From Deficiency to Therapy: Systemic Consequences of ALAS1 Disruption and the Protective Role of 5-ALA
Abstract
1. Heme Biosynthesis and Central Role in Cellular Metabolism
2. Essential Role of ALAS1 and ALAS2 Isozymes in Embryogenesis and Development
3. Systemic Metabolic Dysfunction Caused by Heme Deficiency
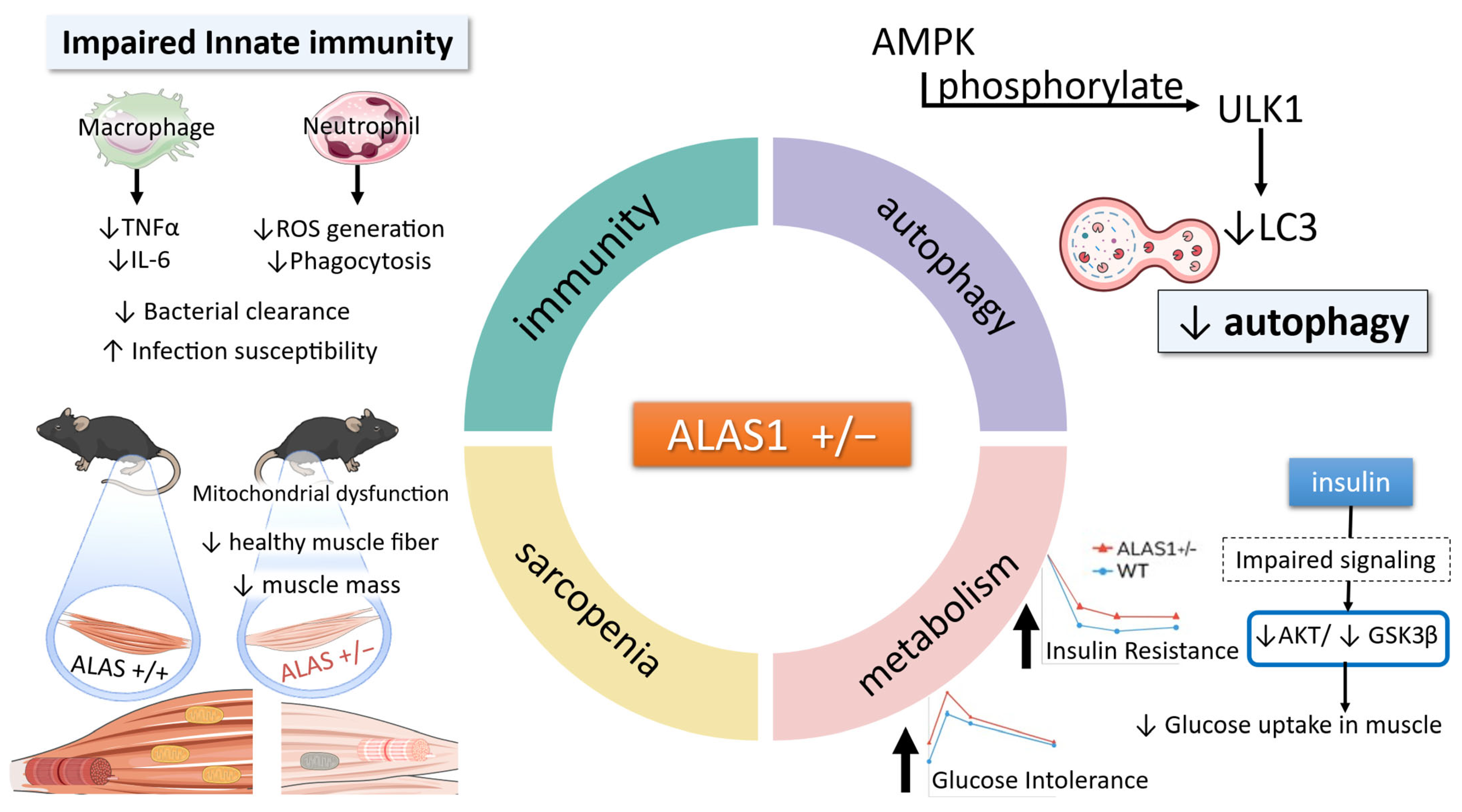
3.1. Insulin Resistance and Glucose Intolerance in ALAS1+/− Mice
3.2. Glycogen Storage Dysregulation via AMPK Suppression in Heme-Deficient Muscle
4. Heme Deficiency-Induced Mitochondrial Defects in Skeletal Muscle
5. Suppressed Autophagy in Heme Deficiency: The Role of AMPK Signaling
6. Heme Deficiency as a Driver of Age-Associated Decline in Muscle Function and Metabolism
7. Multiorgan Impact of Heme Deficiency
7.1. Disrupted Erythropoiesis and Anemia in ALAS2 Deficiency
7.2. Stress Signaling Through the HRI-ISR Axis in Anemic States
8. Innate Immune System Suppression Under Heme-Deficient States and Restoration by 5-ALA
9. Emerging Roles of Heme in Neuronal Health
10. Conclusions and Future Perspectives: Heme as a Multisystem Regulator and Therapeutic Target
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
List of Abbreviations
| 5-ALA | 5-aminolevulinic acid |
| AD | Alzheimer’s disease |
| ALAS | ALA synthase |
| ALAS1+/− | ALAS1 heterozygous |
| ALAS1–/– | Alas1 knockout |
| ALAS2+/− | ALAS2 heterozygous |
| AMPK | AMP-activated protein kinase |
| DEPC5 | DEP domain-containing 5 |
| ETC | electron transport chain |
| GYS1 | glycogen synthase |
| HO-1 | heme oxygenase-1 |
| HRI | heme-regulated inhibitor kinase |
| IL-6 | interleukin-6 |
| ISR | integrated stress response |
| LPS | lipopolysaccharide |
| MHO-1 | skeletal muscle-specific HO-1 deletion |
| mTORC1 | mammalian target of rapamycin complex 1 |
| NMDA | N-methyl-D-aspartate |
| NOS | nitric oxide synthase |
| PD | Parkinson’s disease |
| PGRMC2 | progesterone receptor membrane component 2 |
| ROS | reactive oxygen species |
| SA | succinyl acetone |
| TNF-α | tumor necrosis factor-α |
| UPR | unfolded protein response |
| XLSA | X-linked sideroblastic anemia |
References
- Ponka, P. Cell biology of heme. Am. J. Med. Sci. 1999, 318, 241–256. [Google Scholar] [CrossRef] [PubMed]
- Dunaway, L.S.; Loeb, S.A.; Petrillo, S.; Tolosano, E.; Isakson, B.E. Heme metabolism in nonerythroid cells. J. Biol. Chem. 2024, 300, 107132. [Google Scholar] [CrossRef]
- Blumberger, J. Electron transfer and transport through multi-heme proteins: Recent progress and future directions. Curr. Opin. Chem. Biol. 2018, 47, 24–31. [Google Scholar] [CrossRef]
- Furuyama, K.; Kaneko, K.; Vargas, P.D. Heme as a magnificent molecule with multiple missions: Heme determines its own fate and governs cellular homeostasis. Tohoku J. Exp. Med. 2007, 213, 1–16. [Google Scholar] [CrossRef]
- Chiabrando, D.; Mercurio, S.; Tolosano, E. Heme and erythropoieis: More than a structural role. Haematologica 2014, 99, 973–983. [Google Scholar] [CrossRef]
- Watanabe-Matsui, M.; Kadoya, S.; Segawa, K.; Shima, H.; Nakagawa, T.; Nagasawa, Y.; Hayashi, S.; Matsumoto, M.; Ikeda, M.; Muto, A.; et al. Heme regulates protein interactions and phosphorylation of BACH2 intrinsically disordered region in humoral response. iScience 2025, 28, 111529. [Google Scholar] [CrossRef]
- Murray, M.H.; Valfort, A.C.; Koelblen, T.; Ronin, C.; Ciesielski, F.; Chatterjee, A.; Veerakanellore, G.B.; Elgendy, B.; Walker, J.K.; Hegazy, L.; et al. Structural basis of synthetic agonist activation of the nuclear receptor REV-ERB. Nat. Commun. 2022, 13, 7131. [Google Scholar] [CrossRef]
- Liao, R.; Bresnick, E.H. Heme as a differentiation-regulatory transcriptional cofactor. Int. J. Hematol. 2022, 116, 174–181. [Google Scholar] [CrossRef]
- Fujita, H.; Furuyama, K.; Sasaki, T.; Takagawa, M. Molecular regulation of heme biosynthesis. Nihon Rinsho. 1995, 53, 1339–1348. [Google Scholar]
- Nakajima, O.; Takahashi, S.; Harigae, H.; Furuyama, K.; Hayashi, N.; Sassa, S.; Yamamoto, M. Heme deficiency in erythroid lineage causes differentiation arrest and cytoplasmic iron overload. EMBO J. 1999, 18, 6282–6289. [Google Scholar] [CrossRef]
- Fujita, H. Molecular mechanism of heme biosynthesis. Tohoku J. Exp. Med. 1997, 183, 83–99. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Swenson, S.A.; Moore, C.M.; Marcero, J.R.; Medlock, A.E.; Reddi, A.R.; Khalimonchuk, O. From Synthesis to Utilization: The Ins and Outs of Mitochondrial Heme. Cells 2020, 9, 579. [Google Scholar] [CrossRef] [PubMed]
- Balogun, O.; Nejak-Bowen, K. The Hepatic Porphyrias: Revealing the Complexities of a Rare Disease. Semin. Liver Dis. 2023, 43, 446–459. [Google Scholar] [CrossRef]
- Piel, R.B., 3rd; Dailey, H.A.J.; Medlock, A.E. The mitochondrial heme metabolon: Insights into the complex(ity) of heme synthesis and distribution. Mol. Genet. Metab. 2019, 128, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Kuryata, O.; Akimov, O.; Riabushko, M.; Kostenko, H.; Kostenko, V.; Mishchenko, A.; Nazarenko, S.; Solovyova, N.; Kostenko, V. Therapeutic potential of 5-aminolevulinic acid in metabolic disorders: Current insights and future directions. iScience 2024, 27, 111477. [Google Scholar] [CrossRef]
- Okano, S.; Zhou, L.; Kusaka, T.; Shibata, K.; Shimizu, K.; Gao, X.; Kikuchi, Y.; Togashi, Y.; Hosoya, T.; Takahashi, S.; et al. Indispensable function for embryogenesis, expression and regulation of the nonspecific form of the 5-aminolevulinate synthase gene in mouse. Genes Cells 2010, 15, 77–89. [Google Scholar] [CrossRef]
- Saitoh, S.; Okano, S.; Nohara, H.; Nakano, H.; Shirasawa, N.; Naito, A.; Yamamoto, M.; Kelly, V.P.; Takahashi, K.; Tanaka, T.; et al. 5-aminolevulinic acid (ALA) deficiency causes impaired glucose tolerance and insulin resistance coincident with an attenuation of mitochondrial function in aged mice. PLoS ONE 2018, 13, e0189593. [Google Scholar] [CrossRef]
- van Wijk, K.; Akabane, T.; Kimura, T.; Saitoh, S.; Okano, S.; Kelly, V.P.; Takagi, M.; Kodama, K.; Takahashi, K.; Tanaka, T.; et al. Heterozygous disruption of ALAS1 in mice causes an accelerated age-dependent reduction in free heme, but not total heme, in skeletal muscle and liver. Arch. Biochem. Biophys. 2021, 697, 108721. [Google Scholar] [CrossRef]
- Aivado, M.; Gattermann, N.; Rong, A.; Giagounidis, A.A.N.; Prall, W.C.; Czibere, A.; Hildebrandt, B.; Haas, R.; Bottomley, S.S. X-linked sideroblastic anemia associated with a novel ALAS2 mutation and unfortunate skewed X-chromosome inactivation patterns. Blood Cells. Mol. Dis. 2006, 37, 40–45. [Google Scholar]
- Nakajima, O.; Okano, S.; Harada, H.; Kusaka, T.; Gao, X.; Hosoya, T.; Suzuki, N.; Takahashi, S.; Yamamoto, M. Transgenic rescue of erythroid 5-aminolevulinate synthase-deficient mice results in the formation of ring sideroblasts and siderocytes. Genes Cells 2006, 11, 685–700. [Google Scholar] [CrossRef]
- Ono, K.; Fujiwara, T.; Saito, K.; Nishizawa, H.; Takahashi, N.; Suzuki, C.; Ochi, T.; Kato, H.; Ishii, Y.; Onodera, K.; et al. Congenital sideroblastic anemia model due to ALAS2 mutation is susceptible to ferroptosis. Sci. Rep. 2022, 12, 9024. [Google Scholar] [CrossRef]
- Peoc’h, K.; Nicolas, G.; Schmitt, C.; Mirmiran, A.; Daher, R.; Lefebvre, T.; Gouya, L.; Karim, Z.; Puy, H. Regulation and tissue-specific expression of δ-aminolevulinic acid synthases in non-syndromic sideroblastic anemias and porphyrias. Mol. Genet. Metab. 2019, 128, 190–197. [Google Scholar] [CrossRef]
- Akabane, T.; Sagae, H.; van Wijk, K.; Saitoh, S.; Kimura, T.; Okano, S.; Kodama, K.; Takahashi, K.; Nakajima, M.; Tanaka, T.; et al. Heme deficiency in skeletal muscle exacerbates sarcopenia and impairs autophagy by reducing AMPK signaling. Sci. Rep. 2024, 14, 22147. [Google Scholar] [CrossRef] [PubMed]
- Galmozzi, A.; Kok, B.P.; Kim, A.S.; Montenegro-Burke, J.R.; Lee, J.Y.; Spreafico, R.; Mosure, S.; Albert, V.; Cintron-Colon, R.; Godio, C.; et al. PGRMC2 is an intracellular haem chaperone critical for adipocyte function. Nature 2019, 576, 138–142. [Google Scholar] [CrossRef] [PubMed]
- Ju, T.J.; Kwon, W.Y.; Kim, Y.W.; Kim, J.Y.; Kim, Y.D.; Lee, I.K.; Park, S.Y. Hemin improves insulin sensitivity in skeletal muscle in high fat-fed mice. J. Pharmacol. Sci. 2014, 126, 115–125. [Google Scholar] [CrossRef]
- Fujii, C.; Miyashita, K.; Mitsuishi, M.; Sato, M.; Fujii, K.; Inoue, H.; Hagiwara, A.; Endo, S.; Uto, A.; Ryuzaki, M.; et al. Treatment of sarcopenia and glucose intolerance through mitochondrial activation by 5-aminolevulinic acid. Sci. Rep. 2017, 7, 4013. [Google Scholar] [CrossRef]
- Nakajima, O.; Saitoh, S.; Kimura, T.; Osaki, T.; Vincent, K.P.; Takahashi, K.; Tanaka, T.; Nakajima, M. Heme Deficiency Causes Impaired Glycogen Synthesis in Skeletal Muscle Leading to Insulin Resistance. Diabetes 2018, 67, 1716. [Google Scholar] [CrossRef]
- Murray, B.; Rosenbloom, C. Fundamentals of glycogen metabolism for coaches and athletes. Nutr. Rev. 2018, 76, 243–259. [Google Scholar] [CrossRef]
- Atamna, H.; Killilea, D.W.; Killilea, A.N.; Ames, B.N. Heme deficiency may be a factor in the mitochondrial and neuronal decay of aging. Proc. Natl. Acad. Sci. USA 2002, 99, 14807–14812. [Google Scholar] [CrossRef]
- Atamna, H.; Liu, J.; Ames, B.N. Heme deficiency selectively interrupts assembly of mitochondrial complex IV in human fibroblasts: Relevance to aging. J. Biol. Chem. 2001, 276, 48410–48416. [Google Scholar] [CrossRef]
- Singh, A.; Xu, Y.J. Heme deficiency sensitizes yeast cells to oxidative stress induced by hydroxyurea. J. Biol. Chem. 2017, 292, 9088–9103. [Google Scholar] [CrossRef]
- Yuan, Q.; Zeng, Z.L.; Yang, S.; Li, A.; Zu, X.; Liu, J. Mitochondrial Stress in Metabolic Inflammation: Modest Benefits and Full Losses. Oxid. Med. Cell Longev. 2022, 2022, 8803404. [Google Scholar] [CrossRef]
- Alves de Souza, R.W.; Gallo, D.; Lee, G.R.; Katsuyama, E.; Schaufler, A.; Weber, J.; Csizmadia, E.; Tsokos, G.C.; Koch, L.G.; Britton, S.L.; et al. Skeletal muscle heme oxygenase-1 activity regulates aerobic capacity. Cell Rep. 2021, 35, 109018. [Google Scholar] [CrossRef] [PubMed]
- Mistretta, M.; Fiorito, V.; Allocco, A.L.; Ammirata, G.; Hsu, M.Y.; Digiovanni, S.; Belicchi, M.; Napoli, L.; Ripolone, M.; Trombetta, E.; et al. Flvcr1a deficiency promotes heme-based energy metabolism dysfunction in skeletal muscle. Cell Rep. 2024, 43, 113854. [Google Scholar] [CrossRef] [PubMed]
- Uchitomi, R.; Hatazawa, Y.; Senoo, N.; Yoshioka, K.; Fujita, M.; Shimizu, T.; Miura, S.; Ono, Y.; Kamei, Y. Metabolomic Analysis of Skeletal Muscle in Aged Mice. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Leduc-Gaudet, J.P.; Hussain, S.N.A.; Barreiro, E.; Gouspillou, G. Mitochondrial dynamics and mitophagy in skeletal muscle health and aging. Int. J. Mol. Sci. 2021, 22, 8179. [Google Scholar] [CrossRef]
- Xia, Q.; Huang, X.; Huang, J.; Zheng, Y.; March, M.E.; Li, J.; Wei, Y. The Role of Autophagy in Skeletal Muscle Diseases. Front. Physiol. 2021, 12, 638983. [Google Scholar] [CrossRef]
- Li, A.; Gao, M.; Liu, B.; Qin, Y.; chen, L.; Liu, H.; Wu, H.; Gong, G. Mitochondrial autophagy: Molecular mechanisms and implications for cardiovascular disease. Cell Death Dis. 2022, 13, 1–15. [Google Scholar] [CrossRef]
- Pyo, K.E.; Kim, C.R.; Lee, M.; Kim, J.S.; Kim, K.I.; Baek, S.H. ULK1 O-GlcNAcylation Is Crucial for Activating VPS34 via ATG14L during Autophagy Initiation. Cell Rep. 2018, 25, 2878–2890.e4. [Google Scholar] [CrossRef]
- Graber, T.G.; Fry, C.S.; Brightwell, C.R.; Moro, T.; Maroto, R.; Bhattarai, N.; Porter, C.; Wakamiya, M.; Rasmussen, B.B. Skeletal muscle–specific knockout of DEP domain containing 5 protein increases mTORC1 signaling, muscle cell hypertrophy, and mitochondrial respiration. J. Biol. Chem. 2019, 294, 4091–4102. [Google Scholar] [CrossRef]
- Chen, J.J.; Zhang, S. Heme-regulated eIF2α kinase in erythropoiesis and hemoglobinopathies. Blood 2019, 134, 1697–1707. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; Huo, Y.; Johns, M.; Piper, E.; Mason, J.C.; Carling, D.; Haskard, D.O.; Boyle, J.J. 5′-AMP-activated protein kinase-activating transcription factor 1 cascade modulates human monocyte-derived macrophages to atheroprotective functions in response to heme or metformin. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 2470–2480. [Google Scholar] [CrossRef] [PubMed]
- Stauder, R.; Valent, P.; Theurl, I. Anemia at older age: Etiologies, clinical implications, and management. Blood 2018, 131, 505–514. [Google Scholar] [CrossRef] [PubMed]
- Zeidan, R.S.; Martenson, M.; Tamargo, J.A.; McLaren, C.; Ezzati, A.; Lin, Y.; Yang, J.J.; Yoon, H.S.; McElroy, T.; Collins, J.F.; et al. Iron homeostasis in older adults: Balancing nutritional requirements and health risks. J. Nutr. Health Aging 2024, 28, 100212. [Google Scholar] [CrossRef]
- Immenschuh, S.; Vijayan, V.; Janciauskiene, S.; Gueler, F. Heme as a target for therapeutic interventions. Front. Pharmacol. 2017, 8, 1–15. [Google Scholar] [CrossRef]
- Lv, P.; Gao, S.; Liu, F. Heme-deficient primitive red blood cells induce HSPC ferroptosis by altering iron homeostasis during zebrafish embryogenesis. Development 2023, 150, dev201690. [Google Scholar] [CrossRef]
- Harigae, H.; Nakajima, O.; Suwabe, N.; Yokoyama, H.; Furuyama, K.; Sasaki, T.; Kaku, M.; Yamamoto, M.; Sassa, S. Aberrant iron accumulation and oxidized status of erythroid-specific delta-aminolevulinate synthase (ALAS2)-deficient definitive erythroblasts. Blood 2003, 101, 1188–1193. [Google Scholar] [CrossRef]
- Chung, J.; Chen, C.; Paw, B.H. Heme metabolism and erythropoiesis. Curr. Opin. Hematol. 2012, 19, 156–162. [Google Scholar] [CrossRef]
- Auerbach, M.; Deloughery, T.G.; Tirnauer, J.S. Iron Deficiency in Adults: A Review. Jama 2025, 21237, 1813–1823. [Google Scholar] [CrossRef]
- Dutt, S.; Hamza, I.; Bartnikas, T.B. Molecular Mechanisms of Iron and Heme Metabolism. Annu. Rev. Nutr. 2022, 42, 311–335. [Google Scholar] [CrossRef]
- Horowitz, K.M.; Ingardia, C.J.; Borgida, A.F. Anemia in Pregnancy. Clin. Lab. Med. 2013, 33, 281–291. [Google Scholar] [CrossRef]
- Watanabe, K.; Negoro, R.; Fujita, T. 5-ALA treatment increases intracellular heme levels and enhances CYP3A4 activity in genome-edited Caco-2 cells. Biochem. Biophys. Res. Commun. 2023, 664, 94–99. [Google Scholar] [CrossRef]
- Volin, L. Haem arginate treatment for hereditary sideroblastic anaemia. Eur. J. Haematol. 1989, 42, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Suragani, R.N.V.S.; Zachariah, R.S.; Velazquez, J.G.; Liu, S.; Sun, C.-W.; Townes, T.M.; Chen, J.-J. Heme-regulated eIF2α kinase activated Atf4 signaling pathway in oxidative stress and erythropoiesis. Blood 2012, 119, 5276–5284. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.J. HRI protein kinase in cytoplasmic heme sensing and mitochondrial stress response: Relevance to hematological and mitochondrial diseases. J. Biol. Chem. 2025, 301, 108494. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, W.J.; Tape, K.N.; Shackelford, J.E.; Wikander, T.M.; Richards, M.J.; Fliesler, S.J.; Krisans, S.K.; Faust, P.L. Peroxisome deficiency causes a complex phenotype because of hepatic SREBP/insig dysregulation associated with endoplasmic reticulum stress. J. Biol. Chem. 2009, 284, 7232–7245. [Google Scholar] [CrossRef]
- Peng, Y.; Li, J.; Luo, D.; Zhang, S.; Li, S.; Wang, D.; Wang, X.; Zhang, Z.; Wang, X.; Sun, C.; et al. Muscle atrophy induced by overexpression of ALAS2 is related to muscle mitochondrial dysfunction. Skelet. Muscle 2021, 11, 1–11. [Google Scholar] [CrossRef]
- Panday, A.; Sahoo, M.K.; Osorio, D.; Batra, S. NADPH oxidases: An overview from structure to innate immunity-associated pathologies. Cell Mol. Immunol. 2015, 12, 5–23. [Google Scholar] [CrossRef]
- Tejero, J.; Santolini, J.; Stuehr, D.J. Fast ferrous heme-NO oxidation in nitric oxide synthases. FEBS J. 2009, 276, 4505–4514. [Google Scholar] [CrossRef]
- Pradhan, P.; Vijayan, V.; Gueler, F.; Immenschuh, S. Interplay of heme with macrophages in homeostasis and inflammation. Int. J. Mol. Sci. 2020, 21, 740. [Google Scholar] [CrossRef]
- Saitoh, S.; Takeda, Y.; Araki, A.; Nouchi, Y.; Yamaguchi, R.; Nakajima, O.; Asao, H. 5-Aminolevulinic Acid (5-ALA) Plays an Important Role in the Function of Innate Immune Cells. Inflammation 2024. [Google Scholar] [CrossRef]
- Ma, L.; Wang, L.; Qu, Y.; Wan, X.; Hashimoto, K. A role of splenic heme biosynthesis pathway in the persistent prophylactic actions of arketamine in lipopolysaccharide-treated mice. Transl. Psychiatry 2023, 13, 1–10. [Google Scholar] [CrossRef]
- Otterbein, L.E.; Bach, F.H.; Alam, J.; Soares, M.; Lu, H.T.; Wysk, M.; Davis, R.J.; Flavell, R.A.; Choi, A.M.K. Carbon monoxide has anti-inflammatory effects involving the mitogen- activated protein kinase pathway. Nat. Med. 2000, 6, 422–428. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.F.; Zhu, Y.S.; Jiang, H.; Xu, H.; Sun, Y. Heme oxygenase-1 mediates the anti-inflammatory effect of isoflurane preconditioning in LPS-stimulated macrophages. Acta Pharmacol. Sin. 2009, 30, 228–234. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Simões, R.L.; Arruda, M.A.; Canetti, C.; Serezani, C.H.; Fierro, I.M.; Barja-Fidalgo, C. Proinflammatory responses of heme in alveolar macrophages: Repercussion in lung hemorrhagic episodes. Mediators Inflamm. 2013, 2013, 946878. [Google Scholar] [CrossRef]
- Lian, J.; Chen, J.; Wang, K.; Zhao, L.; Meng, P.; Yang, L.; Wei, J.; Ma, N.; Xu, J.; Zhang, W.; et al. ALAS1 is essential for neutrophil maturation in zebrafish. Haematologica 2018, 103, 1785–1795. [Google Scholar] [CrossRef]
- Pae, H.-O.; Chung, H.-T. Heme Oxygenase-1: Its Therapeutic Roles in Inflammatory Diseases. Immune Netw. 2009, 9, 12. [Google Scholar] [CrossRef]
- Chinta, K.C.; Pacl, H.T.; Agarwal, A.; Steyn, A.J.C. Heme oxygenase-1 as a pharmacological target for host-directed therapy to limit tuberculosis associated immunopathology. Antioxidants 2021, 10, 177. [Google Scholar] [CrossRef]
- Fujino, M.; Nishio, Y.; Ito, H.; Tanaka, T.; Li, X.K. 5-Aminolevulinic acid regulates the inflammatory response and alloimmune reaction. Int. Immunopharmacol. 2016, 37, 71–78. [Google Scholar] [CrossRef]
- Soladogun, A.S.; Zhang, L. The Neural Palette of Heme: Altered Heme Homeostasis Underlies Defective Neurotransmission, Increased Oxidative Stress, and Disease Pathogenesis. Antioxidants 2024, 13, 1441. [Google Scholar] [CrossRef]
- Mammen, P.P.A.; Shelton, J.M.; Ye, Q.; Kanatous, S.B.; McGrath, A.J.; Richardson, J.A.; Garry, D.J. Cytoglobin is a stress-responsive hemoprotein expressed in the developing and adult brain. J. Histochem. Cytochem. 2006, 54, 1349–1361. [Google Scholar] [CrossRef]
- Bilska-Wilkosz, A.; Iciek, M.; Górny, M.; Kowalczyk-Pachel, D. The role of hemoproteins: Hemoglobin, myoglobin and neuroglobin in endogenous thiosulfate production processes. Int. J. Mol. Sci. 2017, 18, 1315. [Google Scholar] [CrossRef]
- Grojean, S.; Koziel, V.; Vert, P.; Daval, J.L. Bilirubin induces apoptosis via activation of NMDA receptors in developing rat brain neurons. Exp. Neurol. 2000, 166, 334–341. [Google Scholar] [CrossRef]
- Stockley, J.H.; Vaquie, A.M.; Xu, Z.; Bartels, T.; Jordan, G.D.; Holmqvist, S.; Gunter, S.; Lam, G.; Yamamoto, D.; Pek, R.H.; et al. Oligodendrocyte Slc48a1 (Hrg1) encodes a functional heme transporter required for myelin integrity. Glia 2024, 73, 399–421. [Google Scholar] [CrossRef]
- Sengupta, A.; Hon, T.; Zhang, L. Heme deficiency suppresses the expression of key neuronal genes and causes neuronal cell death. Mol. Brain Res. 2005, 137, 23–30. [Google Scholar] [CrossRef]
- Chernova, T.; Steinert, J.R.; Guerin, C.J.; Nicotera, P.; Forsythe, I.D.; Smith, A.G. Neurite degeneration induced by heme deficiency mediated via inhibition of NMDA receptor-dependent extracellular signal-regulated kinase 1/2 activation. J. Neurosci. 2007, 27, 8475–8485. [Google Scholar] [CrossRef]
- Matsuo, K.; Asamitsu, S.; Maeda, K.; Suzuki, H.; Kawakubo, K.; Komiya, G.; Kudo, K.; Sakai, Y.; Hori, K.; Ikenoshita, S.; et al. RNA G-quadruplexes form scaffolds that promote neuropathological α-synuclein aggregation. Cell 2024, 187, 6835–6848.e20. [Google Scholar] [CrossRef]
- Hijioka, M.; Kitamura, K.; Yanagisawa, D.; Nishimura, K.; Takata, K.; Inden, M.; Kitamura, Y. Neuroprotective effects of 5-aminolevulinic acid against neurodegeneration in rat models of Parkinson’s disease and stroke. J. Pharmacol. Sci. 2020, 144, 183–187. [Google Scholar] [CrossRef]
- Atamna, H.; Boyle, K. Amyloid-β peptide binds with heme to form a peroxidase: Relationship to the cytopathologies of Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 2006, 103, 3381–3386. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, J.; Liu, L.; Wang, R.; Lai, X.; Xu, M. Interaction between amyloid-β peptide and heme probed by electrochemistry and atomic force microscopy. ACS Chem. Neurosci. 2013, 4, 535–539. [Google Scholar] [CrossRef]
- Sweeny, E.A.; Singh, A.B.; Chakravarti, R.; Martinez-Guzman, O.; Saini, A.; Haque, M.M.; Garee, G.; Dans, P.D.; Hannibal, L.; Reddi, A.R.; et al. Glyceraldehyde-3-phosphate dehydrogenase is a chaperone that allocates labile heme in cells. J. Biol. Chem. 2018, 293, 14557–14568. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Haraguchi, A.; Shigeno, R.; Ito, A.; Horie, I.; Kawakami, A.; Abiru, N. A single-arm, open-label, intervention study to investigate the improvement of glucose tolerance after administration of the 5-aminolevulinic acid (5-ALA) in the patients with mitochondrial diabetes mellitus. Medicine 2021, 100, e25100. [Google Scholar] [CrossRef] [PubMed]
- Shinoda, Y.; Kato, D.; Ando, R.; Endo, H.; Takahashi, T.; Tsuneoka, Y.; Fujiwara, Y. Systematic review and meta-analysis of in vitro anti-human cancer experiments investigating the use of 5-aminolevulinic acid (5-ALA) for photodynamic therapy. Pharmaceuticals 2021, 14, 229. [Google Scholar] [CrossRef] [PubMed]
| Related Gene/Compound | Pathology or Benefit | Article or Study Reference |
|---|---|---|
| ALAS1 | Embryonic lethality (E7.5), developmental arrest | Okano et al., 2010 [16] |
| ALAS1 | Impaired glucose tolerance, insulin resistance, mitochondrial dysfunction | Saitoh et al., 2018 [17] |
| ALAS1 | Reduced autophagy, sarcopenia (AMPK supression) | Akabane et al., 2024 [23] |
| ALAS1 | Suppressed immune response (impaired cytokine production, impaired neutrophil function) | Saitoh et al., 2024; [61] |
| ALAS1 | Glycogen storage (AMPK supression) | Nakajima et al., 2018 [27] |
| ALAS2 | Embryonic lethality (~E11.5), severe anemia, impaired erythropoiesis, iron overload | Nakajima et al., 1999 [10] Ono et al., 2022 [21] |
| ALAS2 | X-linked sideroblastic anemia (XLSA), mitochondrial iron accumulation in erythroblasts | Aivado et al., 2006 [19] Nakajima et al., 2006 [20] |
| ALAS2 | Muscle atrophy, mitochondrial dysfunction (overexpression study) | Peng et al., 2018 [57] |
| 5-ALA | Improved glucose tolerance, insulin sensitivity, mitochondrial function, improved muscle mass and muscle function | Fujii et al., 2017 [26] |
| 5-ALA | Enhanced immune response, restored cytokine production, neutrophil function, and bacterial clearance | Saitoh et al., 2024 [61] |
| 5-ALA | Reduced α-synuclein aggregation and motor decline (Parkinson’s disease model) | Matsuo et al., 2024 [77] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
van Wijk, K.; Nakajima, O. From Deficiency to Therapy: Systemic Consequences of ALAS1 Disruption and the Protective Role of 5-ALA. Life 2025, 15, 1259. https://doi.org/10.3390/life15081259
van Wijk K, Nakajima O. From Deficiency to Therapy: Systemic Consequences of ALAS1 Disruption and the Protective Role of 5-ALA. Life. 2025; 15(8):1259. https://doi.org/10.3390/life15081259
Chicago/Turabian Stylevan Wijk, Koen, and Osamu Nakajima. 2025. "From Deficiency to Therapy: Systemic Consequences of ALAS1 Disruption and the Protective Role of 5-ALA" Life 15, no. 8: 1259. https://doi.org/10.3390/life15081259
APA Stylevan Wijk, K., & Nakajima, O. (2025). From Deficiency to Therapy: Systemic Consequences of ALAS1 Disruption and the Protective Role of 5-ALA. Life, 15(8), 1259. https://doi.org/10.3390/life15081259






