Impact of Sex on Lung Function in Adult Langerhans Cell Histiocytosis
Abstract
1. Introduction
2. Material and Methods
3. Results
4. Discussion
4.1. Small Airway Involvement
4.2. Diffusing Capacity of the Lungs for Carbon Monoxide
4.3. Sex-Based Functional Phenotypes: Clinical Implications
4.4. SEX-Based Functional Phenotypes: Management Implications
4.5. Study Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Goyal, G.; Tazi, A.; Go, R.S.; Rech, K.L.; Picarsic, J.L.; Vassallo, R.; Young, J.R.; Cox, C.W.; Van Laar, J.; Hermiston, M.L.; et al. International expert consensus recommendations for the diagnosis and treatment of Langerhans cell histiocytosis in adults. Blood 2022, 139, 2601–2621. [Google Scholar] [CrossRef]
- Goyal, G.; Shah, M.V.; Hook, C.C.; Wolanskyj, A.P.; Call, T.G.; Rech, K.L.; Go, R.S. Adult disseminated Langerhans cell histiocytosis: Incidence, racial disparities and long-term outcomes. Br. J. Haematol. 2018, 182, 579–581. [Google Scholar] [CrossRef] [PubMed]
- Girschikofsky, M.; Arico, M.; Castillo, D.; Chu, A.; Doberauer, C.; Fichter, J.; Haroche, J.; A Kaltsas, G.; Makras, P.; Marzano, A.V.; et al. Management of adult patients with Langerhans cell histiocytosis: Recommendations from an expert panel on behalf of Euro-Histio-Net. Orphanet J. Rare Dis. 2013, 8, 72. [Google Scholar] [CrossRef] [PubMed]
- Emile, J.-F.; Abla, O.; Fraitag, S.; Horne, A.; Haroche, J.; Donadieu, J.; Requena-Caballero, L.; Jordan, M.B.; Abdel-Wahab, O.; Allen, C.E.; et al. Revised classification of histiocytoses and neoplasms of the macrophage-dendritic cell lineages. Blood 2016, 127, 2672–2681. [Google Scholar] [CrossRef]
- Goyal, G.; Acosta-Medina, A.A.; Abeykoon, J.P.; Dai, C.; Ravindran, A.; Vassallo, R.; Ryu, J.H.; Shah, M.V.; Bennani, N.N.; Young, J.R.; et al. Long-term outcomes among adults with Langerhans cell histiocytosis. Blood Adv. 2023, 7, 6568–6578. [Google Scholar] [CrossRef]
- Jouenne, F.; Chevret, S.; Bugnet, E.; Clappier, E.; Lorillon, G.; Meignin, V.; Sadoux, A.; Cohen, S.; Haziot, A.; How-Kit, A.; et al. Genetic landscape of adult Langerhans cell histiocytosis with lung involvement. Eur. Respir. J. 2020, 55, 1901190. [Google Scholar] [CrossRef]
- Chakraborty, R.; Hampton, O.A.; Shen, X.; Simko, S.J.; Shih, A.; Abhyankar, H.; Lim, K.P.H.; Covington, K.R.; Trevino, L.; Dewal, N.; et al. Mutually exclusive recurrent somatic mutations in MAP2K1 and BRAF support a central role for ERK activation in LCH pathogenesis. Blood 2014, 124, 3007–3015. [Google Scholar] [CrossRef]
- Durham, B.H.; Rodrigo, E.L.; Picarsic, J.; Abramson, D.; Rotemberg, V.; De Munck, S.; Pannecoucke, E.; Lu, S.X.; Pastore, A.; Yoshimi, A.; et al. Activating mutations in CSF1R and additional receptor tyrosine kinases in histiocytic neoplasms. Nat. Med. 2019, 25, 1839–1842. [Google Scholar] [CrossRef]
- Vassallo, R.; Harari, S.; Tazi, A. Current understanding and management of pulmonary Langerhans cell histiocytosis. Thorax 2017, 72, 937–945. [Google Scholar] [CrossRef]
- Liu, H.; Osterburg, A.R.; Flury, J.; Swank, Z.; McGraw, D.W.; Gupta, N.; Wikenheiser-Brokamp, K.A.; Kumar, A.; Tazi, A.; Inoue, Y.; et al. MAPK mutations and cigarette smoke promote the pathogenesis of pulmonary Langerhans cell histiocytosis. JCI Insight 2020, 5, e132048. [Google Scholar] [CrossRef]
- Zahid, I.; Sohail, A.; Tahir, R.; Belardo, M.; Hooks, B. Pulmonary langerhans cell histiocytosis secondary to Marijuana use: A case report and systematic review of the literature. BMC Pulm. Med. 2025, 25, 44. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vassallo, R.; Ryu, J.H.; Schroeder, D.R.; Decker, P.A.; Limper, A.H. Clinical outcomes of pulmonary Langerhans′-cell histiocytosis in adults. N. Engl. J. Med. 2002, 346, 484–490. [Google Scholar] [CrossRef] [PubMed]
- Crausman, R.S.; Jennings, C.A.; Tuder, R.M.; Ackerson, L.M.; Irvin, C.G.; King, T.E., Jr. Pulmonary histiocytosis X: Pulmonary function and exercise pathophysiology. Am. J. Respir. Crit. Care Med. 1996, 153, 426–435. [Google Scholar] [CrossRef]
- Tazi, A.; de Margerie, C.; Naccache, J.M.; Fry, S.; Dominique, S.; Jouneau, S.; Lorillon, G.; Bugnet, E.; Chiron, R.; Wallaert, B.; et al. The natural history of adult pulmonary Langerhans cell histiocytosis: A prospective multicentre study. Orphanet J. Rare Dis. 2015, 10, 30. [Google Scholar] [CrossRef]
- Elia, D.; Torre, O.; Cassandro, R.; Caminati, A.; Harari, S. Pulmonary Langerhans cell histiocytosis: A comprehensive analysis of 40 patients and literature review. Eur. J. Intern. Med. 2015, 26, 351–356. [Google Scholar] [CrossRef]
- Miao, H.L.; Zhao, A.L.; Duan, M.H.; Zhou, D.B.; Cao, X.X.; Li, J. Clinical presentation and prognostic analysis of adult patients with Langerhans cell histiocytosis with pulmonary involvement. BMC Cancer 2020, 20, 911. [Google Scholar] [CrossRef] [PubMed]
- Kalafatis, D.; Gao, J.; Pesonen, I.; Carlson, L.; Sköld, C.M.; Ferrara, G. Gender differences at presentation of idiopathic pulmonary fibrosis in Sweden. BMC Pulm. Med. 2019, 19, 222. [Google Scholar] [CrossRef] [PubMed]
- Kawano-Dourado, L.; Glassberg, M.K.; Assayag, D.; Borie, R.; Johannson, K.A. Sex and gender in interstitial lung diseases. Eur. Respir. Rev. 2021, 30, 210105. [Google Scholar] [CrossRef] [PubMed]
- Tondo, P.; Scioscia, G.; De Pace, C.C.; Murgolo, F.; Maci, F.; Stella, G.M.; Pescatore, D.; Barbaro, M.P.F.; Lacedonia, D. Gender Differences Are a Leading Factor in 5-Year Survival of Patients with Idiopathic Pulmonary Fibrosis over Antifibrotic Therapy Reduction. Life 2025, 15, 106. [Google Scholar] [CrossRef]
- Sesé, L.; Nunes, H.; Cottin, V.; Israel-Biet, D.; Crestani, B.; Guillot-Dudoret, S.; Cadranel, J.; Wallaert, B.; Tazi, A.; Maître, B.; et al. Gender Differences in Idiopathic Pulmonary Fibrosis: Are Men and Women Equal? Front. Med. 2021, 8, 713698. [Google Scholar] [CrossRef]
- Wanat, K.A.; Rosenbach, M. Cutaneous Sarcoidosis. Clin. Chest Med. 2015, 36, 685–702. [Google Scholar] [CrossRef]
- Pasadhika, S.; Rosenbaum, J.T. Ocular Sarcoidosis. Clin. Chest Med. 2015, 36, 669–683. [Google Scholar] [CrossRef]
- De Vries, J.; Lower, E.E.; Drent, M. Quality of life in sarcoidosis: Assessment and management. Semin. Respir. Crit. Care Med. 2010, 31, 485–493. [Google Scholar] [CrossRef]
- Wijsenbeek, M.S.; Culver, D.A. Treatment of Sarcoidosis. Clin. Chest Med. 2015, 36, 751–767. [Google Scholar] [CrossRef] [PubMed]
- Parikh, K.S.; Dahhan, T.; Nicholl, L.; Ruopp, N.; Pomann, G.-M.; Fortin, T.; Tapson, V.F.; Rajagopal, S. Clinical Features and Outcomes of Patients with Sarcoidosis-associated Pulmonary Hypertension. Sci. Rep. 2019, 9, 4061. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.R.; E Shaw, D.; Avoseh, M.; Soomro, I.; Pointon, K.S.; Kokosi, M.; Nicholson, A.G.; Desai, S.R.; George, P.M. Diagnosis of cystic lung diseases: A position statement from the UK Cystic Lung Disease Rare Disease Collaborative Network. Thorax 2024, 79, 366–377. [Google Scholar] [CrossRef] [PubMed]
- Graham, B.L.; Steenbruggen, I.; Miller, M.R.; Barjaktarevic, I.Z.; Cooper, B.G.; Hall, G.L.; Hallstrand, T.S.; Kaminsky, D.A.; McCarthy, K.; McCormack, M.C.; et al. Standardization of Spirometry 2019 Update. An Official American Thoracic Society and European Respiratory Society Technical Statement. Am. J. Respir. Crit. Care Med. 2019, 200, e70–e88. [Google Scholar] [CrossRef]
- Quanjer, P.H.; Stanojevic, S.; Cole, T.J.; Baur, X.; Hall, G.L.; Culver, B.H.; Enright, P.L.; Hankinson, J.L.; Ip, M.S.M.; Zheng, J.; et al. Multi-ethnic reference values for spirometry for the 3-95-yr age range: The global lung function 2012 equations. Eur. Respir. J. 2012, 40, 1324–1343. [Google Scholar] [CrossRef]
- Graham, B.L.; Brusasco, V.; Burgos, F.; Cooper, B.G.; Jensen, R.; Kendrick, A.; MacIntyre, N.R.; Thompson, B.R.; Wanger, J. 2017 ERS/ATS standards for single-breath carbon monoxide uptake in the lung. Eur. Respir. J. 2017, 49, 1600016, Erratum in: Eur. Respir. J. 2018, 52, 1650016. [Google Scholar] [CrossRef] [PubMed]
- Robinson, P.D.; Latzin, P.; Verbanck, S.; Hall, G.L.; Horsley, A.; Gappa, M.; Thamrin, C.; Arets, H.G.; Aurora, P.; Fuchs, S.I.; et al. Consensus statement for inert gas washout measurement using multiple- and single- breath tests. Eur. Respir. J. 2013, 41, 507–522. [Google Scholar] [CrossRef]
- Götz, G.; Fichter, J. Langerhans’-cell histiocytosis in 58 adults. Eur. J. Med. Res. 2004, 9, 510–514. [Google Scholar]
- Doberauer, C.; Bornemann, C. Experiences of a Single Center in One Hundred Ninety-Four Adult Patients with Langerhans Cell Histiocytosis. J. Hematol. 2022, 11, 131–141. [Google Scholar] [CrossRef]
- Hazim, A.Z.; Ruan, G.J.; Hu, M.; Ravindran, A.; Rech, K.L.; Young, J.R.; Cox, C.W.; Abeykoon, J.P.; Scheckel, C.; Vassallo, R.; et al. Langerhans cell histiocytosis with lung involvement in isolation and multisystem disease: Staging, natural history, and comparative survival. Am. J. Hematol. 2021, 96, 1604–1610. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Gao, Y.; Pan, Z.; Jia, X.; Yan, Y.; Min, X.; Huang, K.; Jiang, T. Influence of Emphysema and Air Trapping Heterogeneity on Pulmonary Function in Patients with COPD. Int. J. Chronic Obstr. Pulm. Dis. 2019, 14, 2863–2872. [Google Scholar] [CrossRef] [PubMed]
- Suri, H.S.; Yi, E.S.; Nowakowski, G.S.; Vassallo, R. Pulmonary langerhans cell histiocytosis. Orphanet J. Rare Dis. 2012, 7, 16. [Google Scholar] [CrossRef] [PubMed]
- Yin, C.; Xie, H.; He, X.; Zhang, Y.; Zhang, A.; Li, H. Small airway dysfunction in idiopathic pulmonary fibrosis. Front. Pharmacol. 2022, 13, 1025814. [Google Scholar] [CrossRef]
- Barkas, G.I.; Daniil, Z.; Kotsiou, O.S. The Role of Small Airway Disease in Pulmonary Fibrotic Diseases. J. Pers. Med. 2023, 13, 1600. [Google Scholar] [CrossRef]
- Toumpanakis, D.; Karagiannis, K.; Paredi, P.; Bikov, A.; Bonifazi, M.; Lota, H.K.; Kalsi, H.; Minelli, C.; Dikaios, N.; Kastis, G.A.; et al. Contribution of Peripheral Airways Dysfunction to Poor Quality of Life in Sarcoidosis. Chest 2025. Mar 11:S0012-3692(25)00286-7. [Google Scholar] [CrossRef]
- Polychronopoulos, V.S.; Prakash, U.B.S. Airway involvement in sarcoidosis. Chest 2009, 136, 1371–1380. [Google Scholar] [CrossRef]
- Lill, H.; Kliiman, K.; Altraja, A. Factors signifying gender differences in clinical presentation of sarcoidosis among Estonian population. Clin. Respir. J. 2016, 10, 282–290. [Google Scholar] [CrossRef]
- Lindhe, J.; Brånemark, P.I. Changes in vascular permeability after local application of sex hormones. J. Periodontal Res. 1967, 2, 259–265. [Google Scholar] [CrossRef]
- Hechter, O.; Krohn, L.; Harris, J. The effect of estrogen on the permeability of the uterine capillaries. Endocrinology 1941, 29, 386–392. [Google Scholar] [CrossRef]
- Bouwsema, M.M.; Tedjasaputra, V.; Stickland, M.K. Are there sex differences in the capillary blood volume and diffusing capacity response to exercise? J. Appl. Physiol. 2017, 122, 460–469. [Google Scholar] [CrossRef]
- Lorillon, G.; Tazi, A. How I manage pulmonary Langerhans cell histiocytosis. Eur. Respir. Rev. 2017, 26, 170070. [Google Scholar] [CrossRef]
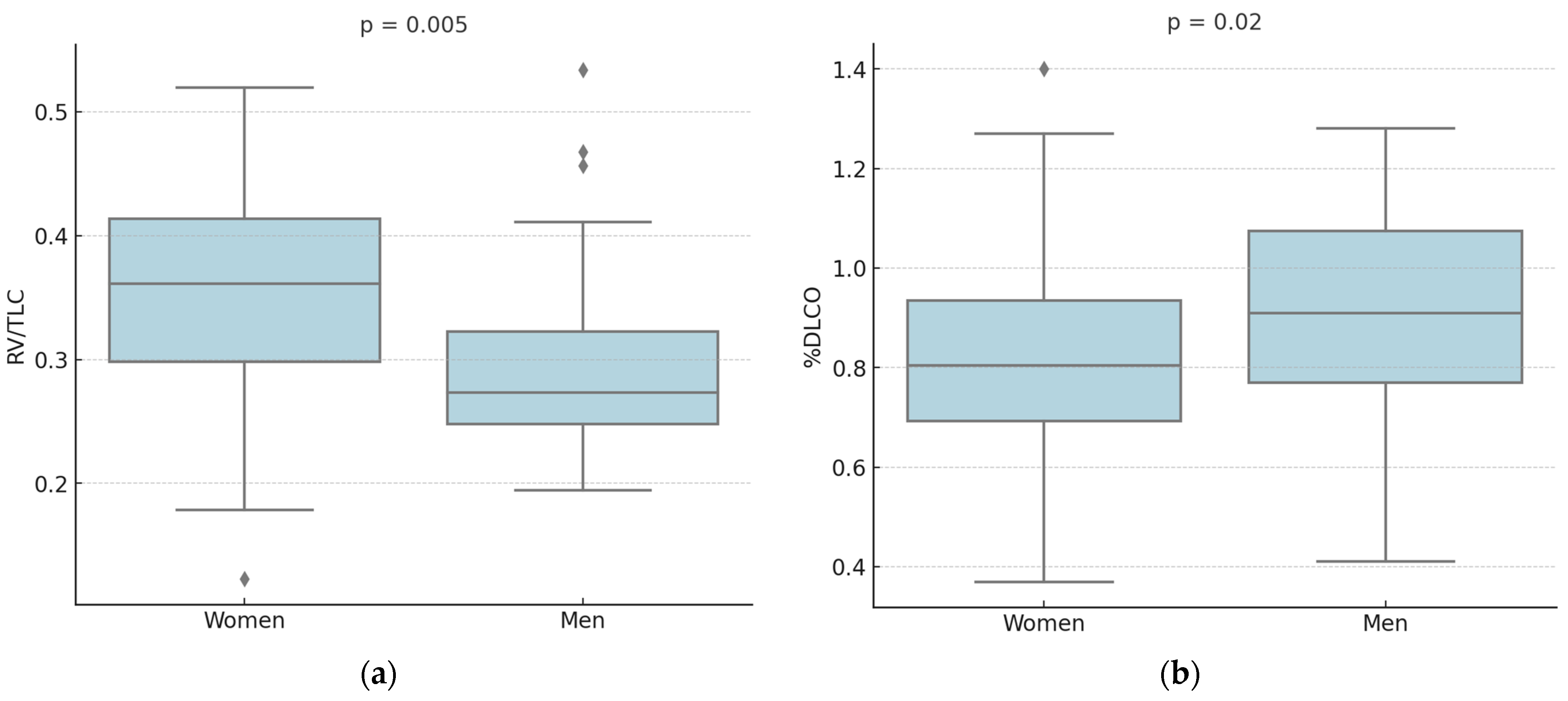

| Women (n = 47) | Men (n = 32) | p-Value | |
|---|---|---|---|
| Age, years | 45 ± 12 | 38 ± 12 | 0.007 |
| Smoking habit, n (%) | smokers: 13 (28%) | smokers: 12 (37%) | NS |
| former smokers: 25 (53%) | former smokers: 14 (44%) | ||
| no smokers: 9 (19%) | no smokers: 6 (19%) | ||
| Lung involvement, n (%) | 34 (72%) | 20 (62%) | 0.7 |
| Pack years | 12 ± 14 | 22 ± 21 | 0.021 |
| Resting dyspnea, n (%) | 12 (25.5%) | 6 (19%) | NS |
| Exertional dyspnea, n (%) | 26 (56.5%) | 11 (34.5%) | NS |
| Cough, n (%) | 18 (38%) | 8 (25%) | NS |
| FEV1, L | 2.7 ± 0.6 | 4.1 ± 1.3 | <0.001 |
| %FEV1 | 100 ± 20 | 100 ± 27 | NS |
| FVC, L | 3.4 ± 0.6 | 5.2 ± 1.2 | <0.001 |
| %FVC | 109 ± 16 | 105 ± 21 | NS |
| FEV1/FVC | 0.8 ± 0.1 | 0.8 ± 0.1 | NS |
| MEF25% | 0.59 ± 0.31 | 0.7 ± 0.4 | 0.075 |
| FEF25–75% | 0.75 ± 0.3 | 0.88 ± 0.35 | 0.08 |
| TLC, L | 5.1 ± 0.9 | 6.9 ± 1.2 | <0.001 |
| %TLC | 111 ± 19 | 109 ± 23 | NS |
| RV, L | 1.6 ± 0.6 | 2.6 ± 1 | <0.001 |
| %RV | 102 ± 36 | 115 ± 40 | NS |
| RV/TLC | 0.35 ± 0.09 | 0.3 ± 0.08 | 0.005 |
| FRC, L | 2.3 ± 0.6 | 3.4 ± 1 | <0.001 |
| %FRC | 98 ± 26 | 111 ± 34 | NS |
| %DLCO | 81 ± 22 | 92 ± 24 | 0.02 |
| %DLCO/VA | 84 ± 18 | 94 ± 18 | 0.01 |
| Independent Variable | β Coefficient | OR (95% CI) | p-Value |
|---|---|---|---|
| Intercept | 5.3 | - | |
| Resting dyspnea | 31.5 | 4.25 × 1013 (3.83 × 10−6–1.76 × 1034) | NS |
| Exertional dyspnea | 1.3 | 3.39 × 100 (3.98 × 10−1–2.89 × 101) | NS |
| %FEV1 | −42 | 3.75 × 10−19 (9.57 × 10−54–1.47 × 1016) | NS |
| %FVC | 16 | 9.41 × 108 (3.03 × 10−12–2.92 × 1025) | NS |
| RV/TLC | 1.47 | 1.35 × 106 (4.65 × 10−5–3.91 × 1016) | NS |
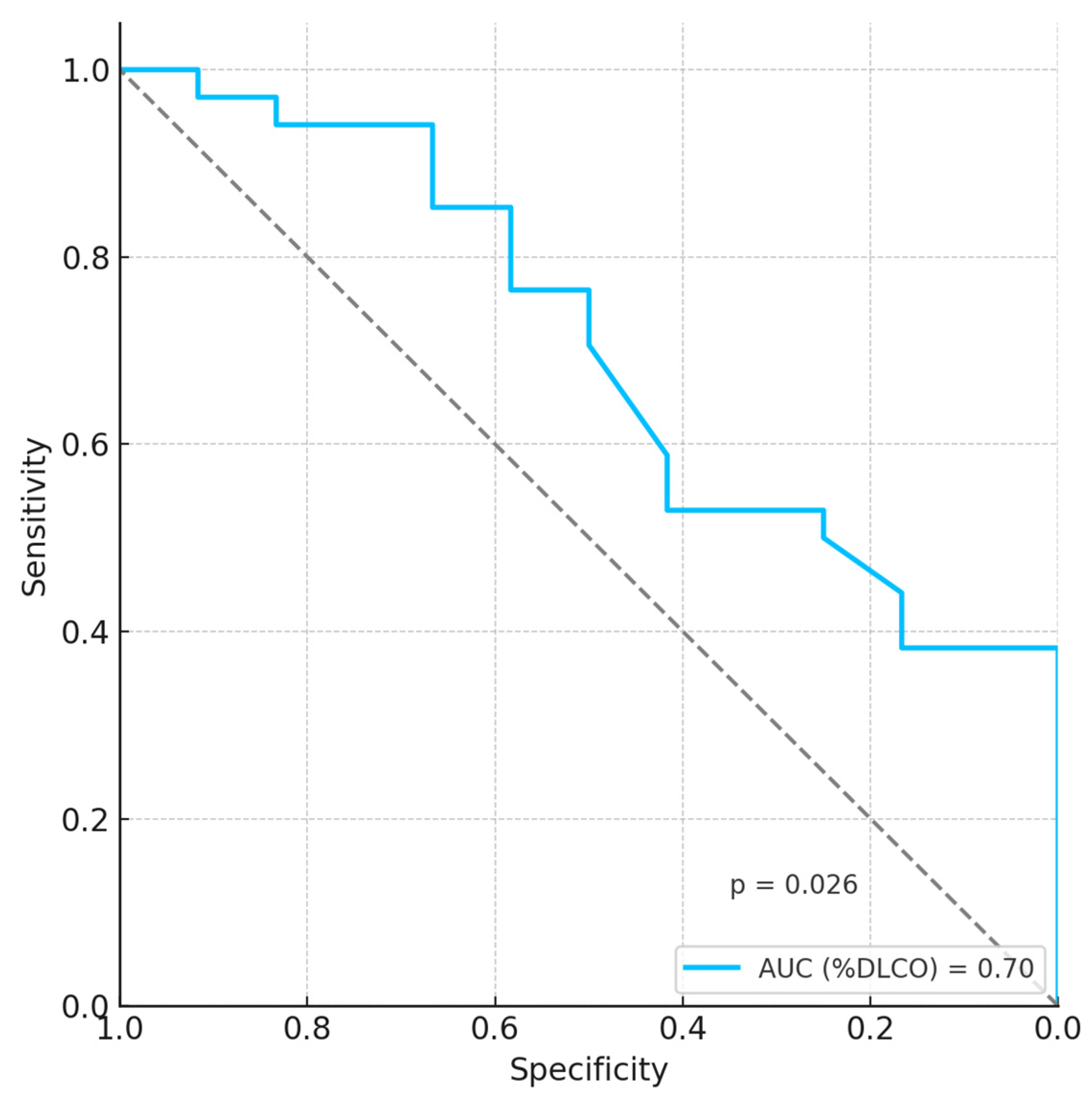
| %DLCO | −18 | 1.60 × 10−8 (2.09 × 10−15–1.22 × 10−1) | 0.02 |
| %DLCO/VA | −4 | 5.91 × 104 (3 × 10−10–1.17 × 1019) | NS |
| Independent Variable | β Coefficient | OR (95% CI) | p-Value |
|---|---|---|---|
| Intercept | 17.17 | - | |
| Resting dyspnea | 2.55 | 1.27 × 1012 (1.30 × 10−2–1.90 × 106) | NS |
| Exertional dyspnea | 28 | 1.28 × 101 (5.74 × 10−1–5.53 × 101) | NS |
| %FEV1 | −69 | 6.78 × 10−31 (4.42 × 10−62–1.04 × 101) | 0.06 |
| %FVC | 45.3 | 4.89 × 1019 (9.59 × 10−6–2.49 × 1044) | NS |
| RV/TLC | 34.4 | 8.87 × 1014 (3.60 × 10−9–2.19 × 1038) | NS |
| %DLCO | −4.4 | 0.012 (2.71 × 10−11–5.50 × 108) | NS |
| %DLCO/VA | −6.7 | 1.88 × 10−6 (5.54 × 10−15–636.36) | NS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fabozzi, A.; Paciucci, G.; de Rose, G.; Romiti, R.; Palumbo, G.; Paone, G.; Bonini, M.; Palange, P. Impact of Sex on Lung Function in Adult Langerhans Cell Histiocytosis. Life 2025, 15, 1258. https://doi.org/10.3390/life15081258
Fabozzi A, Paciucci G, de Rose G, Romiti R, Palumbo G, Paone G, Bonini M, Palange P. Impact of Sex on Lung Function in Adult Langerhans Cell Histiocytosis. Life. 2025; 15(8):1258. https://doi.org/10.3390/life15081258
Chicago/Turabian StyleFabozzi, Antonio, Gianluca Paciucci, Giulia de Rose, Roberto Romiti, Giovanna Palumbo, Gregorino Paone, Matteo Bonini, and Paolo Palange. 2025. "Impact of Sex on Lung Function in Adult Langerhans Cell Histiocytosis" Life 15, no. 8: 1258. https://doi.org/10.3390/life15081258
APA StyleFabozzi, A., Paciucci, G., de Rose, G., Romiti, R., Palumbo, G., Paone, G., Bonini, M., & Palange, P. (2025). Impact of Sex on Lung Function in Adult Langerhans Cell Histiocytosis. Life, 15(8), 1258. https://doi.org/10.3390/life15081258






