Morphological and Functional Analysis of Residual Lung After Pneumonectomy in Lung Cancer Surgery via 3D-CT Method
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Study Protocol
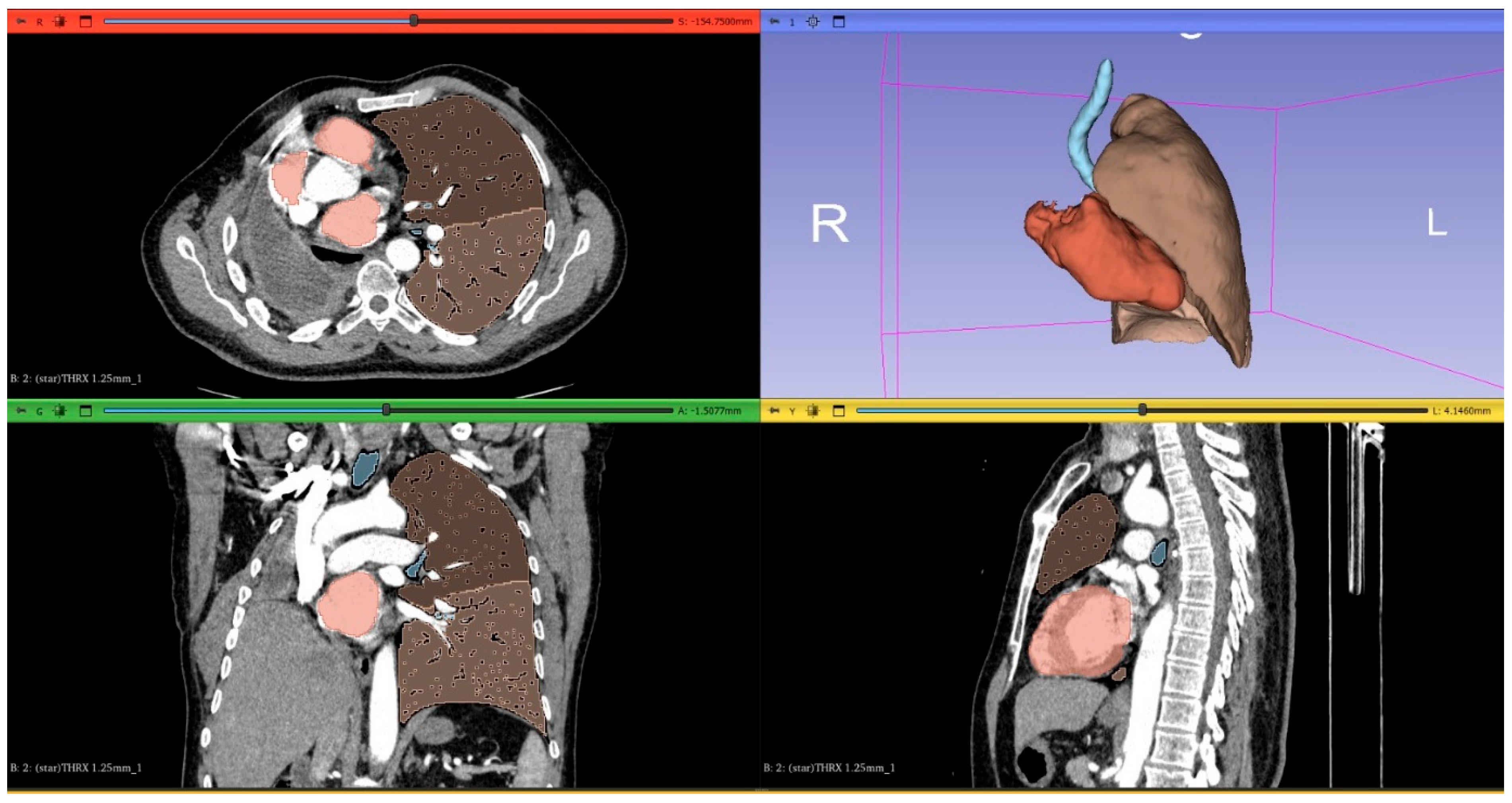
2.2. Computed Tomography Image Acquisition and Postprocessing
2.3. Prediction of Postoperative Lung Function
2.4. Statistical Analysis
3. Results
Postoperative Residual Lung Expansion and Patient Characteristics
4. Discussion
- (a)
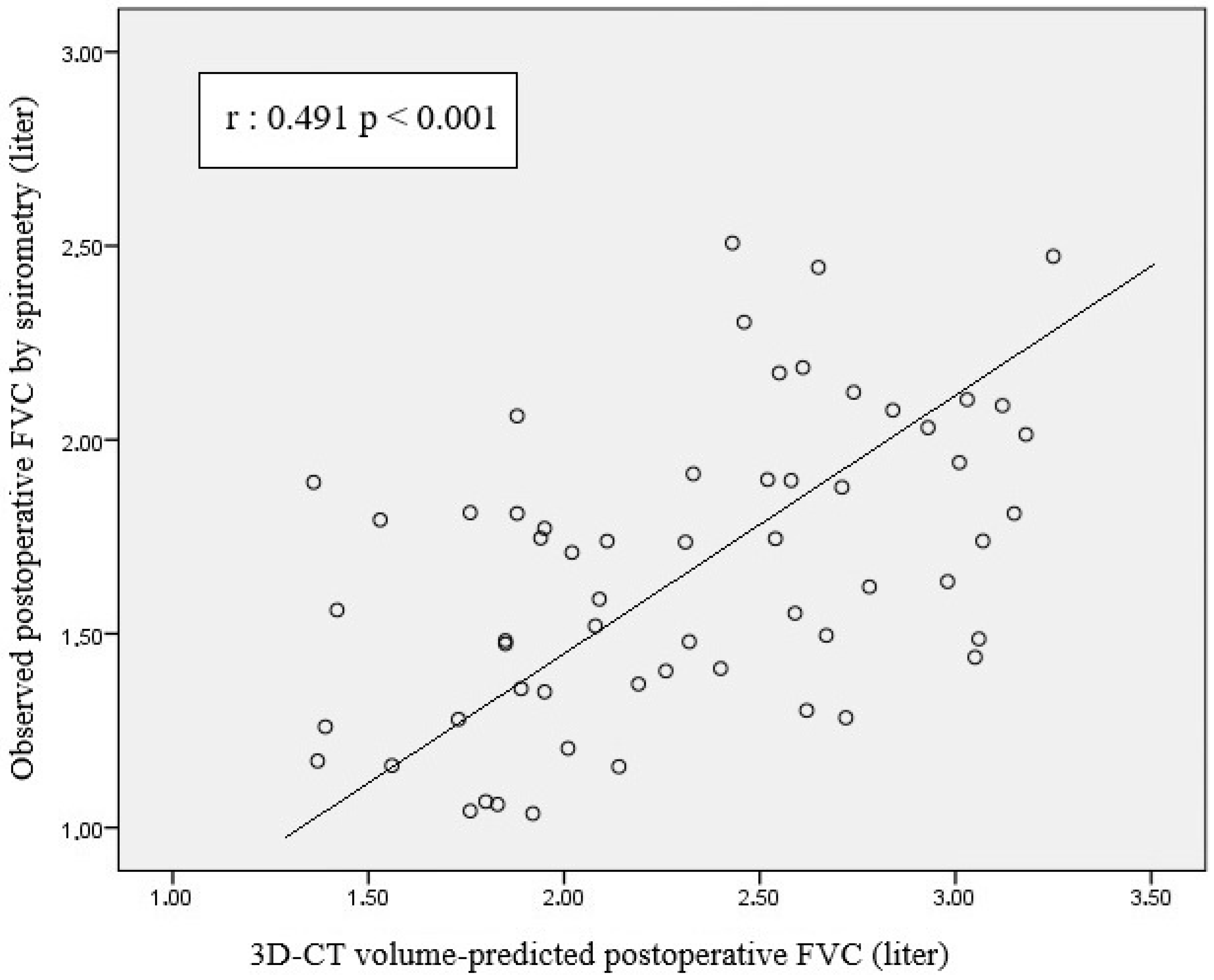
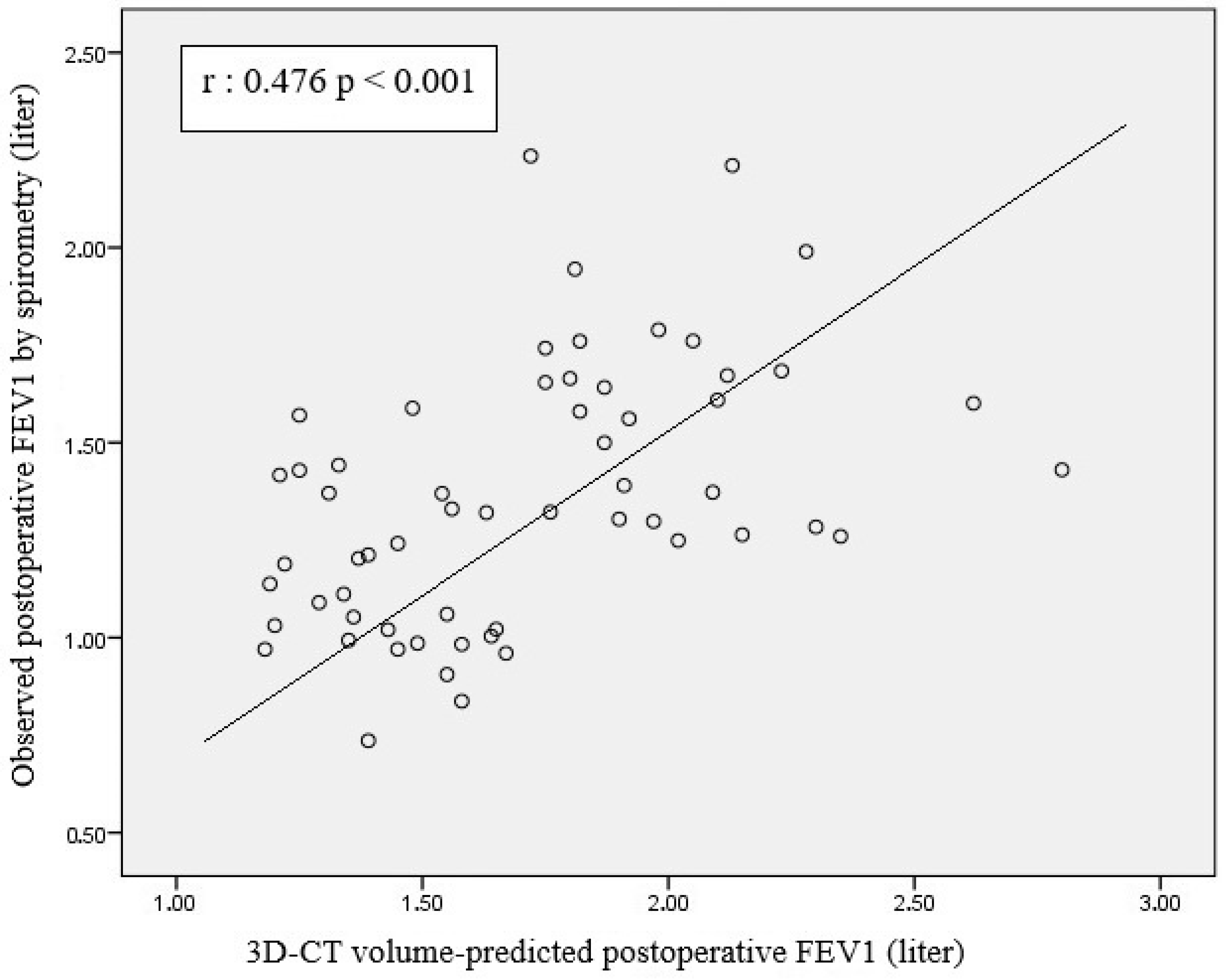
- There was a significant positive correlation between 3D-CT volumetry-based PPO FVC and FEV1 and postoperative FVC and FEV1 values.
- (b)
- There was a moderate-to-strong positive correlation between segment count-based predicted FVC and FEV1 and postoperative FVC and FEV1 values.
- (c)
- The left pneumonectomy group had higher mean FVC and FEV1 values estimated using postoperative 3D-CT volumetry than the right pneumonectomy group.
- (d)
- Cases with a postoperative residual lung volume ratio ≥ 1.2 had significantly higher mean postoperative FVC value than cases with a ratio < 1.2.
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sharma, S.; Beshara, M.; Bora, V. Pneumonectomy. In Medical Management of the Surgical Patient: A Textbook of Perioperative Medicine [Internet]; 2024; pp. 596–597. Available online: https://www.ncbi.nlm.nih.gov/books/NBK555969/ (accessed on 17 January 2025).
- Huang, J.; Osarogiagbon, R.U.; Giroux, D.J.; Nishimura, K.K.; Bille, A.; Cardillo, G.; Detterbeck, F.; Kernstine, K.; Kim, H.K.; Lievens, Y.; et al. The International Association for the Study of Lung Cancer Staging Project for Lung Cancer: Proposals for the Revision of the N Descriptors in the Forthcoming Ninth Edition of the TNM Classification for Lung Cancer. J. Thorac. Oncol. 2024, 19, 766–785. [Google Scholar] [CrossRef] [PubMed]
- Topaloğlu, Ö.; Türkyılmaz, A.; Karapolat, S.; Buran, A.; Tekinbaş, C. Extended resections in the treatment of locally advanced lung cancer. Turk. J. Thorac. Cardiovasc. Surg. 2023, 31, 538. [Google Scholar] [CrossRef] [PubMed]
- Abe, M.; Osoegawa, A.; Karashima, T.; Takamori, S.; Takumi, Y.; Sugio, K. An analysis of residual lung volume changes after segmentectomy based on three-dimensional computed tomography. J. Thorac. Dis. 2024, 16, 3553–3562. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.; Kong, S.; Kang, D.; Lee, G.; Cho, J.H.; Shim, Y.M.; Cho, J.; Kim, H.K.; Park, H.Y. Longitudinal changes in pulmonary function and patient-reported outcomes after lung cancer surgery. Respir. Res. 2022, 23, 224. [Google Scholar] [CrossRef]
- Mizobuchi, T.; Wada, H.; Sakairi, Y.; Suzuki, H.; Nakajima, T.; Tagawa, T.; Iwata, T.; Motoori, K.; Yoshida, S.; Yoshino, I. Spirometric and radiological evaluation of the remnant lung long after major pulmonary resection: Can compensatory phenomena be recognized in clinical cases? Surg. Today 2014, 44, 1735–1743. [Google Scholar] [CrossRef]
- Yokoba, M.; Ichikawa, T.; Harada, S.; Naito, M.; Sato, Y.; Katagiri, M. Postoperative pulmonary function changes according to the resected lobe: A 1-year follow-up study of lobectomized patients. J. Thorac. Dis. 2018, 10, 6891–6902. [Google Scholar] [CrossRef]
- Ueda, K.; Tanaka, T.; Hayashi, M.; Li, T.S.; Tanaka, N.; Hamano, K. Computed tomography-defined functional lung volume after segmentectomy versus lobectomy. Eur. J. Cardiothorac. Surg. 2010, 37, 1433–1437. [Google Scholar] [CrossRef]
- Butler, J.P.; Loring, S.H.; Patz, S.; Tsuda, A.; Yablonskiy, D.A.; Mentzer, S.J. Evidence for Adult Lung Growth in Humans. N. Engl. J. Med. 2012, 367, 244. [Google Scholar] [CrossRef]
- Ohata, K.; Chen-Yoshikawa, T.F.; Hamaji, M.; Kubo, T.; Nakamura, T.; Date, H. Radiologic evaluation of compensatory lung growth using computed tomography by comparison with histological data from a large animal model. Sci. Rep. 2022, 12, 2520. [Google Scholar] [CrossRef]
- Mizobuchi, T.; Chen, F.; Yoshino, I.; Iwata, T.; Yoshida, S.; Bando, T.; Date, H. Radiologic evaluation for volume and weight of remnant lung in living lung donors. J. Thorac. Cardiovasc. Surg. 2013, 146, 1253–1258. [Google Scholar] [CrossRef]
- Fernández-Rodríguez, L.; Torres, I.; Romera, D.; Galera, R.; Casitas, R.; Martínez-Cerón, E.; Díaz-Agero, P.; Utrilla, C.; García-Río, F. Prediction of postoperative lung function after major lung resection for lung cancer using volumetric computed tomography. J. Thorac. Cardiovasc. Surg. 2018, 156, 2297–2308.e5. [Google Scholar] [CrossRef] [PubMed]
- Brunelli, A.; Kim, A.W.; Berger, K.I.; Addrizzo-Harris, D.J. Physiologic evaluation of the patient with lung cancer being considered for resectional surgery: Diagnosis and management of lung cancer, 3rd ed: American college of chest physicians evidence-based clinical practice guidelines. Chest 2013, 143 (Suppl. S5), e166S–e190S. [Google Scholar] [CrossRef]
- Homma, T.; Saji, H.; Shimada, Y.; Tanabe, K.; Kojima, K.; Marushima, H.; Miyazawa, T.; Kimura, H.; Sakai, H.; Otsubo, K.; et al. Effect of Preoperative Single-Inhaler Triple Therapy on Pulmonary Function in Lung Cancer Patients with Chronic Obstructive Pulmonary Disease and FEV1 < 1.5 L. Cancers 2025, 17, 1803. [Google Scholar]
- Park, C.H.; Kim, T.H.; Lee, S.; Paik, H.C.; Haam, S.J. New predictive equation for lung volume using chest computed tomography for size matching in lung transplantation. Transplant. Proc. 2015, 47, 498–503. [Google Scholar] [CrossRef]
- Kim, S.J.; Lee, Y.J.; Park, J.S.; Cho, Y.J.; Cho, S.; Yoon, H.I.; Kim, K.; Lee, J.H.; Jheon, S.; Lee, C.T. Changes in pulmonary function in lung cancer patients after video-assisted thoracic surgery. Ann. Thorac. Surg. 2015, 99, 210–217. [Google Scholar] [CrossRef]
- Korst, R.J.; Ginsberg, R.J.; Ailawadi, M.; Bains, M.S.; Downey, R.J.; Rusch, V.W.; Stover, D. Lobectomy improves ventilatory function in selected patients with severe COPD. Ann. Thorac. Surg. 1998, 66, 898–902. [Google Scholar] [CrossRef]
- Ueda, K.; Tanaka, T.; Li, T.S.; Tanaka, N.; Hamano, K. Quantitative computed tomography for the prediction of pulmonary function after lung cancer surgery: A simple method using simulation software. Eur. J. Cardiothorac. Surg. 2009, 35, 414–418. [Google Scholar] [CrossRef]
- Oizumi, H.; Kanauchi, N.; Kato, H.; Endoh, M.; Suzuki, J.; Fukaya, K.; Sadahiro, M. Anatomic thoracoscopic pulmonary segmentectomy under 3-dimensional multidetector computed tomography simulation: A report of 52 consecutive cases. J. Thorac. Cardiovasc. Surg. 2011, 141, 678–682. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Zhao, S.; Wang, L.; Li, F.; Wang, J.; Gu, C. Comparison between functional lung volume measurement and segment counting for predicting postoperative pulmonary function after pulmonary resection in lung cancer patients. BMC Pulm. Med. 2023, 23, 6. [Google Scholar] [CrossRef] [PubMed]
- Brunelli, A.; Charloux, A.; Bolliger, C.T.; Rocco, G.; Sculier, J.P.; Varela, G.; Licker, M.J.; Ferguson, M.K.; Faivre-Finn, C.; Huber, R.M.; et al. ERS/ESTS clinical guidelines on fitness for radical therapy in lung cancer patients (surgery and chemo-radiotherapy). Eur. Respir. J. 2009, 34, 17–41. [Google Scholar] [CrossRef]
- Brunelli, A.; Ferguson, M.K.; Rocco, G.; Pieretti, P.; Vigneswaran, W.T.; Morgan-Hughes, N.J.; Zanello, M.; Salati, M. A scoring system predicting the risk for intensive care unit admission for complications after major lung resection: A multicenter analysis. Ann. Thorac. Surg. 2008, 86, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.S.; Abboud, R.T.; Wang, L.M. Effect of lung resection on exercise capacity and on carbon monoxide diffusing capacity during exercise. Chest 2006, 129, 863–872. [Google Scholar] [CrossRef][Green Version]
- Bolliger, C.T.; Jordan, P.; Solèr, M.; Stulz, P.; Tamm, M.; Wyser, C.; Gonon, M.; Perruchoud, A.P. Pulmonary function and exercise capacity after lung resection. Eur. Respir. J. 1996, 9, 415–421. [Google Scholar] [CrossRef]
- Fernandez, L.G.; Mehta, C.K.; Kron, I.L.; Laubach, V.E. Reinitiation of compensatory lung growth after subsequent lung resection. J. Thorac. Cardiovasc. Surg. 2007, 134, 1300–1305. [Google Scholar] [CrossRef]
- Suzuki, H.; Morimoto, J.; Mizobuchi, T.; Fujiwara, T.; Nagato, K.; Nakajima, T.; Iwata, T.; Yoshida, S.; Yoshino, I. Does segmentectomy really preserve the pulmonary function better than lobectomy for patients with early-stage lung cancer? Surg. Today 2017, 47, 463–469. [Google Scholar] [CrossRef]
- Wakamatsu, I.; Matsuguma, H.; Nakahara, R.; Chida, M. Factors associated with compensatory lung growth after pulmonary lobectomy for lung malignancy: An analysis of lung weight and lung volume changes based on computed tomography findings. Surg. Today 2020, 50, 144–152. [Google Scholar] [CrossRef]
- Shibazaki, T.; Mori, S.; Suyama, Y.; Arakawa, S.; Tsukamoto, Y.; Kato, D.; Kinoshita, T.; Nakada, T.; Ohtsuka, T. Effect of residual lung expansion on pulmonary function after lobectomy. Gen. Thorac. Cardiovasc. Surg. 2025, 73, 602–608. [Google Scholar] [CrossRef]
- Maciąg, B.; Wojtyś, M.E.; Waloryszak, A.; Wójcik, N.; Pieróg, J.; Safranow, K.; Sulikowski, T.; Grodzki, T.; Wójcik, J. Scintigraphic Assessment of Pulmonary Flow in Patients After Pneumonectomy. Diagnostics 2025, 15, 747. [Google Scholar] [CrossRef] [PubMed]
- Ueda, K.; Tanaka, T.; Hayashi, M.; Li, T.S.; Kaneoka, T.; Tanaka, N.; Hamano, K. Compensation of pulmonary function after upper lobectomy versus lower lobectomy. J. Thorac. Cardiovasc. Surg. 2011, 142, 762–767. [Google Scholar] [CrossRef] [PubMed]
- Shibazaki, T.; Mori, S.; Arakawa, S.; Tsukamoto, Y.; Nakada, T.; Takahashi, Y.; Ohtsuka, T. Compensatory expansion of the right middle lobe: Volumetric and functional analysis of the changes after right upper or lower lobectomy. Updates Surg. 2024, 76, 2313–2320. [Google Scholar] [CrossRef]




| Variable | Left Pneumonectomy (n = 40) | Right Pneumonectomy (n = 19) | p Value |
|---|---|---|---|
| Age | 63.4 ± 7.7 | 59.9 ± 10.2 | 0.151 |
| Preoperative lung volume (mL) | 2342 ± 468 | 2019 ± 348 | |
| Postoperative lung volume (mL) | 2771 ± 524 | 2522 ± 605 | |
| Postoperative residual lung expansion ratio | 1.21 ± 0.29 | 1.25 ± 0.27 | 0.638 |
| 3D-CT predicted FVC (mL) | 1745 ± 372 | 1506 ± 351 | 0.023 |
| 3D-CT predicted FEV1 (mL) | 1434 ± 318 | 1208 ± 317 | 0.013 |
| Volume-predicted FEV1/FVC (%) | 82 ± 9 | 80 ± 7 | 0.385 |
| ASC-predicted FVC (mL) | 1550 ± 326 | 1463 ± 248 | 0.311 |
| ASC-predicted FEV1 (mL) | 1250 ± 292 | 1151 ± 260 | 0.215 |
| Segment-based FEV1/FVC (%) | 81 ± 9 | 78 ± 7 | 0.271 |
| Preoperative spirometric FVC (mL) | 2849 ± 686 | 2927 ± 759 | 0.693 |
| Preoperative spirometric FEV1 (mL) | 2279 ± 617 | 2331 ± 680 | 0.774 |
| Preoperative FEV1/FVC (%) | 80 ± 12 | 79 ± 7 | 0.762 |
| Postoperative spirometric FVC (mL) | 2328 ± 531 | 2293 ± 537 | 0.816 |
| Postoperative spirometric FEV1 (mL) | 1743 ± 409 | 1633 ± 284 | 0.295 |
| Postoperative FEV1/FVC (%) | 76 ± 10 | 74 ± 14 | 0.574 |
| Variable | Postoperative Residual Lung Expansion Rate < 1.2 (n = 31) | Postoperative Residual Lung Expansion Rate ≥ 1.2 (n = 28) | p Value |
|---|---|---|---|
| Age (years) | 62.7 ± 7.9 | 61.8 ± 9.4 | 0.683 |
| Preoperative spirometric FVC (mL) | 2739 ± 729 | 3023 ± 659 | 0.124 |
| Preoperative spirometric FEV1 (mL) | 2154 ± 612 | 2463 ± 624 | 0.053 |
| Preoperative FEV1/FVC (%) | 79 ± 11 | 81 ± 10 | 0.383 |
| ASC-predicted FVC (mL) | 1459 ± 310 | 1591 ± 286 | 0.096 |
| ASC-predicted FEV1 (mL) | 1161 ± 272 | 1282 ± 288 | 0.103 |
| 3D-CT predicted FVC (mL) | 1651 ± 414 | 1687 ± 343 | 0.722 |
| 3D-CT predicted FEV1 (mL) | 1321 ± 347 | 1405 ± 315 | 0.340 |
| Volume-predicted FEV1/FVC (%) | 80 ± 8 | 83 ± 8 | 0.225 |
| Postoperative spirometric FVC (mL) | 2174 ± 511 | 2475 ± 510 | 0.028 |
| Postoperative spirometric FEV1 (mL) | 1634 ± 346 | 1789 ± 395 | 0.116 |
| Postoperative FEV1/FVC (%) | 77 ± 12 | 73 ± 11 | 0.268 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Topaloglu, O.; Aktepe, R.; Kilic, K.N.; Karapolat, S.; Uzun, A.Y.; Senturk Topaloglu, E.; Turkyilmaz, A.; Ozden, S.; Gumus, A.; Tekinbas, C.; et al. Morphological and Functional Analysis of Residual Lung After Pneumonectomy in Lung Cancer Surgery via 3D-CT Method. Life 2025, 15, 1265. https://doi.org/10.3390/life15081265
Topaloglu O, Aktepe R, Kilic KN, Karapolat S, Uzun AY, Senturk Topaloglu E, Turkyilmaz A, Ozden S, Gumus A, Tekinbas C, et al. Morphological and Functional Analysis of Residual Lung After Pneumonectomy in Lung Cancer Surgery via 3D-CT Method. Life. 2025; 15(8):1265. https://doi.org/10.3390/life15081265
Chicago/Turabian StyleTopaloglu, Omer, Rıza Aktepe, Kubra Nur Kilic, Sami Karapolat, Ali Yavuz Uzun, Elvan Senturk Topaloglu, Atila Turkyilmaz, Serkan Ozden, Aziz Gumus, Celal Tekinbas, and et al. 2025. "Morphological and Functional Analysis of Residual Lung After Pneumonectomy in Lung Cancer Surgery via 3D-CT Method" Life 15, no. 8: 1265. https://doi.org/10.3390/life15081265
APA StyleTopaloglu, O., Aktepe, R., Kilic, K. N., Karapolat, S., Uzun, A. Y., Senturk Topaloglu, E., Turkyilmaz, A., Ozden, S., Gumus, A., Tekinbas, C., & Turut, H. (2025). Morphological and Functional Analysis of Residual Lung After Pneumonectomy in Lung Cancer Surgery via 3D-CT Method. Life, 15(8), 1265. https://doi.org/10.3390/life15081265






