Impact of Chromosomal Structural Rearrangements on IVF Laboratory Outcomes in PGT-SR Cycles: A Propensity Score Matching-Based Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
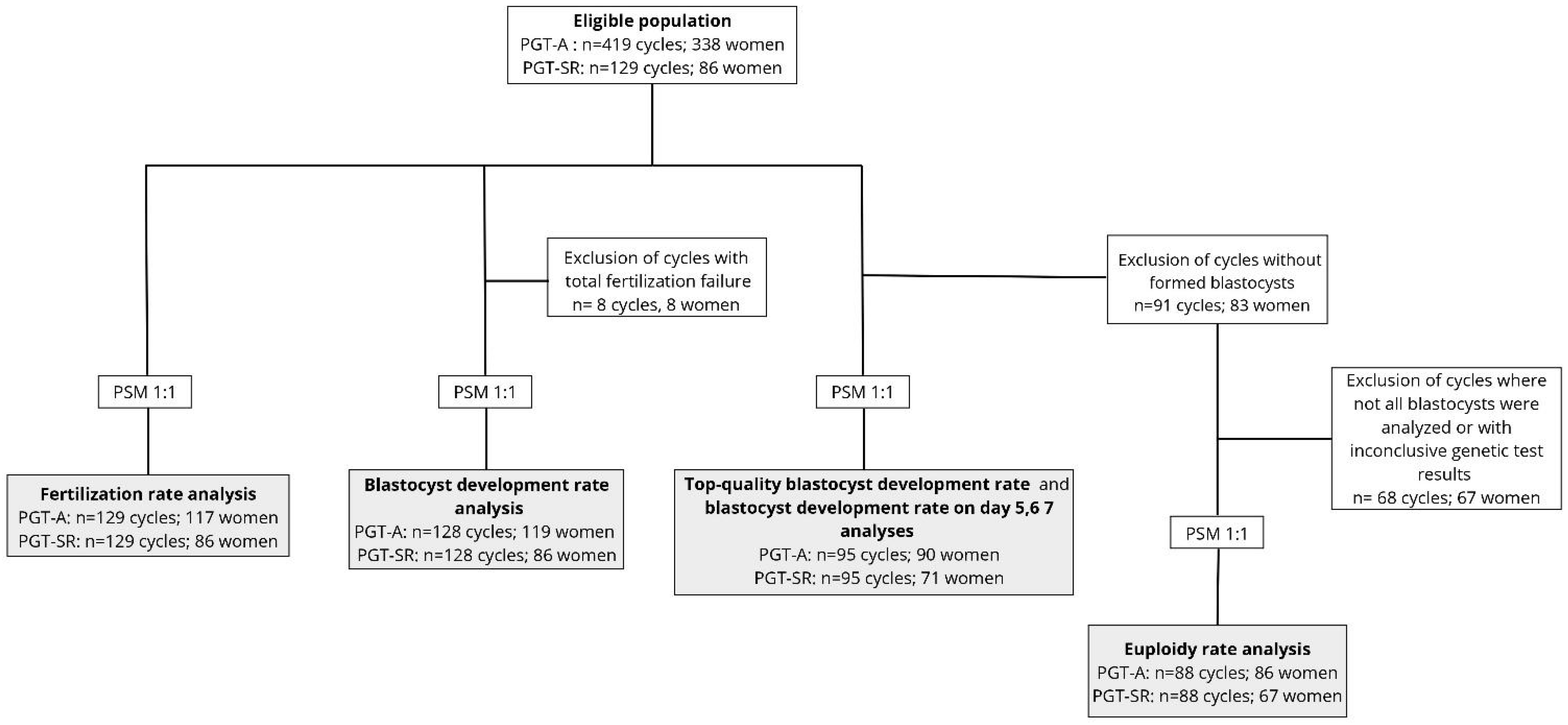
2.2. Participants
2.3. Assisted Reproductive Technology Procedures
2.4. Outcome Measures
2.5. Covariates
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ART | Assisted Reproduction Technology |
| BMI | Body mass index |
| CI | Confidence interval |
| ICM | Inner cell mass |
| Inv | Inversions |
| IVF | In Vitro Fertilization |
| OR | Odds ratio |
| PGT-A | Preimplantation genetic testing for aneuploidies |
| PGT-M | Preimplantation genetic testing for monogenic/single-gene defects |
| PGT-SR | Preimplantation genetic testing for structural rearrangements |
| PSM | Propensity score matching |
| RecT | Reciprocal translocations |
| RobT | Robertsonian translocations |
| SMD | Standardized mean difference |
| SR | Structural rearrangement |
| TE | Trophectoderm |
| TMSC | Total Motile Sperm Count |
References
- Jacobs, P.A.; Browne, C.; Gregson, N.; Joyce, C.; White, H. Estimates of the frequency of chromosome abnormalities detectable in unselected newborns using moderate levels of banding. J. Med. Genet. 1992, 29, 103–108. [Google Scholar] [CrossRef]
- Sampson, J.E.; Ouhibi, N.; Lawce, H.; Patton, P.E.; Battaglia, D.E.; Burry, K.A.; Olson, S.B. The role for preimplantation genetic diagnosis in balanced translocation carriers. Am. J. Obstet. Gynecol. 2004, 190, 1707–1711. [Google Scholar] [CrossRef]
- Clementini, E.; Palka, C.; Iezzi, I.; Stuppia, L.; Guanciali-Franchi, P.; Tiboni, G.M. Prevalence of chromosomal abnormalities in 2078 infertile couples referred for assisted reproductive techniques. Hum. Reprod. 2005, 20, 437–442. [Google Scholar] [CrossRef]
- Sugiura-Ogasawara, M.; Ozaki, Y.; Sato, T.; Suzumori, N.; Suzumori, K. Poor prognosis of recurrent aborters with either maternal or paternal reciprocal translocations. Fertil. Steril. 2004, 81, 367–373. [Google Scholar] [CrossRef]
- Pei, Z.; Deng, K.; Lei, C.; Du, D.; Yu, G.; Sun, X.; Xu, C.; Zhang, S. Identifying Balanced Chromosomal Translocations in Human Embryos by Oxford Nanopore Sequencing and Breakpoints Region Analysis. Front. Genet. 2022, 12, 810900. [Google Scholar] [CrossRef]
- Sugiura-Ogasawara, M.; Aoki, K.; Fujii, T.; Fujita, T.; Kawaguchi, R.; Maruyama, T.; Ozawa, N.; Sugi, T.; Takeshita, T.; Saito, S. Subsequent pregnancy outcomes in recurrent miscarriage patients with a paternal or maternal carrier of a structural chromosome rearrangement. J. Hum. Genet. 2008, 53, 622–628. [Google Scholar] [CrossRef]
- Ozawa, N.; Maruyama, T.; Nagashima, T.; Ono, M.; Arase, T.; Ishimoto, H.; Yoshimura, Y. Pregnancy outcomes of reciprocal translocation carriers who have a history of repeated pregnancy loss. Fertil. Steril. 2008, 90, 1301–1304. [Google Scholar] [CrossRef]
- FitzSimmons, J.; Jackson, D.; Wapner, R.; Jackson, L. Subsequent reproductive outcome in couples with repeated pregnancy loss. Am. J. Med. Genet. 1983, 16, 583–587. [Google Scholar] [CrossRef]
- Fortuny, A.; Carrio, A.; Soler, A.; Cararach, J.; Fuster, J.; Salami, C. Detection of balanced chromosome rearrangements in 445 couples with repeated abortion and cytogenetic prenatal testing in carriers. Fertil. Steril. 1988, 49, 774–779. [Google Scholar] [CrossRef]
- ESHRE PGT-SR/PGT-A Working Group; Coonen, E.; Rubio, C.; Christopikou, D.; Dimitriadou, E.; Gontar, J.; Goossens, V.; Maurer, M.; Spinella, F.; Vermeulen, N. ESHRE PGT Consortium good practice recommendations for the detection of structural and numerical chromosomal aberrations†. Hum. Reprod. Open. 2020, 3, hoaa017. [Google Scholar] [CrossRef]
- Insogna, I.G.; Lanes, A.; Dobson, L.; Ginsburg, E.S.; Racowsky, C.; Yanushpolsky, E. Blastocyst conversion rate and ploidy in patients with structural rearrangements. J. Assist. Reprod. Genet. 2021, 38, 1143–1151. [Google Scholar] [CrossRef]
- Liu, M.; Bu, Z.; Liu, Y.; Liu, J.; Dai, S. Are ovarian responses and the number of transferable embryos different in females and partners of male balanced translocation carriers? J. Assist. Reprod. Genet. 2022, 39, 2019–2026. [Google Scholar] [CrossRef]
- Tong, J.; Niu, Y.; Wan, A.; Zhang, T. Effect of parental origin and predictors for obtaining a euploid embryo in balanced translocation carriers. Reprod. Biomed. Online 2022, 44, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Dang, T.; Xie, P.; Zhang, Z.; Hu, L.; Tang, Y.; Tan, Y.; Luo, K.; Gong, F.; Lu, G.; Lin, G. The effect of carrier characteristics and female age on preimplantation genetic testing results of blastocysts from Robertsonian translocation carriers. J. Assist. Reprod. Genet. 2023, 40, 1995–2002. [Google Scholar] [CrossRef]
- Villanacci, R.; Buzzaccarini, G.; Marzanati, D.; Vanni, V.S.; De Santis, L.; Alteri, A.; Candiani, M.; Pagliardini, L.; Papaleo, E. Delayed blastocyst development is influenced by the level of progesterone on the day of trigger. J. Assist. Reprod. Genet. 2023, 40, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Cermisoni, G.C.; Minetto, S.; Marzanati, D.; Alteri, A.; Salmeri, N.; Rabellotti, E.; Nova, A.; Salonia, A.; Pozzi, E.; Candiani, M.; et al. Effect of ejaculatory abstinence period on fertilization and clinical outcomes in ICSI cycles: A retrospective analysis. Reprod. Biomed. Online 2024, 48, 103401. [Google Scholar] [CrossRef]
- Vanni, V.S.; Alteri, A.; De Santis, L.; Cermisoni, G.C.; Rabellotti, E.; Delprato, D.; Parma, M.; Papaleo, E.; Fedele, L.; Candiani, M. Laparoscopic Oocyte Retrieval and Cryopreservation during Vaginoplasty for Treatment of Mayer-Rokitansky-Kuster-Hauser Syndrome. J. Vis. Exp. 2022, 183, 63634. [Google Scholar]
- Fiorentino, F.; Biricik, A.; Bono, S.; Spizzichino, L.; Cotroneo, E.; Cottone, G.; Kokocinski, F.; Michel, C.-E. Development and validation of a next-generation sequencing-based protocol for 24-chromosome aneuploidy screening of embryos. Fertil. Steril. 2014, 101, 1375–1382. [Google Scholar] [CrossRef]
- Bono, S.; Biricik, A.; Spizzichino, L.; Nuccitelli, A.; Minasi, M.G.; Greco, E.; Spinella, F.; Fiorentino, F. Validation of a semiconductor next-generation sequencing-based protocol for preimplantation genetic diagnosis of reciprocal translocations. Prenat. Diagn. 2015, 35, 938–944. [Google Scholar] [CrossRef]
- Alteri, A.; Campo, G.; Pagliardini, L.; Privitera, L.; Cavoretto, P.I.; Candiani, M.; Papaleo, E.; Viganò, P. The effect of vitrified–warmed blastocyst transfer on postnatal growth: A 1-year follow-up questionnaire study. Reprod. Biomed. Online 2022, 44, 907–914. [Google Scholar] [CrossRef]
- Ho, D.; Imai, K.; King, G.; Stuart, E.A. MatchIt: Nonparametric Preprocessing for Parametric Causal Inference. J. Stat. Softw. 2011, 42, 1–28. [Google Scholar] [CrossRef]
- The Working Group on the update of the ESHRE/ALPHA Istanbul Consensus; Coticchio, G.; Ahlström, A.; Arroyo, G.; Balaban, B.; Campbell, A.; Santos, M.J.D.L.; Ebner, T.; Gardner, D.K.; Kovačič, B.; et al. The Istanbul consensus update: A revised ESHRE/ALPHA consensus on oocyte and embryo static and dynamic morphological assessment. Hum. Reprod. 2025, 40, 989–1035. [Google Scholar] [CrossRef]
- Munné, S.; Sandalinas, M.; Escudero, T.; Fung, J.; Gianaroli, L.; Cohen, J. Outcome of preimplantation genetic diagnosis of translocations. Fertil. Steril. 2000, 73, 1209–1218. [Google Scholar] [CrossRef]
- Ko, D.S.; Cho, J.W.; Park, S.Y.; Kim, J.Y.; Koong, M.K.; Song, I.O.; Kang, I.S.; Lim, C.K. Clinical outcomes of preimplantation genetic diagnosis (PGD) and analysis of meiotic segregation modes in reciprocal translocation carriers. Am. J. Med. Genet. A. 2010, 152A, 1428–1433. [Google Scholar] [CrossRef]
- Beyer, C.E.; Willats, E. Natural selection between day 3 and day 5/6 PGD embryos in couples with reciprocal or Robertsonian translocations. J. Assist. Reprod. Genet. 2017, 34, 1483–1492. [Google Scholar] [CrossRef]
- Sfakianoudis, K.; Maziotis, E.; Karantzali, E.; Kokkini, G.; Grigoriadis, S.; Pantou, A.; Giannelou, P.; Petroutsou, K.; Markomichali, C.; Fakiridou, M.; et al. Molecular Drivers of Developmental Arrest in the Human Preimplantation Embryo: A Systematic Review and Critical Analysis Leading to Mapping Future Research. Int. J. Mol. Sci. 2021, 22, 8353. [Google Scholar] [CrossRef]
- McCoy, R.C.; Summers, M.C.; McCollin, A.; Ottolini, C.S.; Ahuja, K.; Handyside, A.H. Meiotic and mitotic aneuploidies drive arrest of in vitro fertilized human preimplantation embryos. Genome Med. 2023, 15, 77. [Google Scholar] [CrossRef]
- Tian, Z.; Lian, W.; Xu, L.; Long, Y.; Tang, L.; Wang, H. Robust evidence reveals the reliable rate of normal/balanced embryos for identifying reciprocal translocation and Robertsonian translocation carriers. Zygote 2024, 32, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Boynukalin, F.K.; Gultomruk, M.; Turgut, N.E.; Rubio, C.; Rodrigo, L.; Yarkiner, Z.; Ecemis, S.; Karlikaya, G.; Findikli, N.; Bahceci, M. The impact of patient, embryo, and translocation characteristics on the ploidy status of young couples undergoing preimplantation genetic testing for structural rearrangements (PGT-SR) by next generation sequencing (NGS). J. Assist. Reprod. Genet. 2021, 38, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Mateu-Brull, E.; Rodrigo, L.; Peinado, V.; Mercader, A.; Campos-Galindo, I.; Bronet, F.; García-Herrero, S.; Florensa, M.; Milán, M.; Rubio, C. Interchromosomal effect in carriers of translocations and inversions assessed by preimplantation genetic testing for structural rearrangements (PGT-SR). J. Assist. Reprod. Genet. 2019, 36, 2547–2555. [Google Scholar] [CrossRef] [PubMed]
- Halvaei, I.; Litzky, J.; Esfandiari, N. Advanced paternal age: Effects on sperm parameters, assisted reproduction outcomes and offspring health. Reprod. Biol. Endocrinol. 2020, 18, 110. [Google Scholar] [CrossRef]
- Ogilvie, C.M.; Scriven, P.N. Meiotic outcomes in reciprocal translocation carriers ascertained in 3-day human embryos. Eur. J. Hum. Genet. 2002, 10, 801–806. [Google Scholar] [CrossRef]
- Benn, P.; Merrion, K. Chromosome segregation of human nonhomologous Robertsonian translocations: Insights from preimplantation genetic testing. Eur. J. Hum. Genet. 2025, 33, 711–717. [Google Scholar] [CrossRef] [PubMed]
- Yuan, P.; Zheng, L.; Ou, S.; Zhao, H.; Li, R.; Luo, H.; Tan, X.; Zhang, Q.; Wang, W. Evaluation of chromosomal abnormalities from preimplantation genetic testing to the reproductive outcomes: A comparison between three different structural rearrangements based on next-generation sequencing. J. Assist. Reprod. Genet. 2021, 38, 709–718. [Google Scholar] [CrossRef] [PubMed]
- Van Der Schoot, V.; Dondorp, W.; Dreesen, J.C.F.M.; Coonen, E.; Paulussen, A.D.C.; De Wert, G.; de Die-Smulders, C.E.M. Preimplantation genetic testing for more than one genetic condition: Clinical and ethical considerations and dilemmas. Hum. Reprod. 2019, 34, 1146–1154. [Google Scholar] [CrossRef] [PubMed]
- Butler, R.; Nakhuda, G.; Guimond, C.; Jing, C.; Lee, N.; Hitkari, J.; Tallon, N.; Taylor, B.; Yuzpe, A. Analysis of PGT-M and PGT-SR outcomes at a Canadian fertility clinic. Prenat. Diagn. 2019, 39, 866–870. [Google Scholar] [CrossRef]
| Outcome | PGT-A (Ref.) | PGT-SR | OR | 95%CI | p-Value |
|---|---|---|---|---|---|
| Fertilization rate | 83.3% [66.7%, 92.3%] | 85.7% [75%, 100%] | 1.25 | 1.00–1.55 | 0.077 |
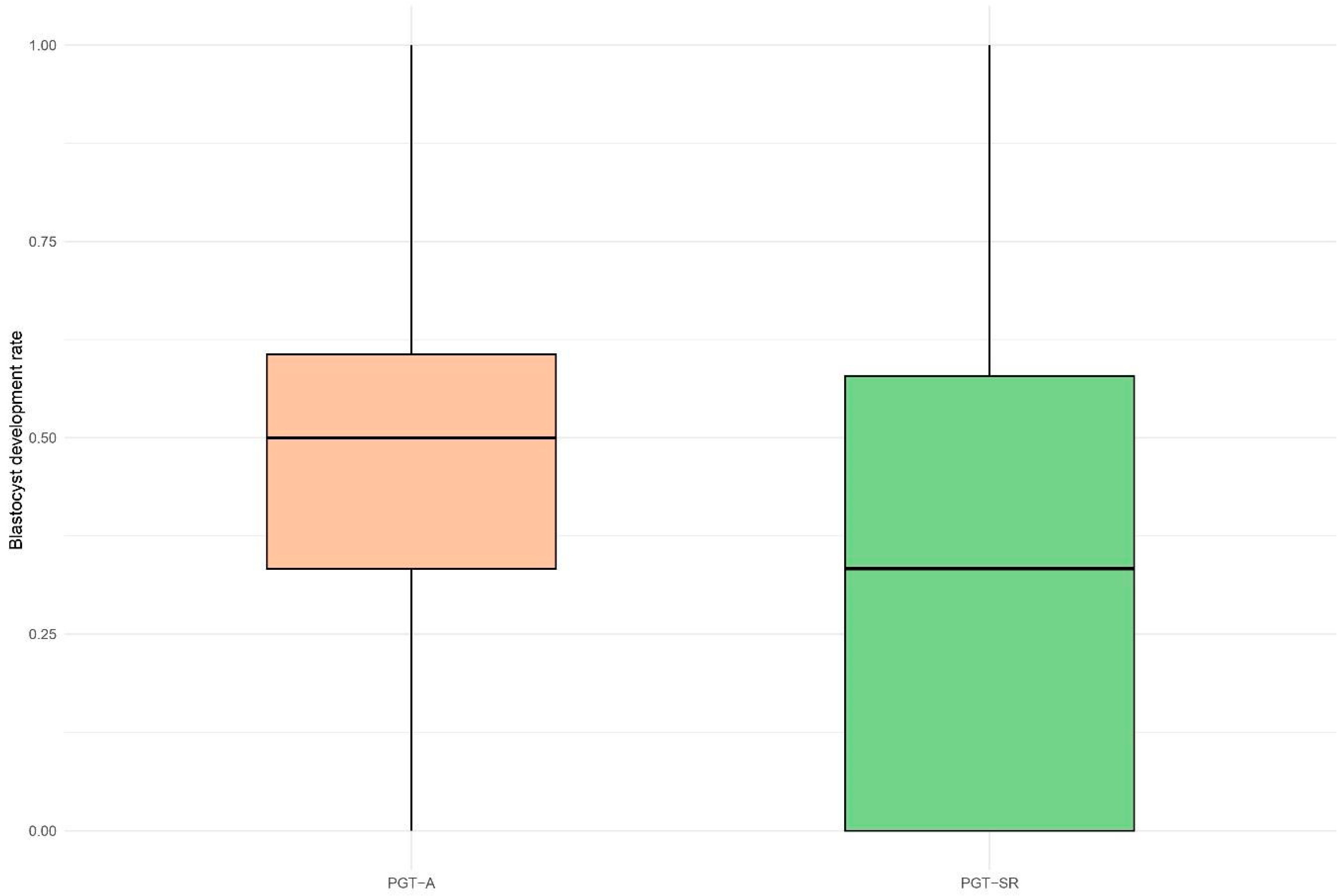
| blastocyst development rate | 50.0% [33.3%, 60.6%] | 33.3% [0.0%, 57.9%] | 0.74 | 0.61–0.89 | 0.008 |
| Top-quality blastocyst development rate | 10% [0.0%, 33.3%] | 0.0% [0.0%, 0.0%] | 0.42 | 0.27–0.65 | 0.002 |
| Euploidy rate | 33.3% [0.0%, 50.0%] | 33.3% [0.0%, 66.7%] | 1.24 | 0.88–1.75 | 0.258 |
| Characteristics | Female (n = 45) | Male (n = 43) | p-Value |
|---|---|---|---|
| Maternal age at oocytes retrieval, years; mean (sd) | 35.1 (4.4) | 36.1 (3.9) | 0.277 |
| Maternal BMI, kg/m2; mean (sd) | 22.1 (2.7) | 22.6 (3.3) | 0.477 |
| Paternal age, years; mean (sd) | 37.3 (5.2) | 40.2 (5.7) | 0.023 |
| Seminal quality based on TMSC; n (%) | 0.001 | ||
| normal male factor | 33 (73.3) | 15 (34.9) | |
| moderate male factor | 7 (15.6) | 15 (34.9) | |
| severe male factor | 5 (11.1) | 13 (30.2) | |
| Length of stimulation, days; (median [IQR]) | 10.0 [9.0, 11.0] | 10.0 [9.0, 11.0] | 0.685 |
| Progesterone concentration on day of ovulation trigger (ng/mL); (median [IQR]) | 1.1 [0.7, 1.5] | 0.9 [0.8, 1.3] | 0.420 |
| Mature (metaphase II) oocyte rate; (median [IQR]) | 0.8 [0.7, 1.0] | 0.8 [0.7, 0.9] | 0.624 |
| Structural rearrangement; n(%) | 0.698 | ||
| Other structural rearrangement | 16 (35.6) | 18 (41.9) | |
| Insertion | 1 (6.2) | 0 (0.0) | |
| Inversion | 6 (37.5) | 6 (33.3) | |
| Robertsonian translocation | 9 (56.3) | 12 (66.7) | |
| Reciprocal translocation | 29 (64.4) | 25 (58.1) |
| OR | 95%CI | p-Value | |
|---|---|---|---|
| Carrier | |||
| Female | Ref. | ||
| Male | 1.62 | 0.76–3.43 | 0.207 |
| Structural rearrangement | |||
| Other types of SR | Ref. | ||
| Reciprocal translocation | 0.08 | 0.04–0.18 | <0.001 |
| Maternal characteristics | |||
| Maternal BMI (kg/m2) | 1.12 | 0.99–1.26 | 0.061 |
| Mature (metaphase II) oocyte rate | 2.39 | 0.25–22.40 | 0.447 |
| Progesterone concentration on day of ovulation trigger (ng/mL) | 1.08 | 0.72–1.62 | 0.720 |
| Length of stimulation (days) | 0.91 | 0.73–1.13 | 0.381 |
| Paternal characteristics | |||
| Paternal age at oocytes retrieval (years) | 0.99 | 0.93–1.06 | 0.887 |
| Seminal quality based on TMSC | |||
| normal male factor | Ref. | ||
| moderate male factor | 0.99 | 0.47–2.10 | 0.989 |
| severe male factor | 0.43 | 0.16–1.14 | 0.089 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marzanati, D.; D’Alessandro, S.; Gentilini, D.; Rabellotti, E.; Privitera, L.; Faulisi, S.; Spinella, F.; Biricik, A.; Cotroneo, E.; Candiani, M.; et al. Impact of Chromosomal Structural Rearrangements on IVF Laboratory Outcomes in PGT-SR Cycles: A Propensity Score Matching-Based Study. Life 2025, 15, 1266. https://doi.org/10.3390/life15081266
Marzanati D, D’Alessandro S, Gentilini D, Rabellotti E, Privitera L, Faulisi S, Spinella F, Biricik A, Cotroneo E, Candiani M, et al. Impact of Chromosomal Structural Rearrangements on IVF Laboratory Outcomes in PGT-SR Cycles: A Propensity Score Matching-Based Study. Life. 2025; 15(8):1266. https://doi.org/10.3390/life15081266
Chicago/Turabian StyleMarzanati, Daria, Sara D’Alessandro, Davide Gentilini, Elisa Rabellotti, Laura Privitera, Sonia Faulisi, Francesca Spinella, Anil Biricik, Ettore Cotroneo, Massimo Candiani, and et al. 2025. "Impact of Chromosomal Structural Rearrangements on IVF Laboratory Outcomes in PGT-SR Cycles: A Propensity Score Matching-Based Study" Life 15, no. 8: 1266. https://doi.org/10.3390/life15081266
APA StyleMarzanati, D., D’Alessandro, S., Gentilini, D., Rabellotti, E., Privitera, L., Faulisi, S., Spinella, F., Biricik, A., Cotroneo, E., Candiani, M., Pagliardini, L., Papaleo, E., & Alteri, A. (2025). Impact of Chromosomal Structural Rearrangements on IVF Laboratory Outcomes in PGT-SR Cycles: A Propensity Score Matching-Based Study. Life, 15(8), 1266. https://doi.org/10.3390/life15081266







