Maternal Lifestyle During Pregnancy and Its Influence on Offspring’s Telomere Length
Abstract
1. Introduction
2. Biological Role and Regulation of Telomeres in Fetal Life
2.1. Telomere Dynamics in Fetal Development
2.2. Genetic and Maternal Inheritance of TL
3. Maternal Lifestyle Factors Influencing Telomere Length
3.1. Nutrition and Dietary Patterns
3.2. Physical Activity
4. Maternal Stressors and Systemic Inflammation
4.1. Obesity
4.2. Maternal Psychological Stress
4.3. Sleep
4.4. Maternal Infection
5. Smoking, Alcohol, and Caffeine
5.1. Smoking
5.2. Alcohol
5.3. Caffeine
6. Environmental Exposures
7. Strength of Evidence Across Maternal Factors
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PA | Physical activity |
| PAHs | Polycyclic aromatic hydrocarbons |
| MD | Mediterranean diet |
| TL | Telomere length |
| ROS | Reactive oxygen species |
| SSBs | Sugar-sweetened beverages |
| PTB | Pre-term birth |
| BMI | Body mass index |
| CO | Carbon monoxide |
| COX | Cytochrome-c oxidase |
| IPF | Idiopathic pulmonary fibrosis |
| COPD | Chronic obstructive pulmonary disease |
| HPA | Hypothalamic–pituitary–adrenal |
| ACTH | Adrenocorticotropic hormone |
| ADHD | Attention-deficit/hyperactivity disorder |
| CRH | Corticotropin-releasing hormone |
| MDD | Major Depressive Disorder |
| Q-PCR | Quantitative polymerase chain reaction |
| ACOG | American College of Obstetricians and Gynecologists |
| RANZCOG | Royal Australian and New Zealand College of Obstetricians and Gynecologists |
| SOGC | Society of Obstetricians and Gynecologists of Canada |
| ADA | American Dietetic Association |
| PUFA | Polyunsaturated fatty acid |
| TAC | Total antioxidant capacity |
| WHO | World Health Organization |
References
- Moyzis, R.K.; Buckingham, J.M.; Cram, L.S.; Dani, M.; Deaven, L.L.; Jones, M.D.; Meyne, J.; Ratliff, R.L.; Wu, J.-R. A highly conserved repetitive DNA sequence, (TTAGGG)n, present at the telomeres of human chromosomes. Proc. Natl. Acad. Sci. USA 1988, 85, 6622–6626. [Google Scholar] [CrossRef]
- Gorenjak, V.; Petrelis, A.M.; Stathopoulou, M.G.; Visvikis-Siest, S. Telomere length determinants in childhood. Clin. Chem. Lab. Med. 2020, 58, 162–177. [Google Scholar] [CrossRef]
- Sanders, J.L.; Newman, A.B. Telomere length in epidemiology: A biomarker of aging, age-related disease, both, or neither? Epidemiol. Rev. 2013, 35, 112–131. [Google Scholar] [CrossRef] [PubMed]
- Entringer, S.; de Punder, K.; Buss, C.; Wadhwa, P.D. The fetal programming of telomere biology hypothesis: An update. Philos. Trans. R. Soc. B Biol. Sci. 2018, 373, 20170151. [Google Scholar] [CrossRef]
- Yu, H.J.; Byun, Y.H.; Park, C.-K. Techniques for assessing telomere length: A methodological review. Comput. Struct. Biotechnol. J. 2024, 23, 1489–1498. [Google Scholar] [CrossRef]
- Wang, Y.; Savage, S.A.; Alsaggaf, R.; Aubert, G.; Dagnall, C.L.; Spellman, S.R.; Lee, S.J.; Hicks, B.; Jones, K.; Katki, H.A. Telomere length calibration from qPCR measurement: Limitations of current method. Cells 2018, 7, 183. [Google Scholar] [CrossRef]
- De Meyer, T.; Rietzschel, E.R.; De Buyzere, M.L.; De Bacquer, D.; Van Criekinge, W.; De Backer, G.G.; Gillebert, T.C.; Van Oostveldt, P.; Bekaert, S. Paternal age at birth is an important determinant of offspring telomere length. Hum. Mol. Genet. 2007, 16, 3097–3102. [Google Scholar] [CrossRef]
- Vaiserman, A.; Krasnienkov, D. Telomere Length as a Marker of Biological Age: State-of-the-Art, Open Issues, and Future Perspectives. Front. Genet. 2020, 11, 630186. [Google Scholar] [CrossRef]
- Montemurro, T.; Lavazza, C.; Montelatici, E.; Budelli, S.; La Rosa, S.; Barilani, M.; Mei, C.; Manzini, P.; Ratti, I.; Cimoni, S.; et al. Off-the-Shelf Cord-Blood Mesenchymal Stromal Cells: Production, Quality Control, and Clinical Use. Cells 2024, 13, 1066. [Google Scholar] [CrossRef]
- Talwadekar, M.D.; Kale, V.P.; Limaye, L.S. Placenta-derived mesenchymal stem cells possess better immunoregulatory properties compared to their cord-derived counterparts-a paired sample study. Sci. Rep. 2015, 5, 15784. [Google Scholar] [CrossRef]
- Vahter, M.; Broberg, K.; Harari, F. Placental and Cord Blood Telomere Length in Relation to Maternal Nutritional Status. J. Nutr. 2020, 150, 2646–2655. [Google Scholar] [CrossRef] [PubMed]
- Gluckman, P.D.; Hanson, M.A.; Cooper, C.; Thornburg, K.L. Effect of in utero and early-life conditions on adult health and disease. N. Engl. J. Med. 2008, 359, 61–73. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Tan, K.M.L.; Gong, M.; Chong, M.F.F.; Tan, K.H.; Chong, Y.S.; Meaney, M.J.; Gluckman, P.D.; Eriksson, J.G.; Karnani, N. Variability in newborn telomere length is explained by inheritance and intrauterine environment. BMC Med. 2022, 20, 20. [Google Scholar] [CrossRef]
- Entringer, S.; Buss, C.; Wadhwa, P.D. Prenatal stress, telomere biology, and fetal programming of health and disease risk. Sci. Signal. 2012, 5, pt12. [Google Scholar] [CrossRef]
- Entringer, S.; Buss, C.; Wadhwa, P.D. Prenatal stress and developmental programming of human health and disease risk: Concepts and integration of empirical findings. Curr. Opin. Endocrinol. Diabetes Obes. 2010, 17, 507–516. [Google Scholar] [CrossRef]
- Martens, D.S.; Van Der Stukken, C.; Derom, C.; Thiery, E.; Bijnens, E.M.; Nawrot, T.S. Newborn telomere length predicts later life telomere length: Tracking telomere length from birth to child- and adulthood. eBioMedicine 2021, 63, 103164. [Google Scholar] [CrossRef]
- Okuda, K.; Bardeguez, A.; Gardner, J.P.; Rodriguez, P.; Ganesh, V.; Kimura, M.; Skurnick, J.; Awad, G.; Aviv, A. Telomere length in the newborn. Pediatr. Res. 2002, 52, 377–381. [Google Scholar] [CrossRef]
- Abu-Awwad, S.-A.; Craina, M.; Gluhovschi, A.; Ciordas, P.D.; Marian, C.; Boscu, L.; Bernad, E.; Iurciuc, M.; Abu-Awwad, A.; Iurciuc, S. Linking Pregnancy and Long-Term Health: The Impact of Cardiovascular Risk on Telomere Shortening in Pregnant Women. Medicina 2023, 59, 1012. [Google Scholar] [CrossRef]
- Levstek, T.; Kozjek, E.; Dolžan, V.; Trebušak Podkrajšek, K. Telomere Attrition in Neurodegenerative Disorders. Front. Cell. Neurosci. 2020, 14, 219. [Google Scholar] [CrossRef]
- Zheng, B.; Fu, J. Telomere dysfunction in some pediatric congenital and growth-related diseases. Front. Pediatr. 2023, 11, 1133102. [Google Scholar] [CrossRef]
- Latifovic, L.; Peacock, S.D.; Massey, T.E.; King, W.D. The Influence of Alcohol Consumption, Cigarette Smoking, and Physical Activity on Leukocyte Telomere Length. Cancer Epidemiol. Biomark. Prev. 2016, 25, 374–380. [Google Scholar] [CrossRef]
- Tsatsakis, A.; Oikonomopoulou, T.; Nikolouzakis, T.K.; Vakonaki, E.; Tzatzarakis, M.; Flamourakis, M.; Renieri, E.; Fragkiadaki, P.; Iliaki, E.; Bachlitzanaki, M. Role of telomere length in human carcinogenesis. Int. J. Oncol. 2023, 63, 78. [Google Scholar] [CrossRef]
- Baliou, S.; Pelagiadis, I.; Apetroaei, M.-M.; Vakonaki, E.; Arsene, A.L.; Hatzidaki, E.; Tzatzarakis, M.N.; Ioannou, P.; Tsatsakis, A.; Stiakaki, E. The Telomere Length Signature in Leukemias—From Molecular Mechanisms Underlying Telomere Shortening to Immunotherapeutic Options Against Telomerase. Cancers 2025, 17, 1936. [Google Scholar] [CrossRef]
- Barker, D. Fetal origins of coronary heart disease. Br. Heart J. 1993, 69, 195. [Google Scholar] [CrossRef]
- Lowensohn, R.I.; Stadler, D.D.; Naze, C. Current Concepts of Maternal Nutrition. Obstet. Gynecol. Surv. 2016, 71, 413–426. [Google Scholar] [CrossRef]
- Bischoff, C.; Graakjaer, J.; Petersen, H.C.; Hjelmborg, J.; Vaupel, J.W.; Bohr, V.; Koelvraa, S.; Christensen, K. The heritability of telomere length among the elderly and oldest-old. Twin Res. Hum. Genet. 2005, 8, 433–439. [Google Scholar] [CrossRef]
- Vasa-Nicotera, M.; Brouilette, S.; Mangino, M.; Thompson, J.R.; Braund, P.; Clemitson, J.R.; Mason, A.; Bodycote, C.L.; Raleigh, S.M.; Louis, E.; et al. Mapping of a major locus that determines telomere length in humans. Am. J. Hum. Genet. 2005, 76, 147–151. [Google Scholar] [CrossRef]
- Andrew, T.; Aviv, A.; Falchi, M.; Surdulescu, G.L.; Gardner, J.P.; Lu, X.; Kimura, M.; Kato, B.S.; Valdes, A.M.; Spector, T.D. Mapping genetic loci that determine leukocyte telomere length in a large sample of unselected female sibling pairs. Am. J. Hum. Genet. 2006, 78, 480–486. [Google Scholar] [CrossRef] [PubMed]
- Cesare, A.J.; Reddel, R.R. Alternative lengthening of telomeres: Models, mechanisms and implications. Nat. Rev. Genet. 2010, 11, 319–330. [Google Scholar] [CrossRef] [PubMed]
- Broer, L.; Codd, V.; Nyholt, D.R.; Deelen, J.; Mangino, M.; Willemsen, G.; Albrecht, E.; Amin, N.; Beekman, M.; De Geus, E.J. Meta-analysis of telomere length in 19 713 subjects reveals high heritability, stronger maternal inheritance and a paternal age effect. Eur. J. Hum. Genet. 2013, 21, 1163–1168. [Google Scholar] [CrossRef] [PubMed]
- Nawrot, T.S.; Staessen, J.A.; Gardner, J.P.; Aviv, A. Telomere length and possible link to X chromosome. Lancet 2004, 363, 507–510. [Google Scholar] [CrossRef]
- Fagan, E.; Sun, F.; Bae, H.; Elo, I.; Andersen, S.L.; Lee, J.; Christensen, K.; Thyagarajan, B.; Sebastiani, P.; Perls, T.; et al. Telomere length is longer in women with late maternal age. Menopause 2017, 24, 497–501. [Google Scholar] [CrossRef] [PubMed]
- Ye, Q.; Apsley, A.T.; Etzel, L.; Hastings, W.J.; Kozlosky, J.T.; Walker, C.; Wolf, S.E.; Shalev, I. Telomere length and chronological age across the human lifespan: A systematic review and meta-analysis of 414 study samples including 743,019 individuals. Ageing Res. Rev. 2023, 90, 102031. [Google Scholar] [CrossRef] [PubMed]
- Flor-Alemany, M.; Acosta-Manzano, P.; Migueles, J.H.; Varela-López, A.; Baena-García, L.; Quiles, J.L.; Aparicio, V.A. Influence of an exercise intervention plus an optimal Mediterranean diet adherence during pregnancy on the telomere length of the placenta. The GESTAFIT project. Placenta 2023, 136, 42–45. [Google Scholar] [CrossRef] [PubMed]
- Moshfeghinia, R.; Torabi, A.; Mostafavi, S.; Rahbar, S.; Moradi, M.S.; Sadeghi, E.; Mootz, J.; Vardanjani, H.M. Maternal psychological stress during pregnancy and newborn telomere length: A systematic review and meta-analysis. BMC Psychiatry 2023, 23, 947. [Google Scholar] [CrossRef]
- Habibi, N.; Bianco-Miotto, T.; Phoi, Y.Y.; Jankovic-Karasoulos, T.; Roberts, C.T.; Grieger, J.A. Maternal diet and offspring telomere length: A systematic review. Nutr. Rev. 2021, 79, 148–159. [Google Scholar] [CrossRef]
- Baliou, S.; Ioannou, P.; Apetroaei, M.-M.; Vakonaki, E.; Fragkiadaki, P.; Kirithras, E.; Tzatzarakis, M.N.; Arsene, A.L.; Docea, A.O.; Tsatsakis, A. The Impact of the Mediterranean Diet on Telomere Biology: Implications for Disease Management—A Narrative Review. Nutrients 2024, 16, 2525. [Google Scholar] [CrossRef]
- Herlin, M.; Broberg, K.; Igra, A.M.; Li, H.; Harari, F.; Vahter, M. Exploring telomere length in mother-newborn pairs in relation to exposure to multiple toxic metals and potential modifying effects by nutritional factors. BMC Med. 2019, 17, 77. [Google Scholar] [CrossRef]
- Andreu-Sánchez, S.; Aubert, G.; Ripoll-Cladellas, A.; Henkelman, S.; Zhernakova, D.V.; Sinha, T.; Kurilshikov, A.; Cenit, M.C.; Jan Bonder, M.; Franke, L.; et al. Genetic, parental and lifestyle factors influence telomere length. Commun. Biol. 2022, 5, 565. [Google Scholar] [CrossRef]
- Galiè, S.; Canudas, S.; Muralidharan, J.; García-Gavilán, J.; Bulló, M.; Salas-Salvadó, J. Impact of Nutrition on Telomere Health: Systematic Review of Observational Cohort Studies and Randomized Clinical Trials. Adv. Nutr. 2020, 11, 576–601. [Google Scholar] [CrossRef]
- Myers, K.O.; Boubakari, I.; Yusuf, K.K.; Mauck, D.E.; Salihu, H.M. The effect of maternal vitamin C intake on fetal telomere length. J. Matern. Fetal Neonatal Med. 2021, 34, 1143–1148. [Google Scholar] [CrossRef] [PubMed]
- Rumbold, A.; Ota, E.; Nagata, C.; Shahrook, S.; Crowther, C.A. Vitamin C supplementation in pregnancy. Cochrane Database Syst. Rev. 2015, 2015, Cd004072. [Google Scholar] [PubMed]
- Cai, Y.; Zhong, Y.D.; Zhang, H.; Lu, P.L.; Liang, Y.Y.; Hu, B.; Wu, H. Association between dietary vitamin C and telomere length: A cross-sectional study. Front. Nutr. 2023, 10, 1025936. [Google Scholar] [CrossRef] [PubMed]
- Marcon, F.; Siniscalchi, E.; Crebelli, R.; Saieva, C.; Sera, F.; Fortini, P.; Simonelli, V.; Palli, D. Diet-related telomere shortening and chromosome stability. Mutagenesis 2012, 27, 49–57. [Google Scholar] [CrossRef]
- Wagner, C.L.; Taylor, S.N.; Johnson, D.D.; Hollis, B.W. The role of vitamin D in pregnancy and lactation: Emerging concepts. Women’s Health 2012, 8, 323–340. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, G.J.; Lee, D.; Ko, J.H.; Lim, I.; Bang, H.; Koes, B.W.; Seong, B.; Lee, D.C. Higher maternal vitamin D concentrations are associated with longer leukocyte telomeres in newborns. Matern. Child Nutr. 2018, 14, e12475. [Google Scholar] [CrossRef]
- Entringer, S.; Epel, E.S.; Lin, J.; Blackburn, E.H.; Buss, C.; Shahbaba, B.; Gillen, D.L.; Venkataramanan, R.; Simhan, H.N.; Wadhwa, P.D. Maternal Folate Concentration in Early Pregnancy and Newborn Telomere Length. Ann. Nutr. Metab. 2015, 66, 202–208. [Google Scholar] [CrossRef]
- Magnano San Lio, R.; Maugeri, A.; La Rosa, M.C.; Giunta, G.; Panella, M.; Cianci, A.; Caruso, M.A.T.; Agodi, A.; Barchitta, M. Nutrient intakes and telomere length of cell-free circulating DNA from amniotic fluid: Findings from the Mamma & Bambino cohort. Sci. Rep. 2022, 12, 11671. [Google Scholar] [CrossRef]
- Zhou, D.; Li, Z.; Sun, Y.; Yan, J.; Huang, G.; Li, W. Early Life Stage Folic Acid Deficiency Delays the Neurobehavioral Development and Cognitive Function of Rat Offspring by Hindering De Novo Telomere Synthesis. Int. J. Mol. Sci. 2022, 23, 6948. [Google Scholar] [CrossRef]
- Leung, C.W.; Laraia, B.A.; Needham, B.L.; Rehkopf, D.H.; Adler, N.E.; Lin, J.; Blackburn, E.H.; Epel, E.S. Soda and cell aging: Associations between sugar-sweetened beverage consumption and leukocyte telomere length in healthy adults from the National Health and Nutrition Examination Surveys. Am. J. Public Health 2014, 104, 2425–2431. [Google Scholar] [CrossRef]
- García-Calzón, S.; Moleres, A.; Martínez-González, M.A.; Martínez, J.A.; Zalba, G.; Marti, A. Dietary total antioxidant capacity is associated with leukocyte telomere length in a children and adolescent population. Clin. Nutr. 2015, 34, 694–699. [Google Scholar] [CrossRef]
- Salihu, H.M.; Adegoke, K.K.; King, L.M.; Daas, R.; Paothong, A.; Pradhan, A.; Aliyu, M.H.; Whiteman, V.E. Effects of Maternal Carbohydrate and Fat Intake on Fetal Telomere Length. South. Med. J. 2018, 111, 591–596. [Google Scholar] [CrossRef]
- Sweeting, A.; Mijatovic, J.; Brinkworth, G.D.; Markovic, T.P.; Ross, G.P.; Brand-Miller, J.; Hernandez, T.L. The carbohydrate threshold in pregnancy and gestational diabetes: How low can we go? Nutrients 2021, 13, 2599. [Google Scholar] [CrossRef]
- Tucker, L.A. Milk Fat Intake and Telomere Length in U.S. Women and Men: The Role of the Milk Fat Fraction. Oxidative Med. Cell. Longev. 2019, 2019, 1574021. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; You, N.C.; Song, Y.; Kang, M.K.; Hou, L.; Wallace, R.; Eaton, C.B.; Tinker, L.F.; Liu, S. Intake of small-to-medium-chain saturated fatty acids is associated with peripheral leukocyte telomere length in postmenopausal women. J. Nutr. 2013, 143, 907–914. [Google Scholar] [CrossRef] [PubMed]
- Xiong, T.; Wu, Y.; Huang, L.; Chen, X.; Zhang, Y.; Zhong, C.; Gao, Q.; Hong, M.; Hu, X.; Yang, X. Association between the maternal protein nutrition status during pregnancy and the risk of preterm birth. Matern. Child Nutr. 2021, 17, e13043. [Google Scholar] [CrossRef] [PubMed]
- Switkowski, K.M.; Jacques, P.F.; Must, A.; Kleinman, K.P.; Gillman, M.W.; Oken, E. Maternal protein intake during pregnancy and linear growth in the offspring. Am. J. Clin. Nutr. 2016, 104, 1128–1136. [Google Scholar] [CrossRef]
- Kasielski, M.; Eusebio, M.O.; Pietruczuk, M.; Nowak, D. The relationship between peripheral blood mononuclear cells telomere length and diet—unexpected effect of red meat. Nutr. J. 2016, 15, 68. [Google Scholar] [CrossRef]
- Lis, N.; Lamnisos, D.; Bograkou-Tzanetakou, A.; Hadjimbei, E.; Tzanetakou, I.P. Preterm Birth and Its Association with Maternal Diet, and Placental and Neonatal Telomere Length. Nutrients 2023, 15, 4975. [Google Scholar] [CrossRef]
- Blumfield, M.L.; Collins, C.E. High-protein diets during pregnancy: healthful or harmful for offspring? Am. J. Clin. Nutr. 2014, 100, 993–995. [Google Scholar] [CrossRef]
- Zaragoza-Martí, A.; Ruiz-Ródenas, N.; Herranz-Chofre, I.; Sánchez-SanSegundo, M.; Serrano Delgado, V.C.; Hurtado-Sánchez, J.A. Adherence to the Mediterranean Diet in Pregnancy and Its Benefits on Maternal-Fetal Health: A Systematic Review of the Literature. Front. Nutr. 2022, 9, 813942. [Google Scholar] [CrossRef]
- Assaf-Balut, C.; García de la Torre, N.; Fuentes, M.; Durán, A.; Bordiú, E.; Del Valle, L.; Valerio, J.; Jiménez, I.; Herraiz, M.A.; Izquierdo, N. A high adherence to six food targets of the mediterranean diet in the late first trimester is associated with a reduction in the risk of materno-foetal outcomes: The St. Carlos gestational diabetes mellitus prevention study. Nutrients 2018, 11, 66. [Google Scholar] [CrossRef] [PubMed]
- Boccardi, V.; Esposito, A.; Rizzo, M.R.; Marfella, R.; Barbieri, M.; Paolisso, G. Mediterranean diet, telomere maintenance and health status among elderly. PLoS ONE 2013, 8, e62781. [Google Scholar] [CrossRef]
- García-Calzón, S.; Martínez-González, M.A.; Razquin, C.; Arós, F.; Lapetra, J.; Martínez, J.A.; Zalba, G.; Marti, A. Mediterranean diet and telomere length in high cardiovascular risk subjects from the PREDIMED-NAVARRA study. Clin. Nutr. 2016, 35, 1399–1405. [Google Scholar] [CrossRef]
- Meinilä, J.; Perälä, M.M.; Kautiainen, H.; Männistö, S.; Kanerva, N.; Shivappa, N.; Hébert, J.R.; Iozzo, P.; Guzzardi, M.A.; Eriksson, J.G. Healthy diets and telomere length and attrition during a 10-year follow-up. Eur. J. Clin. Nutr. 2019, 73, 1352–1360. [Google Scholar] [CrossRef]
- Sebastiani, G.; Herranz Barbero, A.; Borrás-Novell, C.; Alsina Casanova, M.; Aldecoa-Bilbao, V.; Andreu-Fernández, V.; Pascual Tutusaus, M.; Ferrero Martínez, S.; Gómez Roig, M.D.; García-Algar, O. The Effects of Vegetarian and Vegan Diet during Pregnancy on the Health of Mothers and Offspring. Nutrients 2019, 11, 557. [Google Scholar] [CrossRef]
- Agnoli, C.; Baroni, L.; Bertini, I.; Ciappellano, S.; Fabbri, A.; Papa, M.; Pellegrini, N.; Sbarbati, R.; Scarino, M.L.; Siani, V.; et al. Position paper on vegetarian diets from the working group of the Italian Society of Human Nutrition. Nutr. Metab. Cardiovasc. Dis. 2017, 27, 1037–1052. [Google Scholar] [CrossRef]
- Dwaraka, V.B.; Aronica, L.; Carreras-Gallo, N.; Robinson, J.L.; Hennings, T.; Carter, M.M.; Corley, M.J.; Lin, A.; Turner, L.; Smith, R.; et al. Unveiling the epigenetic impact of vegan vs. omnivorous diets on aging: Insights from the Twins Nutrition Study (TwiNS). BMC Med. 2024, 22, 301. [Google Scholar] [CrossRef]
- Tucker, L.A. Fruit and Vegetable Intake and Telomere Length in a Random Sample of 5448 U.S. Adults. Nutrients 2021, 13, 1415. [Google Scholar] [CrossRef]
- Tucker, L.A. Dietary Fiber and Telomere Length in 5674 U.S. Adults: An NHANES Study of Biological Aging. Nutrients 2018, 10, 400. [Google Scholar] [CrossRef]
- Bountziouka, V.; Nelson, C.P.; Wang, Q.; Musicha, C.; Codd, V.; Samani, N.J. Dietary Patterns and Practices and Leucocyte Telomere Length: Findings from the UK Biobank. J. Acad. Nutr. Diet. 2023, 123, 912–922.e26. [Google Scholar] [CrossRef] [PubMed]
- Cinegaglia, N.; Antoniazzi, L.; Rosa, D.; Miranda, D.; Acosta-Navarro, J.; Bortolotto, L.; Hong, V.; Sandrim, V. Shortening telomere is associated with subclinical atherosclerosis biomarker in omnivorous but not in vegetarian healthy men. Aging 2019, 11, 5070–5080. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, M.; Cheng, J.; Guan, S.; Hou, L.; Zu, S.; Yang, L.; Wu, H.; Li, H.; Fan, Y.; et al. Association of healthy and unhealthy plant-based diets with telomere length. Clin. Nutr. 2024, 43, 1694–1701. [Google Scholar] [CrossRef] [PubMed]
- Craig, W.J.; Mangels, A.R. Position of the American Dietetic Association: Vegetarian diets. J. Am. Diet. Assoc. 2009, 109, 1266–1282. [Google Scholar] [CrossRef]
- Richter, M.; Boeing, H.; Grünewald-Funk, D.; Heseker, H.; Kroke, A.; Leschik-Bonnet, E.; Oberritter, H.; Strohm, D.; Watzl, B. Vegan diet. Position of the German nutrition society (DGE). Ernahr. Umsch. 2016, 63, 92–102. [Google Scholar]
- Bauer, I.; Hartkopf, J.; Kullmann, S.; Schleger, F.; Hallschmid, M.; Pauluschke-Fröhlich, J.; Fritsche, A.; Preissl, H. Spotlight on the fetus: How physical activity during pregnancy influences fetal health: A narrative review. BMJ Open Sport Exerc. Med. 2020, 6, e000658. [Google Scholar] [CrossRef]
- May, L.E.; Allen, J.J.; Gustafson, K.M. Fetal and maternal cardiac responses to physical activity and exercise during pregnancy. Early Hum. Dev. 2016, 94, 49–52. [Google Scholar] [CrossRef]
- Clapp, J.F.; Kim, H., 3rd; Burciu, B.; Lopez, B. Beginning regular exercise in early pregnancy: Effect on fetoplacental growth. Am. J. Obstet. Gynecol. 2000, 183, 1484–1488. [Google Scholar] [CrossRef]
- Gomez-Cabrera, M.C.; Domenech, E.; Viña, J. Moderate exercise is an antioxidant: Upregulation of antioxidant genes by training. Free. Radic. Biol. Med. 2008, 44, 126–131. [Google Scholar] [CrossRef]
- Akay, G.G. Telomeres and Psychological Stress: Perspective on Psychopathologies. Noro Psikiyatr. Ars. 2022, 59, 330–337. [Google Scholar]
- De Lange, T. Shelterin-mediated telomere protection. Annu. Rev. Genet. 2018, 52, 223–247. [Google Scholar] [CrossRef]
- Semeraro, M.D.; Smith, C.; Kaiser, M.; Levinger, I.; Duque, G.; Gruber, H.-J.; Herrmann, M. Physical activity, a modulator of aging through effects on telomere biology. Aging 2020, 12, 13803. [Google Scholar] [CrossRef]
- Pérez, L.M.; Amaral, M.A.; Mundstock, E.; Barbé-Tuana, F.M.; Guma, F.; Jones, M.H.; Machado, D.C.; Sarria, E.E.; Marques, E.M.M.; Preto, L.T.; et al. Effects of Diet on Telomere Length: Systematic Review and Meta-Analysis. Public Health Genom. 2017, 20, 286–292. [Google Scholar] [CrossRef]
- Physical Activity and Exercise During Pregnancy and the Postpartum Period: ACOG Committee Opinion, Number 804. Obstet. Gynecol. 2020, 135, e178–e188. [CrossRef] [PubMed]
- Piercy, K.L.; Troiano, R.P.; Ballard, R.M.; Carlson, S.A.; Fulton, J.E.; Galuska, D.A.; George, S.M.; Olson, R.D. The Physical Activity Guidelines for Americans. Jama 2018, 320, 2020–2028. [Google Scholar] [CrossRef] [PubMed]
- Brown, W.J.; Hayman, M.; Haakstad, L.A.; Lamerton, T.; Mena, G.P.; Green, A.; Keating, S.E.; Gomes, G.A.; Coombes, J.S.; Mielke, G.I. Australian guidelines for physical activity in pregnancy and postpartum. J. Sci. Med. Sport 2022, 25, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Mottola, M.F.; Davenport, M.H.; Ruchat, S.M.; Davies, G.A.; Poitras, V.J.; Gray, C.E.; Jaramillo Garcia, A.; Barrowman, N.; Adamo, K.B.; Duggan, M.; et al. 2019 Canadian guideline for physical activity throughout pregnancy. Br. J. Sports Med. 2018, 52, 1339–1346. [Google Scholar] [CrossRef]
- Berghella, V.; Saccone, G. Exerc. Pregnancy! Am. J. Obstet. Gynecol. 2017, 216, 335–337. [Google Scholar] [CrossRef]
- Hinman, S.K.; Smith, K.B.; Quillen, D.M.; Smith, M.S. Exercise in Pregnancy: A Clinical Review. Sports Health 2015, 7, 527–531. [Google Scholar] [CrossRef]
- Davenport, M.H.; Ruchat, S.M.; Poitras, V.J.; Jaramillo Garcia, A.; Gray, C.E.; Barrowman, N.; Skow, R.J.; Meah, V.L.; Riske, L.; Sobierajski, F.; et al. Prenatal exercise for the prevention of gestational diabetes mellitus and hypertensive disorders of pregnancy: A systematic review and meta-analysis. Br. J. Sports Med. 2018, 52, 1367–1375. [Google Scholar] [CrossRef]
- Tsakiridis, I.; Bakaloudi, D.R.; Oikonomidou, A.C.; Dagklis, T.; Chourdakis, M. Exercise during pregnancy: A comparative review of guidelines. J. Perinat. Med. 2020, 48, 519–525. [Google Scholar] [CrossRef]
- Lazarides, C.; Epel, E.S.; Lin, J.; Blackburn, E.H.; Voelkle, M.C.; Buss, C.; Simhan, H.N.; Wadhwa, P.D.; Entringer, S. Maternal pro-inflammatory state during pregnancy and newborn leukocyte telomere length: A prospective investigation. Brain Behav. Immun. 2019, 80, 419–426. [Google Scholar] [CrossRef]
- Send, T.S.; Gilles, M.; Codd, V.; Wolf, I.; Bardtke, S.; Streit, F.; Strohmaier, J.; Frank, J.; Schendel, D.; Sütterlin, M.W. Telomere length in newborns is related to maternal stress during pregnancy. Neuropsychopharmacology 2017, 42, 2407–2413. [Google Scholar] [CrossRef]
- Tung, K.T.S.; Hung, C.M.W.; Chan, K.L.; Wong, R.S.; Tsang, H.W.; Wong, W.H.S.; Lo, C.K.M.; Tso, W.W.Y.; Chua, G.T.; Yee, B.K.; et al. Influence of Maternal Infection and Pregnancy Complications on Cord Blood Telomere Length. Oxidative Med. Cell. Longev. 2021, 2021, 3339456. [Google Scholar] [CrossRef]
- Leddy, M.A.; Power, M.L.; Schulkin, J. The impact of maternal obesity on maternal and fetal health. Rev. Obstet. Gynecol. 2008, 1, 170–178. [Google Scholar] [PubMed]
- Martens, D.S.; Plusquin, M.; Gyselaers, W.; De Vivo, I.; Nawrot, T.S. Maternal pre-pregnancy body mass index and newborn telomere length. BMC Med. 2016, 14, 148. [Google Scholar] [CrossRef] [PubMed]
- Whitaker, R.C. Predicting preschooler obesity at birth: The role of maternal obesity in early pregnancy. Pediatrics 2004, 114, e29–e36. [Google Scholar] [CrossRef] [PubMed]
- Wei, B.; Shao, Y.; Liang, J.; Tang, P.; Mo, M.; Liu, B.; Huang, H.; Tan, H.J.J.; Huang, D.; Liu, S.; et al. Maternal overweight but not paternal overweight before pregnancy is associated with shorter newborn telomere length: Evidence from Guangxi Zhuang birth cohort in China. BMC Pregnancy Childbirth 2021, 21, 283. [Google Scholar] [CrossRef]
- Maugeri, A.; Magnano San Lio, R.; La Rosa, M.C.; Giunta, G.; Panella, M.; Cianci, A.; Caruso, M.A.T.; Agodi, A.; Barchitta, M. The Relationship between Telomere Length and Gestational Weight Gain: Findings from the Mamma & Bambino Cohort. Biomedicines 2022, 10, 67. [Google Scholar]
- Reyes, F.I.; Boroditsky, R.S.; Winter, J.S.; Faiman, C. Studies on human sexual development. II. Fetal Matern. Serum Gonadotropin Sex Steroid concentrations. J. Clin. Endocrinol. Metab. 1974, 38, 612–617. [Google Scholar] [CrossRef]
- Vina, J.; Gambini, J.; Lopez-Grueso, R.; Abdelaziz, K.M.; Jove, M.; Borras, C. Females live longer than males: Role of oxidative stress. Curr. Pharm. Des. 2011, 17, 3959–3965. [Google Scholar] [CrossRef] [PubMed]
- Dolatian, M.; Sharifi, N.; Mahmoodi, Z.; Fathnezhad-kazemi, A.; Bahrami-vazir, E.; Rashidian, T. Weight gain during pregnancy and its associated factors: A Path analysis. Nurs. Open 2020, 7, 1568–1577. [Google Scholar] [CrossRef] [PubMed]
- Entringer, S.; Epel, E.S.; Lin, J.; Buss, C.; Shahbaba, B.; Blackburn, E.H.; Simhan, H.N.; Wadhwa, P.D. Maternal psychosocial stress during pregnancy is associated with newborn leukocyte telomere length. Am. J. Obstet. Gynecol. 2013, 208, e1–e7. [Google Scholar] [CrossRef] [PubMed]
- Mayer, S.E.; Guan, J.; Lin, J.; Hamlat, E.; Parker, J.E.; Brownell, K.; Price, C.; Mujahid, M.; Tomiyama, A.J.; Slavich, G.M. Intergenerational effects of maternal lifetime stressor exposure on offspring telomere length in Black and White women. Psychol. Med. 2023, 53, 6171–6182. [Google Scholar] [CrossRef]
- Leistner, C.; Menke, A. Hypothalamic-pituitary-adrenal axis and stress. Handb. Clin. Neurol. 2020, 175, 55–64. [Google Scholar]
- Lin, J.; Epel, E. Stress and telomere shortening: Insights from cellular mechanisms. Ageing Res. Rev. 2022, 73, 101507. [Google Scholar] [CrossRef]
- Salihu, H.M.; King, L.M.; Nwoga, C.; Paothong, A.; Pradhan, A.; Marty, P.J.; Daas, R.; Whiteman, V.E. Association Between Maternal-Perceived Psychological Stress and Fetal Telomere Length. South. Med. J. 2016, 109, 767–772. [Google Scholar] [CrossRef]
- Dowell, J.; Elser, B.A.; Schroeder, R.E.; Stevens, H.E. Cellular stress mechanisms of prenatal maternal stress: Heat shock factors and oxidative stress. Neurosci. Lett. 2019, 709, 134368. [Google Scholar] [CrossRef]
- Marchetto, N.M.; Glynn, R.A.; Ferry, M.L.; Ostojic, M.; Wolff, S.M.; Yao, R.; Haussmann, M.F. Prenatal stress and newborn telomere length. Am. J. Obstet. Gynecol. 2016, 215, e1–e8. [Google Scholar] [CrossRef]
- Verner, G.; Epel, E.; Lahti-Pulkkinen, M.; Kajantie, E.; Buss, C.; Lin, J.; Blackburn, E.; Räikkönen, K.; Wadhwa, P.D.; Entringer, S. Maternal Psychological Resilience During Pregnancy and Newborn Telomere Length: A Prospective Study. Am. J. Psychiatry 2021, 178, 183–192. [Google Scholar] [CrossRef]
- Ämmälä, A.-J.; Vitikainen, E.I.K.; Hovatta, I.; Paavonen, J.; Saarenpää-Heikkilä, O.; Kylliäinen, A.; Pölkki, P.; Porkka-Heiskanen, T.; Paunio, T. Maternal stress or sleep during pregnancy are not reflected on telomere length of newborns. Sci. Rep. 2020, 10, 13986. [Google Scholar] [CrossRef]
- Bosquet Enlow, M.; Petty, C.R.; Hacker, M.R.; Burris, H.H. Maternal psychosocial functioning, obstetric health history, and newborn telomere length. Psychoneuroendocrinology 2021, 123, 105043. [Google Scholar] [CrossRef] [PubMed]
- Izano, M.A.; Cushing, L.J.; Lin, J.; Eick, S.M.; Goin, D.E.; Epel, E.; Woodruff, T.J.; Morello-Frosch, R. The association of maternal psychosocial stress with newborn telomere length. PLoS ONE 2020, 15, e0242064. [Google Scholar] [CrossRef] [PubMed]
- Sedov, I.D.; Cameron, E.E.; Madigan, S.; Tomfohr-Madsen, L.M. Sleep quality during pregnancy: A meta-analysis. Sleep Med. Rev. 2018, 38, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Song, L.; Fan, G.; Wu, M.; Bi, J.; Xu, L.; Xiong, C.; Xia, W.; Cao, Z.; Xu, S.; et al. Associations of self-reported sleep duration and sleep quality during pregnancy with newborn telomere length. Sleep Health 2023, 9, 475–481. [Google Scholar] [CrossRef]
- Salihu, H.M.; King, L.; Patel, P.; Paothong, A.; Pradhan, A.; Louis, J.; Naik, E.; Marty, P.J.; Whiteman, V. Association between maternal symptoms of sleep disordered breathing and fetal telomere length. Sleep 2015, 38, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Auriti, C.; De Rose, D.U.; Santisi, A.; Martini, L.; Piersigilli, F.; Bersani, I.; Ronchetti, M.P.; Caforio, L. Pregnancy and viral infections: Mechanisms of fetal damage, diagnosis and prevention of neonatal adverse outcomes from cytomegalovirus to SARS-CoV-2 and Zika virus. Biochim. Et Biophys. Acta Mol. Basis Dis. 2021, 1867, 166198. [Google Scholar] [CrossRef]
- Liu, B.; Maekawa, T.; Chatton, B.; Ishii, S. In utero TNF-α treatment induces telomere shortening in young adult mice in an ATF7-dependent manner. FEBS Open Bio. 2016, 6, 56–63. [Google Scholar] [CrossRef]
- Maekawa, T.; Liu, B.; Nakai, D.; Yoshida, K.; Nakamura, K.-i.; Yasukawa, M.; Koike, M.; Takubo, K.; Chatton, B.; Ishikawa, F. ATF7 mediates TNF-α–induced telomere shortening. Nucleic Acids Res. 2018, 46, 4487–4504. [Google Scholar] [CrossRef]
- Banerjee, S.; Deacon, A.; Suter, M.A.; Aagaard, K.M. Understanding the Placental Biology of Tobacco Smoke, Nicotine, and Marijuana (THC) Exposures During Pregnancy. Clin. Obstet. Gynecol. 2022, 65, 347–359. [Google Scholar] [CrossRef]
- Garrabou, G.; Hernàndez, A.S.; Catalán García, M.; Morén, C.; Tobías, E.; Córdoba, S.; López, M.; Figueras, F.; Grau, J.M.; Cardellach, F. Molecular basis of reduced birth weight in smoking pregnant women: Mitochondrial dysfunction and apoptosis. Addict. Biol. 2016, 21, 159–170. [Google Scholar] [CrossRef]
- Chełchowska, M.; Gajewska, J.; Ambroszkiewicz, J.; Mazur, J.; Ołtarzewski, M.; Maciejewski, T.M. Influence of Oxidative Stress Generated by Smoking during Pregnancy on Glutathione Status in Mother-Newborn Pairs. Antioxidants 2021, 10, 1866. [Google Scholar] [CrossRef]
- Valdes, A.M.; Andrew, T.; Gardner, J.P.; Kimura, M.; Oelsner, E.; Cherkas, L.F.; Aviv, A.; Spector, T.D. Obesity, cigarette smoking, and telomere length in women. Lancet 2005, 366, 662–664. [Google Scholar] [CrossRef]
- Liu, B.; Song, L.; Zhang, L.; Wu, M.; Wang, L.; Cao, Z.; Xiong, C.; Zhang, B.; Li, Y.; Xia, W.; et al. Prenatal second-hand smoke exposure and newborn telomere length. Pediatr. Res. 2020, 87, 1081–1085. [Google Scholar] [CrossRef]
- Mirzakhani, H.; De Vivo, I.; Leeder, J.S.; Gaedigk, R.; Vyhlidal, C.A.; Weiss, S.T.; Tantisira, K. Early pregnancy intrauterine fetal exposure to maternal smoking and impact on fetal telomere length. Eur. J. Obstet. Gynecol. Reprod. Biol. 2017, 218, 27–32. [Google Scholar] [CrossRef]
- Salihu, H.M.; Pradhan, A.; King, L.; Paothong, A.; Nwoga, C.; Marty, P.J.; Whiteman, V. Impact of intrauterine tobacco exposure on fetal telomere length. Am. J. Obstet. Gynecol. 2015, 212, e1–e8. [Google Scholar] [CrossRef] [PubMed]
- Ip, P.; Chung, B.H.; Ho, F.K.; Chan, G.C.; Deng, W.; Wong, W.H.; Lee, S.L.; Chan, P.Y.; Ying, D.; Wong, W.L.; et al. Prenatal Tobacco Exposure Shortens Telomere Length in Children. Nicotine Tob. Res. 2017, 19, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Theall, K.P.; McKasson, S.; Mabile, E.; Dunaway, L.F.; Drury, S.S. Early hits and long-term consequences: Tracking the lasting impact of prenatal smoke exposure on telomere length in children. Am. J. Public Health 2013, 103, S133–S135. [Google Scholar] [CrossRef] [PubMed]
- Almanzar, G.; Eberle, G.; Lassacher, A.; Specht, C.; Koppelstaetter, C.; Heinz-Erian, P.; Trawöger, R.; Bernhard, D.; Prelog, M. Maternal cigarette smoking and its effect on neonatal lymphocyte subpopulations and replication. BMC Pediatr. 2013, 13, 57. [Google Scholar] [CrossRef]
- Wadhwa, P.D.; Buss, C.; Entringer, S.; Swanson, J.M. Developmental origins of health and disease: Brief history of the approach and current focus on epigenetic mechanisms. Semin. Reprod. Med. 2009, 27, 358–368. [Google Scholar] [CrossRef]
- Howell, M.P.; Jones, C.W.; Herman, C.A.; Mayne, C.V.; Fernandez, C.; Theall, K.P.; Esteves, K.C.; Drury, S.S. Impact of prenatal tobacco smoking on infant telomere length trajectory and ADHD symptoms at 18 months: A longitudinal cohort study. BMC Med. 2022, 20, 153. [Google Scholar] [CrossRef]
- Maugeri, A.; Barchitta, M.; Magnano San Lio, R.; La Rosa, M.C.; La Mastra, C.; Favara, G.; Ferlito, M.; Giunta, G.; Panella, M.; Cianci, A. The effect of alcohol on telomere length: A systematic review of epidemiological evidence and a pilot study during pregnancy. Int. J. Environ. Res. Public Health 2021, 18, 5038. [Google Scholar] [CrossRef]
- Topiwala, A.; Taschler, B.; Ebmeier, K.P.; Smith, S.; Zhou, H.; Levey, D.F.; Codd, V.; Samani, N.J.; Gelernter, J.; Nichols, T.E.; et al. Alcohol consumption and telomere length: Mendelian randomization clarifies alcohol’s effects. Mol. Psychiatry 2022, 27, 4001–4008. [Google Scholar] [CrossRef] [PubMed]
- Schölin, L.; Watson, J.; Dyson, J.; Smith, L.A. Alcohol Guidelines for Pregnant Women: Barriers and Enablers for Midwives to Deliver Advice; Institute of Alcohol Studies: London, UK, 2019. [Google Scholar]
- Lui, S.; Jones, R.L.; Robinson, N.J.; Greenwood, S.L.; Aplin, J.D.; Tower, C.L. Detrimental effects of ethanol and its metabolite acetaldehyde, on first trimester human placental cell turnover and function. PLoS ONE 2014, 9, e87328. [Google Scholar] [CrossRef] [PubMed]
- Harpaz, T.; Abumock, H.; Beery, E.; Edel, Y.; Lahav, M.; Rozovski, U.; Uziel, O. The Effect of Ethanol on Telomere Dynamics and Regulation in Human Cells. Cells 2018, 7, 169. [Google Scholar] [CrossRef] [PubMed]
- Griffin, I.; Ibrahimou, B.; Navejar, N.; Aggarwal, A.; Myers, K.; Mauck, D.; Yusuf, K.K.; Wudil, U.J.; Aliyu, M.H.; Salihu, H.M. Maternal Caffeine Consumption and Racial Disparities in Fetal Telomere Length. Int. J. MCH AIDS 2020, 9, 14–21. [Google Scholar] [CrossRef]
- Knutti, R.; Rothweiler, H.; Schlatter, C. Effect of pregnancy on the pharmacokinetics of caffeine. Eur. J. Clin. Pharmacol. 1981, 21, 121–126. [Google Scholar] [CrossRef]
- Rhee, J.; Kim, R.; Kim, Y.; Tam, M.; Lai, Y.; Keum, N.; Oldenburg, C.E. Maternal Caffeine Consumption during Pregnancy and Risk of Low Birth Weight: A Dose-Response Meta-Analysis of Observational Studies. PLoS ONE 2015, 10, e0132334. [Google Scholar] [CrossRef]
- World Health Organization. WHO Recommendations on Antenatal Care for a Positive Pregnancy Experience; World Health Organization: Geneva, Switzerland, 2016. [Google Scholar]
- Gynecologists, ACoOa. ACOG CommitteeOpinion No. 462: Moderate caffeine consumption during pregnancy. Obstet. Gynecol. 2010, 116 Pt 1, 467–468. [Google Scholar] [CrossRef]
- Hlisníková, H.; Petrovičová, I.; Kolena, B.; Šidlovská, M.; Sirotkin, A. Effects and Mechanisms of Phthalates’ Action on Reproductive Processes and Reproductive Health: A Literature Review. Int. J. Environ. Res. Public Health 2020, 17, 6811. [Google Scholar] [CrossRef]
- Drwal, E.; Rak, A.; Gregoraszczuk, E.L. Review: Polycyclic aromatic hydrocarbons (PAHs)-Action on placental function and health risks in future life of newborns. Toxicology 2019, 411, 133–142. [Google Scholar] [CrossRef]
- Michels, K.B.; De Vivo, I.; Calafat, A.M.; Binder, A.M. In utero exposure to endocrine-disrupting chemicals and telomere length at birth. Environ. Res. 2020, 182, 109053. [Google Scholar] [CrossRef] [PubMed]
- Khoshhali, M.; Amin, M.M.; Fatehizadeh, A.; Ebrahimi, A.; Taheri, E.; Kelishadi, R. Impact of prenatal triclosan exposure on gestational age and anthropometric measures at birth: A systematic review and meta-analysis. J. Res. Med. Sci. 2020, 25, 61. [Google Scholar] [PubMed]
- Zhang, Y.; Dong, S.; Wang, H.; Tao, S.; Kiyama, R. Biological impact of environmental polycyclic aromatic hydrocarbons (ePAHs) as endocrine disruptors. Environ. Pollut. 2016, 213, 809–824. [Google Scholar] [CrossRef] [PubMed]
- Nie, J.; Li, J.; Cheng, L.; Deng, Y.; Li, Y.; Yan, Z.; Duan, L.; Niu, Q.; Tang, D. Prenatal polycyclic aromatic hydrocarbons metabolites, cord blood telomere length, and neonatal neurobehavioral development. Environ. Res. 2019, 174, 105–113. [Google Scholar] [CrossRef]
- Song, L.; Liu, B.; Wu, M.; Zhang, L.; Wang, L.; Zhang, B.; Xiong, C.; Li, Y.; Cao, Z.; Wang, Y.; et al. Prenatal Exposure to Phthalates and Newborn Telomere Length: A Birth Cohort Study in Wuhan, China. Environ. Health Perspect 2019, 127, 87007. [Google Scholar] [CrossRef]
- Weatherly, L.M.; Gosse, J.A. Triclosan exposure, transformation, and human health effects. J. Toxicol. Environ. Health Part B Crit. Rev. 2017, 20, 447–469. [Google Scholar] [CrossRef]
- Ma, R.; Tang, N.; Feng, L.; Wang, X.; Zhang, J.; Ren, X.; Du, Y.; Ouyang, F. Effects of triclosan exposure on placental extravillous trophoblast motility, relevant IGF2/H19 signaling and DNA methylation-related enzymes of HTR-8/SVneo cell line. Ecotoxicol. Environ. Saf. 2021, 228, 113051. [Google Scholar] [CrossRef]
- Parolini, M.; De Felice, B.; Mondellini, S.; Caprioli, M.; Possenti, C.D.; Rubolini, D. Prenatal exposure to triclosan induced brain telomere shortening in a wild bird species. Environ. Toxicol. Pharmacol. 2021, 87, 103718. [Google Scholar] [CrossRef]
- Rosen, E.M.; Muñoz, M.I.; McElrath, T.; Cantonwine, D.E.; Ferguson, K.K. Environmental contaminants and preeclampsia: A systematic literature review. J. Toxicol. Environ. Health 2018, 21, 291–319. [Google Scholar] [CrossRef]
- Canudas, S.; Becerra-Tomás, N.; Hernández-Alonso, P.; Galié, S.; Leung, C.; Crous-Bou, M.; De Vivo, I.; Gao, Y.; Gu, I.; Meinilä, C.; et al. Mediterranean Diet and Telomere Length: A Systematic Review and Meta-Analysis. Adv. Nutr. 2020, 11, 1544–1554. [Google Scholar] [CrossRef]
- Zhang, R.; Du, J.; Xiao, Z.; Jiang, Y.; Jin, L.; Weng, Q. Association between the peripartum maternal and fetal telomere lengths and mitochondrial DNA copy numbers and preeclampsia: A prospective case–control study. BMC Pregnancy Childbirth 2022, 22, 483. [Google Scholar] [CrossRef]
| Organization | Recommended Duration of Physical Activity |
|---|---|
| American College of Obstetricians and Gynecologists (ACOG) [84] | 150 min of moderate-intensity aerobic exercise each week |
| U.S. Department of Health and Human Services [85] | 150 min of moderate-intensity aerobic exercise each week |
| Royal Australian and New Zealand College of Obstetricians and Gynecologists (RAZCOG) [86] | 150–300 min of regular exercise weekly or 70–150 min of vigorous exercise weekly (with sessions not exceeding 60 min) |
| Society of Obstetricians and Gynecologists of Canada (SOGC) [87] | No official exercise duration recommendation |
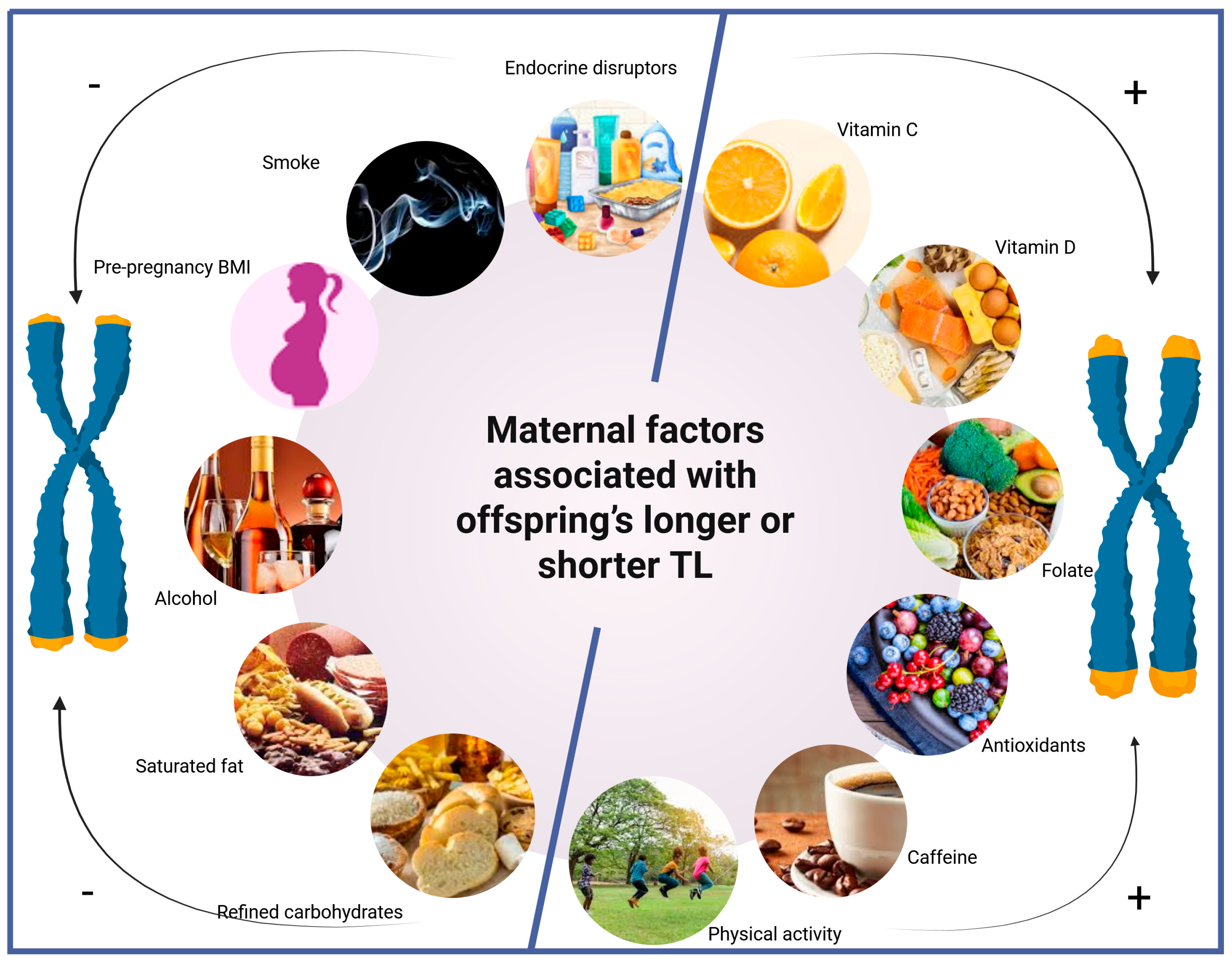
| Maternal Factor | Association with Offspring’s TL | Key Studies * |
|---|---|---|
| Vitamin C intake | + | [41,43] |
| Vitamin D status | + | [36,46] |
| Folate intake | + | [47,49,133] |
| Magnesium | ± | [11] |
| Mediterranean-style diet (MD) | + (female), ± (male) | [40,64,153] |
| High-antioxidant foods | + | [51] |
| Refined carbohydrates/high glycemic load | – | [50,51,52] |
| Saturated fat intake | – | [36,54,55] |
| Alcohol consumption | – | [127,132] |
| Caffeine intake | + | [36,137] |
| Physical activity + MD | + | [34] |
| Maternal pre-pregnancy BMI | – | [96,98] |
| Adequate gestational weight gain | + | [91] |
| Maternal stress | –/± | [93,103,111] |
| Poor sleep/sleep-disordered breathing | –/± | [111,115,116] |
| Smoking (active) | –/+ | [124,125,126,127,128]; outlier [129] |
| Second-hand smoke | – | [124] |
| Triclosan exposure | – (male-specific) | [144,151] |
| Phthalate exposure | ± | [144,148] |
| PAH exposure | – | [147] |
| Maternal infection (bacterial or viral) | – | [94,118] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vakonaki, E.; Vitiadou, M.T.; Panteris, E.; Tzatzarakis, M.; Tsatsakis, A.; Hatzidaki, E. Maternal Lifestyle During Pregnancy and Its Influence on Offspring’s Telomere Length. Life 2025, 15, 1250. https://doi.org/10.3390/life15081250
Vakonaki E, Vitiadou MT, Panteris E, Tzatzarakis M, Tsatsakis A, Hatzidaki E. Maternal Lifestyle During Pregnancy and Its Influence on Offspring’s Telomere Length. Life. 2025; 15(8):1250. https://doi.org/10.3390/life15081250
Chicago/Turabian StyleVakonaki, Elena, Maria Theodora Vitiadou, Eleftherios Panteris, Manolis Tzatzarakis, Aristides Tsatsakis, and Eleftheria Hatzidaki. 2025. "Maternal Lifestyle During Pregnancy and Its Influence on Offspring’s Telomere Length" Life 15, no. 8: 1250. https://doi.org/10.3390/life15081250
APA StyleVakonaki, E., Vitiadou, M. T., Panteris, E., Tzatzarakis, M., Tsatsakis, A., & Hatzidaki, E. (2025). Maternal Lifestyle During Pregnancy and Its Influence on Offspring’s Telomere Length. Life, 15(8), 1250. https://doi.org/10.3390/life15081250








