Association Between Serum per- and Polyfluoroalkyl Substances and Iron Status Biomarkers in a Representative Sample of U.S. Adults: NHANES 2013–2018
Abstract
1. Introduction
2. Materials and Methods
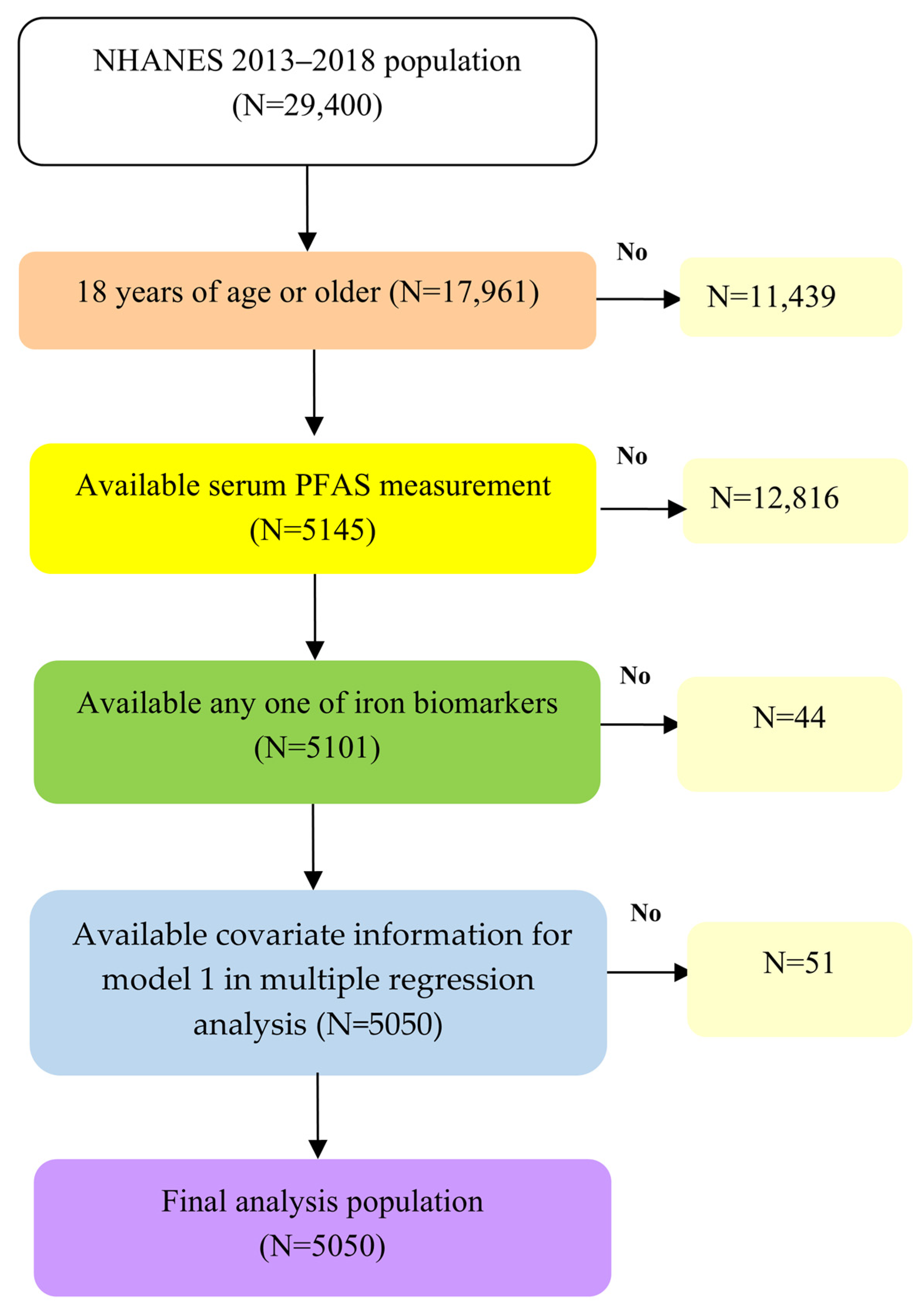
2.1. Study Population
2.2. Measurement of Serum PFAS Levels
2.3. Measurement of Biomarkers of Iron Status
2.4. Covariates
2.5. Statistics
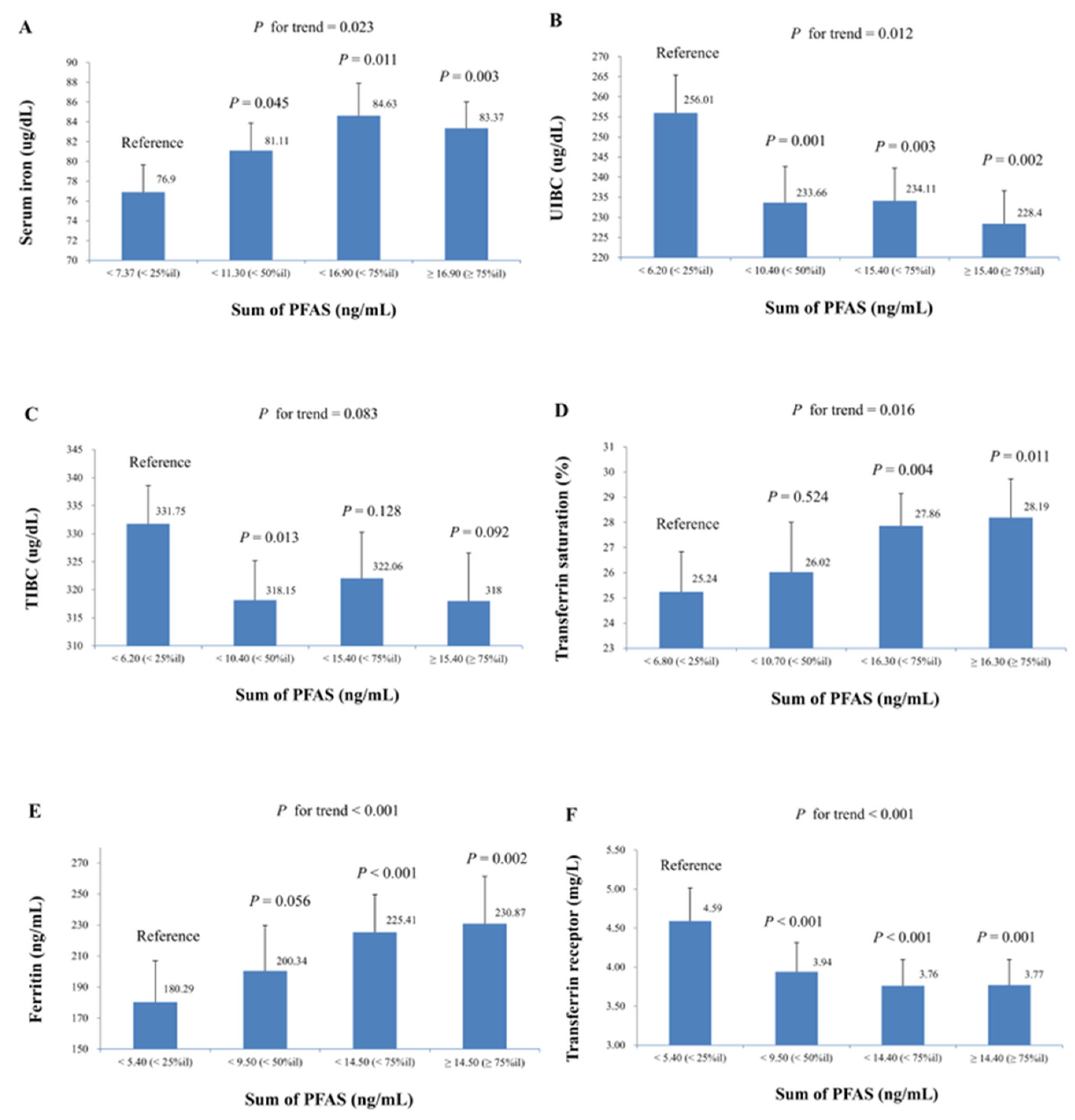
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BMI | Body mass index |
| Hct | Hematocrit |
| Ln | Natural logarithm |
| LOD | Limit of detection |
| NHANES | National Health and Nutrition Examination Survey |
| n-PFOA | n-Perfluorooctanoic acid |
| n-PFOS | n-Perfluorooctane sulfonic acid |
| RDA | Recommended Dietary Allowance |
| PFAS | Per- and polyfluoroalkyl substances |
| PFDeA | Perfluorodecanoic acid |
| PFHxS | Perfluorohexane sulfonic acid |
| PFNA | Perfluorononanoic acid |
| sm-PFOS | Perfluoromethylheptane sulfonic acid isomers |
| TIBC | Total iron-binding capacity |
| UIBC | Unsaturated iron-binding capacity |
References
- Gaines, L.G.T. Historical and current usage of per- and polyfluoroalkyl substances (PFAS): A literature review. Am. J. Ind. Med. 2023, 66, 353–378. [Google Scholar] [CrossRef]
- De Silva, A.O.; Armitage, J.M.; Bruton, T.A.; Dassuncao, C.; Heiger-Bernays, W.; Hu, X.C.; Kärrman, A.; Kelly, B.; Ng, C.; Robuck, A.; et al. PFAS Exposure Pathways for Humans and Wildlife: A Synthesis of Current Knowledge and Key Gaps in Understanding. Environ. Toxicol. Chem. 2021, 40, 631–657. [Google Scholar] [CrossRef] [PubMed]
- Haug, M.; Dunder, L.; Lind, P.M.; Lind, L.; Salihovic, S. Associations of perfluoroalkyl substances (PFAS) with lipid and lipoprotein profiles. J. Expo. Sci. Environ. Epidemiol. 2023, 33, 757–765. [Google Scholar] [CrossRef] [PubMed]
- Ojo, A.F.; Peng, C.; Ng, J.C. Assessing the human health risks of per- and polyfluoroalkyl substances: A need for greater focus on their interactions as mixtures. J. Hazard. Mater. 2021, 407, 124863. [Google Scholar] [CrossRef]
- Klingelhöfer, D.; Braun, M.; Groneberg, D.A.; Brüggmann, D. The “forever” per- and polyfluoroalkyl substances (PFAS): A critical accounting of global research on a major threat under changing regulations. Chemosphere 2024, 354, 141694. [Google Scholar] [CrossRef]
- Seo, S.-H.; Son, M.-H.; Choi, S.-D.; Lee, D.-H.; Chang, Y.-S. Influence of exposure to perfluoroalkyl substances (PFASs) on the Korean general population: 10-year trend and health effects. Environ. Int. 2018, 113, 149–161. [Google Scholar] [CrossRef]
- Hull, S.D.; Deen, L.; Petersen, K.U.; Jensen, T.K.; Hammer, P.; Wils, R.S.; Frankel, H.N.; Ostrowski, S.R.; Tøttenborg, S.S. Time trends in per- and polyfluoroalkyl substances (PFAS) concentrations in the Danish population: A review based on published and newly analyzed data. Environ. Res. 2023, 237, 117036. [Google Scholar] [CrossRef] [PubMed]
- Sonnenberg, N.K.; Ojewole, A.E.; Ojewole, C.O.; Lucky, O.P.; Kusi, J. Trends in Serum Per- and Polyfluoroalkyl Substance (PFAS) Concentrations in Teenagers and Adults, 1999–2018 NHANES. Int. J. Environ. Res. Public Health 2023, 20, 6984. [Google Scholar] [CrossRef]
- Galy, B.; Conrad, M.; Muckenthaler, M. Mechanisms controlling cellular and systemic iron homeostasis. Nat. Rev. Mol. Cell Biol. 2024, 25, 133–155. [Google Scholar] [CrossRef]
- Katsarou, A.; Pantopoulos, K. Basics and principles of cellular and systemic iron homeostasis. Mol. Asp. Med. 2020, 75, 100866. [Google Scholar] [CrossRef]
- Wang, W.; Knovich, M.A.; Coffman, L.G.; Torti, F.M.; Torti, S.V. Serum ferritin: Past, present and future. Biochim. Biophys. Acta 2010, 1800, 760–769. [Google Scholar] [CrossRef] [PubMed]
- Fisher, A.L.; Wang, C.Y.; Xu, Y.; Joachim, K.; Xiao, X.; Phillips, S.; Moschetta, G.A.; Alfaro-Magallanes, V.M.; Babitt, J.L. Functional role of endothelial transferrin receptor 1 in iron sensing and homeostasis. Am. J. Hematol. 2022, 97, 1548–1559. [Google Scholar] [CrossRef]
- Yang, L.; Chen, Y.; Ji, H.; Zhang, X.; Zhou, Y.; Li, J.; Wang, Y.; Xie, Z.; Yuan, W.; Liang, H.; et al. Per- and Poly-fluoroalkyl Substances and Bile Acid Profiles in Pregnant Women. Environ. Sci. Technol. 2023, 57, 15869–15881. [Google Scholar] [CrossRef]
- Sen, P.; Qadri, S.; Luukkonen, P.K.; Ragnarsdottir, O.; McGlinchey, A.; Jäntti, S.; Juuti, A.; Arola, J.; Schlezinger, J.J.; Webster, T.F.; et al. Exposure to environmental contaminants is associated with altered hepatic lipid metabolism in non-alcoholic fatty liver disease. J. Hepatol. 2022, 76, 283–293. [Google Scholar] [CrossRef]
- Li, J.; Wang, L.; Zhang, X.; Liu, P.; Deji, Z.; Xing, Y.; Zhou, Y.; Lin, X.; Huang, Z. Per- and polyfluoroalkyl substances exposure and its influence on the intestinal barrier: An overview on the advances. Sci. Total Environ. 2022, 852, 158362. [Google Scholar] [CrossRef]
- Malesza, I.J.; Bartkowiak-Wieczorek, J.; Winkler-Galicki, J.; Nowicka, A.; Dzięciołowska, D.; Błaszczyk, M.; Gajniak, P.; Słowińska, K.; Niepolski, L.; Walkowiak, J.; et al. The Dark Side of Iron: The Relationship between Iron, Inflammation and Gut Microbiota in Selected Diseases Associated with Iron Deficiency Anaemia-A Narrative Review. Nutrients 2022, 14, 3478. [Google Scholar] [CrossRef]
- Axling, U.; Önning, G.; Combs, M.A.; Bogale, A.; Högström, M.; Svensson, M. The Effect of Lactobacillus plantarum 299v on Iron Status and Physical Performance in Female Iron-Deficient Athletes: A Randomized Controlled Trial. Nutrients 2020, 12, 1279. [Google Scholar] [CrossRef] [PubMed]
- Omoike, O.E.; Pack, R.P.; Mamudu, H.M.; Liu, Y.; Strasser, S.; Zheng, S.; Okoro, J.; Wang, L. Association between per and polyfluoroalkyl substances and markers of inflammation and oxidative stress. Environ. Res. 2021, 196, 110361. [Google Scholar] [CrossRef]
- Zhou, R.; Peng, J.; Zhang, L.; Sun, Y.; Yan, J.; Jiang, H. Association between the dietary inflammatory index and serum perfluoroalkyl and polyfluoroalkyl substance concentrations: Evidence from NANHES 2007–2018. Food Funct. 2024, 15, 7375. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.Y.; Lee, H.L.; Wang, C.; Sung, F.C.; Su, T.C. Examining the impact of polyfluoroalkyl substance exposure on erythrocyte profiles and its related nutrients: Insights from a prospective study on young Taiwanese. Environ. Pollut. 2024, 359, 124576. [Google Scholar] [CrossRef]
- CDC. NHANES 2017-2018. Available online: https://wwwn.cdc.gov/nchs/nhanes/continuousnhanes/default.aspx?BeginYear=2017 (accessed on 18 May 2024).
- CDC. Online Solid Phase Extraction-High Performance Liquid Chromatography-Turbo Ion Spray-Tandem Mass Spectrometry (Online SPE-HPLC-TIS-MS/MS). Available online: https://wwwn.cdc.gov/nchs/data/nhanes/public/2017/labmethods/PFAS-J-MET-508.pdf (accessed on 6 August 2025).
- CDC. Standard Biochemistry Profile. Available online: https://wwwn.cdc.gov/Nchs/Data/Nhanes/Public/2017/DataFiles/BIOPRO_J.htm (accessed on 6 August 2025).
- CDC. Iron Status-Serum. Available online: https://wwwn.cdc.gov/Nchs/Data/Nhanes/Public/2017/DataFiles/FETIB_J.htm (accessed on 6 August 2025).
- CDC. Ferritin. Available online: https://wwwn.cdc.gov/Nchs/Data/Nhanes/Public/2017/DataFiles/FERTIN_J.htm (accessed on 6 August 2025).
- CDC. Transferrin Receptor. Available online: https://wwwn.cdc.gov/Nchs/Data/Nhanes/Public/2017/DataFiles/TFR_J.htm (accessed on 6 August 2025).
- CDC. National Health and Nutrition Examination Survey (NHANES): Smoking. Available online: http://wwwn.cdc.gov/nchs/nhanes/search/DataPage.aspx?Component=Questionnaire&CycleBeginYear=2013 (accessed on 27 July 2024).
- CDC. 2017–2018 Dietary Data-Continuous NHANES. Available online: https://wwwn.cdc.gov/nchs/nhanes/search/datapage.aspx?Component=Dietary&CycleBeginYear=2017 (accessed on 18 May 2024).
- Lin, C.Y.; Lee, H.L.; Chen, C.W.; Wang, C.; Sung, F.C.; Su, T.C. Global DNA methylation mediates the association between serum perfluorooctane sulfonate and carotid intima-media thickness in young and middle-aged Taiwanese populations. Ecotoxicol. Environ. Saf. 2022, 241, 113782. [Google Scholar] [CrossRef]
- Yan, M.T.; Chao, C.T.; Lin, S.H. Chronic Kidney Disease: Strategies to Retard Progression. Int. J. Mol. Sci. 2021, 22, 10084. [Google Scholar] [CrossRef]
- CDC. Analytic and Reporting Guidelines: The National Health and Nutrition Examination Survey (NHANES). Available online: https://wwwn.cdc.gov/nchs/data/nhanes/analyticguidelines/11-16-analytic-guidelines.pdf (accessed on 6 August 2025).
- NIH. Iron. Available online: https://ods.od.nih.gov/factsheets/Iron-Consumer/ (accessed on 23 June 2024).
- Brennan, N.M.; Evans, A.T.; Fritz, M.K.; Peak, S.A.; von Holst, H.E. Trends in the Regulation of Per- and Polyfluoroalkyl Substances (PFAS): A Scoping Review. Int. J. Environ. Res. Public Health 2021, 18, 10900. [Google Scholar] [CrossRef]
- Sun, H.; Weaver, C.M. Decreased Iron Intake Parallels Rising Iron Deficiency Anemia and Related Mortality Rates in the US Population. J. Nutr. 2021, 151, 1947–1955. [Google Scholar] [CrossRef]
- Kim, D.-H.; Lee, J.-H.; Oh, J.-E. Assessment of individual-based perfluoroalkly substances exposure by multiple human exposure sources. J. Hazard. Mater. 2019, 365, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Wieringa, F.T.; Dijkhuizen, M.A.; Berger, J. Sources of Iron: Diet, Supplemental, and Environmental. In Nutritional Anemia; Karakochuk, C.D., Zimmermann, M.B., Moretti, D., Kraemer, K., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 127–140. [Google Scholar]
- Dignass, A.; Farrag, K.; Stein, J. Limitations of Serum Ferritin in Diagnosing Iron Deficiency in Inflammatory Conditions. Int. J. Chronic Dis. 2018, 2018, 9394060. [Google Scholar] [CrossRef] [PubMed]
- Cui, F.; Liu, H.; Li, Y.; Zheng, T.Z.; Xu, S.; Xia, W.; Sheng, X. Association of exposure to per- and polyfluoroalkyl substances with hemoglobin and hematocrit during pregnancy. Ecotoxicol. Environ. Saf. 2022, 248, 114319. [Google Scholar] [CrossRef]
- Zeidan, R.S.; Martenson, M.; Tamargo, J.A.; McLaren, C.; Ezzati, A.; Lin, Y.; Yang, J.J.; Yoon, H.-S.; McElroy, T.; Collins, J.F.; et al. Iron homeostasis in older adults: Balancing nutritional requirements and health risks. J. Nutr. Health Aging 2024, 28, 100212. [Google Scholar] [CrossRef]
- Botta, A.; Barra, N.G.; Lam, N.H.; Chow, S.; Pantopoulos, K.; Schertzer, J.D.; Sweeney, G. Iron Reshapes the Gut Microbiome and Host Metabolism. J. Lipid Atheroscler. 2021, 10, 160–183. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhang, L.; Li, Q.; Wang, P.; Wang, H.; Shi, H.; Lu, W.; Zhang, Y. Prenatal PFAS exposure, gut microbiota dysbiosis, and neurobehavioral development in childhood. J. Hazard. Mater. 2024, 469, 133920. [Google Scholar] [CrossRef]
- Lamichhane, S.; Härkönen, T.; Vatanen, T.; Hyötyläinen, T.; Knip, M.; Orešič, M. Impact of exposure to per- and polyfluoroalkyl substances on fecal microbiota composition in mother-infant dyads. Environ. Int. 2023, 176, 107965. [Google Scholar] [CrossRef]
- Sen, P.; Fan, Y.; Schlezinger, J.J.; Ehrlich, S.D.; Webster, T.F.; Hyötyläinen, T.; Pedersen, O.; Orešič, M. Exposure to environmental toxicants is associated with gut microbiome dysbiosis, insulin resistance and obesity. Environ. Int. 2024, 186, 108569. [Google Scholar] [CrossRef] [PubMed]
- Xing, J.; Wang, F.; Xu, Q.; Yin, B.; Fang, D.; Zhao, J.; Zhang, H.; Chen, Y.Q.; Wang, G.; Chen, W. Screening of potential probiotic lactic acid bacteria based on gastrointestinal properties and perfluorooctanoate toxicity. Appl. Microbiol. Biotechnol. 2016, 100, 6755–6766. [Google Scholar] [CrossRef]
- Di Vincenzo, F.; Del Gaudio, A.; Petito, V.; Lopetuso, L.R.; Scaldaferri, F. Gut microbiota, intestinal permeability, and systemic inflammation: A narrative review. Intern. Emerg. Med. 2024, 19, 275–293. [Google Scholar] [CrossRef]
- Rickard, B.P.; Rizvi, I.; Fenton, S.E. Per- and poly-fluoroalkyl substances (PFAS) and female reproductive outcomes: PFAS elimination, endocrine-mediated effects, and disease. Toxicology 2022, 465, 153031. [Google Scholar] [CrossRef]
- Roth, K.; Yang, Z.; Agarwal, M.; Liu, W.; Peng, Z.; Long, Z.; Birbeck, J.; Westrick, J.; Liu, W.; Petriello, M.C. Exposure to a mixture of legacy, alternative, and replacement per- and polyfluoroalkyl substances (PFAS) results in sex-dependent modulation of cholesterol metabolism and liver injury. Environ. Int. 2021, 157, 106843. [Google Scholar] [CrossRef]
- Camaschella, C.; Nai, A.; Silvestri, L. Iron metabolism and iron disorders revisited in the hepcidin era. Haematologica 2020, 105, 260–272. [Google Scholar] [CrossRef] [PubMed]
- Pagani, A.; Nai, A.; Silvestri, L.; Camaschella, C. Hepcidin and Anemia: A Tight Relationship. Front. Physiol. 2019, 10, 1249. [Google Scholar] [CrossRef] [PubMed]
| Variables | N | % |
|---|---|---|
| Age (year) | ||
| 18–39 | 1798 | 35.6 |
| 40–59 | 1584 | 31.4 |
| ≥60 | 1668 | 33.0 |
| Men | 2416 | 47.8 |
| Ethnicity | ||
| Mexican-American | 771 | 15.3 |
| Other Hispanic | 536 | 10.6 |
| Non-Hispanic white | 1852 | 36.7 |
| Non-Hispanic black | 1080 | 21.4 |
| Non-Hispanic Asian | 599 | 11.9 |
| Other ethnicity | 212 | 4.2 |
| Smoking status | ||
| Active smokers | 1163 | 23.0 |
| Environmental tobacco smoke | 802 | 15.9 |
| Non-smokers | 3085 | 61.1 |
| ≥12 alcoholic drinks/year | 2733 | 54.1 |
| Diabetes Mellitus | 970 | 19.2 |
| Chronic renal failure | 269 | 5.3 |
| Body mass index | 3598 | 71.2 |
| <25 | 1452 | 28.8 |
| 25–29 | 1594 | 31.2 |
| ≥30 | 2004 | 40.0 |
| Variables | N | Mean ± SD | Geometric Mean | Minimum | Maximum |
|---|---|---|---|---|---|
| PFAS (ng/mL) | |||||
| n-PFOA | 5050 | 1.98 ± 2.36 | 1.49 | 0.07 | 85.20 |
| n-PFOS | 5050 | 5.74 ± 19.22 | 3.51 | 0.07 | 1270.00 |
| sm-PFOS | 5050 | 2.05 ± 2.62 | 1.38 | 0.07 | 133.00 |
| PFNA | 5050 | 0.77 ± 0.73 | 0.57 | 0.07 | 16.30 |
| PFHxS | 5050 | 1.80 ± 2.18 | 1.19 | 0.07 | 48.80 |
| PFDeA | 5050 | 0.30 ± 0.86 | 0.19 | 0.07 | 51.30 |
| Oral iron intake (mg/day) | 4031 | 17.18 ± 14.78 | 14.18 | 0.91 | 369.42 |
| Iron status biomarkers | |||||
| Serum iron (ug/dL) | 5044 | 84 ± 36 | 76 | 7 | 325 |
| UIBC (ug/dL) | 1670 | 236 ± 65 | 227 | 12 | 540 |
| TIBC (ug/dL) | 1669 | 324 ± 53 | 320 | 169 | 605 |
| Transferrin saturation (%) | 1669 | 27.63 ± 11.96 | 25 | 3 | 92 |
| Ferritin (ng/mL) | 2147 | 131.7 ± 160.6 | 77.1 | 1.8 | 2030.0 |
| Transferrin receptor (mg/L) | 2139 | 3.5 ± 2.2 | 3.2 | 1.2 | 39.5 |
| PFAS (ng/mL) | n-PFOA | n-PFOS | sm-PFOS | PFNA | PFHxS | PFDeA | Sum of PFAS | ||
|---|---|---|---|---|---|---|---|---|---|
| N | Adj. β (SE) | Adj. β (SE) | Adj. β (SE) | Adj. β (SE) | Adj. β (SE) | Adj. β (SE) | Adj. β (SE) | ||
| Oral iron intake (mg/day) | Model 1 | 4031 | −1.07 (0.33) | −1.54 (0.41) | −1.67 (0.42) | −1.72 (0.45) | −1.05 (0.36) | −1.33 (0.44) | −1.77 (0.46) |
| p | 0.002 | 0.001 | <0.001 | <0.001 | 0.006 | 0.004 | <0.001 | ||
| Serum iron (ug/dL) | Model 1 | 5044 | 5.66 (0.92) | 3.94 (0.96) | 4.69 (1.12) | 3.50 (1.15) | 4.05 (0.92) | 4.28 (0.91) | 5.79 (1.11) |
| p | <0.001 | <0.001 | <0.001 | 0.004 | <0.001 | <0.001 | <0.001 | ||
| Model 2 | 4026 | 4.36 (0.99) | 3.39 (1.03) | 3.80 (1.16) | 2.88 (1.13) | 3.02 (0.98) | 3.90 (1.10) | 4.91 (1.18) | |
| p | <0.001 | 0.002 | 0.002 | 0.014 | 0.004 | 0.001 | <0.001 | ||
| UIBC (ug/dL) | Model 1 | 1670 | −12.11 (2.42) | −13.47 (2.39) | −14.57 (2.66) | −9.58 (3.05) | −11.98 (2.67) | −12.78 (2.30) | −16.36 (2.69) |
| p | <0.001 | <0.001 | <0.001 | 0.007 | <0.001 | <0.001 | <0.001 | ||
| Model 2 | 1332 | −12.47 (2.55) | −14.58 (3.37) | −15.23 (2.99) | −10.94 (3.26) | −11.45 (2.74) | −13.45 (2.94) | −17.83 (3.36) | |
| p | <0.001 | 0.001 | <0.001 | 0.004 | 0.001 | <0.001 | <0.001 | ||
| TIBC (ug/dL) | Model 1 | 1669 | −4.00 (2.66) | −7.92 (1.89) | −8.94 (1.69) | −5.72 (2.92) | −7.22 (1.85) | −6.32 (2.80) | −8.96 (2.28) |
| p | 0.154 | 0.001 | <0.001 | 0.069 | 0.001 | 0.039 | 0.001 | ||
| Model 2 | 1331 | −4.23 (2.74) | −8.25 (2.48) | −9.70 (1.94) | −6.08 (3.13) | −6.85 (2.00) | −5.95 (3.40) | −9.66 (2.52) | |
| p | 0.143 | 0.005 | <0.001 | 0.071 | 0.004 | 0.101 | 0.002 | ||
| Transferrin saturation (%) | Model 1 | 1669 | 2.55 (0.33) | 2.13 (0.46) | 2.19 (0.63) | 1.44 (0.42) | 1.84 (0.47) | 2.34 (0.30) | 2.70 (0.52) |
| p | <0.001 | <0.001 | 0.003 | 0.004 | 0.001 | <0.001 | <0.001 | ||
| Model 2 | 1331 | 2.57 (0.39) | 2.31 (0.57) | 2.20 (0.69) | 1.75 (0.43) | 1.75 (0.52) | 2.54 (0.38) | 2.90 (0.64) | |
| p | <0.001 | 0.001 | 0.006 | 0.001 | 0.004 | <0.001 | <0.001 | ||
| Ferritin (ng/mL) | Model 1 | 2147 | 11.15 (6.05) | 14.51 (5.10) | 16.70 (4.49) | 15.80 (3.98) | 12.61 (5.26) | 16.45 (3.55) | 18.74 (6.22) |
| p | 0.075 | 0.008 | 0.001 | <0.001 | 0.023 | <0.001 | 0.005 | ||
| Model 2 | 1695 | 12.62 (7.13) | 15.40 (6.33) | 17.64 (4.93) | 16.97 (5.53) | 13.27 (5.21) | 15.56 (4.60) | 20.19 (6.87) | |
| p | 0.087 | 0.021 | 0.001 | 0.005 | 0.016 | 0.002 | 0.006 | ||
| Transferrin receptor (mg/L) | Model 1 | 2139 | −0.59 (0.17) | −0.52 (0.17) | −0.62 (0.17) | −0.32 (0.13) | −0.44 (0.12) | −0.30 (0.08) | −0.58 (0.13) |
| p | 0.001 | 0.004 | 0.001 | 0.016 | 0.001 | 0.001 | <0.001 | ||
| Model 2 | 1688 | −0.42 (0.10) | −0.45 (0.10) | −0.52 (0.13) | −0.28 (0.10) | −0.45 (0.11) | −0.30 (0.07) | −0.57 (0.12) | |
| p | <0.001 | <0.001 | <0.001 | 0.008 | <0.001 | <0.001 | <0.001 | ||
| Serum Iron (ug/dL) | UIBC (ug/dL) | TIBC (ug/dL) | Transferrin Saturation (%) | Ferritin (ng/mL) | Transferrin Receptor (mg/L) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adj. β(SE) | p | Adj. β(SE) | p | Adj. β(SE) | p | Adj. β(SE) | p | Adj. β(SE) | p | Adj. β(SE) | p | |
| Gender | ||||||||||||
| Men | 4.07 (2.11) | 0.060 | −13.71 (4.98) | 0.015 | −3.53 (3.59) | 0.341 | 3.08 (1.08) | 0.012 | 20.50 (15.29) | 0.200 | −0.23 (0.09) | 0.026 |
| Women | 5.37 (1.32) | <0.001 | −20.06 (3.66) | <0.001 | −13.9 (3.67) | 0.002 | 2.63 (0.71) | 0.002 | 18.81 (7.39) | 0.016 | −0.74 (0.16) | <0.001 |
| Age, years | ||||||||||||
| 18–40 | 7.79 (1.75) | <0.001 | −20.92 (6.70) | 0.007 | −10.15 (4.54) | 0.041 | 3.80 (1.21) | 0.007 | 14.94 (8.00) | 0.072 | −0.51 (0.10) | <0.001 |
| 40–59 | 4.16 (2.42) | 0.093 | −14.53 (5.23) | 0.014 | −8.51 (4.01) | 0.051 | 2,12 (1.16) | 0.088 | 42.68 (7.91) | <0.001 | −0.87 (0.28) | 0.004 |
| ≥60 | 2.32 (1.72) | 0.184 | −12.81 (3.77) | 0.004 | −6.19 (3.76) | 0.121 | 2.43 (0.90) | 0.016 | −7.04 (17.76) | 0.697 | −0.17 (0.10) | 0.114 |
| Ethnicity | ||||||||||||
| Non-Hispanic white | 3.93 (1.67) | 0.023 | −14.41 (4.59) | 0.007 | −7.69 (4.11) | 0.081 | 2.24 (0.80) | 0.014 | 18.21 (10.04) | 0.081 | −0.29 (0.10) | 0.009 |
| Other | 6.63 (1.33) | <0.001 | −22.94 (3.36) | <0.001 | −12.10 (1.87) | <0.001 | 3.96 (0.80) | <0.001 | 19.08 (12.24) | 0.129 | −0.93 (0.19) | <0.001 |
| BMI (Kg/m2) | ||||||||||||
| <25 | 6.78 (2.40) | 0.007 | −17.98 (6.38) | 0.013 | −5.42 (5.12) | 0.306 | 4.15 (1.17) | 0.003 | 27.11 (11.25) | 0.023 | −0.47 (0.10) | <0.001 |
| ≥25 | 4.05 (1.40) | 0.006 | −16.39 (4.13) | 0.001 | −10.40 (2.63) | 0.001 | 2.23 (0.94) | 0.031 | 16.62 (8.67) | 0.065 | −0.58 (0.16) | 0.001 |
| Total iron intake (mg/day) | ||||||||||||
| <13.94 | 7.07 (1.59) | <0.001 | −21.18 (3.87) | <0.001 | −7.68 (3.99) | 0.073 | 4.44 (0.46) | <0.001 | 22.35 (11.27) | 0.056 | −0.61 (0.09) | <0.001 |
| ≥13.94 | 2.90 (1.73) | 0.010 | −13.99 (5.85) | 0.030 | −11.34 (4.72) | 0.029 | 1.23 (1.07) | 0.269 | 23.04 (8.74) | 0.013 | −0.53 (0.21) | 0.014 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.-J.; Lin, Y.-L.; Su, T.-C.; Wang, C.; Lin, C.-Y. Association Between Serum per- and Polyfluoroalkyl Substances and Iron Status Biomarkers in a Representative Sample of U.S. Adults: NHANES 2013–2018. Life 2025, 15, 1274. https://doi.org/10.3390/life15081274
Wang W-J, Lin Y-L, Su T-C, Wang C, Lin C-Y. Association Between Serum per- and Polyfluoroalkyl Substances and Iron Status Biomarkers in a Representative Sample of U.S. Adults: NHANES 2013–2018. Life. 2025; 15(8):1274. https://doi.org/10.3390/life15081274
Chicago/Turabian StyleWang, Wei-Jie, Yu-Ling Lin, Ta-Chen Su, Chikang Wang, and Chien-Yu Lin. 2025. "Association Between Serum per- and Polyfluoroalkyl Substances and Iron Status Biomarkers in a Representative Sample of U.S. Adults: NHANES 2013–2018" Life 15, no. 8: 1274. https://doi.org/10.3390/life15081274
APA StyleWang, W.-J., Lin, Y.-L., Su, T.-C., Wang, C., & Lin, C.-Y. (2025). Association Between Serum per- and Polyfluoroalkyl Substances and Iron Status Biomarkers in a Representative Sample of U.S. Adults: NHANES 2013–2018. Life, 15(8), 1274. https://doi.org/10.3390/life15081274






