Possible Role of Novel Mitochondrial Subsets in Migraine
Abstract
1. Introduction
2. Overview of Migraine Models
2.1. Dural Stimulation
2.2. Algogenic Substances
2.2.1. Calcitonin Gene-Related Peptide (CGRP)
2.2.2. Pituitary Adenylate Cyclase-Activating Polypeptide (PACAP)
2.2.3. Cilostazol
2.2.4. Nitroglycerin (NTG)
2.3. Medication Overuse
Triptans and Opioids
2.4. Genetic Models
2.5. Specific Channel Activation
2.5.1. ATP-Sensitive Potassium (KATP) Channels
2.5.2. Transient Receptor Potential Ankyrin 1 (TRPA1)
2.6. Hormone Manipulation
2.7. Optogenic Model
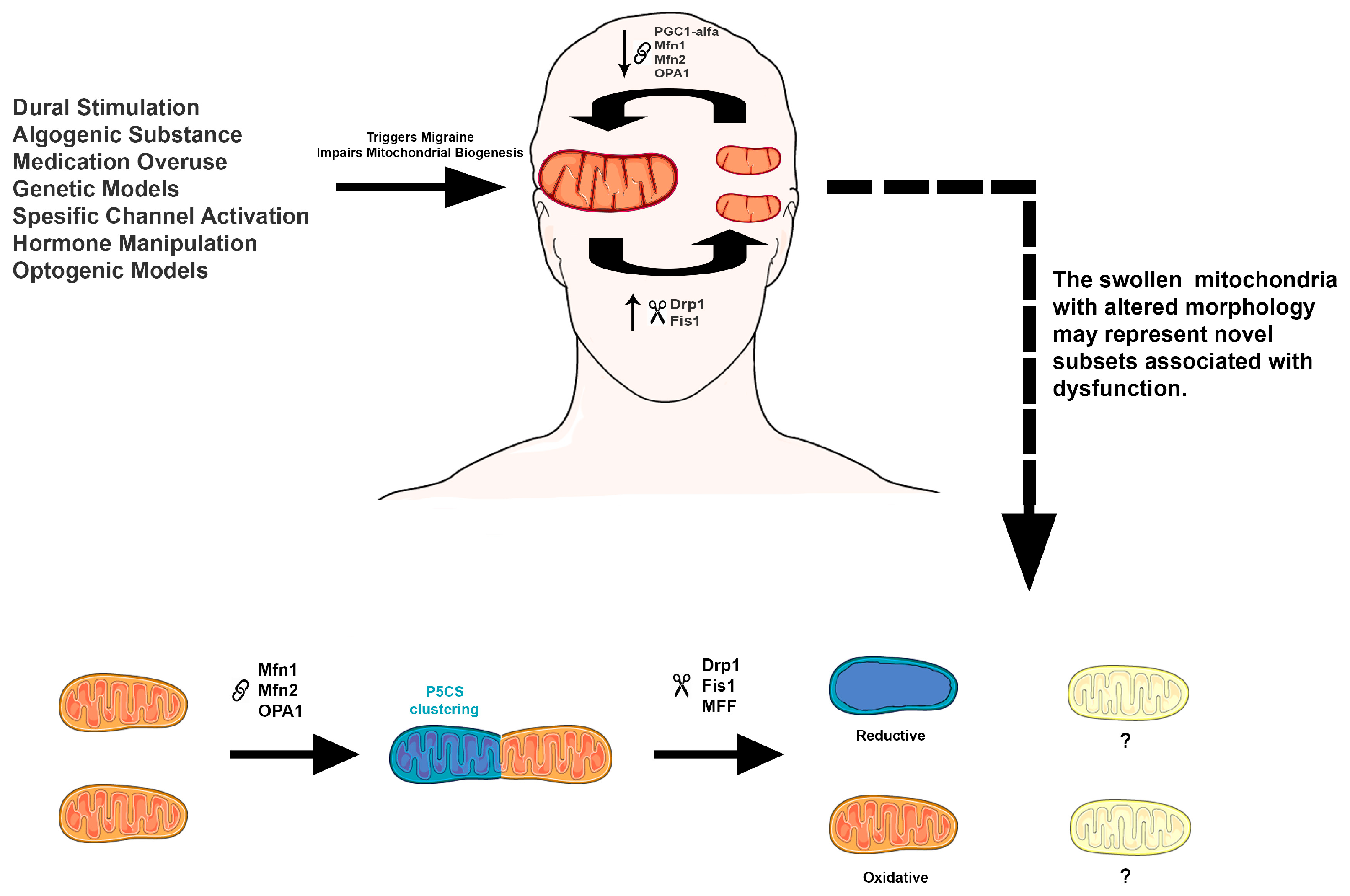
3. Mitochondrial Dysfunction in Migraine
4. Animal Studies Investigating the Role of Mitochondria in Migraine
4.1. Mitochondrial Function in Nitroglycerin (NTG)-Induced Migraine
4.2. Mitochondrial Function in Dural Stimulation-Induced Migraine
4.2.1. KCl-Induced Cortical Spreading Depression (CSD)
4.2.2. Inflammatory Soup-Induced Migraine
4.3. Mitochondrial Function in Pituitary Adenylate Cyclase-Activating Polypeptide (PACAP)-Induced Migraine
4.4. Mitochondrial Function in Genetic Models of Migraine
4.5. Mitochondrial Function Following Facial Capsaicin Application
5. Metabolic Subtypes of Mitochondria
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| ATP | Adenosine Triphosphate |
| cAMP | Cyclic Adenosine Monophosphate |
| Cenpb | Centromere Protein B |
| CGRP | Calcitonin Gene-Related Peptide |
| COX IV | Cytochrome c Oxidase Subunit IV |
| CSD | Cortical Spreading Depression |
| Drp1 | Dynamin-Related Protein 1 |
| ERRs | Estrogen-Related Receptors |
| ETC | Electron Transport Chain |
| FASP | Familial Advanced Sleep Phase |
| Fbl | Fibrillarin |
| Fhl2 | Four and a Half LIM Domains Protein 2 |
| FHM | Familial Hemiplegic Migraine |
| Fis1 | Fission 1 Protein |
| Gnai1 | Guanine Nucleotide-Binding Protein G(I) Subunit Alpha 1 |
| Gnal | Guanine Nucleotide-Binding Protein G(olf) Subunit Alpha |
| Hmga1 | High Mobility Group AT-Hook1 |
| Hsp90aa1 | Heat Shock Protein 90 Alpha Family Class A Member 1 |
| IL-1β | Interleukin-1 Beta |
| IMM | Inner Mitochondrial Membrane |
| KATP | ATP- Sensitive Potassium Channel |
| KCl | Potassium Chloride |
| MA | Migraine with Aura |
| MDA | Malondialdehyde |
| MFF | Mitochondrial Fission Factor |
| MFN1 | Mitofusin 1 |
| MFN2 | Mitofusin 2 |
| Mief1 | Mitochondrial Elongation Factor 1 |
| Mief2 | Mitochondrial Elongation Factor 2 |
| MMP | Mitochondrial Membrane Potential |
| mtDNA | Mitochondrial DNA |
| NADH | Nicotinamide Adenine Dinucleotide (Reduced Form) |
| NF-κB | Nuclear Factor Kappa-Light-Chain-Enhancer of Activated B Cells |
| nNOS | Neural Nitric Oxide Synthase |
| NO | Nitric Oxide |
| NRF1 | Nuclear Respiratory Factor 1 |
| NRF2 | Nuclear Respiratory Factor 2 |
| NSAIDs | Non-Steroidal Anti-Inflammatory Drugs |
| NTG | Nitroglycerin |
| OMM | Outer Mitochondrial Membrane |
| OPA1 | Optic Atrophy 1 Protein |
| OXPHOS | Oxidative Phosphorylation |
| P5CS | Pyrroline-5-Carboxylate Synthase |
| PACAP | Pituitary Adenylate Cyclase-Activating Polypeptide |
| PAG | Periaqueductal Graey |
| PBMC | Peripheral Blood Mononuclear Cells |
| PGC-1α | Peroxisome Proliferator-Activated Receptor Gamma Coactivator 1-Alpha |
| PPARs | Peroxisome Proliferator-Activated Receptors |
| PPARγ | Peroxisome Proliferator-Activated Receptor Gamma |
| RCR | Respiratory Control Ratio |
| ROS | Reactive Oxygen Species |
| RT-PCR | Reverse Transcription Polymerase Chain Reaction |
| SIRT1 | Sirtuin 1 |
| SIRT3 | Sirtuin 3 |
| Slc25a5 | Solute Carrier Family 25 Member 5 |
| ssEM | Serial Section Electron Microscopy |
| TCC | Trigeminocervical Complex |
| TEM | Transmission Electron Microscopy |
| TFAM | Transcription Factor A Mitochondrial |
| TG | Trigeminal Ganglion |
| TNC | Trigeminal Nucleus Caudalis |
| Tomm34 | Translocase of Outer Mitochondrial Membrane 34 |
| Tomm6 | Translocase of Outer Mitochondrial Membrane 6 |
| Tomm70 | Translocase of Outer Mitochondrial Membrane 70 |
| TRPA1 | Transient Receptor Potential Ankyrin 1 |
| TRPV1 | Transient Receptor Potential Vanilloid 1 |
| VDAC1 | Voltage- Dependent Anion Channel 1 |
References
- Ashina, M. Migraine. N. Engl. J. Med. 2020, 383, 1866–1876. [Google Scholar] [CrossRef]
- Linde, M.; Gustavsson, A.; Stovner, L.J.; Steiner, T.J.; Barre, J.; Katsarava, Z.; Lainez, J.M.; Lampl, C.; Lanteri-Minet, M.; Rastenyte, D.; et al. The cost of headache disorders in Europe: The Eurolight project. Eur. J. Neurol. 2012, 19, 703–711. [Google Scholar] [CrossRef]
- Ho, T.W.; Edvinsson, L.; Goadsby, P.J. CGRP and its receptors provide new insights into migraine pathophysiology. Nat. Rev. Neurol. 2010, 6, 573–582. [Google Scholar] [CrossRef] [PubMed]
- Noseda, R.; Burstein, R. Migraine pathophysiology: Anatomy of the trigeminovascular pathway and associated neurological symptoms, cortical spreading depression, sensitization, and modulation of pain. Pain 2013, 154 (Suppl. 1), S44–S53. [Google Scholar] [CrossRef] [PubMed]
- Gross, E.C.; Lisicki, M.; Fischer, D.; Sandor, P.S.; Schoenen, J. The metabolic face of migraine-from pathophysiology to treatment. Nat. Rev. Neurol. 2019, 15, 627–643. [Google Scholar] [CrossRef]
- Ryu, K.W.; Fung, T.S.; Baker, D.C.; Saoi, M.; Park, J.; Febres-Aldana, C.A.; Aly, R.G.; Cui, R.; Sharma, A.; Fu, Y.; et al. Cellular ATP demand creates metabolically distinct subpopulations of mitochondria. Nature 2024, 635, 746–754. [Google Scholar] [CrossRef] [PubMed]
- Chou, T.M.; Chen, S.P. Animal Models of Chronic Migraine. Curr. Pain Headache Rep. 2018, 22, 44. [Google Scholar] [CrossRef]
- Vuralli, D.; Wattiez, A.S.; Russo, A.F.; Bolay, H. Behavioral and cognitive animal models in headache research. J. Headache Pain 2019, 20, 11. [Google Scholar] [CrossRef]
- Lauritzen, M.; Jorgensen, M.B.; Diemer, N.H.; Gjedde, A.; Hansen, A.J. Persistent oligemia of rat cerebral cortex in the wake of spreading depression. Ann. Neurol. 1982, 12, 469–474. [Google Scholar] [CrossRef]
- Bolay, H.; Reuter, U.; Dunn, A.K.; Huang, Z.; Boas, D.A.; Moskowitz, M.A. Intrinsic brain activity triggers trigeminal meningeal afferents in a migraine model. Nat. Med. 2002, 8, 136–142. [Google Scholar] [CrossRef]
- Zhang, X.; Levy, D.; Noseda, R.; Kainz, V.; Jakubowski, M.; Burstein, R. Activation of meningeal nociceptors by cortical spreading depression: Implications for migraine with aura. J. Neurosci. 2010, 30, 8807–8814. [Google Scholar] [CrossRef]
- Fioravanti, B.; Kasasbeh, A.; Edelmayer, R.; Skinner, D.P., Jr.; Hartings, J.A.; Burklund, R.D.; De Felice, M.; French, E.D.; Dussor, G.O.; Dodick, D.W.; et al. Evaluation of cutaneous allodynia following induction of cortical spreading depression in freely moving rats. Cephalalgia 2011, 31, 1090–1100. [Google Scholar] [CrossRef] [PubMed]
- Strassman, A.M.; Raymond, S.A.; Burstein, R. Sensitization of meningeal sensory neurons and the origin of headaches. Nature 1996, 384, 560–564. [Google Scholar] [CrossRef]
- Avona, A.; Burgos-Vega, C.; Burton, M.D.; Akopian, A.N.; Price, T.J.; Dussor, G. Dural Calcitonin Gene-Related Peptide Produces Female-Specific Responses in Rodent Migraine Models. J. Neurosci. 2019, 39, 4323–4331. [Google Scholar] [CrossRef]
- Burgos-Vega, C.C.; Quigley, L.D.; Trevisan Dos Santos, G.; Yan, F.; Asiedu, M.; Jacobs, B.; Motina, M.; Safdar, N.; Yousuf, H.; Avona, A.; et al. Non-invasive dural stimulation in mice: A novel preclinical model of migraine. Cephalalgia 2019, 39, 123–134. [Google Scholar] [CrossRef]
- Wattiez, A.S.; Wang, M.; Russo, A.F. CGRP in Animal Models of Migraine. Handb. Exp. Pharmacol. 2019, 255, 85–107. [Google Scholar] [CrossRef] [PubMed]
- Akerman, S.; Goadsby, P.J. Neuronal PAC1 receptors mediate delayed activation and sensitization of trigeminocervical neurons: Relevance to migraine. Sci. Transl. Med. 2015, 7, 308ra157. [Google Scholar] [CrossRef]
- Waschek, J.A.; Baca, S.M.; Akerman, S. PACAP and migraine headache: Immunomodulation of neural circuits in autonomic ganglia and brain parenchyma. J. Headache Pain 2018, 19, 23. [Google Scholar] [CrossRef]
- Do, T.P.; Deligianni, C.; Amirguliyev, S.; Snellman, J.; Lopez, C.L.; Al-Karagholi, M.A.; Guo, S.; Ashina, M. Second messenger signalling bypasses CGRP receptor blockade to provoke migraine attacks in humans. Brain 2023, 146, 5224–5234. [Google Scholar] [CrossRef] [PubMed]
- Christensen, S.L.; Petersen, S.; Sorensen, D.B.; Olesen, J.; Jansen-Olesen, I. Cilostazol induces C-fos expression in the trigeminal nucleus caudalis and behavioural changes suggestive of headache with the migraine-like feature photophobia in female rats. Cephalalgia 2018, 38, 452–465. [Google Scholar] [CrossRef]
- Demartini, C.; Greco, R.; Zanaboni, A.M.; Sances, G.; De Icco, R.; Borsook, D.; Tassorelli, C. Nitroglycerin as a comparative experimental model of migraine pain: From animal to human and back. Prog. Neurobiol. 2019, 177, 15–32. [Google Scholar] [CrossRef]
- Dagidir, H.G.; Topa, E.; Vuralli, D.; Bolay, H. Medication overuse headache is associated with elevated lipopolysaccharide binding protein and pro-inflammatory molecules in the bloodstream. J. Headache Pain 2023, 24, 150. [Google Scholar] [CrossRef]
- De Felice, M.; Ossipov, M.H.; Porreca, F. Persistent medication-induced neural adaptations, descending facilitation, and medication overuse headache. Curr. Opin. Neurol. 2011, 24, 193–196. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pietrobon, D.; Brennan, K.C. Mechanisms underlying CSD initiation implicated by genetic mouse models of migraine. J. Headache Pain 2025, 26, 17. [Google Scholar] [CrossRef] [PubMed]
- Al-Karagholi, M.A.; Hansen, J.M.; Guo, S.; Olesen, J.; Ashina, M. Opening of ATP-sensitive potassium channels causes migraine attacks: A new target for the treatment of migraine. Brain 2019, 142, 2644–2654. [Google Scholar] [CrossRef] [PubMed]
- Mei, H.R.; Lam, M.; Kulkarni, S.R.; Ashina, H.; Ashina, M.; Dussor, G. Meningeal K ATP channels contribute to behavioral responses in preclinical migraine models. Pain 2025, 166, 398–407. [Google Scholar] [CrossRef]
- Alpay, B.; Cimen, B.; Akaydin, E.; Bolay, H.; Sara, Y. Levcromakalim provokes an acute rapid-onset migraine-like phenotype without inducing cortical spreading depolarization. J. Headache Pain 2023, 24, 93. [Google Scholar] [CrossRef]
- Jiang, L.; Wang, Y.; Xu, Y.; Ma, D.; Wang, M. The Transient Receptor Potential Ankyrin Type 1 Plays a Critical Role in Cortical Spreading Depression. Neuroscience 2018, 382, 23–34. [Google Scholar] [CrossRef]
- Nassini, R.; Materazzi, S.; Vriens, J.; Prenen, J.; Benemei, S.; De Siena, G.; la Marca, G.; Andre, E.; Preti, D.; Avonto, C.; et al. The ‘headache tree’ via umbellulone and TRPA1 activates the trigeminovascular system. Brain 2012, 135, 376–390. [Google Scholar] [CrossRef]
- Sandweiss, A.J.; Cottier, K.E.; McIntosh, M.I.; Dussor, G.; Davis, T.P.; Vanderah, T.W.; Largent-Milnes, T.M. 17-beta-Estradiol induces spreading depression and pain behavior in alert female rats. Oncotarget 2017, 8, 114109–114122. [Google Scholar] [CrossRef]
- Chung, D.Y.; Sadeghian, H.; Qin, T.; Lule, S.; Lee, H.; Karakaya, F.; Goins, S.; Oka, F.; Yaseen, M.A.; Houben, T.; et al. Determinants of Optogenetic Cortical Spreading Depolarizations. Cereb. Cortex 2019, 29, 1150–1161. [Google Scholar] [CrossRef]
- Pieczenik, S.R.; Neustadt, J. Mitochondrial dysfunction and molecular pathways of disease. Exp. Mol. Pathol. 2007, 83, 84–92. [Google Scholar] [CrossRef]
- Tatsuta, T.; Langer, T. Quality control of mitochondria: Protection against neurodegeneration and ageing. EMBO J. 2008, 27, 306–314. [Google Scholar] [CrossRef]
- Scarpulla, R.C.; Vega, R.B.; Kelly, D.P. Transcriptional integration of mitochondrial biogenesis. Trends Endocrinol. Metab. 2012, 23, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Tiehuis, L.H.; Koene, S.; Saris, C.G.J.; Janssen, M.C.H. Mitochondrial migraine; a prevalence, impact and treatment efficacy cohort study. Mitochondrion 2020, 53, 128–132. [Google Scholar] [CrossRef] [PubMed]
- Borkum, J.M. Brain Energy Deficit as a Source of Oxidative Stress in Migraine: A Molecular Basis for Migraine Susceptibility. Neurochem. Res. 2021, 46, 1913–1932. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Guan, X.; Chen, K.; Jin, S.; Wang, C.; Yan, L.; Shi, Z.; Zhang, X.; Chen, L.; Wan, Q. Abnormal mitochondrial dynamics and impaired mitochondrial biogenesis in trigeminal ganglion neurons in a rat model of migraine. Neurosci. Lett. 2017, 636, 127–133. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Yue, G.; Lin, J.; Liu, X.; Wang, L.; Zhao, Y. Effects of ligustrazine on energy metabolism in migraine rats based on mitochondria-inflammation pathway. Neurosci. Lett. 2025, 844, 138035. [Google Scholar] [CrossRef]
- Vafaei, A.; Vafaeian, A.; Iranmehr, A.; Nassireslami, E.; Hasannezhad, B.; Hosseini, Y. Effects of beta-sitosterol on anxiety in migraine-induced rats: The role of oxidative/nitrosative stress and mitochondrial function. CNS Neurosci. Ther. 2024, 30, e14892. [Google Scholar] [CrossRef]
- Xie, W.; Li, R.; Tang, W.; Ma, Z.; Miao, S.; Li, C.; Yang, C.; Li, B.; Wang, T.; Gong, Z.; et al. Proteomics profiling reveals mitochondrial damage in the thalamus in a mouse model of chronic migraine. J. Headache Pain 2023, 24, 122. [Google Scholar] [CrossRef]
- Barbosa, I.R.; Cunha, G.; Luft, C.; Rubensam, G.; Freitas, R.D.S.; Greggio, S.; Venturin, G.; Oliveira, J.R.; Costa, J.C.; Campos, M.M. Fructose supplementation shifts rat brain metabolism in experimental migraine. Brain Res. Bull. 2023, 200, 110694. [Google Scholar] [CrossRef]
- Li, R.; Liu, Y.; Chen, N.; Zhang, Y.; Song, G.; Zhang, Z. Valproate Attenuates Nitroglycerin-Induced Trigeminovascular Activation by Preserving Mitochondrial Function in a Rat Model of Migraine. Med. Sci. Monit. 2016, 22, 3229–3237. [Google Scholar] [CrossRef] [PubMed]
- Sword, J.; Fomitcheva, I.V.; Kirov, S.A. Spreading depolarization causes reversible neuronal mitochondria fragmentation and swelling in healthy, normally perfused neocortex. J. Cereb. Blood Flow Metab. 2024, 44, 1561–1579. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Qiu, E.; Dong, Z.; Liu, R.; Wu, S.; Yu, S. Protection of flunarizine on cerebral mitochondria injury induced by cortical spreading depression under hypoxic conditions. J. Headache Pain 2011, 12, 47–53. [Google Scholar] [CrossRef]
- Shan, Z.; Wang, Y.; Qiu, T.; Zhou, Y.; Zhang, Y.; Hu, L.; Zhang, L.; Liang, J.; Ding, M.; Fan, S.; et al. SS-31 alleviated nociceptive responses and restored mitochondrial function in a headache mouse model via Sirt3/Pgc-1alpha positive feedback loop. J. Headache Pain 2023, 24, 65. [Google Scholar] [CrossRef]
- Liang, J.; Zhou, X.; Wang, J.; Fei, Z.Y.; Qin, G.C.; Zhang, D.K.; Zhou, J.Y.; Chen, L.X. Upregulation of silent information regulator 1 alleviates mitochondrial dysfunction in the trigeminal nucleus caudalis in a rat model of chronic migraine. Neuroreport 2021, 32, 144–156. [Google Scholar] [CrossRef]
- Fried, N.T.; Moffat, C.; Seifert, E.L.; Oshinsky, M.L. Functional mitochondrial analysis in acute brain sections from adult rats reveals mitochondrial dysfunction in a rat model of migraine. Am. J. Physiol. Cell Physiol. 2014, 307, C1017–C1030. [Google Scholar] [CrossRef]
- Takacs-Lovasz, K.; Kun, J.; Aczel, T.; Urban, P.; Gyenesei, A.; Bolcskei, K.; Szoke, E.; Helyes, Z. PACAP-38 Induces Transcriptomic Changes in Rat Trigeminal Ganglion Cells Related to Neuroinflammation and Altered Mitochondrial Function Presumably via PAC1/VPAC2 Receptor-Independent Mechanism. Int. J. Mol. Sci. 2022, 23, 2120. [Google Scholar] [CrossRef]
- Bawa, B.; Abbott, L.C. Analysis of calcium ion homeostasis and mitochondrial function in cerebellar granule cells of adult CaV 2.1 calcium ion channel mutant mice. Neurotox. Res. 2008, 13, 1–18. [Google Scholar] [CrossRef]
- Shibata, M.; Kayama, Y.; Takizawa, T.; Ibata, K.; Shimizu, T.; Yuzaki, M.; Suzuki, N.; Nakahara, J. Resilience to capsaicin-induced mitochondrial damage in trigeminal ganglion neurons. Mol. Pain 2020, 16, 1744806920960856. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Zhao, X.; Shang, W.; Liu, Y.; Ji, J.F.; Liu, J.P.; Tong, C. Pyrroline-5-carboxylate synthase senses cellular stress and modulates metabolism by regulating mitochondrial respiration. Cell Death Differ. 2021, 28, 303–319. [Google Scholar] [CrossRef]
- Ren, C.; Liu, J.; Zhou, J.; Liang, H.; Wang, Y.; Sun, Y.; Ma, B.; Yin, Y. Low levels of serum serotonin and amino acids identified in migraine patients. Biochem. Biophys. Res. Commun. 2018, 496, 267–273. [Google Scholar] [CrossRef]
- Martami, F.; Holton, K.F. Targeting Glutamate Neurotoxicity through Dietary Manipulation: Potential Treatment for Migraine. Nutrients 2023, 15, 3952. [Google Scholar] [CrossRef] [PubMed]
- Rollins, B.; Martin, M.V.; Morgan, L.; Vawter, M.P. Analysis of whole genome biomarker expression in blood and brain. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2010, 153B, 919–936. [Google Scholar] [CrossRef] [PubMed]
- Aczel, T.; Benczik, B.; Agg, B.; Kortesi, T.; Urban, P.; Bauer, W.; Gyenesei, A.; Tuka, B.; Tajti, J.; Ferdinandy, P.; et al. Disease- and headache-specific microRNA signatures and their predicted mRNA targets in peripheral blood mononuclear cells in migraineurs: Role of inflammatory signalling and oxidative stress. J. Headache Pain 2022, 23, 113. [Google Scholar] [CrossRef] [PubMed]
- Aczel, T.; Kortesi, T.; Kun, J.; Urban, P.; Bauer, W.; Herczeg, R.; Farkas, R.; Kovacs, K.; Vasarhelyi, B.; Karvaly, G.B.; et al. Identification of disease- and headache-specific mediators and pathways in migraine using blood transcriptomic and metabolomic analysis. J. Headache Pain 2021, 22, 117. [Google Scholar] [CrossRef]
| Model | Species | Tissues | Key Findings | Research Team (Year) |
|---|---|---|---|---|
| Nitroglycerin | Sprague Dawley rats | Medulla oblongata, trigeminal nucleus caudalis (TNC) | Reduced mitochondrial membrane potential (MMP) Decreased ATP production Increased ROS levels Mitochondrial swelling and cristae disruption | Wang et al. (2025) [38] |
| Wistar rats | Frontal cortex | Elevated oxidative stress Decreased ATP levels | Vafaei et al. (2024) [39] | |
| C57BL/6J mice | Thalamus, hypothalamus, periaqueductal gray (PAG), trigeminal ganglion (TG), trigeminocervical complex (TCC) | Altered Complex I activity Decreased ATP production Increased mitochondrial fragmentation (fission) | Xie et al. (2023) [40] | |
| Wistar rats | Hypothalamus, inferior colliculus | Increased glucose uptake in hypothalamus and inferior colliculus Minor changes in PGC1α levels | Barbosa et al. (2023) [41] | |
| Sprague Dawley rats | Spinal TN | Decreased mtDNA copy number Reduced PGC1α, TFAM, PPARγ Decreased ATP and MMP Increased Bax, decreased Bcl-2 | Li et al. (2016) [42] | |
| KCl-Induced Cortical Spreading Depression | C57BL/6J mice | Brain | Mitochondrial fragmentation Shorter tubular mitochondria Mitochondrial swelling | Sword et al. (2024) [43] |
| Sprague Dawley rats | Cerebral cortex | Decreased state 3 respiration Increased state 4 respiration Lower respiratory control ratio (RCR) | Li et al. (2011) [44] | |
| Inflammatory Soup | C57BL/6J mice | TNC | Reduced MMP and ATP Increased ROS and MDA Decreased PGC1α and TFAM Increased Drp1 and Fis1 (enhanced fission) Smaller, swollen mitochondria with fewer cristae Impaired mitophagy (increased p62, decreased Pink1) | Shan et al. (2023) [45] |
| Sprague Dawley rats | TG | Small, fragmented mitochondria with altered ultrastructure Increased Drp1, decreased Mfn1 Reduced mtDNA, PGC1α, NRF1, NRF2, TFAM mRNA | Dong et al. (2017) [37] | |
| Sprague Dawley rats | TNC | Decreased SIRT1, TFAM, NRF1, NRF2 Reduced ATP and MMP Decreased mtDNA Mitochondrial swelling and disrupted cristae | Liang et al. (2021) [46] | |
| Sprague Dawley rats | TNC | Decreased spare respiratory capacity Reduced oxygen consumption rate | Fried et al. (2014) [47] | |
| PACAP stimulation | Cultured rat TG neurons | TG neurons | Downregulation of Complex I B6 subunit, Fbl, Fhl2, Slc25a5, Tomm6 Upregulation of Cenpb, Gnal, Hsp90aa1, Hmga1, Tomm70, Gnai1, Tomm34 | Takacs-Lovasz et al. (2022) [48] |
| Genetic Model | Cav2.1 transgenic mice | Cerebellar granule cells | Reduced MMP | Bawa and Abbott (2008) [49] |
| Facial Capsaicin Application | C57BL/6 mice | TG neurons | Mitochondrial swelling, cristae loss, reduced mitochondrial number Increased COX IV, Mic60/Mitofilin mRNA, VDAC1 | Shibata et al. (2020) [50] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Savran, O.Y.; Tuncer, M. Possible Role of Novel Mitochondrial Subsets in Migraine. Life 2025, 15, 1273. https://doi.org/10.3390/life15081273
Savran OY, Tuncer M. Possible Role of Novel Mitochondrial Subsets in Migraine. Life. 2025; 15(8):1273. https://doi.org/10.3390/life15081273
Chicago/Turabian StyleSavran, Ozgur Yildirim, and Meltem Tuncer. 2025. "Possible Role of Novel Mitochondrial Subsets in Migraine" Life 15, no. 8: 1273. https://doi.org/10.3390/life15081273
APA StyleSavran, O. Y., & Tuncer, M. (2025). Possible Role of Novel Mitochondrial Subsets in Migraine. Life, 15(8), 1273. https://doi.org/10.3390/life15081273






