fibrosis.
- Arterial stiffness and calcification;
- Endothelial dysfunction via suppression of EET-mediated nitric oxide signaling;
- Myocardial remodeling and left ventricular hypertrophy;
- Enhanced oxidative stress and vascular inflammation.
4.2.3. EPHX2 Inhibitors: Preclinical and Early Clinical Insights
4.3. AOPPs: A Marker and Mediator of Oxidative Protein Damage in CKD
4.3.1. Overview
4.3.2. Formation and Biochemical Characteristics
- Oxidized albumin and other plasma proteins;
- Interaction with MPO-derived oxidants, which accumulate due to immune activation and poor clearance in CKD.
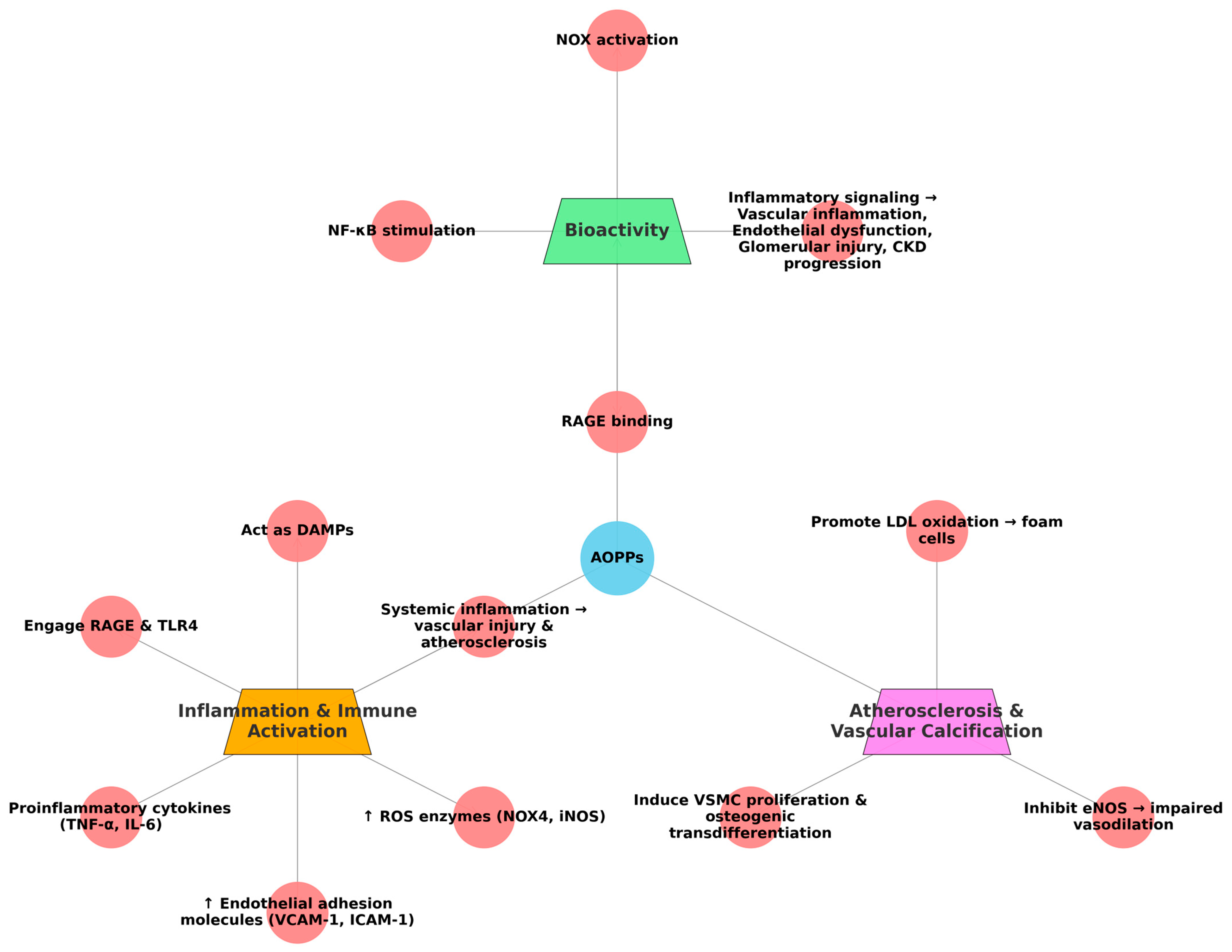
- NOX activation;
- NF-κB pathway stimulation;
- RAGE binding.
4.3.3. Inflammation and Immune Activation
- Proinflammatory cytokines (e.g., TNF-α, IL-6);
- Endothelial adhesion molecules (VCAM-1, ICAM-1);
- ROS-generating enzymes (e.g., NOX4, iNOS).
4.3.4. Atherosclerosis and Vascular Calcification
- Promoting LDL oxidation, enhancing foam cell formation;
- Inducing vascular smooth muscle cell (VSMC) proliferation and osteogenic transdifferentiation;
- Inhibiting eNOS, impairing vasodilation.
4.4. TBARSs: A Classical Marker of Lipid Peroxidation in CKD
4.4.1. Overview
4.4.2. Biological Significance
4.5. Other Noteworthy CKD-Related Biomarkers: FGF23, Soluble Klotho, and Indoxyl Sulfate
5. Discussion and Clinical Implications
5.1. Multi-Biomarker Approach in CKD: Complementary Pathways
- PCSK9 represents dysregulated lipid metabolism and vascular inflammation;
- EPHX2 reflects endothelial dysfunction and impaired vasoprotective signaling via EET degradation;
- AOPPs and TBARSs quantify oxidative damage to proteins and lipids, respectively, and reflect both uremic toxicity and immune activation.
5.2. Clinical Utility and Risk Stratification
- Prognostic Risk Models
- Renal progression, including ESKD risk;
- MACEs;
- All-cause and cardiovascular mortality, particularly in dialysis.
- Nutritional and Inflammatory Monitoring
- Therapeutic Response Monitoring
- PCSK9 levels decrease in response to evolocumab or inclisiran; a 2025 editorial proposed that PCSK9 inhibition may also preserve tubular megalin expression and reduce proteinuria, opening renal therapeutic indications [144];
- EPHX2 inhibitors (e.g., GSK2256294) are under development, and methylation profiling may identify responders vs. non-responders, laying the groundwork for precision nephrology [227].
5.3. Toward Personalized Nephrology
- Stratify patients by molecular phenotype;
- Monitor biochemical responses to interventions;
- Select targeted therapies (e.g., PCSK9 or EPHX2 inhibitors) for the right patients;
- Detect residual cardiovascular or inflammatory risk not captured by traditional markers;
- In parallel, possibly use these biomarkers to help uncover novel therapeutic targets, such as RAGE antagonists for AOPP-mediated damage or NOX4 inhibitors for TBARS-driven lipid injury.
5.4. Prognostic Value Beyond Traditional Markers
6. Limitations and Future Directions
6.1. Analytical and Methodological Challenges
6.2. Limited Longitudinal and Interventional Data
6.3. Clinical Implementation Barriers
6.4. Future Research Directions
- Standardization of assays for oxidative and lipid-associated biomarkers in CKD;
- Prospective, multi-ethnic, and adequately powered cohort studies;
- Interventional trials testing biomarker-guided therapy, including PCSK9 and EPHX2 inhibitors;
- Integration of multi-omics approaches with machine learning to personalize CKD management;
- Development of regulatory pathways and clinical practice guidelines recognizing these markers. Recent advances in epigenetics also open new possibilities. A study by Gao et al. [72] reported EPHX2 methylation patterns associated with diabetic kidney disease severity, suggesting that epigenetic modifications may serve as both diagnostic tools and therapeutic targets.
7. Conclusions
- Standardizing assays for oxidative and inflammatory biomarkers;
- Conducting prospective, diverse, and adequately powered cohort studies;
- Integrating multi-omics and machine learning models for personalized prediction;
- Exploring biomarker-guided interventional trials targeting lipid metabolism, oxidative stress, and endothelial dysfunction.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kovesdy, C.P. Epidemiology of chronic kidney disease: An update 2022. Kidney Int. Suppl. 2022, 12, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Sundström, J.; Bodegard, J.; Bollmann, A.; Vervloet, M.G.; Mark, P.B.; Karasik, A.; Taveira-Gomes, T.; Botana, M.; Birkeland, K.I.; Thuresson, M.; et al. Prevalence, outcomes, and cost of chronic kidney disease in a contemporary population of 2·4 million patients from 11 countries: The CaReMe CKD study. Lancet Reg. Health-Eur. 2022, 20, 100438. [Google Scholar] [CrossRef]
- Zoccali, C.; Mallamaci, F.; Adamczak, M.; de Oliveira, R.B.; Massy, Z.A.; Sarafidis, P.; Agarwal, R.; Mark, P.B.; Kotanko, P.; Ferro, C.J.; et al. Cardiovascular complications in chronic kidney disease: A review from the European Renal and Cardiovascular Medicine Working Group of the European Renal Association. Cardiovasc. Res. 2023, 119, 2017–2032. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, J.; Ren, Y.; Sun, R.; Zhai, X. Unveiling the pathogenesis and therapeutic approaches for diabetic nephropathy: Insights from panvascular diseases. Front. Endocrinol. 2024, 15, 1368481. [Google Scholar] [CrossRef]
- Jha, R.; Lopez-Trevino, S.; Kankanamalage, H.R.; Jha, J.C. Diabetes and Renal Complications: An Overview on Pathophysiology, Biomarkers and Therapeutic Interventions. Biomedicines 2024, 12, 1098. [Google Scholar] [CrossRef]
- Efiong, E.E.; Maedler, K.; Effa, E.; Osuagwu, U.L.; Peters, E.; Ikebiuro, J.O.; Soremekun, C.; Ihediwa, U.; Niu, J.; Fuchs, M.; et al. Decoding diabetic kidney disease: A comprehensive review of interconnected pathways, molecular mediators, and therapeutic insights. Diabetol. Metab. Syndr. 2025, 17, 192. [Google Scholar] [CrossRef]
- Cho, M.E.; Brunt, V.E.; Shiu, Y.-T.; Bunsawat, K. Endothelial dysfunction in chronic kidney disease: A clinical perspective. Am. J. Physiol. -Heart Circ. Physiol. 2025, 329, H135–H153. [Google Scholar] [CrossRef] [PubMed]
- Coyle, M.; Flaherty, G.; Jennings, C. A critical review of chronic kidney disease as a risk factor for coronary artery disease. Int. J. Cardiol. Heart Vasc. 2021, 35, 100822. [Google Scholar] [CrossRef]
- Said, S.; Hernandez, G.T. The link between chronic kidney disease and cardiovascular disease. J. Nephropathol. 2014, 3, 99–104. [Google Scholar] [CrossRef]
- Bikbov, B.; Purcell, C.A.; Levey, A.S.; Smith, M.; Abdoli, A.; Abebe, M.; Adebayo, O.M.; Afarideh, M.; Agarwal, S.K.; Agudelo-Botero, M.; et al. Global, regional, and national burden of chronic kidney disease, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2020, 395, 709–733. [Google Scholar] [CrossRef] [PubMed]
- Maringhini, S.; Zoccali, C. Chronic Kidney Disease Progression-A Challenge. Biomedicines 2024, 12, 2203. [Google Scholar] [CrossRef]
- Xie, K.; Cao, H.; Ling, S.; Zhong, J.; Chen, H.; Chen, P.; Huang, R. Global, regional, and national burden of chronic kidney disease, 1990–2021: A systematic analysis for the global burden of disease study 2021. Front. Endocrinol. 2025, 16, 1526482. [Google Scholar] [CrossRef]
- Francis, A.; Harhay, M.N.; Ong, A.C.M.; Tummalapalli, S.L.; Ortiz, A.; Fogo, A.B.; Fliser, D.; Roy-Chaudhury, P.; Fontana, M.; Nangaku, M.; et al. Chronic kidney disease and the global public health agenda: An international consensus. Nat. Rev. Nephrol. 2024, 20, 473–485. [Google Scholar] [CrossRef]
- Li, Y.; Ning, Y.; Shen, B.; Shi, Y.; Song, N.; Fang, Y.; Ding, X. Temporal trends in prevalence and mortality for chronic kidney disease in China from 1990 to 2019: An analysis of the Global Burden of Disease Study 2019. Clin. Kidney J. 2022, 16, 312–321. [Google Scholar] [CrossRef] [PubMed]
- Jankowski, J.; Floege, J.; Fliser, D.; Böhm, M.; Marx, N. Cardiovascular Disease in Chronic Kidney Disease. Circulation 2021, 143, 1157–1172. [Google Scholar] [CrossRef]
- Kofod, D.H.; Carlson, N.; Ballegaard, E.F.; Almdal, T.P.; Torp-Pedersen, C.; Gislason, G.; Svendsen, J.H.; Feldt-Rasmussen, B.; Hornum, M. Cardiovascular mortality in patients with advanced chronic kidney disease with and without diabetes: A nationwide cohort study. Cardiovasc. Diabetol. 2023, 22, 140. [Google Scholar] [CrossRef]
- Stevens, P.E.; Ahmed, S.B.; Carrero, J.J.; Foster, B.; Francis, A.; Hall, R.K.; Herrington, W.G.; Hill, G.; Inker, L.A.; Kazancıoğlu, R.; et al. KDIGO 2024 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int. 2024, 105, S117–S314. [Google Scholar] [CrossRef]
- Faro, D.C.; Di Pino, F.L.; Monte, I.P. Inflammation, Oxidative Stress, and Endothelial Dysfunction in the Pathogenesis of Vascular Damage: Unraveling Novel Cardiovascular Risk Factors in Fabry Disease. Int. J. Mol. Sci. 2024, 25, 8273. [Google Scholar] [CrossRef] [PubMed]
- Duni, A.; Liakopoulos, V.; Roumeliotis, S.; Peschos, D.; Dounousi, E. Oxidative Stress in the Pathogenesis and Evolution of Chronic Kidney Disease: Untangling Ariadne’s Thread. Int. J. Mol. Sci. 2019, 20, 3711. [Google Scholar] [CrossRef] [PubMed]
- Ajibowo, A.O.; Okobi, O.E.; Emore, E.; Soladoye, E.; Sike, C.G.; Odoma, V.A.; Bakare, I.O.; Kolawole, O.A.; Afolayan, A.; Okobi, E.; et al. Cardiorenal Syndrome: A Literature Review. Cureus 2023, 15, e41252. [Google Scholar] [CrossRef]
- Nashar, K.; Egan, B.M. Relationship between chronic kidney disease and metabolic syndrome: Current perspectives. Diabetes Metab. Syndr. Obes. 2014, 7, 421–435. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Chen, C.; Li, M.; Yan, G.; Tang, C. Sex Differences in the Associations among Insulin Resistance Indexes with Metabolic Syndrome: A Large Cross-Sectional Study. Int. J. Endocrinol. 2024, 2024, 3352531. [Google Scholar] [CrossRef] [PubMed]
- Paniagua, J.A. Nutrition, insulin resistance and dysfunctional adipose tissue determine the different components of metabolic syndrome. World J. Diabetes 2016, 7, 483–514. [Google Scholar] [CrossRef] [PubMed]
- Mitroi Sakizlian, D.D.; Boldeanu, L.; Mitrea, A.; Clenciu, D.; Vladu, I.M.; Ciobanu Plasiciuc, A.E.; Șarla, A.V.; Siloși, I.; Boldeanu, M.V.; Assani, M.-Z.; et al. The Interplay of Cardiometabolic Syndrome Phenotypes and Cardiovascular Risk Indices in Patients Diagnosed with Diabetes Mellitus. Int. J. Mol. Sci. 2025, 26, 6227. [Google Scholar] [CrossRef]
- Ahrițculesei, R.-V.; Boldeanu, L.; Vladu, I.M.; Clenciu, D.; Mitrea, A.; Cîmpeanu, R.C.; Mustață, M.-L.; Siloși, I.; Boldeanu, M.V.; Vere, C.C. Correlation Between Prognostic Nutritional Index, Glasgow Prognostic Score, and Different Obesity-Related Indices in People with Diabetes or Prediabetes. Diagnostics 2024, 14, 2661. [Google Scholar] [CrossRef]
- Zencirkiran Agus, H.; Kahraman, S. Prognostic nutritional index predicts one-year outcome in heart failure with preserved ejection fraction. Acta Cardiol. 2020, 75, 450–455. [Google Scholar] [CrossRef]
- Keskin, M.; Hayıroğlu, M.İ.; Keskin, T.; Kaya, A.; Tatlısu, M.A.; Altay, S.; Uzun, A.O.; Börklü, E.B.; Güvenç, T.S.; Avcı, İ.İ.; et al. A novel and useful predictive indicator of prognosis in ST-segment elevation myocardial infarction, the prognostic nutritional index. Nutr. Metab. Cardiovasc. Dis. 2017, 27, 438–446. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.L.; Sung, S.H.; Cheng, H.M.; Hsu, P.F.; Guo, C.Y.; Yu, W.C.; Chen, C.H. Prognostic Nutritional Index and the Risk of Mortality in Patients With Acute Heart Failure. J. Am. Heart Assoc. 2017, 6, e004876. [Google Scholar] [CrossRef]
- Tak, B.T.; Cay, S.; Pamukcu, H.E.; Ekizler, F.A.; Kafes, H.; Cetin, E.H.O.; Ulvan, N.; Ozeke, O.; Ozcan, F.; Topaloglu, S.; et al. Prognostic nutritional index as a novel marker for prediction of prognosis in patients with peripartum cardiomyopathy. Medicine 2020, 99, e19524. [Google Scholar] [CrossRef]
- Adam, A.-M.; Popa, R.-F.; Vaduva, C.; Georgescu, C.V.; Adam, G.; Melinte-Popescu, A.-S.; Popa, C.; Socolov, D.; Nechita, A.; Vasilache, I.-A.; et al. Pregnancy Outcomes, Immunophenotyping and Immunohistochemical Findings in a Cohort of Pregnant Patients with COVID-19—A Prospective Study. Diagnostics 2023, 13, 1345. [Google Scholar] [CrossRef]
- Barutcu Atas, D.; Tugcu, M.; Asicioglu, E.; Velioglu, A.; Arikan, H.; Koc, M.; Tuglular, S. Prognostic nutritional index is a predictor of mortality in elderly patients with chronic kidney disease. Int. Urol. Nephrol. 2022, 54, 1155–1162. [Google Scholar] [CrossRef]
- Popescu, M.; Terzea, D.C.; Carsote, M.; Ghenea, A.E.; Costache, A.; Popescu, I.A.S.; Biciuşcă, V.; Busuioc, C.J.; Ghemigian, A.M. COVID-19 infection: From stress-related cortisol levels to adrenal glands infarction. Rom. J. Morphol. Embryol. 2022, 63, 39. [Google Scholar] [CrossRef] [PubMed]
- Assani, M.Z.; Novac, M.B.; Dijmărescu, A.L.; Văduva, C.C.; Vladu, I.M.; Clenciu, D.; Mitrea, A.; Ahrițculesei, R.V.; Stroe-Ionescu, A.; Assani, A.D.; et al. Potential Association Between Atherogenic Coefficient, Prognostic Nutritional Index, and Various Obesity Indices in Diabetic Nephropathy. Nutrients 2025, 17, 1339. [Google Scholar] [CrossRef]
- Dounousi, E.; Tellis, C.; Pavlakou, P.; Duni, A.; Liakopoulos, V.; Mark, P.B.; Papagianni, A.; Tselepis, A.D. Association between PCSK9 Levels and Markers of Inflammation, Oxidative Stress, and Endothelial Dysfunction in a Population of Nondialysis Chronic Kidney Disease Patients. Oxidative Med. Cell Longev. 2021, 2021, 6677012. [Google Scholar] [CrossRef]
- Roumeliotis, S.; Liakopoulos, V.; Dounousi, E.; Mark, P.B. Oxidative Stress in End-Stage Renal Disease: Pathophysiology and Potential Interventions. Oxidative Med. Cell. Longev. 2023, 2023, 9870138. [Google Scholar] [CrossRef]
- Xue, K.; Wang, Y.; Wang, Y.; Fang, H. Advanced Oxidation Protein Product Promotes Oxidative Accentuation in Renal Epithelial Cells via the Soluble (Pro)renin Receptor-Mediated Intrarenal Renin-Angiotensin System and Nox4-H(2)O(2) Signaling. Oxidative Med. Cell Longev. 2021, 2021, 5710440. [Google Scholar] [CrossRef]
- Maciejczyk, M.; Szulimowska, J.; Skutnik, A.; Taranta-Janusz, K.; Wasilewska, A.; Wiśniewska, N.; Zalewska, A. Salivary Biomarkers of Oxidative Stress in Children with Chronic Kidney Disease. J. Clin. Med. 2018, 7, 209. [Google Scholar] [CrossRef] [PubMed]
- Vostálová, J.; Galandáková, A.; Svobodová, A.R.; Orolinová, E.; Kajabová, M.; Schneiderka, P.; Zapletalová, J.; Štrebl, P.; Zadražil, J. Time-Course Evaluation of Oxidative Stress-Related Biomarkers after Renal Transplantation. Ren. Fail. 2012, 34, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Stenvinkel, P.; Alvestrand, A. Inflammation in end-stage renal disease: Sources, consequences, and therapy. Semin. Dial. 2002, 15, 329–337. [Google Scholar] [CrossRef]
- London, G.M. Arterial Stiffness in Chronic Kidney Disease and End-Stage Renal Disease. Blood Purif. 2018, 45, 154–158. [Google Scholar] [CrossRef]
- Baaten, C.; Vondenhoff, S.; Noels, H. Endothelial Cell Dysfunction and Increased Cardiovascular Risk in Patients With Chronic Kidney Disease. Circ. Res. 2023, 132, 970–992. [Google Scholar] [CrossRef]
- Gansevoort, R.T.; Correa-Rotter, R.; Hemmelgarn, B.R.; Jafar, T.H.; Heerspink, H.J.; Mann, J.F.; Matsushita, K.; Wen, C.P. Chronic kidney disease and cardiovascular risk: Epidemiology, mechanisms, and prevention. Lancet 2013, 382, 339–352. [Google Scholar] [CrossRef]
- Perone, F.; Bernardi, M.; Spadafora, L.; Betti, M.; Cacciatore, S.; Saia, F.; Fogacci, F.; Jaiswal, V.; Asher, E.; Paneni, F.; et al. Non-Traditional Cardiovascular Risk Factors: Tailored Assessment and Clinical Implications. J. Cardiovasc. Dev. Dis. 2025, 12, 171. [Google Scholar] [CrossRef] [PubMed]
- El Chamieh, C.; Liabeuf, S.; Massy, Z. Uremic Toxins and Cardiovascular Risk in Chronic Kidney Disease: What Have We Learned Recently beyond the Past Findings? Toxins 2022, 14, 280. [Google Scholar] [CrossRef] [PubMed]
- Kadatane, S.P.; Satariano, M.; Massey, M.; Mongan, K.; Raina, R. The Role of Inflammation in CKD. Cells 2023, 12, 1581. [Google Scholar] [CrossRef]
- Tinti, F.; Lai, S.; Noce, A.; Rotondi, S.; Marrone, G.; Mazzaferro, S.; Di Daniele, N.; Mitterhofer, A.P. Chronic Kidney Disease as a Systemic Inflammatory Syndrome: Update on Mechanisms Involved and Potential Treatment. Life 2021, 11, 419. [Google Scholar] [CrossRef] [PubMed]
- Townsend, R.R.; Anderson, A.H.; Chirinos, J.A.; Feldman, H.I.; Grunwald, J.E.; Nessel, L.; Roy, J.; Weir, M.R.; Wright, J.T., Jr.; Bansal, N.; et al. Association of Pulse Wave Velocity With Chronic Kidney Disease Progression and Mortality: Findings From the CRIC Study (Chronic Renal Insufficiency Cohort). Hypertension 2018, 71, 1101–1107. [Google Scholar] [CrossRef]
- Kyriakidis, N.C.; Cobo, G.; Dai, L.; Lindholm, B.; Stenvinkel, P. Role of Uremic Toxins in Early Vascular Ageing and Calcification. Toxins 2021, 13, 26. [Google Scholar] [CrossRef]
- Wang, J.H.; Lin, Y.L.; Hsu, B.G. Endothelial dysfunction in chronic kidney disease: Mechanisms, biomarkers, diagnostics, and therapeutic strategies. Tzu Chi Med. J. 2025, 37, 125–134. [Google Scholar] [CrossRef]
- Jha, P.K.; Nakano, T.; Itto, L.Y.U.; Barbeiro, M.C.; Lupieri, A.; Aikawa, E.; Aikawa, M. Vascular inflammation in chronic kidney disease: The role of uremic toxins in macrophage activation. Front. Cardiovasc. Med. 2025, 12, 1574489. [Google Scholar] [CrossRef]
- Diaz-Ricart, M.; Torramade-Moix, S.; Pascual, G.; Palomo, M.; Moreno-Castaño, A.B.; Martinez-Sanchez, J.; Vera, M.; Cases, A.; Escolar, G. Endothelial Damage, Inflammation and Immunity in Chronic Kidney Disease. Toxins 2020, 12, 361. [Google Scholar] [CrossRef]
- Harlacher, E.; Wollenhaupt, J.; Baaten, C.C.F.M.J.; Noels, H. Impact of Uremic Toxins on Endothelial Dysfunction in Chronic Kidney Disease: A Systematic Review. Int. J. Mol. Sci. 2022, 23, 531. [Google Scholar] [CrossRef]
- Topçiu-Shufta, V.; Haxhibeqiri, V.; Begolli, L.; Baruti-Gafurri, Z.; Vesel, S.; Haxhibeqiri Md, S.; Miftari, R.; Kurti, L.; Avdi, D. Correlation of Inflammation and Lipoprotein (a) with Hypercoagulability in Hemodialysis Patients. Med. Arch. 2015, 69, 232–235. [Google Scholar] [CrossRef]
- Istanbuly, O.; Belcher, J.; Tabinor, M.; Solis-Trapala, I.; Lambie, M.; Davies, S.J. Estimating the association between systemic Interleukin-6 and mortality in the dialysis population. Re-analysis of the global fluid study, systematic review and meta-analysis. BMC Nephrol. 2023, 24, 312. [Google Scholar] [CrossRef]
- Fine, A. Relevance of C-reactive protein levels in peritoneal dialysis patients. Kidney Int. 2002, 61, 615–620. [Google Scholar] [CrossRef] [PubMed]
- Md Dom, Z.I.; Pipino, C.; Krolewski, B.; O’Neil, K.; Satake, E.; Krolewski, A.S. Effect of TNFα stimulation on expression of kidney risk inflammatory proteins in human umbilical vein endothelial cells cultured in hyperglycemia. Sci. Rep. 2021, 11, 11133. [Google Scholar] [CrossRef] [PubMed]
- da Costa, R.M.; Neves, K.B.; Mestriner, F.L.; Louzada-Junior, P.; Bruder-Nascimento, T.; Tostes, R.C. TNF-α induces vascular insulin resistance via positive modulation of PTEN and decreased Akt/eNOS/NO signaling in high fat diet-fed mice. Cardiovasc. Diabetol. 2016, 15, 119. [Google Scholar] [CrossRef] [PubMed]
- Si, S.; Liu, H.; Xu, L.; Zhan, S. Identification of novel therapeutic targets for chronic kidney disease and kidney function by integrating multi-omics proteome with transcriptome. Genome Med. 2024, 16, 84. [Google Scholar] [CrossRef]
- Tanase, D.M.; Valasciuc, E.; Gosav, E.M.; Ouatu, A.; Buliga-Finis, O.N.; Floria, M.; Maranduca, M.A.; Serban, I.L. Portrayal of NLRP3 Inflammasome in Atherosclerosis: Current Knowledge and Therapeutic Targets. Int. J. Mol. Sci. 2023, 24, 8162. [Google Scholar] [CrossRef]
- Wang, L.; Zhu, Y.; Zhang, N.; Xian, Y.; Tang, Y.; Ye, J.; Reza, F.; He, G.; Wen, X.; Jiang, X. The multiple roles of interferon regulatory factor family in health and disease. Signal Transduct. Target. Ther. 2024, 9, 282. [Google Scholar] [CrossRef]
- Granata, S.; Masola, V.; Zoratti, E.; Scupoli, M.T.; Baruzzi, A.; Messa, M.; Sallustio, F.; Gesualdo, L.; Lupo, A.; Zaza, G. NLRP3 Inflammasome Activation in Dialyzed Chronic Kidney Disease Patients. PLoS ONE 2015, 10, e0122272. [Google Scholar] [CrossRef]
- Zhang, C.; Yang, Y. Targeting toll-like receptor 4 (TLR4) and the NLRP3 inflammasome: Novel and emerging therapeutic targets for hyperuricaemia nephropathy. Biomol. Biomed. 2023, 24, 688–697. [Google Scholar] [CrossRef]
- Islamuddin, M.; Qin, X. Renal macrophages and NLRP3 inflammasomes in kidney diseases and therapeutics. Cell Death Discov. 2024, 10, 229. [Google Scholar] [CrossRef]
- Ji, Y.; Hua, H.; Jia, Z.; Zhang, A.; Ding, G. Therapy Targeted to the NLRP3 Inflammasome in Chronic Kidney Disease. Kidney Dis. 2024, 10, 369–383. [Google Scholar] [CrossRef]
- Jin, L.; Jin, F.; Guo, S.; Liu, W.; Wei, B.; Fan, H.; Li, G.; Zhang, X.; Su, S.; Li, R.; et al. Metformin Inhibits NLR Family Pyrin Domain Containing 3 (NLRP)-Relevant Neuroinflammation via an Adenosine-5′-Monophosphate-Activated Protein Kinase (AMPK)-Dependent Pathway to Alleviate Early Brain Injury After Subarachnoid Hemorrhage in Mice. Front. Pharmacol. 2022, 13, 796616. [Google Scholar] [CrossRef]
- Kunnumakkara, A.B.; Hegde, M.; Parama, D.; Girisa, S.; Kumar, A.; Daimary, U.D.; Garodia, P.; Yenisetti, S.C.; Oommen, O.V.; Aggarwal, B.B. Role of Turmeric and Curcumin in Prevention and Treatment of Chronic Diseases: Lessons Learned from Clinical Trials. ACS Pharmacol. Transl. Sci. 2023, 6, 447–518. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Zhang, Y.; He, J.; Yang, Z.; Zhang, R.; Li, L.; Luo, Z.; Ye, Y.; Sun, Q. Hydroxychloroquine Inhibits Macrophage Activation and Attenuates Renal Fibrosis After Ischemia-Reperfusion Injury. Front. Immunol. 2021, 12, 645100. [Google Scholar] [CrossRef] [PubMed]
- Momtazi-Borojeni, A.A.; Sabouri-Rad, S.; Gotto, A.M.; Pirro, M.; Banach, M.; Awan, Z.; Barreto, G.E.; Sahebkar, A. PCSK9 and inflammation: A review of experimental and clinical evidence. Eur. Heart J. Cardiovasc. Pharmacother. 2019, 5, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Liu, M.M.; Jin, J.L.; Cao, Y.X.; Guo, Y.L.; Wu, N.Q.; Zhu, C.G.; Dong, Q.; Sun, J.; Xu, R.X.; et al. Association of circulating PCSK9 concentration with cardiovascular metabolic markers and outcomes in stable coronary artery disease patients with or without diabetes: A prospective, observational cohort study. Cardiovasc. Diabetol. 2020, 19, 167. [Google Scholar] [CrossRef]
- Schlieper, G.; Schurgers, L.; Brandenburg, V.; Reutelingsperger, C.; Floege, J. Vascular calcification in chronic kidney disease: An update. Nephrol. Dial. Transpl. 2016, 31, 31–39. [Google Scholar] [CrossRef]
- Lee, H.Y.; Lim, S.; Park, S. Role of Inflammation in Arterial Calcification. Korean Circ. J. 2021, 51, 114–125. [Google Scholar] [CrossRef]
- Gao, P.; Cao, Y.; Ma, L. Regulation of soluble epoxide hydrolase in renal-associated diseases: Insights from potential mechanisms to clinical researches. Front. Endocrinol. 2024, 15, 1304547. [Google Scholar] [CrossRef]
- Imig, J.D. Epoxyeicosatrienoic acids, 20-hydroxyeicosatetraenoic acid, and renal microvascular function. Prostaglandins Other Lipid Mediat. 2013, 104–105, 2–7. [Google Scholar] [CrossRef]
- Ahrițculesei, R.V.; Boldeanu, L.; Caragea, D.C.; Vladu, I.M.; Clenciu, D.; Mitrea, A.; Ungureanu, A.M.; Văduva, C.C.; Dijmărescu, A.L.; Popescu, A.I.S.; et al. Association Between Pentraxins and Obesity in Prediabetes and Newly Diagnosed Type 2 Diabetes Mellitus Patients. Int. J. Mol. Sci. 2025, 26, 3661. [Google Scholar] [CrossRef]
- Miyasato, Y.; Hanna, R.M.; Morinaga, J.; Mukoyama, M.; Kalantar-Zadeh, K. Prognostic Nutritional Index as a Predictor of Mortality in 101,616 Patients Undergoing Hemodialysis. Nutrients 2023, 15, 311. [Google Scholar] [CrossRef] [PubMed]
- Podkowińska, A.; Formanowicz, D. Chronic Kidney Disease as Oxidative Stress- and Inflammatory-Mediated Cardiovascular Disease. Antioxidants 2020, 9, 752. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Zhang, C. Oxidative Stress: A Culprit in the Progression of Diabetic Kidney Disease. Antioxidants 2024, 13, 455. [Google Scholar] [CrossRef]
- Daenen, K.; Andries, A.; Mekahli, D.; Van Schepdael, A.; Jouret, F.; Bammens, B. Oxidative stress in chronic kidney disease. Pediatr. Nephrol. 2019, 34, 975–991. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Singh, P.; Khurana, S.; Ganguly, N.K.; Kukreti, R.; Saso, L.; Rana, D.S.; Taneja, V.; Bhargava, V. Implications of oxidative stress in chronic kidney disease: A review on current concepts and therapies. Kidney Res. Clin. Pract. 2021, 40, 183–193. [Google Scholar] [CrossRef]
- Ling, X.C.; Kuo, K.-L. Oxidative stress in chronic kidney disease. Ren. Replace. Ther. 2018, 4, 53. [Google Scholar] [CrossRef]
- Lee, W.C.; Li, L.C.; Chen, J.B.; Chang, H.W. Indoxyl sulfate-induced oxidative stress, mitochondrial dysfunction, and impaired biogenesis are partly protected by vitamin C and N-acetylcysteine. ScientificWorldJournal 2015, 2015, 620826. [Google Scholar] [CrossRef]
- Hall, A.M.; Unwin, R.J. The not so ‘mighty chondrion’: Emergence of renal diseases due to mitochondrial dysfunction. Nephron Physiol. 2007, 105, p1–p10. [Google Scholar] [CrossRef]
- Liu, S.; Yuan, Y.; Xue, Y.; Xing, C.; Zhang, B. Podocyte Injury in Diabetic Kidney Disease: A Focus on Mitochondrial Dysfunction. Front. Cell Dev. Biol. 2022, 10, 832887. [Google Scholar] [CrossRef]
- Vodošek Hojs, N.; Bevc, S.; Ekart, R.; Hojs, R. Oxidative Stress Markers in Chronic Kidney Disease with Emphasis on Diabetic Nephropathy. Antioxidants 2020, 9, 925. [Google Scholar] [CrossRef]
- Vida, C.; Oliva, C.; Yuste, C.; Ceprián, N.; Caro, P.J.; Valera, G.; González de Pablos, I.; Morales, E.; Carracedo, J. Oxidative Stress in Patients with Advanced CKD and Renal Replacement Therapy: The Key Role of Peripheral Blood Leukocytes. Antioxidants 2021, 10, 1155. [Google Scholar] [CrossRef]
- Pellegrino, D.; La Russa, D.; Marrone, A. Oxidative Imbalance and Kidney Damage: New Study Perspectives from Animal Models to Hospitalized Patients. Antioxidants 2019, 8, 594. [Google Scholar] [CrossRef]
- Cordiano, R.; Di Gioacchino, M.; Mangifesta, R.; Panzera, C.; Gangemi, S.; Minciullo, P.L. Malondialdehyde as a Potential Oxidative Stress Marker for Allergy-Oriented Diseases: An Update. Molecules 2023, 28, 5979. [Google Scholar] [CrossRef]
- Florens, N.; Calzada, C.; Lyasko, E.; Juillard, L.; Soulage, C.O. Modified Lipids and Lipoproteins in Chronic Kidney Disease: A New Class of Uremic Toxins. Toxins 2016, 8, 376. [Google Scholar] [CrossRef] [PubMed]
- Liakopoulos, V.; Roumeliotis, S.; Gorny, X.; Dounousi, E.; Mertens, P.R. Oxidative Stress in Hemodialysis Patients: A Review of the Literature. Oxidative Med. Cell Longev. 2017, 2017, 3081856. [Google Scholar] [CrossRef] [PubMed]
- Roumeliotis, S.; Mallamaci, F.; Zoccali, C. Endothelial Dysfunction in Chronic Kidney Disease, from Biology to Clinical Outcomes: A 2020 Update. J. Clin. Med. 2020, 9, 2359. [Google Scholar] [CrossRef] [PubMed]
- Martens, C.R.; Edwards, D.G. Peripheral vascular dysfunction in chronic kidney disease. Cardiol. Res. Pract. 2011, 2011, 267257. [Google Scholar] [CrossRef]
- Witko-Sarsat, V.; Friedlander, M.; Capeillère-Blandin, C.; Nguyen-Khoa, T.; Nguyen, A.T.; Zingraff, J.; Jungers, P.; Descamps-Latscha, B. Advanced oxidation protein products as a novel marker of oxidative stress in uremia. Kidney Int. 1996, 49, 1304–1313. [Google Scholar] [CrossRef] [PubMed]
- Binder, V.; Ljubojevic, S.; Haybaeck, J.; Holzer, M.; El-Gamal, D.; Schicho, R.; Pieske, B.; Heinemann, A.; Marsche, G. The myeloperoxidase product hypochlorous acid generates irreversible high-density lipoprotein receptor inhibitors. Arter. Thromb. Vasc. Biol. 2013, 33, 1020–1027. [Google Scholar] [CrossRef]
- Collado, S.; Coll, E.; Nicolau, C.; Azqueta, M.; Pons, M.; Cruzado, J.M.; de la Torre, B.; Deulofeu, R.; Mojal, S.; Pascual, J.; et al. Serum osteoprotegerin in prevalent hemodialysis patients: Associations with mortality, atherosclerosis and cardiac function. BMC Nephrol. 2017, 18, 290. [Google Scholar] [CrossRef][Green Version]
- Heidari, B. C-reactive protein and other markers of inflammation in hemodialysis patients. Casp. J. Intern. Med. 2013, 4, 611–616. [Google Scholar]
- Stenvinkel, P.; Carrero, J.J.; Axelsson, J.; Lindholm, B.; Heimbürger, O.; Massy, Z. Emerging biomarkers for evaluating cardiovascular risk in the chronic kidney disease patient: How do new pieces fit into the uremic puzzle? Clin. J. Am. Soc. Nephrol. 2008, 3, 505–521. [Google Scholar] [CrossRef] [PubMed]
- Descamps-Latscha, B.; Drüeke, T.; Witko-Sarsat, V. Dialysis-induced oxidative stress: Biological aspects, clinical consequences, and therapy. Semin. Dial. 2001, 14, 193–199. [Google Scholar] [CrossRef]
- Himmelfarb, J.; Ikizler, T.A.; Ellis, C.; Wu, P.; Shintani, A.; Dalal, S.; Kaplan, M.; Chonchol, M.; Hakim, R.M. Provision of antioxidant therapy in hemodialysis (PATH): A randomized clinical trial. J. Am. Soc. Nephrol. 2014, 25, 623–633. [Google Scholar] [CrossRef]
- Gewin, L.; Zent, R. How does TGF-β mediate tubulointerstitial fibrosis? Semin. Nephrol. 2012, 32, 228–235. [Google Scholar] [CrossRef]
- Kim, H.J.; Vaziri, N.D. Contribution of impaired Nrf2-Keap1 pathway to oxidative stress and inflammation in chronic renal failure. Am. J. Physiol. -Ren. Physiol. 2010, 298, F662–F671. [Google Scholar] [CrossRef] [PubMed]
- Zoja, C.; Benigni, A.; Remuzzi, G. The Nrf2 pathway in the progression of renal disease. Nephrol. Dial. Transplant. 2013, 29, i19–i24. [Google Scholar] [CrossRef]
- Nezu, M.; Suzuki, N.; Yamamoto, M. Targeting the KEAP1-NRF2 System to Prevent Kidney Disease Progression. Am. J. Nephrol. 2017, 45, 473–483. [Google Scholar] [CrossRef]
- Tanase, D.M.; Gosav, E.M.; Anton, M.I.; Floria, M.; Seritean Isac, P.N.; Hurjui, L.L.; Tarniceriu, C.C.; Costea, C.F.; Ciocoiu, M.; Rezus, C. Oxidative Stress and NRF2/KEAP1/ARE Pathway in Diabetic Kidney Disease (DKD): New Perspectives. Biomolecules 2022, 12, 1227. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhou, Q.; Su, L.; Ni, L. Mitochondrial dysfunction: The hidden catalyst in chronic kidney disease progression. Ren. Fail. 2025, 47, 2506812. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Shang, J.; Inagi, R. Control of Mitochondrial Quality: A Promising Target for Diabetic Kidney Disease Treatment. Kidney Int. Rep. 2025, 10, 994–1010. [Google Scholar] [CrossRef]
- Aranda-Rivera, A.K.; Cruz-Gregorio, A.; Amador-Martínez, I.; Hernández-Cruz, E.Y.; Tapia, E.; Pedraza-Chaverri, J. Antioxidants targeting mitochondria function in kidney diseases. Mitochondrial Commun. 2024, 2, 21–37. [Google Scholar] [CrossRef]
- Linder, B.A.; Stute, N.L.; Hutchison, Z.J.; Barnett, A.M.; Tharpe, M.A.; Kavazis, A.N.; Kirkman, D.L.; Gutierrez, O.M.; Robinson, A.T. Acute high-dose MitoQ does not increase urinary kidney injury markers in healthy adults: A randomized crossover trial. Am. J. Physiol. Ren. Physiol. 2024, 326, F135–F142. [Google Scholar] [CrossRef]
- Gottwald, E.M.; Duss, M.; Bugarski, M.; Haenni, D.; Schuh, C.D.; Landau, E.M.; Hall, A.M. The targeted anti-oxidant MitoQ causes mitochondrial swelling and depolarization in kidney tissue. Physiol. Rep. 2018, 6, e13667. [Google Scholar] [CrossRef]
- Nezu, M.; Suzuki, N. Roles of Nrf2 in Protecting the Kidney from Oxidative Damage. Int. J. Mol. Sci. 2020, 21, 2951. [Google Scholar] [CrossRef]
- Pergola, P.E.; Raskin, P.; Toto, R.D.; Meyer, C.J.; Huff, J.W.; Grossman, E.B.; Krauth, M.; Ruiz, S.; Audhya, P.; Christ-Schmidt, H.; et al. Bardoxolone methyl and kidney function in CKD with type 2 diabetes. N. Engl. J. Med. 2011, 365, 327–336. [Google Scholar] [CrossRef]
- Nagasu, H.; Sogawa, Y.; Kidokoro, K.; Itano, S.; Yamamoto, T.; Satoh, M.; Sasaki, T.; Suzuki, T.; Yamamoto, M.; Wigley, W.C.; et al. Bardoxolone methyl analog attenuates proteinuria-induced tubular damage by modulating mitochondrial function. FASEB J. 2019, 33, 12253–12263. [Google Scholar] [CrossRef]
- Arendshorst, W.J.; Vendrov, A.E.; Kumar, N.; Ganesh, S.K.; Madamanchi, N.R. Oxidative Stress in Kidney Injury and Hypertension. Antioxidants 2024, 13, 1454. [Google Scholar] [CrossRef] [PubMed]
- Jin, Q.; Liu, T.; Qiao, Y.; Liu, D.; Yang, L.; Mao, H.; Ma, F.; Wang, Y.; Peng, L.; Zhan, Y. Oxidative stress and inflammation in diabetic nephropathy: Role of polyphenols. Front. Immunol. 2023, 14, 1185317. [Google Scholar] [CrossRef] [PubMed]
- Fakhruddin, S.; Alanazi, W.; Jackson, K.E. Diabetes-Induced Reactive Oxygen Species: Mechanism of Their Generation and Role in Renal Injury. J. Diabetes Res. 2017, 2017, 8379327. [Google Scholar] [CrossRef]
- Tasić, D.; Dimitrijević, Z. The Role of Oxidative Stress as a Mechanism in the Pathogenesis of Acute Heart Failure in Acute Kidney Injury. Diagnostics 2024, 14, 2094. [Google Scholar] [CrossRef]
- Khalil, S.K.; Amer, H.A.; El Behairy, A.M.; Warda, M. Oxidative stress during erythropoietin hyporesponsiveness anemia at end stage renal disease: Molecular and biochemical studies. J. Adv. Res. 2016, 7, 348–358. [Google Scholar] [CrossRef]
- Bissinger, R.; Schaefer, L.; Bohnert, B.N.; Schork, A.; Hoerber, S.; Peter, A.; Qadri, S.M.; Birkenfeld, A.L.; Heyne, N.; Bakchoul, T.; et al. GFR is a Key Determinant of Red Blood Cell Survival in Anemia Associated With Progressive CKD. Kidney Int. Rep. 2025, 10, 730–742. [Google Scholar] [CrossRef]
- Chuang, P.Y.; Yu, Q.; Fang, W.; Uribarri, J.; He, J.C. Advanced glycation endproducts induce podocyte apoptosis by activation of the FOXO4 transcription factor. Kidney Int. 2007, 72, 965–976. [Google Scholar] [CrossRef]
- Nangaku, M.; Kanda, H.; Takama, H.; Ichikawa, T.; Hase, H.; Akizawa, T. Randomized Clinical Trial on the Effect of Bardoxolone Methyl on GFR in Diabetic Kidney Disease Patients (TSUBAKI Study). Kidney Int. Rep. 2020, 5, 879–890. [Google Scholar] [CrossRef]
- Chin, M.P.; Reisman, S.A.; Bakris, G.L.; O’Grady, M.; Linde, P.G.; McCullough, P.A.; Packham, D.; Vaziri, N.D.; Ward, K.W.; Warnock, D.G.; et al. Mechanisms contributing to adverse cardiovascular events in patients with type 2 diabetes mellitus and stage 4 chronic kidney disease treated with bardoxolone methyl. Am. J. Nephrol. 2014, 39, 499–508. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.U.; Yeom, J.-h.; Kim, W. Beneficial Effects of Vitamin E Supplementation on Endothelial Dysfunction, Inflammation, and Oxidative Stress Biomarkers in Patients Receiving Hemodialysis: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Int. J. Mol. Sci. 2021, 22, 11923. [Google Scholar] [CrossRef]
- Jiang, X.S.; Xiang, X.Y.; Chen, X.M.; He, J.L.; Liu, T.; Gan, H.; Du, X.G. Inhibition of soluble epoxide hydrolase attenuates renal tubular mitochondrial dysfunction and ER stress by restoring autophagic flux in diabetic nephropathy. Cell Death Dis. 2020, 11, 385. [Google Scholar] [CrossRef]
- Ho, H.-J.; Shirakawa, H. Oxidative Stress and Mitochondrial Dysfunction in Chronic Kidney Disease. Cells 2023, 12, 88. [Google Scholar] [CrossRef]
- Colombijn, J.M.; Hooft, L.; Jun, M.; Webster, A.C.; Bots, M.L.; Verhaar, M.C.; Vernooij, R.W. Antioxidants for adults with chronic kidney disease. Cochrane Database Syst. Rev. 2023, 11, Cd008176. [Google Scholar] [CrossRef]
- Elmarakby, A.A.; Faulkner, J.; Al-Shabrawey, M.; Wang, M.H.; Maddipati, K.R.; Imig, J.D. Deletion of soluble epoxide hydrolase gene improves renal endothelial function and reduces renal inflammation and injury in streptozotocin-induced type 1 diabetes. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 301, R1307–1317. [Google Scholar] [CrossRef]
- Suárez-Santisteban, M.A.; Santos-Díaz, G.; García-Bernalt, V.; Pérez-Pico, A.M.; Mingorance, E.; Mayordomo, R.; Dorado, P. Association between CYP4A11 and EPHX2 genetic polymorphisms and chronic kidney disease progression in hypertensive patients. Nefrología 2024, 44, 382–395. [Google Scholar] [CrossRef]
- Shapiro, M.D.; Fazio, S. PCSK9 and Atherosclerosis-Lipids and Beyond. J. Atheroscler. Thromb. 2017, 24, 462–472. [Google Scholar] [CrossRef]
- Hwang, H.S.; Kim, J.S.; Kim, Y.G.; Lee, S.Y.; Ahn, S.Y.; Lee, H.J.; Lee, D.Y.; Lee, S.H.; Moon, J.Y.; Jeong, K.H. Circulating PCSK9 Level and Risk of Cardiovascular Events and Death in Hemodialysis Patients. J. Clin. Med. 2020, 9, 244. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.-J.; Liu, S.; Lee, J.; Zheng, H.; Gurung, R.L.; Ang, K.; Wu, H.; Lim, S.C. Plasma proprotein convertase subtilisin/kexin type 9 (PCSK9) and the risk of chronic kidney disease progression in patients with type 2 diabetes. Diabetes Obes. Metab. 2025, 27, 4446–4453. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.H.; Peng, J.; Ren, Z.; Yang, J.; Li, T.T.; Li, T.H.; Wang, Z.; Wei, D.H.; Liu, L.S.; Zheng, X.L.; et al. New role of PCSK9 in atherosclerotic inflammation promotion involving the TLR4/NF-κB pathway. Atherosclerosis 2017, 262, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Kheirkhah, A.; Lamina, C.; Kollerits, B.; Schachtl-Riess, J.F.; Schultheiss, U.T.; Forer, L.; Sekula, P.; Kotsis, F.; Eckardt, K.U.; Kronenberg, F. PCSK9 and Cardiovascular Disease in Individuals with Moderately Decreased Kidney Function. Clin. J. Am. Soc. Nephrol. 2022, 17, 809–818. [Google Scholar] [CrossRef]
- Cao, M.; Li, M.; Li, X.; Li, Y.; Chen, Y.; Drekolia, M.-K.; Cheng, X.; Lagos, F.D.; Bibli, S.-I.; Hu, J. Endothelial soluble epoxide hydrolase links polyunsaturated fatty acid metabolism to oxidative stress and atherosclerosis progression. Redox Biol. 2025, 85, 103730. [Google Scholar] [CrossRef]
- Vinereanu, I.-V.; Peride, I.; Niculae, A.; Andreea Taisia, T.; Caragheorgheopol, A.; Manda, D.; Checherita, I. The Relationship between Advanced Oxidation Protein Products, Vascular Calcifications and Arterial Stiffness in Predialysis Chronic Kidney Disease Patients. Medicina 2021, 57, 452. [Google Scholar] [CrossRef]
- Bavi Behbahani, H.; Alipour, M.; Zare Javid, A.; Razmi, H.; Tofighzadeh, P.; Fayazfar, F.; Keramatzadeh, S.; Shokri, S.; Soltaniyan Dehkordi, H.; Khosravi, K.; et al. The association Malnutrition-Inflammation Score with chronic kidney disease-associated pruritus and quality of life in hemodialysis patients: A multicenter cross-sectional study. Sci. Rep. 2024, 14, 31811. [Google Scholar] [CrossRef]
- Garay-Sevilla, M.E.; Beeri, M.S.; de la Maza, M.P.; Rojas, A.; Salazar-Villanea, S.; Uribarri, J. The potential role of dietary advanced glycation endproducts in the development of chronic non-infectious diseases: A narrative review. Nutr. Res. Rev. 2020, 33, 298–311. [Google Scholar] [CrossRef] [PubMed]
- Zeinalabedini, M.; Shapouri, M.; Mirzaee, P.; Kamali, M.; Mahmoudi, Z.; Noriani, N.; Saeedirad, Z.; Adabi, S.B.; Mobarakeh, K.A.; Shamsi-Goushki, A.; et al. The Effect of Omega-3 Supplements on Renal Function Indices in Chronic Kidney Patients Undergoing Hemodialysis. Nutr. Metab. Insights 2025, 18, 11786388251345518. [Google Scholar] [CrossRef] [PubMed]
- Chedid, M.; Kaidbay, H.D.; Wigerinck, S.; Mkhaimer, Y.; Smith, B.; Zubidat, D.; Sekhon, I.; Prajwal, R.; Duriseti, P.; Issa, N.; et al. Cardiovascular Outcomes in Kidney Transplant Recipients With ADPKD. Kidney Int. Rep. 2022, 7, 1991–2005. [Google Scholar] [CrossRef]
- Gianazza, E.; Brioschi, M.; Fernandez, A.M.; Banfi, C. Lipoxidation in cardiovascular diseases. Redox Biol. 2019, 23, 101119. [Google Scholar] [CrossRef]
- Ho, E.; Karimi Galougahi, K.; Liu, C.-C.; Bhindi, R.; Figtree, G.A. Biological markers of oxidative stress: Applications to cardiovascular research and practice. Redox Biol. 2013, 1, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Luquero, A.; Badimon, L.; Borrell-Pages, M. PCSK9 Functions in Atherosclerosis Are Not Limited to Plasmatic LDL-Cholesterol Regulation. Front. Cardiovasc. Med. 2021, 8, 639727. [Google Scholar] [CrossRef]
- Abifadel, M.; Varret, M.; Rabès, J.P.; Allard, D.; Ouguerram, K.; Devillers, M.; Cruaud, C.; Benjannet, S.; Wickham, L.; Erlich, D.; et al. Mutations in PCSK9 cause autosomal dominant hypercholesterolemia. Nat. Genet. 2003, 34, 154–156. [Google Scholar] [CrossRef]
- Luo, Y.; Warren, L.; Xia, D.; Jensen, H.; Sand, T.; Petras, S.; Qin, W.; Miller, K.S.; Hawkins, J. Function and distribution of circulating human PCSK9 expressed extrahepatically in transgenic mice. J. Lipid Res. 2009, 50, 1581–1588. [Google Scholar] [CrossRef]
- Liu, H. Association between PCSK9 inhibitors and acute kidney injury: A pharmacovigilance study. Front. Pharmacol. 2024, 15, 1353848. [Google Scholar] [CrossRef]
- Hummelgaard, S.; Kresse, J.C.; Jensen, M.S.; Glerup, S.; Weyer, K. Emerging roles of PCSK9 in kidney disease: Lipid metabolism, megalin regulation and proteinuria. Pflug. Arch. 2025, 477, 773–786. [Google Scholar] [CrossRef]
- Wang, Z.; Yu, X.; Ma, H.; Yao, S.; Li, Z.; Zhang, R.; Liang, H.; Jiao, J. Proprotein convertase subtilisin/kexin type 9 contributes to cisplatin-induced acute kidney injury by interacting with cyclase-associated protein 1 to promote megalin lysosomal degradation. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2025, 1872, 119984. [Google Scholar] [CrossRef]
- Wu, D.; Zhou, Y.; Pan, Y.; Li, C.; Wang, Y.; Chen, F.; Chen, X.; Yang, S.; Zhou, Z.; Liao, Y.; et al. Vaccine Against PCSK9 Improved Renal Fibrosis by Regulating Fatty Acid β-Oxidation. J. Am. Heart Assoc. 2020, 9, e014358. [Google Scholar] [CrossRef]
- Liu, C.; Chen, J.; Chen, H.; Zhang, T.; He, D.; Luo, Q.; Chi, J.; Hong, Z.; Liao, Y.; Zhang, S.; et al. PCSK9 Inhibition: From Current Advances to Evolving Future. Cells 2022, 11, 2972. [Google Scholar] [CrossRef] [PubMed]
- Nozue, T. Lipid Lowering Therapy and Circulating PCSK9 Concentration. J. Atheroscler. Thromb. 2017, 24, 895–907. [Google Scholar] [CrossRef] [PubMed]
- Taylor, B.A.; Thompson, P.D. Statins and Their Effect on PCSK9-Impact and Clinical Relevance. Curr. Atheroscler. Rep. 2016, 18, 46. [Google Scholar] [CrossRef] [PubMed]
- Katsuki, S.; Jha, P.K.; Aikawa, E.; Aikawa, M. The role of proprotein convertase subtilisin/kexin 9 (PCSK9) in macrophage activation: A focus on its LDL receptor-independent mechanisms. Front. Cardiovasc. Med. 2024, 11, 1431398. [Google Scholar] [CrossRef]
- Bagheri, B.; Khatibiyan Feyzabadi, Z.; Nouri, A.; Azadfallah, A.; Mahdizade Ari, M.; Hemmati, M.; Darban, M.; Alavi Toosi, P.; Banihashemian, S.Z. Atherosclerosis and Toll-Like Receptor4 (TLR4), Lectin-Like Oxidized Low-Density Lipoprotein-1 (LOX-1), and Proprotein Convertase Subtilisin/Kexin Type9 (PCSK9). Mediat. Inflamm. 2024, 2024, 5830491. [Google Scholar] [CrossRef] [PubMed]
- Yurtseven, E.; Ural, D.; Baysal, K.; Tokgözoğlu, L. An Update on the Role of PCSK9 in Atherosclerosis. J. Atheroscler. Thromb. 2020, 27, 909–918. [Google Scholar] [CrossRef]
- Dutka, M.; Zimmer, K.; Ćwiertnia, M.; Ilczak, T.; Bobiński, R. The role of PCSK9 in heart failure and other cardiovascular diseases—Mechanisms of action beyond its effect on LDL cholesterol. Heart Fail. Rev. 2024, 29, 917–937. [Google Scholar] [CrossRef]
- Kong, P.; Cui, Z.-Y.; Huang, X.-F.; Zhang, D.-D.; Guo, R.-J.; Han, M. Inflammation and atherosclerosis: Signaling pathways and therapeutic intervention. Signal Transduct. Target. Ther. 2022, 7, 131. [Google Scholar] [CrossRef]
- Feng, Z.; Liao, X.; Zhang, H.; Peng, J.; Huang, Z.; Yi, B. Increased serum PCSK9 levels are associated with renal function impairment in patients with type 2 diabetes mellitus. Ren. Fail. 2023, 45, 2215880. [Google Scholar] [CrossRef]
- Suh, S.H.; Kim, S.W. Dyslipidemia in Patients with Chronic Kidney Disease: An Updated Overview. Diabetes Metab. J. 2023, 47, 612–629. [Google Scholar] [CrossRef]
- Duan, H.; Shi, Y.; Zhang, Q.; Shi, X.; Zhang, Y.; Liu, J.; Zhang, Y. Causal relationship between PCSK9 inhibitor and primary glomerular disease: A drug target Mendelian randomization study. Front. Endocrinol. 2024, 15, 1335489. [Google Scholar] [CrossRef] [PubMed]
- Elewa, U.; Fernández-Fernández, B.; Mahillo-Fernández, I.; Martin-Cleary, C.; Sanz, A.B.; Sanchez-Niño, M.D.; Ortiz, A. PCSK9 in diabetic kidney disease. Eur. J. Clin. Invest. 2016, 46, 779–786. [Google Scholar] [CrossRef]
- Wang, J.; Yue, X.; Meng, C.; Wang, Z.; Jin, X.; Cui, X.; Yang, J.; Shan, C.; Gao, Z.; Yang, Y.; et al. Acute Hyperglycemia May Induce Renal Tubular Injury Through Mitophagy Inhibition. Front. Endocrinol. 2020, 11, 536213. [Google Scholar] [CrossRef]
- Pressly, J.; Fornoni, A. The Many Lives of PCSK9: Therapeutic Implications. Kidney360 2022, 3, 1296–1298. [Google Scholar] [CrossRef] [PubMed]
- Lupo, M.G.; Bressan, A.; Donato, M.; Canzano, P.; Camera, M.; Poggio, P.; Greco, M.F.; Garofalo, M.; De Martin, S.; Panighel, G.; et al. PCSK9 promotes arterial medial calcification. Atherosclerosis 2022, 346, 86–97. [Google Scholar] [CrossRef]
- Tan, D.; Yang, X.; Yang, J.; Fan, G.; Xiong, G. PCSK9 in Vascular Aging and Age-Related Diseases. Aging Dis. 2025. [Google Scholar] [CrossRef] [PubMed]
- Ruscica, M.; Ferri, N.; Fogacci, F.; Rosticci, M.; Botta, M.; Marchiano, S.; Magni, P.; D’Addato, S.; Giovannini, M.; Borghi, C.; et al. Circulating Levels of Proprotein Convertase Subtilisin/Kexin Type 9 and Arterial Stiffness in a Large Population Sample: Data From the Brisighella Heart Study. J. Am. Heart Assoc. 2017, 6, e005764. [Google Scholar] [CrossRef] [PubMed]
- Karakoyun, A.; Akkubak, Y.; Göktepe, M.H.; Yılmaz, P.D.; Kadıyoran, C.; Oğul, M.; Kucuk, A. Increased PCSK9 associated with cIMT in AS: A useful marker for subclinical atherosclerosis in patients with ankylosing spondylitis. Arch. Rheumatol. 2024, 39, 652–661. [Google Scholar] [CrossRef]
- Ruscica, M.; Macchi, C.; Giuliani, A.; Rizzuto, A.S.; Ramini, D.; Sbriscia, M.; Carugo, S.; Bonfigli, A.R.; Corsini, A.; Olivieri, F.; et al. Circulating PCSK9 as a prognostic biomarker of cardiovascular events in individuals with type 2 diabetes: Evidence from a 16.8-year follow-up study. Cardiovasc. Diabetol. 2023, 22, 222. [Google Scholar] [CrossRef]
- PCSK9 Inhibitors. Available online: https://www.ncbi.nlm.nih.gov/books/NBK448100/ (accessed on 15 June 2025).
- Jeswani, B.M.; Sharma, S.; Rathore, S.S.; Nazir, A.; Bhatheja, R.; Kapoor, K. PCSK9 Inhibitors: The Evolving Future. Health Sci. Rep. 2024, 7, e70174. [Google Scholar] [CrossRef] [PubMed]
- Sabatine, M.S.; Giugliano, R.P.; Keech, A.C.; Honarpour, N.; Wiviott, S.D.; Murphy, S.A.; Kuder, J.F.; Wang, H.; Liu, T.; Wasserman, S.M.; et al. Evolocumab and Clinical Outcomes in Patients with Cardiovascular Disease. N. Engl. J. Med. 2017, 376, 1713–1722. [Google Scholar] [CrossRef]
- Roumeliotis, S.; Roumeliotis, A.; Stamou, A.; Panagoutsos, S.; Manolopoulos, V.G.; Tsetsos, F.; Georgitsi, M.; Liakopoulos, V. Association of rs11780592 Polymorphism in the Human Soluble Epoxide Hydrolase Gene (EPHX2) with Oxidized LDL and Mortality in Patients with Diabetic Chronic Kidney Disease. Oxidative Med. Cell. Longev. 2021, 2021, 8817502. [Google Scholar] [CrossRef]
- Charytan, D.M.; Sabatine, M.S.; Pedersen, T.R.; Im, K.; Park, J.G.; Pineda, A.L.; Wasserman, S.M.; Deedwania, P.; Olsson, A.G.; Sever, P.S.; et al. Efficacy and Safety of Evolocumab in Chronic Kidney Disease in the FOURIER Trial. J. Am. Coll. Cardiol. 2019, 73, 2961–2970. [Google Scholar] [CrossRef]
- Quiroga, B.; Ramos, P.M.; Chiva, V.Á. Efficacy and safety of the PCSK9 inhibitors in the treatment of dyslipidemia in chronic kidney disease. Nefrología 2020, 40, 499–505. [Google Scholar] [CrossRef]
- Amaro, J.M.; Villanego, F.; Naranjo, J.; Orellana, C.; Vigara, L.A.; Narváez, C.E.; Torrado, J.; Cazorla, J.M.; Rodríguez, C.; Mazuecos, A. Treatment with PCSK9 inhibitors in patients with chronic kidney disease at very high cardiovascular risk. Nefrología 2023, 43, 133–135. [Google Scholar] [CrossRef]
- Igweonu-Nwakile, E.O.; Ali, S.; Paul, S.; Yakkali, S.; Teresa Selvin, S.; Thomas, S.; Bikeyeva, V.; Abdullah, A.; Radivojevic, A.; Abu Jad, A.A.; et al. A Systematic Review on the Safety and Efficacy of PCSK9 Inhibitors in Lowering Cardiovascular Risks in Patients With Chronic Kidney Disease. Cureus 2022, 14, e29140. [Google Scholar] [CrossRef]
- Muñoz Ramos, P.; Gil Giraldo, Y.; Álvarez-Chiva, V.; Arroyo, D.; Sango Merino, C.; Moncho Francés, F.; Ocaña, J.; Reque, J.; Sánchez-Álvarez, E.; Górriz, J.L.; et al. Proteinuria-Lowering Effects of Proprotein Convertase Subtilisin/Kexin Type 9 Inhibitors in Chronic Kidney Disease Patients: A Real-World Multicentric Study. Metabolites 2021, 11, 760. [Google Scholar] [CrossRef]
- Skeby, C.K.; Hummelgaard, S.; Gustafsen, C.; Petrillo, F.; Frederiksen, K.P.; Olsen, D.; Kristensen, T.; Ivarsen, P.; Madsen, P.; Christensen, E.I.; et al. Proprotein convertase subtilisin/kexin type 9 targets megalin in the kidney proximal tubule and aggravates proteinuria in nephrotic syndrome. Kidney Int. 2023, 104, 754–768. [Google Scholar] [CrossRef]
- Marfella, R.; Prattichizzo, F.; Sardu, C.; Paolisso, P.; D’Onofrio, N.; Scisciola, L.; La Grotta, R.; Frigé, C.; Ferraraccio, F.; Panarese, I.; et al. Evidence of an anti-inflammatory effect of PCSK9 inhibitors within the human atherosclerotic plaque. Atherosclerosis 2023, 378, 117180. [Google Scholar] [CrossRef] [PubMed]
- Hummelgaard, S.; Hvid, H.; Birn, H.; Glerup, S.; Tom, N.; Bilgin, M.; Kirchhoff, J.E.; Weyer, K. Lack of renoprotective effects by long-term PCSK9 and SGLT2 inhibition using alirocumab and empagliflozin in obese ZSF1 rats. Am. J. Physiol. -Ren. Physiol. 2025, 328, F48–F67. [Google Scholar] [CrossRef] [PubMed]
- Mayne, J.; Dewpura, T.; Raymond, A.; Cousins, M.; Chaplin, A.; Lahey, K.A.; LaHaye, S.A.; Mbikay, M.; Ooi, T.C.; Chrétien, M. Plasma PCSK9 levels are significantly modified by statins and fibrates in humans. Lipids Health Dis. 2008, 7, 22. [Google Scholar] [CrossRef]
- Node, K.; Huo, Y.; Ruan, X.; Yang, B.; Spiecker, M.; Ley, K.; Zeldin, D.C.; Liao, J.K. Anti-inflammatory properties of cytochrome P450 epoxygenase-derived eicosanoids. Science 1999, 285, 1276–1279. [Google Scholar] [CrossRef]
- Imig, J.D. Epoxides and soluble epoxide hydrolase in cardiovascular physiology. Physiol. Rev. 2012, 92, 101–130. [Google Scholar] [CrossRef] [PubMed]
- Noh, M.R.; Jang, H.S.; Salem, F.E.; Ferrer, F.A.; Kim, J.; Padanilam, B.J. Epoxyeicosatrienoic acid administration or soluble epoxide hydrolase inhibition attenuates renal fibrogenesis in obstructive nephropathy. Am. J. Physiol. Ren. Physiol. 2023, 324, F138–f151. [Google Scholar] [CrossRef]
- Imig, J.D.; Hammock, B.D. Soluble epoxide hydrolase as a therapeutic target for cardiovascular diseases. Nat. Rev. Drug Discov. 2009, 8, 794–805. [Google Scholar] [CrossRef]
- Morisseau, C.; Hammock, B.D. Impact of soluble epoxide hydrolase and epoxyeicosanoids on human health. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 37–58. [Google Scholar] [CrossRef]
- Michaelis, U.R.; Fleming, I. From endothelium-derived hyperpolarizing factor (EDHF) to angiogenesis: Epoxyeicosatrienoic acids (EETs) and cell signaling. Pharmacol. Ther. 2006, 111, 584–595. [Google Scholar] [CrossRef]
- Kim, J.; Imig, J.D.; Yang, J.; Hammock, B.D.; Padanilam, B.J. Inhibition of soluble epoxide hydrolase prevents renal interstitial fibrosis and inflammation. Am. J. Physiol. Ren. Physiol. 2014, 307, F971–F980. [Google Scholar] [CrossRef] [PubMed]
- Simpkins, A.N.; Rudic, R.D.; Roy, S.; Tsai, H.J.; Hammock, B.D.; Imig, J.D. Soluble epoxide hydrolase inhibition modulates vascular remodeling. Am. J. Physiol. Heart Circ. Physiol. 2010, 298, H795–H806. [Google Scholar] [CrossRef] [PubMed]
- Niu, Q.; Guo, Z.; Liang, Y.; Zuo, L. Soluble Epoxide Hydrolase Inhibition Attenuates Proteinuria by Alleviating Renal Inflammation and Podocyte Injuries in Adriamycin-Induced Nephropathy. Int. J. Mol. Sci. 2024, 25, 10629. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-J.; Suo, P.; Wang, Y.-N.; Zou, L.; Nie, X.-L.; Zhao, Y.-Y.; Miao, H. Arachidonic acid metabolism as a therapeutic target in AKI-to-CKD transition. Front. Pharmacol. 2024, 15, 1365802. [Google Scholar] [CrossRef]
- Iliff, J.J.; Wang, R.; Zeldin, D.C.; Alkayed, N.J. Epoxyeicosanoids as mediators of neurogenic vasodilation in cerebral vessels. Am. J. Physiol. Heart Circ. Physiol. 2009, 296, H1352–1363. [Google Scholar] [CrossRef]
- He, W.; Huang, J.; Liu, Y.; Xie, C.; Zhang, K.; Zhu, X.; Chen, J.; Huang, H. Deletion of soluble epoxide hydrolase suppressed chronic kidney disease-related vascular calcification by restoring Sirtuin 3 expression. Cell Death Dis. 2021, 12, 992. [Google Scholar] [CrossRef]
- Hamzaoui, M.; Roche, C.; Coquerel, D.; Duflot, T.; Brunel, V.; Mulder, P.; Richard, V.; Bellien, J.; Guerrot, D. Soluble Epoxide Hydrolase Inhibition Prevents Experimental Type 4 Cardiorenal Syndrome. Front. Mol. Biosci. 2021, 7, 604042. [Google Scholar] [CrossRef]
- Li, L.; Li, N.; Pang, W.; Zhang, X.; Hammock, B.D.; Ai, D.; Zhu, Y. Opposite Effects of Gene Deficiency and Pharmacological Inhibition of Soluble Epoxide Hydrolase on Cardiac Fibrosis. PLoS ONE 2014, 9, e94092. [Google Scholar] [CrossRef]
- Deng, G.; Feng, Z.; Kong, X.; Gao, P.; Jiang, Y.; Liu, Y.; Zhao, M.; Ma, L. Dynamic DNA methylation of EPHX2 in diabetic kidney disease progression: A potential biomarker. Gene Rep. 2025, 40, 102245. [Google Scholar] [CrossRef]
- Liu, J.Y. Inhibition of Soluble Epoxide Hydrolase for Renal Health. Front. Pharmacol. 2018, 9, 1551. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Hou, F.F.; Nie, J. AOPPs and the progression of kidney disease. Kidney Int. Suppl. 2014, 4, 102–106. [Google Scholar] [CrossRef]
- Marsche, G.; Frank, S.; Hrzenjak, A.; Holzer, M.; Dirnberger, S.; Wadsack, C.; Scharnagl, H.; Stojakovic, T.; Heinemann, A.; Oettl, K. Plasma-advanced oxidation protein products are potent high-density lipoprotein receptor antagonists in vivo. Circ. Res. 2009, 104, 750–757. [Google Scholar] [CrossRef]
- Kisic, B.; Miric, D.; Dragojevic, I.; Rasic, J.; Popovic, L. Role of Myeloperoxidase in Patients with Chronic Kidney Disease. Oxidative Med. Cell Longev. 2016, 2016, 1069743. [Google Scholar] [CrossRef] [PubMed]
- Gergő, A.M.; Szilárd, K.; Eszter, S.; Melinda, K.; Lívia, S.; Csaba, C.; Katalin, B.; Lajos, B.; Attila, M.; István, W. Role of Tyrosine Isomers in Acute and Chronic Diseases Leading to Oxidative Stress—A Review. Curr. Med. Chem. 2016, 23, 667–685. [Google Scholar] [CrossRef]
- Bayarsaikhan, G.; Bayarsaikhan, D.; Lee, J.; Lee, B. Targeting Scavenger Receptors in Inflammatory Disorders and Oxidative Stress. Antioxidants 2022, 11, 936. [Google Scholar] [CrossRef]
- Verberk, S.G.S.; Hahn, N.; Heister, D.; Haverkamp, J.; Snelder, K.S.; de Goede, K.E.; Gorki, F.S.; Hendriks, J.J.A.; Houtkooper, R.H.; Visser, G.; et al. Monocyte and macrophage profiles in patients with inherited long-chain fatty acid oxidation disorders. Biochim. Biophys. Acta Mol. Basis Dis. 2025, 1871, 167524. [Google Scholar] [CrossRef]
- Valdivielso, J.M.; Rodríguez-Puyol, D.; Pascual, J.; Barrios, C.; Bermúdez-López, M.; Sánchez-Niño, M.D.; Pérez-Fernández, M.; Ortiz, A. Atherosclerosis in Chronic Kidney Disease: More, Less, or Just Different? Arter. Thromb. Vasc. Biol. 2019, 39, 1938–1966. [Google Scholar] [CrossRef]
- Ganesan, R.; Henkels, K.M.; Wrenshall, L.E.; Kanaho, Y.; Di Paolo, G.; Frohman, M.A.; Gomez-Cambronero, J. Oxidized LDL phagocytosis during foam cell formation in atherosclerotic plaques relies on a PLD2-CD36 functional interdependence. J. Leukoc. Biol. 2018, 103, 867–883. [Google Scholar] [CrossRef] [PubMed]
- Cao, G.; Xuan, X.; Hu, J.; Zhang, R.; Jin, H.; Dong, H. How vascular smooth muscle cell phenotype switching contributes to vascular disease. Cell Commun. Signal. 2022, 20, 180. [Google Scholar] [CrossRef] [PubMed]
- Tsinari, A.; Roumeliotis, S.; Neofytou, I.E.; Varouktsi, G.; Veljkovic, A.; Stamou, A.; Leivaditis, K.; Liakopoulos, V. The Clinical Utility and Plausibility of Oxidative and Antioxidant Variables in Chronic and End-Stage Kidney Disease: A Review of the Literature. Int. J. Mol. Sci. 2025, 26, 3376. [Google Scholar] [CrossRef]
- Aguilar Diaz De Leon, J.; Borges, C.R. Evaluation of Oxidative Stress in Biological Samples Using the Thiobarbituric Acid Reactive Substances Assay. J. Vis. Exp. 2020. [Google Scholar] [CrossRef]
- Ito, F.; Sono, Y.; Ito, T. Measurement and Clinical Significance of Lipid Peroxidation as a Biomarker of Oxidative Stress: Oxidative Stress in Diabetes, Atherosclerosis, and Chronic Inflammation. Antioxidants 2019, 8, 72. [Google Scholar] [CrossRef]
- Warade, J.P. Malondialdehyde as a Biomarker for Oxidative Stress in Dialysis Patients: A Predictor of Complications and Treatment Efficacy. Ann. Med. Med. Sci. 2025, 4, 517–522. [Google Scholar]
- Gyurászová, M.; Gurecká, R.; Bábíčková, J.; Tóthová, Ľ. Oxidative Stress in the Pathophysiology of Kidney Disease: Implications for Noninvasive Monitoring and Identification of Biomarkers. Oxidative Med. Cell Longev. 2020, 2020, 5478708. [Google Scholar] [CrossRef]
- Himmelfarb, J.; McMonagle, E.; Freedman, S.; Klenzak, J.; McMenamin, E.; Le, P.; Pupim, L.B.; Ikizler, T.A.; The, P.G. Oxidative stress is increased in critically ill patients with acute renal failure. J. Am. Soc. Nephrol. 2004, 15, 2449–2456. [Google Scholar] [CrossRef]
- Modaresi, A.; Nafar, M.; Sahraei, Z. Oxidative Stress in Chronic Kidney Disease. Iran. J. Kidney Dis. 2015, 9, 165–179. [Google Scholar] [PubMed]
- Su, L.-J.; Zhang, J.-H.; Gomez, H.; Murugan, R.; Hong, X.; Xu, D.; Jiang, F.; Peng, Z.-Y. Reactive Oxygen Species-Induced Lipid Peroxidation in Apoptosis, Autophagy, and Ferroptosis. Oxidative Med. Cell. Longev. 2019, 2019, 5080843. [Google Scholar] [CrossRef]
- Mookambika, R.V.; Nitin, R.F. A Retrospective Study on Lipid Profile and Lipid Peroxidation in Chronic Kidney Disease, Focus- Ing on Hemodialysis. Res. J. Med. Sci. 2025, 19, 18–24. [Google Scholar]
- Han, X.; Cai, C.; Xiao, Z.; Quarles, L.D. FGF23 induced left ventricular hypertrophy mediated by FGFR4 signaling in the myocardium is attenuated by soluble Klotho in mice. J. Mol. Cell. Cardiol. 2020, 138, 66–74. [Google Scholar] [CrossRef]
- Faul, C.; Amaral, A.P.; Oskouei, B.; Hu, M.C.; Sloan, A.; Isakova, T.; Gutiérrez, O.M.; Aguillon-Prada, R.; Lincoln, J.; Hare, J.M.; et al. FGF23 induces left ventricular hypertrophy. J. Clin. Investig. 2011, 121, 4393–4408. [Google Scholar] [CrossRef]
- Nakano, T.; Kishimoto, H.; Tokumoto, M. Direct and indirect effects of fibroblast growth factor 23 on the heart. Front. Endocrinol. 2023, 14, 1059179. [Google Scholar] [CrossRef]
- Kitagawa, M.; Sugiyama, H.; Morinaga, H.; Inoue, T.; Takiue, K.; Ogawa, A.; Yamanari, T.; Kikumoto, Y.; Uchida, H.A.; Kitamura, S.; et al. A decreased level of serum soluble Klotho is an independent biomarker associated with arterial stiffness in patients with chronic kidney disease. PLoS ONE 2013, 8, e56695. [Google Scholar] [CrossRef]
- Barreto, F.C.; Barreto, D.V.; Liabeuf, S.; Meert, N.; Glorieux, G.; Temmar, M.; Choukroun, G.; Vanholder, R.; Massy, Z.A. Serum indoxyl sulfate is associated with vascular disease and mortality in chronic kidney disease patients. Clin. J. Am. Soc. Nephrol. 2009, 4, 1551–1558. [Google Scholar] [CrossRef]
- Niwa, T. Indoxyl Sulfate Is a Nephro-Vascular Toxin. J. Ren. Nutr. 2010, 20, S2–S6. [Google Scholar] [CrossRef]
- Hung, S.C.; Kuo, K.L.; Wu, C.C.; Tarng, D.C. Indoxyl Sulfate: A Novel Cardiovascular Risk Factor in Chronic Kidney Disease. J. Am. Heart Assoc. 2017, 6, e005022. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Cao, X.; Zou, J.; Shen, B.; Zhang, X.; Liu, Z.; Lv, W.; Teng, J.; Ding, X. Indoxyl sulfate, a valuable biomarker in chronic kidney disease and dialysis. Hemodial. Int. 2017, 21, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Dopierała, M.; Nitz, N.; Król, O.; Wasicka-Przewoźna, K.; Schwermer, K.; Pawlaczyk, K. New and Emerging Biomarkers in Chronic Kidney Disease. Biomedicines 2025, 13, 1423. [Google Scholar] [CrossRef] [PubMed]
- Scalise, V.; Sanguinetti, C.; Neri, T.; Cianchetti, S.; Lai, M.; Carnicelli, V.; Celi, A.; Pedrinelli, R. PCSK9 Induces Tissue Factor Expression by Activation of TLR4/NFkB Signaling. Int. J. Mol. Sci. 2021, 22, 12640. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Han, W.; Zhang, Y.; Zong, Y.; Tan, N.; Zhang, Y.; Li, L.; Liu, C.; Liu, L. Soluble Epoxide Hydrolase Inhibitor t-AUCB Ameliorates Vascular Endothelial Dysfunction by Influencing the NF-κB/miR-155-5p/eNOS/NO/IκB Cycle in Hypertensive Rats. Antioxidants 2022, 11, 1372. [Google Scholar] [CrossRef]
- Witko-Sarsat, V.; Gausson, V.; Nguyen, A.-T.; Touam, M.; Drüeke, T.; Santangelo, F.; Descamps-Latscha, B. AOPP-induced activation of human neutrophil and monocyte oxidative metabolism: A potential target for N-acetylcysteine treatment in dialysis patients. Kidney Int. 2003, 64, 82–91. [Google Scholar] [CrossRef]
- Signorini, L.; Granata, S.; Lupo, A.; Zaza, G. Naturally Occurring Compounds: New Potential Weapons against Oxidative Stress in Chronic Kidney Disease. Int. J. Mol. Sci. 2017, 18, 1481. [Google Scholar] [CrossRef]
- Dai, S.; Tian, Z.; Zhao, D.; Liang, Y.; Liu, M.; Liu, Z.; Hou, S.; Yang, Y. Effects of Coenzyme Q10 Supplementation on Biomarkers of Oxidative Stress in Adults: A GRADE-Assessed Systematic Review and Updated Meta-Analysis of Randomized Controlled Trials. Antioxidants 2022, 11, 1360. [Google Scholar] [CrossRef] [PubMed]
- Luther, J.M.; Ray, J.; Wei, D.; Koethe, J.R.; Hannah, L.; DeMatteo, A.; Manning, R.; Terker, A.S.; Peng, D.; Nian, H.; et al. GSK2256294 Decreases sEH (Soluble Epoxide Hydrolase) Activity in Plasma, Muscle, and Adipose and Reduces F2-Isoprostanes but Does Not Alter Insulin Sensitivity in Humans. Hypertension 2021, 78, 1092–1102. [Google Scholar] [CrossRef]
- Alobaidi, S. Emerging Biomarkers and Advanced Diagnostics in Chronic Kidney Disease: Early Detection Through Multi-Omics and AI. Diagnostics 2025, 15, 1225. [Google Scholar] [CrossRef]
- Thallas-Bonke, V.; Coughlan, M.; Tan, A.; Harcourt, B.; Morgan, P.; Davies, M.; Cooper, M.; Forbes, J. Targeting the AGE-RAGE axis improves renal function in the context of a healthy diet low in advanced glycation end-product content. Nephrology 2013, 18, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Vlad, C.-E.; Foia, L.; Pavel-Tanasa, M.; Toma, V.; Florea, L.; Voroneanu, L.; Apetrii, M.; Dodi, G.; Covic, A. Evaluation of cardiovascular events and progression to end-stage renal disease in patients with dyslipidemia and chronic kidney disease from the North-Eastern area of Romania. Int. Urol. Nephrol. 2022, 54, 647–659. [Google Scholar] [CrossRef]
- Tu, Q.M.; Jin, H.M.; Yang, X.H. Lipid abnormality in diabetic kidney disease and potential treatment advancements. Front. Endocrinol. 2025, 16, 1503711. [Google Scholar] [CrossRef]
- Camilla, R.; Suzuki, H.; Daprà, V.; Loiacono, E.; Peruzzi, L.; Amore, A.; Ghiggeri, G.M.; Mazzucco, G.; Scolari, F.; Gharavi, A.G.; et al. Oxidative stress and galactose-deficient IgA1 as markers of progression in IgA nephropathy. Clin. J. Am. Soc. Nephrol. 2011, 6, 1903–1911. [Google Scholar] [CrossRef] [PubMed]
- Descamps-Latscha, B.; Witko-Sarsat, V.; Nguyen-Khoa, T.; Nguyen, A.T.; Gausson, V.; Mothu, N.; Cardoso, C.; Noël, L.H.; Guérin, A.P.; London, G.M.; et al. Early prediction of IgA nephropathy progression: Proteinuria and AOPP are strong prognostic markers. Kidney Int. 2004, 66, 1606–1612. [Google Scholar] [CrossRef] [PubMed]
| Biomarker | Main Role | CKD Relevance | Pathway | Clinical Utility |
|---|---|---|---|---|
| PCSK9 | LDL metabolism, inflammation | ↑ in diabetic nephropathy; predicts MACEs | TLR4/NF-κB | Lipid + inflammatory risk stratification |
| EPHX2 | EET degradation, endothelial injury | Correlates with proteinuria, fibrosis | EET/NO signaling | Renal progression marker; targetable |
| AOPP | Protein oxidation, immune activation | High in dialysis; predicts malnutrition, CV risk | RAGE, NOX | Oxidative–inflammatory nexus marker |
| TBARS | Lipid peroxidation | Tracks lipid damage, vascular risk | MDA-DNA/protein adducts | Cardiovascular outcome predictor |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Assani, M.-Z.; Novac, M.B.; Dijmărescu, A.L.; Stroe-Ionescu, A.-Ș.; Boldeanu, M.V.; Siloși, I.; Boldeanu, L. Intersecting Pathways of Inflammation, Oxidative Stress, and Atherogenesis in the Evaluation of CKD: Emerging Biomarkers PCSK9, EPHX2, AOPPs, and TBARSs. Life 2025, 15, 1287. https://doi.org/10.3390/life15081287
Assani M-Z, Novac MB, Dijmărescu AL, Stroe-Ionescu A-Ș, Boldeanu MV, Siloși I, Boldeanu L. Intersecting Pathways of Inflammation, Oxidative Stress, and Atherogenesis in the Evaluation of CKD: Emerging Biomarkers PCSK9, EPHX2, AOPPs, and TBARSs. Life. 2025; 15(8):1287. https://doi.org/10.3390/life15081287
Chicago/Turabian StyleAssani, Mohamed-Zakaria, Marius Bogdan Novac, Anda Lorena Dijmărescu, Alexandra-Ștefania Stroe-Ionescu, Mihail Virgil Boldeanu, Isabela Siloși, and Lidia Boldeanu. 2025. "Intersecting Pathways of Inflammation, Oxidative Stress, and Atherogenesis in the Evaluation of CKD: Emerging Biomarkers PCSK9, EPHX2, AOPPs, and TBARSs" Life 15, no. 8: 1287. https://doi.org/10.3390/life15081287
APA StyleAssani, M.-Z., Novac, M. B., Dijmărescu, A. L., Stroe-Ionescu, A.-Ș., Boldeanu, M. V., Siloși, I., & Boldeanu, L. (2025). Intersecting Pathways of Inflammation, Oxidative Stress, and Atherogenesis in the Evaluation of CKD: Emerging Biomarkers PCSK9, EPHX2, AOPPs, and TBARSs. Life, 15(8), 1287. https://doi.org/10.3390/life15081287










