Antifatigue Effects of 5-Aminolevulinic Acid Chronic Treatment on Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Drugs
2.3. The Animal Model of Fatigue
2.4. Treadmill Fatigue Test
2.5. Measurement of Adipose Tissue Mass and Blood Glucose Levels
2.6. Measurement of Monoamine Levels
2.7. Measurement of MAO Activities in Mouse FCX
2.8. Measurement of Protoporphyrin IX (PpIX) Levels
2.9. Statistical Analysis
3. Results
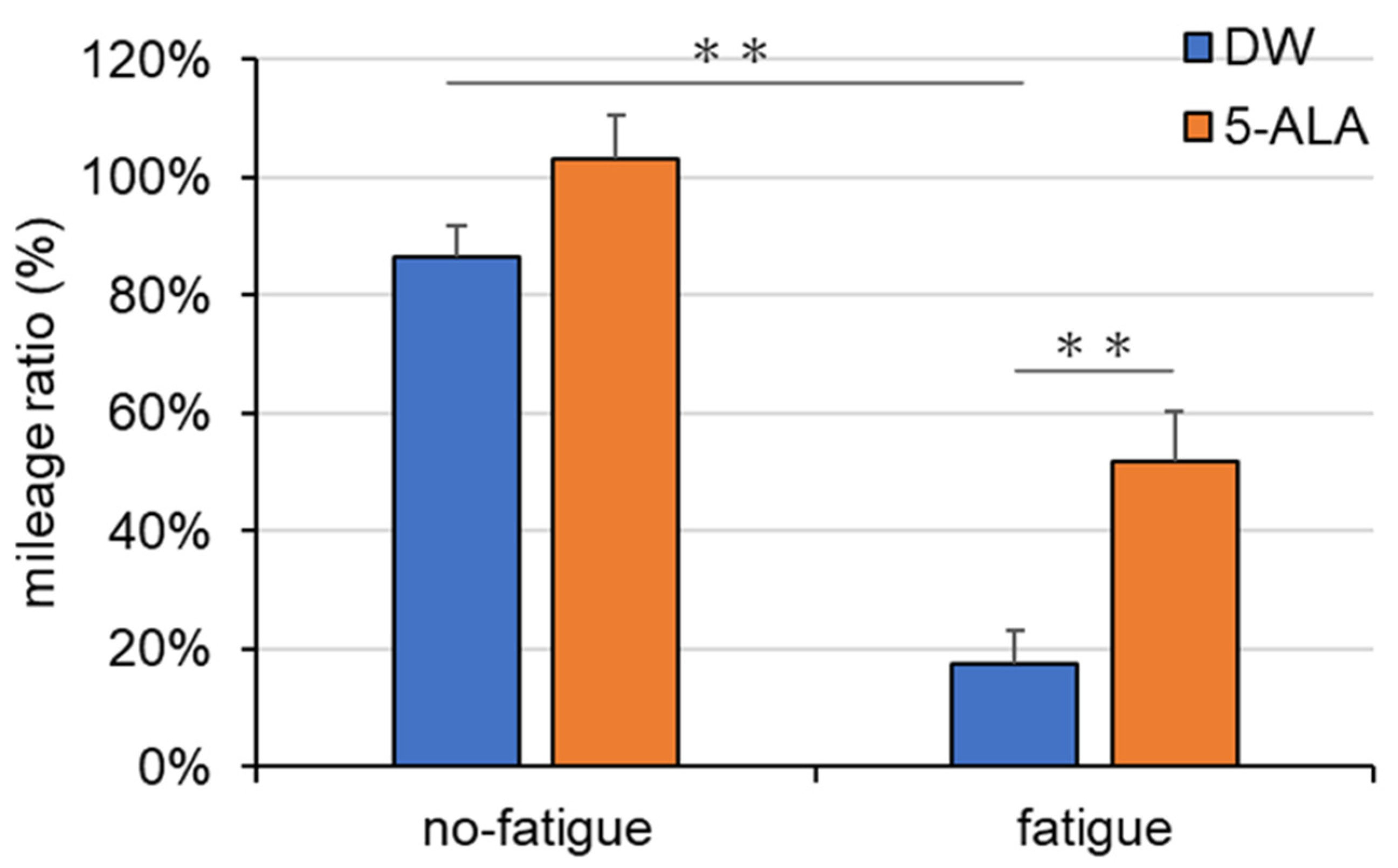
3.1. Mileage Ratio Assessment in the Treadmill Fatigue Test
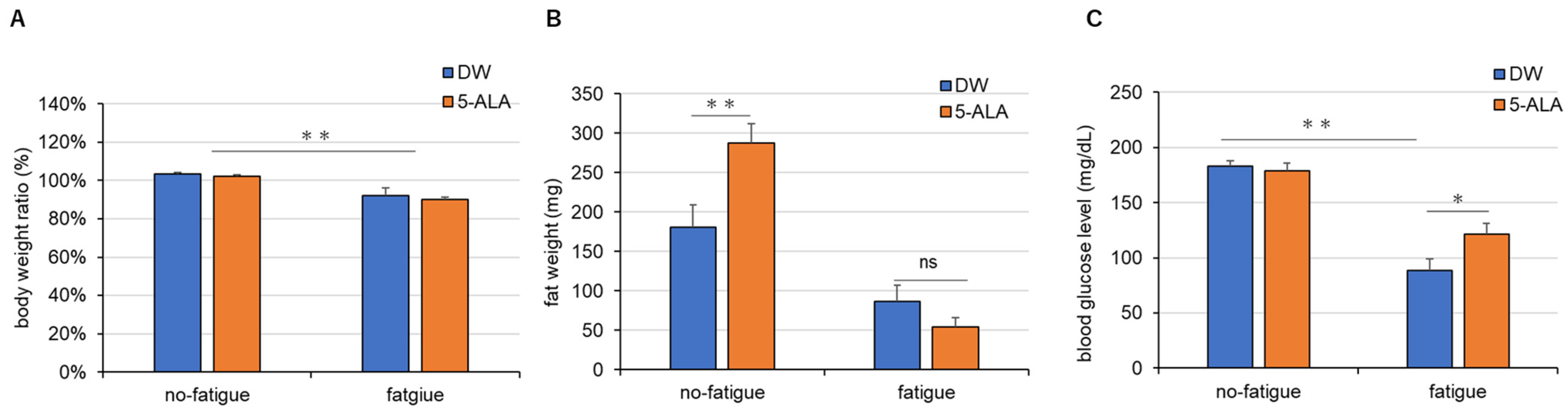
3.2. Body Weight, Adipose Mass, and Blood Glucose Levels
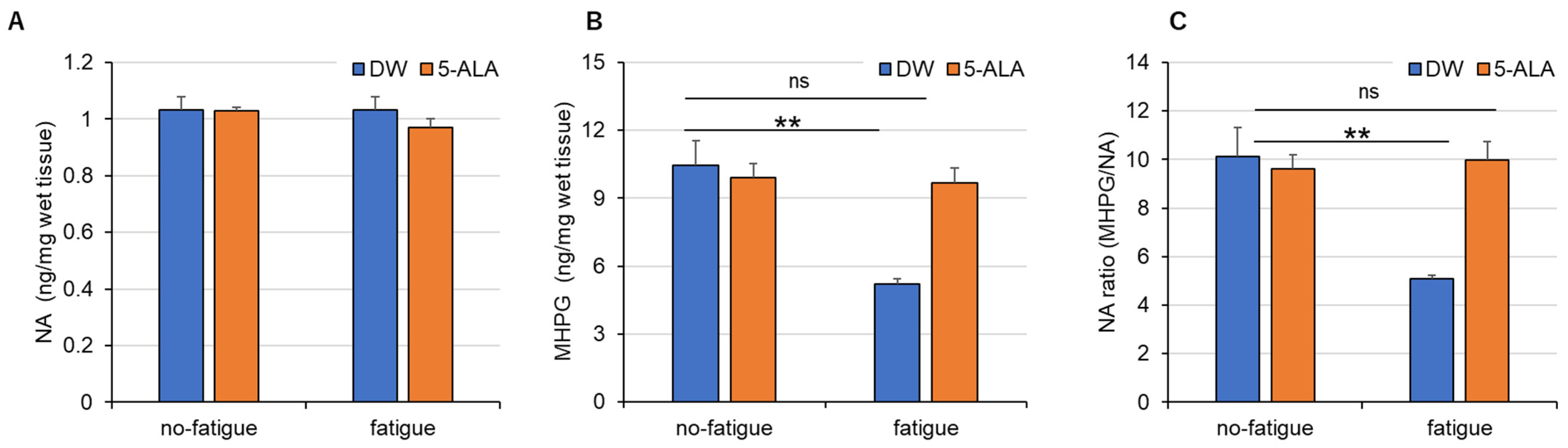
3.3. Levels of Monoamine and Its Metabolite in the FCX of Fatigued Mice
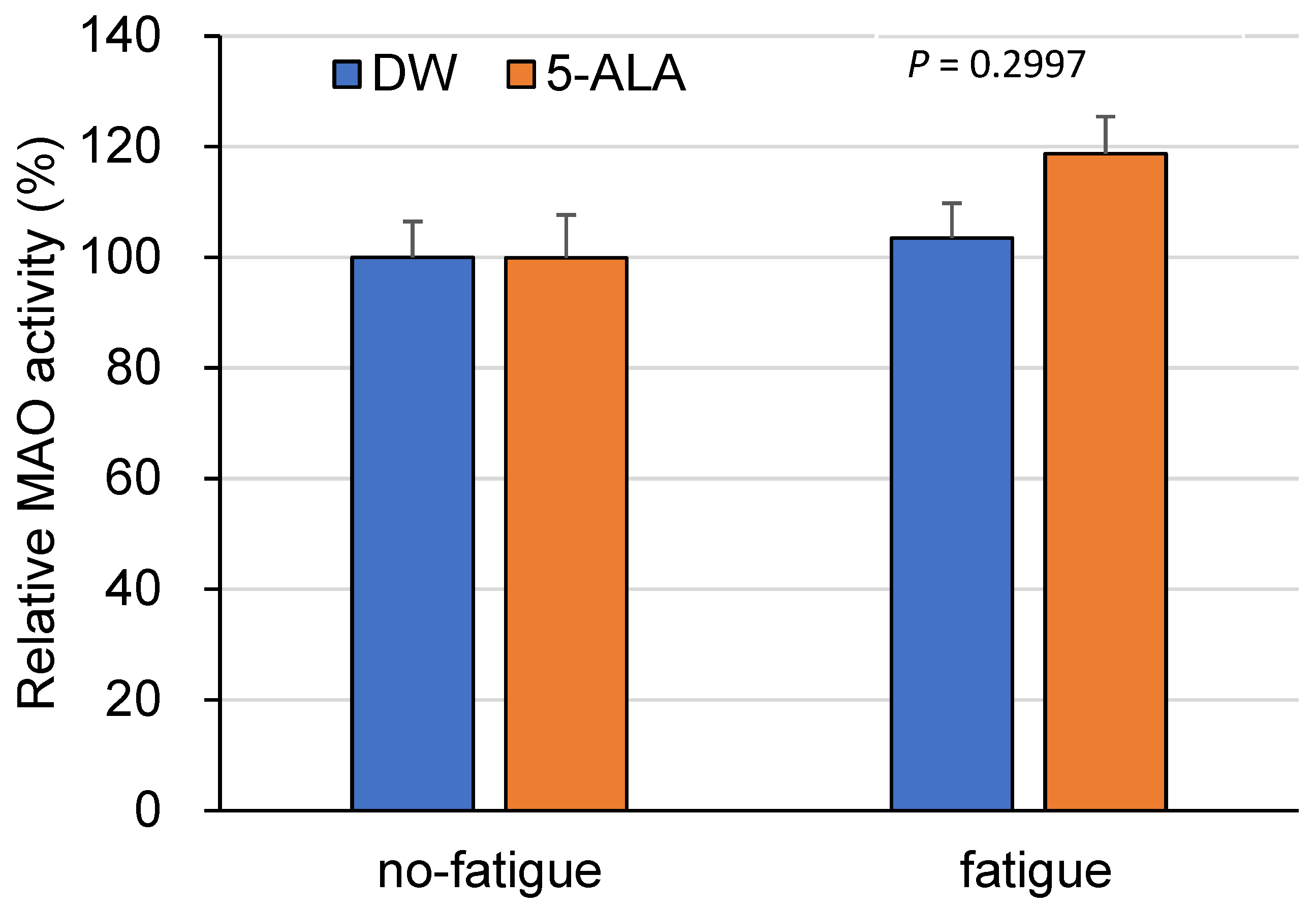
3.4. Measurement of MAO Activities in the FCX
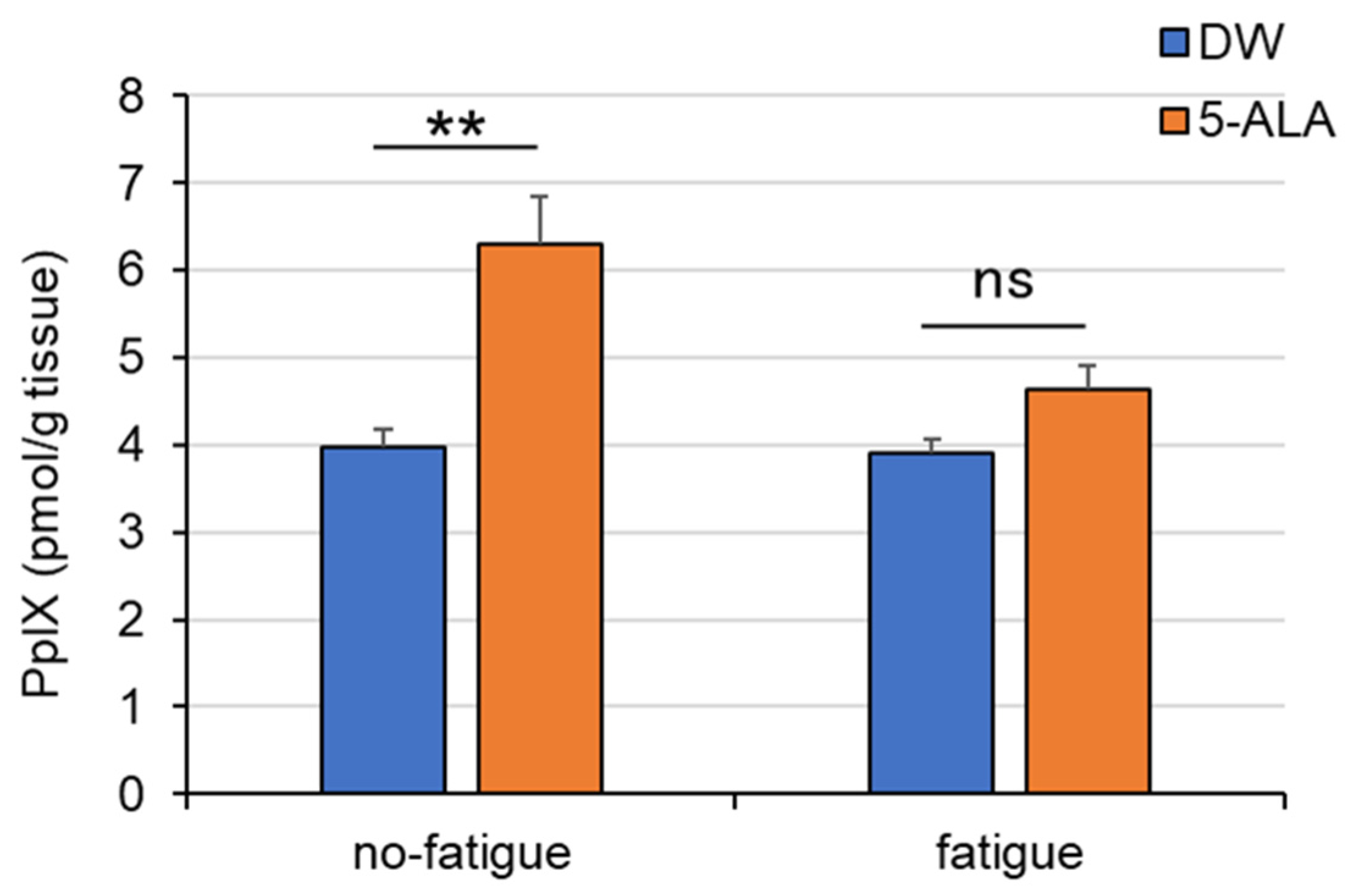
3.5. PpIX Levels in the FCX
4. Discussion
4.1. 5-ALA Has Antifatigue Effects on Fatigued Mice
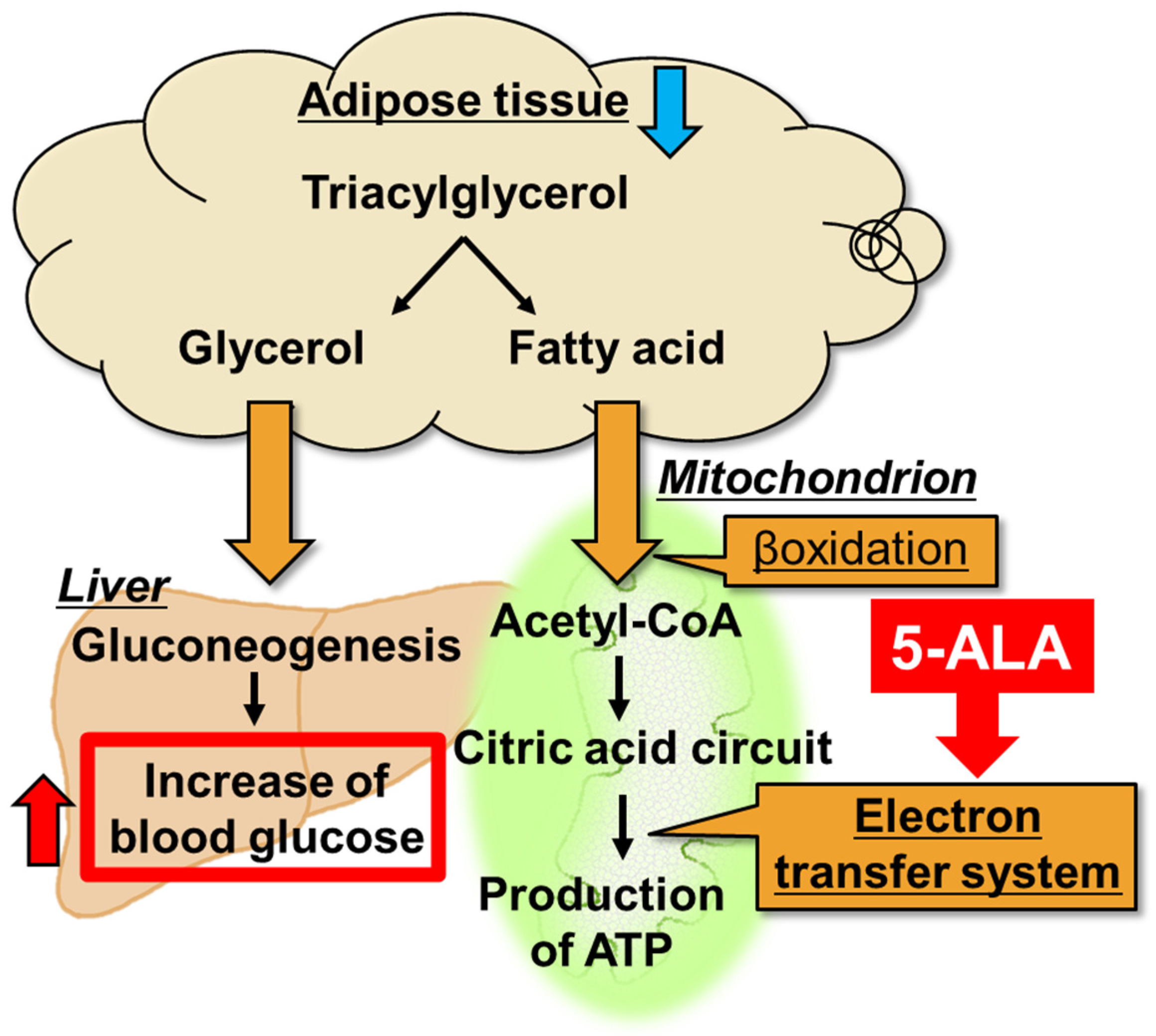
4.2. 5-ALA Improves Fatigue-Induced Hypoglycemia by Promoting the Use of Fatty Acids
4.3. 5-ALA Improves Fatigue-Induced Dysregulation of NA Systems in the FCX
4.4. 5-ALA Improves Fatigue-Induced Decreases in PpIX Concentration in the FCX
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ogura, S.; Maruyama, K.; Hagiya, Y.; Sugiyama, Y.; Tsuchiya, K.; Takahashi, K.; Abe, F.; Tabata, K.; Okura, I.; Nakajima, M.; et al. The effect of 5-aminolevulinic acid on cytochrome c oxidase activity in mouse liver. BMC Res. Notes 2011, 4, 66. [Google Scholar] [CrossRef] [PubMed]
- Higashikawa, F.; Kanno, K.; Ogata, A.; Sugiyama, M. Reduction of fatigue and anger-hostility by the oral administration of 5-aminolevulinic acid phosphate: A randomized, double-blind, placebo-controlled, parallel study. Sci. Rep. 2020, 10, 16004. [Google Scholar] [CrossRef]
- Masuki, S.; Morita, A.; Kamijo, Y.; Ikegawa, S.; Kataoka, Y.; Ogawa, Y.; Sumiyoshi, E.; Takahashi, K.; Tanaka, T.; Nakajima, M.; et al. Impact of 5-aminolevulinic acid with iron supplementation on exercise efficiency and home-based walking training achievement in older women. J. Appl. Physiol. 2016, 120, 87–96. [Google Scholar] [CrossRef]
- Suzuki, H.; Masuki, S.; Morikawa, A.; Ogawa, Y.; Kamijo, Y.I.; Takahashi, K.; Nakajima, M.; Nose, H. Effects of 5-aminolevulinic acid supplementation on home-based walking training achievement in middle-aged depressive women: Randomized, double-blind, crossover pilot study. Sci. Rep. 2018, 8, 7151. [Google Scholar] [CrossRef]
- Trounce, I.; Byrne, E.; Marzuki, S. Decline in skeletal muscle mitochondrial respiratory chain function: Possible factor in ageing. Lancet 1989, 1, 637–639. [Google Scholar] [CrossRef]
- Newsholme, E.A.; Blomstrand, E.; Ekblom, B. Physical and mental fatigue: Metabolic mechanisms and importance of plasma amino acids. Br. Med. Bull. 1992, 48, 477–495. [Google Scholar] [CrossRef] [PubMed]
- Pagliari, R.; Peyrin, L. Physical conditioning in rats influences the central and peripheral catecholamine responses to sustained exercise. Eur. J. Appl. Physiol. Occup. Physiol. 1995, 71, 41–52. [Google Scholar] [CrossRef]
- Matsui, T.; Soya, S.; Okamoto, M.; Ichitani, Y.; Kawanaka, K.; Soya, H. Brain glycogen decreases during prolonged exercise. J. Physiol. 2011, 589, 3383–3393. [Google Scholar] [CrossRef]
- Komori, T.; Nomura, J.; Inoue, K.; Kitayama, I. Tyrosine hydroxylase activity in discrete brain regions of depression model rats. Jpn. J. Psychiatry Neurol. 1990, 44, 747–754. [Google Scholar] [CrossRef] [PubMed]
- Hellriegel, E.T.; D’Mello, A.P. The effect of acute, chronic and chronic intermittent stress on the central noradrenergic system. Pharmacol. Biochem. Behav. 1997, 57, 207–214. [Google Scholar] [CrossRef]
- Redmond, D.E., Jr.; Katz, M.M.; Maas, J.W.; Swann, A.; Casper, R.; Davis, J.M. Cerebrospinal fluid amine metabolites. Relationships with behavioral measurements in depressed, manic, and healthy control subjects. Arch. Gen. Psychiatry 1986, 43, 938–947. [Google Scholar] [CrossRef] [PubMed]
- Fiedorowicz, J.G.; Swarts, K.L. The role of monoamine oxidase inhibitors in current psychiatric practice. J. Psychiatr. Pract. 2004, 10, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Nakamura, F.; Mizokawa, S.; Matsumura, A.; Nozaki, S.; Watanabe, Y. Establishment and assessment of a rat model of fatigue. Neurosci. Lett. 2003, 352, 159–162. [Google Scholar] [CrossRef]
- Komiya, S.; Takekawa, Y.; Ohmori, C.; Takahashi, J.; Koga, E.; Yamauchi, M.; Takahashi, K.; Kamiya, A.; Ishizuka, M.; Nakajima, M.; et al. 5-Aminolevulinic acid improves spatial recognition memory in mice. Eur. J. Pharmacol. 2025, 15, 177658. [Google Scholar] [CrossRef] [PubMed]
- Dougherty, J.P.; Springer, D.A.; Gershengorn, M.C. The Treadmill Fatigue Test: A Simple, High-throughput Assay of Fatigue-like Behavior for the Mouse. J. Vis. Exp. 2016, 31, e54052. [Google Scholar]
- Yoshizawa, K.; Kurono, R.; Sato, H.; Ishijima, E.; Nasu, H.; Ferdaos, N.; Suzuki, H.; Negishi, K. Effect of Sucrose on Cisplatin-induced Fatigue-like Behavior in Mice: Comparison With Fructose and Glucose. Cancer Diagn. Progn. 2021, 1, 95–102. [Google Scholar] [CrossRef]
- Saitoh, A.; Nagayama, Y.; Yamada, D.; Makino, K.; Yoshioka, T.; Yamanaka, N.; Nakatani, M.; Takahashi, Y.; Yamazaki, M.; Shigemoto, C.; et al. Disulfiram Produces Potent Anxiolytic-Like Effects Without Benzodiazepine Anxiolytics-Related Adverse Effects in Mice. Front. Pharmacol. 2022, 13, 826783. [Google Scholar] [CrossRef]
- Ota, U.; Fukuhara, H.; Ishizuka, M.; Abe, F.; Kawada, C.; Tamura, K.; Tanaka, T.; Inoue, K.; Ogura, S.I.; Shuin, T. Plasma protoporphyrin IX following administration of 5-aminolevulinic acid as a potential tumor marker. Mol. Clin. Oncol. 2015, 3, 797–801. [Google Scholar] [CrossRef]
- Bateman, L.; Bested, A.C.; Bonilla, H.F.; Chheda, B.V.; Chu, L.; Curtin, J.M.; Dempsey, T.T.; Dimmock, M.E.; Dowell, T.G.; Felsenstein, D.; et al. Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: Essentials of Diagnosis and Management. Mayo Clin. Proc. 2021, 96, 2861–2878. [Google Scholar] [CrossRef]
- Perez, M.; Shintani, T.; Rodriguez, B.; Davis, J.; Harrigan, R. The Role of 5-Aminolevulinic Acid (5-ALA) and Sleep. Int. J. Clin. Med. 2013, 4, 1–7. [Google Scholar] [CrossRef]
- Ohba, T.; Domoto, S.; Tanaka, M.; Nakamura, S.; Shimazawa, M.; Hara, H. Myalgic Encephalomyelitis/Chronic Fatigue Syndrome Induced by Repeated Forced Swimming in Mice. Biol. Pharm. Bull. 2019, 42, 1140–1145. [Google Scholar] [CrossRef]
- Hou, L.; Li, K.E.; Hu, Y.; Bian, Y.; Ji, W.; Shi, K.; Li, Y.; Chen, M.; Li, J.; Liu, X.; et al. Evaluation of a rat model of exercise-induced fatigue using treadmill running with progressively increasing load. An. Da Acad. Bras. De Cienc. 2019, 91, e20180957. [Google Scholar] [CrossRef]
- Kuryata, O.; Akimov, O.; Riabushko, M.; Kostenko, H.; Kostenko, V.; Mishchenko, A.; Nazarenko, S.; Solovyova, N.; Kostenko, V. Therapeutic potential of 5-aminolevulinic acid in metabolic disorders: Current insights and future directions. iScience 2024, 27, 111477. [Google Scholar] [CrossRef]
- Kuroda, Y.; Kamiya, A.; Ishii, T.; Ishizuka, M.; Yamashita, Y.; Ashida, H. 5-Aminolevulinic acid combined with ferrous iron improves glucose tolerance in high-fat diet-fed mice via upregulation of glucose transporter 1. Exp. Ther. Med. 2021, 22, 1454. [Google Scholar] [CrossRef]
- Ota, U.; Hara, T.; Nakagawa, H.; Tsuru, E.; Tsuda, M.; Kamiya, A.; Kuroda, Y.; Kitajima, Y.; Koda, A.; Ishizuka, M.; et al. 5-aminolevulinic acid combined with ferrous ion reduces adiposity and improves glucose tolerance in diet-induced obese mice via enhancing mitochondrial function. BMC Pharmacol. Toxicol. 2017, 18, 7. [Google Scholar] [CrossRef]
- Ahmadian, M.; Duncan, R.E.; Jaworski, K.; Sarkadi-Nagy, E.; Sul, H.S. Triacylglycerol metabolism in adipose tissue. Future Lipidol. 2007, 2, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Anand, A.; Charney, D.S. Norepinephrine dysfunction in depression. J. Clin. Psychiatry 2000, 61, 16–24. [Google Scholar] [PubMed]
- Duval, F.; Mokrani, M.C.; Monreal-ortiz, J.A.; Fattah, S.; Champeval, C.; Schulz, P.; Macher, J.P. Cortisol hypersecretion in unipolar major depression with melancholic and psychotic features: Dopaminergic, noradrenergic and thyroid correlates. Psychoneuroendocrinology 2006, 31, 876–888. [Google Scholar] [CrossRef] [PubMed]
- Fujii, C.; Miyashita, K.; Mitsuishi, M.; Sato, M.; Fujii, K.; Inoue, H.; Hagiwara, A.; Endo, S.; Uto, A.; Ryuzaki, M.; et al. Treatment of sarcopenia and glucose intolerance through mitochondrial activation by 5-aminolevulinic acid. Sci. Rep. 2017, 7, 4013. [Google Scholar] [CrossRef]
- Wang, P.; Kitayama, I.; Nomura, J. Tyrosine hydroxylase gene expression in the locus coeruleus of depression-model rats and rats exposed to short-and long-term forced walking stress. Life Sci. 1998, 62, 2083–2092. [Google Scholar] [CrossRef]
- Atamna, H.; Killilea, D.W.; Killilea, A.N.; Ames, B.N. Heme deficiency may be a factor in the mitochondrial and neuronal decay of aging. Proc. Natl. Acad. Sci. USA 2002, 99, 14807–14812. [Google Scholar] [CrossRef] [PubMed]

| Speed (m/min) × Time (min) | Inclination | |
|---|---|---|
| Warm-up | 10 m/min × 5 min | 0° |
| 15 m/min × 5 min | ||
| Test | 20 m/min × 5 min | 10° |
| 22 m/min × 5 min | ||
| 24 m/min × 5 min | ||
| 26 m/min × 5 min | ||
| 28 m/min × 5 min | ||
| 30 m/min × 5 min | ||
| 32 m/min × 5 min | ||
| 34 m/min × 5 min |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ohmori, C.; Kumamoto, E.; Kasai, S.; Okano, K.; Ota, U.; Kamiya, A.; Yamauchi, M.; Takahashi, K.; Ishizuka, M.; Yoshizawa, K.; et al. Antifatigue Effects of 5-Aminolevulinic Acid Chronic Treatment on Mice. Life 2025, 15, 1465. https://doi.org/10.3390/life15091465
Ohmori C, Kumamoto E, Kasai S, Okano K, Ota U, Kamiya A, Yamauchi M, Takahashi K, Ishizuka M, Yoshizawa K, et al. Antifatigue Effects of 5-Aminolevulinic Acid Chronic Treatment on Mice. Life. 2025; 15(9):1465. https://doi.org/10.3390/life15091465
Chicago/Turabian StyleOhmori, Chinatsu, Eiko Kumamoto, Satoka Kasai, Kotaro Okano, Urara Ota, Atsuko Kamiya, Mitsugu Yamauchi, Kiwamu Takahashi, Masahiro Ishizuka, Kazumi Yoshizawa, and et al. 2025. "Antifatigue Effects of 5-Aminolevulinic Acid Chronic Treatment on Mice" Life 15, no. 9: 1465. https://doi.org/10.3390/life15091465
APA StyleOhmori, C., Kumamoto, E., Kasai, S., Okano, K., Ota, U., Kamiya, A., Yamauchi, M., Takahashi, K., Ishizuka, M., Yoshizawa, K., Yamada, D., & Saitoh, A. (2025). Antifatigue Effects of 5-Aminolevulinic Acid Chronic Treatment on Mice. Life, 15(9), 1465. https://doi.org/10.3390/life15091465







