Breaking Down Osteoarthritis: Exploring Inflammatory and Mechanical Signaling Pathways
Abstract
1. Introduction
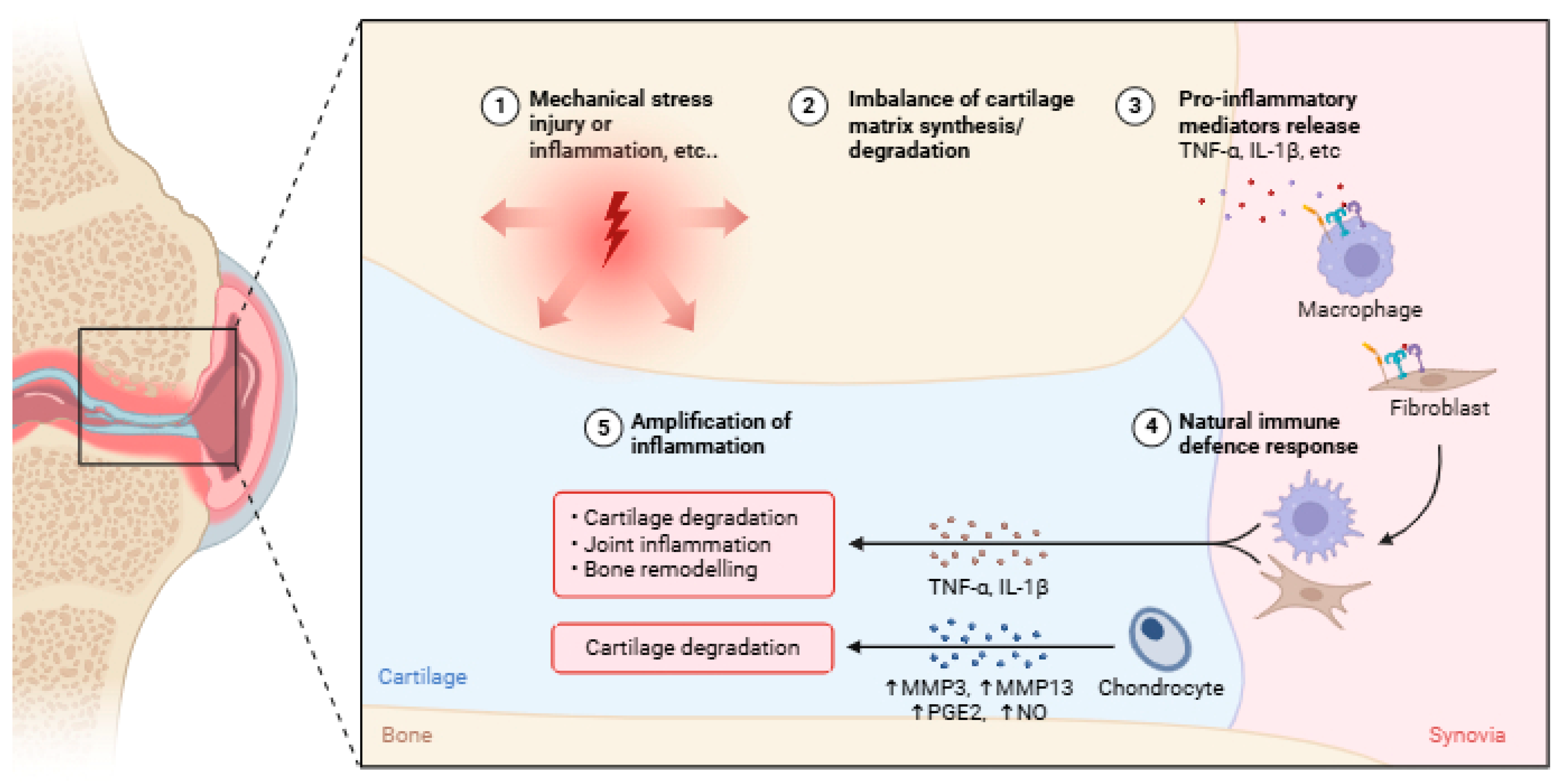
2. Unravelling the Pathophysiology of OA
3. Inflammatory Response in OA
4. Inflammatory Signaling Pathways in OA
4.1. NF-kB Signaling Pathway
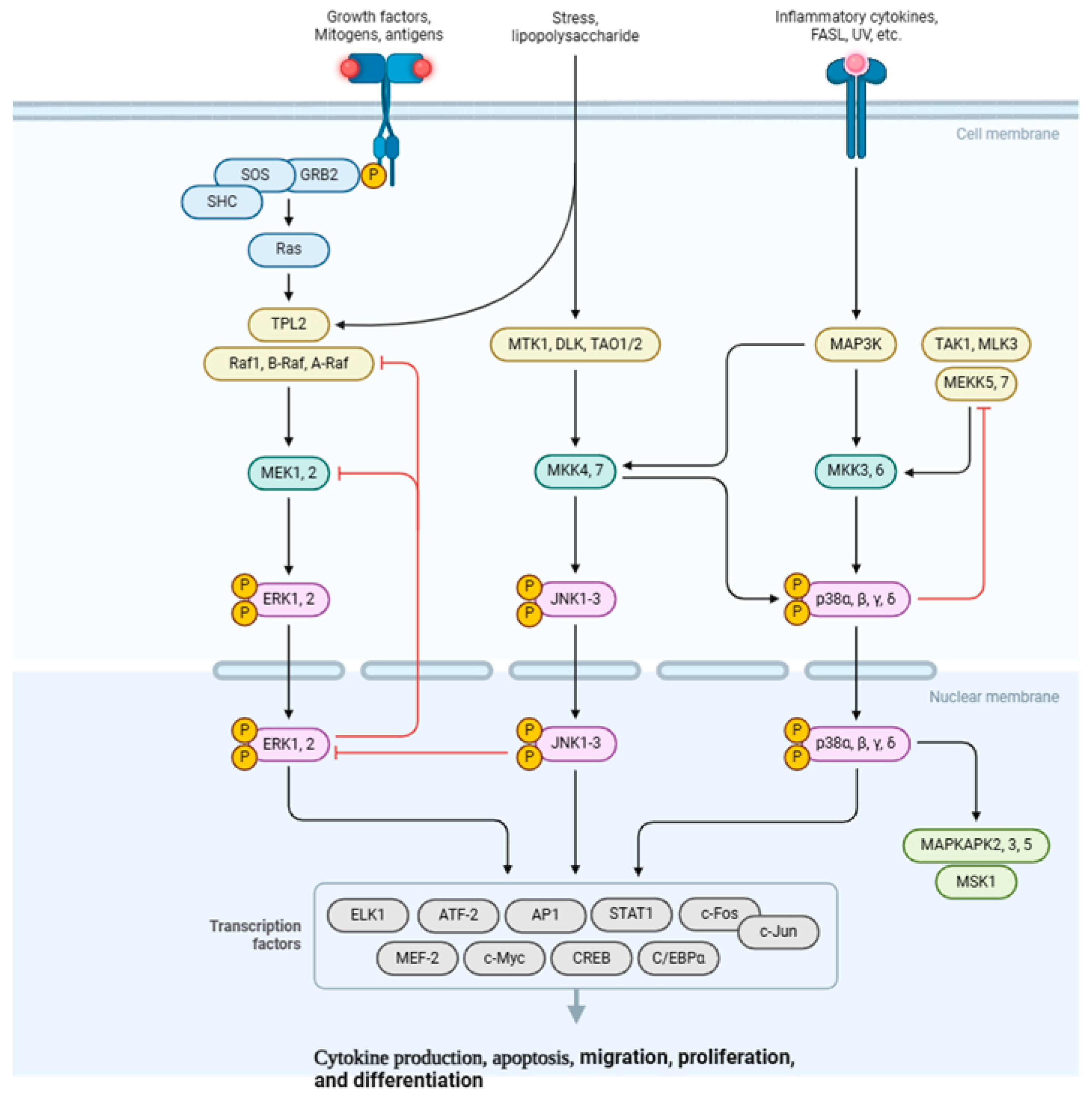
4.2. MAPK Signaling Pathway
4.3. PI3K/AKT/mTOR Signaling Pathway
4.4. JAK/STAT Signaling Pathway
5. Mechanical Stress in OA
6. Mechanotransduction Signaling Pathways in OA
6.1. Wnt/β-Catenin Pathway
6.2. Integrin-FAK Pathway
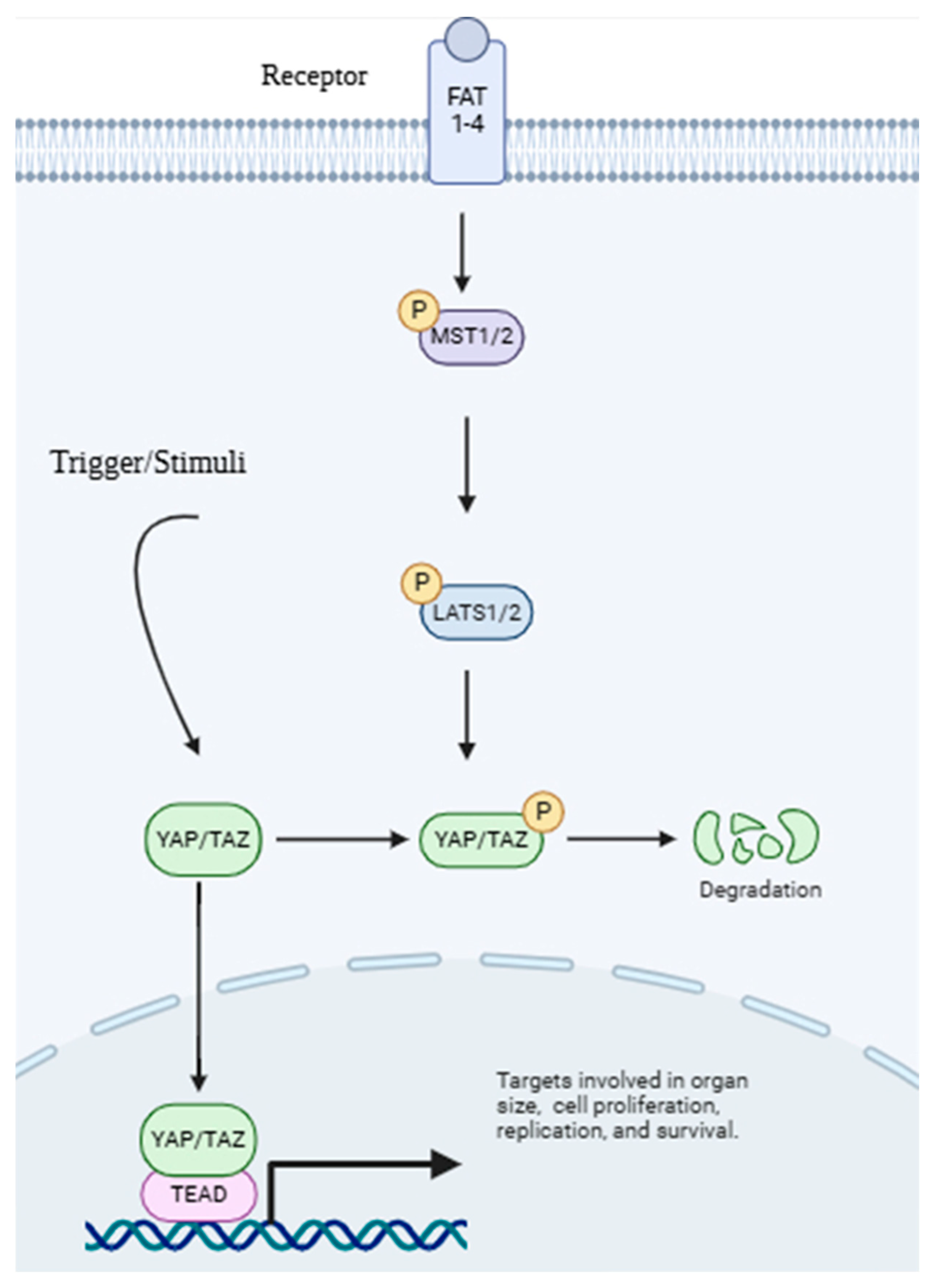
6.3. Hippo–YAP/TAZ Pathway
7. Interplay Between Inflammatory and Mechanical Signaling
8. Emerging Therapeutic Strategies Targeting Inflammatory and Mechanical Pathways in OA
8.1. Small-Molecule Inhibitors
8.2. Biologics
8.3. Regenerative Medicine
8.3.1. Cell-Based Therapies
8.3.2. Tissue Engineering
8.3.3. Gene Therapy
8.4. Non-Pharmacological Approaches
8.4.1. Mechanical Unloading
8.4.2. Joint Offloading Devices
8.4.3. Controlled Joint Disuse
8.4.4. Mechanotherapy
8.4.5. Structured Physical Rehabilitation
9. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhong, Y.; Xu, Y.; Xue, S.; Zhu, L.; Lu, H.; Wang, C.; Chen, H.; Sang, W.; Ma, J. Nangibotide attenuates osteoarthritis by inhibiting osteoblast apoptosis and TGF-β activity in subchondral bone. Inflammopharmacology 2022, 30, 1107–1117. [Google Scholar] [CrossRef]
- Pang, Z.; Jiang, Z.; Zhu, R.; Song, C.; Tang, H.; Cao, L.; Guo, C. Bardoxolone-methyl prevents oxidative stress-mediated apoptosis and extracellular matrix degradation in vitro and alleviates osteoarthritis in vivo. Drug Des. Dev. Ther. 2021, 4, 3735–3747. [Google Scholar] [CrossRef]
- Katz, J.N.; Arant, K.R.; Loeser, R.F. Diagnosis and treatment of hip and knee osteoarthritis: A review. JAMA 2021, 325, 568–578. [Google Scholar] [CrossRef]
- Cui, A.; Li, H.; Wang, D.; Zhong, J.; Chen, Y.; Lu, H. Global, regional prevalence, incidence and risk factors of knee osteoarthritis in population-based studies. EClinicalMedicine 2020, 29. [Google Scholar] [CrossRef]
- Lee, Y.T.; Mohd Yunus, M.H.; Yazid, M.D.; Ugusman, A. Unraveling the path to osteoarthritis management: Targeting chondrocyte apoptosis for therapeutic intervention. Front. Cell Dev. Biol. 2024, 12, 1347126. [Google Scholar] [CrossRef]
- Hunter, D.J.; Schofield, D.; Callander, E. The individual and socioeconomic impact of osteoarthritis. Nat. Rev. Rheumatol. 2014, 10, 437–441. [Google Scholar] [CrossRef] [PubMed]
- Latourte, A.; Kloppenburg, M.; Richette, P. Emerging pharmaceutical therapies for osteoarthritis. Nat. Rev. Rheumatol. 2020, 16, 673–688. [Google Scholar] [CrossRef]
- Bhagat, R.; Saudagar, R. Osteoarthritis: Pathophysiology and current treatment modalities. J. Drug Deliv. Ther. 2019, 9, 661–668. [Google Scholar] [CrossRef]
- Ibounig, T.; Simons, T.; Launonen, A.; Paavola, M. Glenohumeral osteoarthritis: An overview of etiology and diagnostics. Scand. J. Surg. 2021, 110, 441–451. [Google Scholar] [CrossRef]
- Mao, L.; Wu, W.; Wang, M.; Guo, J.; Li, H.; Zhang, S.; Xu, J.; Zou, J. Targeted treatment for osteoarthritis: Drugs and delivery system. Drug Deliv. 2021, 28, 1861–1876. [Google Scholar] [CrossRef] [PubMed]
- Kuyinu, E.L.; Narayanan, G.; Nair, L.S.; Laurencin, C.T. Animal models of osteoarthritis: Classification, update, and measurement of outcomes. J. Orthop. Surg. Res. 2016, 11, 19. [Google Scholar] [CrossRef]
- Musumeci, G.; Aiello, F.C.; Szychlinska, M.A.; Di Rosa, M.; Castrogiovanni, P.; Mobasheri, A. Osteoarthritis in the XXIst century: Risk factors and behaviours that influence disease onset and progression. Int. J. Mol. Sci. 2015, 16, 6093–6112. [Google Scholar] [CrossRef]
- Sukhikh, S.; Noskova, S.; Ivanova, S.; Ulrikh, E.; Izgaryshev, A.; Babich, O. Chondroprotection and molecular mechanism of action of phytonutraceuticals on osteoarthritis. Molecules 2021, 26, 2391. [Google Scholar] [CrossRef]
- Mohd Yunus, M.H.; Lee, Y.; Nordin, A.; Chua, K.H.; Bt Hj Idrus, R. Remodeling Osteoarthritic Articular Cartilage under Hypoxic Conditions. Int. J. Mol. Sci. 2022, 23, 5356. [Google Scholar] [CrossRef]
- Lee, Y.T.; Yunus, M.H.M.; Ugusman, A.; Yazid, M.D. Natural Compounds Affecting Inflammatory Pathways of Osteoarthritis. Antioxidants 2022, 11, 1722. [Google Scholar] [CrossRef]
- Man, G.; Mologhianu, G. Osteoarthritis pathogenesis—A complex process that involves the entire joint. J. Med. Life 2014, 7, 37. [Google Scholar]
- Eschweiler, J.; Horn, N.; Rath, B.; Betsch, M.; Baroncini, A.; Tingart, M.; Migliorini, F. The biomechanics of cartilage—An overview. Life 2021, 11, 302. [Google Scholar] [CrossRef]
- Yunus, M.H.M.; Shuid, A.N.; Busra, M.F.; Chua, K.H.; Ghafar, N.A.; Rani, R.A. The effect of stichopus chloronotus aqueous extract on human osteoarthritis articular chondrocytes in three-dimensional collagen Type I hydrogel in vitro. Sains Malays. 2019, 48, 1671–1683. [Google Scholar] [CrossRef]
- Choi, M.-C.; Jo, J.; Park, J.; Kang, H.K.; Park, Y. NF-κB signaling pathways in osteoarthritic cartilage destruction. Cells 2019, 8, 734. [Google Scholar] [CrossRef]
- Yunus, M.H.M.; Nordin, A.; Kamal, H. Pathophysiological perspective of osteoarthritis. Medicina 2020, 56, 614. [Google Scholar] [CrossRef]
- Walimbe, T.; Panitch, A. Proteoglycans in biomedicine: Resurgence of an underexploited class of ECM molecules. Front. Pharmacol. 2020, 10, 1661. [Google Scholar] [CrossRef]
- Yang, H.; Huang, J.; Mao, Y.; Wang, L.; Li, R.; Ha, C. Vitexin alleviates interleukin-1β-induced inflammatory responses in chondrocytes from osteoarthritis patients: Involvement of HIF-1α pathway. Scand. J. Immunol. 2019, 90, e12773. [Google Scholar] [CrossRef]
- Vilá, S. Inflammation in osteoarthritis. Puerto Rico Health Sci. J. 2017, 36, 123–129. [Google Scholar]
- Kalaitzoglou, E.; Griffin, T.M.; Humphrey, M.B. Innate immune responses and osteoarthritis. Curr. Rheumatol. Rep. 2017, 19, 45. [Google Scholar] [CrossRef]
- Chow, Y.Y.; Chin, K.-Y. The role of inflammation in the pathogenesis of osteoarthritis. Mediat. Inflamm. 2020, 2020, 8293921. [Google Scholar] [CrossRef]
- Li, H.; Xie, S.; Qi, Y.; Li, H.; Zhang, R.; Lian, Y. TNF-α increases the expression of inflammatory factors in synovial fibroblasts by inhibiting the PI3K/AKT pathway in a rat model of monosodium iodoacetate-induced osteoarthritis. Exp. Ther. Med. 2018, 16, 4737–4744. [Google Scholar] [CrossRef]
- Malathi, R.; Kothari, S.; Chattopadhyay, A.; Agrawal, P.K.; Banerjee, U.; Sahu, R.K. Raised serum IL 6 and CRP in radiographic knee osteoarthritis in Eastern India. J. Musculoskelet. Surg. Res. 2017, 5, 21687–21692. [Google Scholar] [CrossRef]
- Loef, M.; Schoones, J.W.; Kloppenburg, M.; Ioan-Facsinay, A. Fatty acids and osteoarthritis: Different types, different effects. Jt. Bone Spine 2019, 86, 451–458. [Google Scholar] [CrossRef]
- Ahmad, N.; Ansari, M.Y.; Haqqi, T.M. Role of iNOS in osteoarthritis: Pathological and therapeutic aspects. J. Cell. Physiol. 2020, 235, 6366–6376. [Google Scholar] [CrossRef]
- Tian, Z.; Zhang, X.; Sun, M. Phytochemicals mediate autophagy against osteoarthritis by maintaining cartilage homeostasis. Front. Pharmacol. 2021, 12, 795058. [Google Scholar] [CrossRef]
- Wang, M.; Shen, J.; Jin, H.; Im, H.J.; Sandy, J.; Chen, D. Recent progress in understanding molecular mechanisms of cartilage degeneration during osteoarthritis. Ann. Acad. Sci. 2011, 1240, 61–69. [Google Scholar] [CrossRef]
- Qu, R.; Chen, X.; Wang, W.; Qiu, C.; Ban, M.; Guo, L.; Vasilev, K.; Chen, J.; Li, W.; Zhao, Y. Ghrelin protects against osteoarthritis through interplay with Akt and NF-κB signaling pathways. Fed. Am. Soc. Exp. Biol. J. 2018, 32, 1044–1058. [Google Scholar] [CrossRef]
- Pérez-García, S.; Carrión, M.; Gutiérrez-Cañas, I.; Villanueva-Romero, R.; Castro, D.; Martínez, C.; González-Álvaro, I.; Blanco, F.J.; Juarranz, Y.; Gomariz, R.P. Profile of matrix-remodeling proteinases in osteoarthritis: Impact of fibronectin. Cells 2019, 9, 40. [Google Scholar] [CrossRef]
- van Hoolwerff, M.; Tuerlings, M.; Wijnen, I.J.; Suchiman, H.E.D.; Cats, D.; Mei, H.; Nelissen, R.G.; van der Linden-van der Zwaag, H.M.; Ramos, Y.F.; Coutinho de Almeida, R. Identification and functional characterization of imbalanced osteoarthritis-associated fibronectin splice variants. Rheumatology 2023, 62, 894–904. [Google Scholar] [CrossRef]
- Barreto, G.; Manninen, M.; Eklund, K.K. Osteoarthritis and toll-like receptors: When innate immunity meets chondrocyte apoptosis. Biology 2020, 9, 65. [Google Scholar] [CrossRef]
- Sun, Q.; Zhang, Y.; Ding, Y.; Xie, W.; Li, H.; Li, S.; Li, Y.; Cai, M. Inhibition of PGE2 in Subchondral Bone Attenuates Osteoarthritis. Cells 2022, 11, 2760. [Google Scholar] [CrossRef]
- Lin, Z.; Wu, D.; Huang, L.; Jiang, C.; Pan, T.; Kang, X.; Pan, J. Nobiletin inhibits il-1β-induced inflammation in chondrocytes via suppression of nf-κb signaling and attenuates osteoarthritis in mice. Front. Pharmacol. 2019, 10, 570. [Google Scholar] [CrossRef]
- Li, H.; Peng, Y.; Wang, X.; Sun, X.; Yang, F.; Sun, Y.; Wang, B. Astragaloside inhibits IL-1β-induced inflammatory response in human osteoarthritis chondrocytes and ameliorates the progression of osteoarthritis in mice. Immunopharmacol. Immunotoxicol. 2019, 41, 497–503. [Google Scholar] [CrossRef]
- Wang, H.; Jiang, Z.; Pang, Z.; Qi, G.; Hua, B.; Yan, Z.; Yuan, H. Engeletin protects against TNF-α-induced apoptosis and reactive oxygen species generation in chondrocytes and alleviates osteoarthritis in vivo. J. Inflamm. Res. 2021, 14, 745. [Google Scholar] [CrossRef]
- Zahan, O.-M.; Serban, O.; Gherman, C.; Fodor, D. The evaluation of oxidative stress in osteoarthritis. Med. Pharm. Rep. 2020, 93, 12. [Google Scholar] [CrossRef]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2016, 1863, 2977–2992. [Google Scholar] [CrossRef]
- Rigoglou, S.; Papavassiliou, A.G. The NF-κB signalling pathway in osteoarthritis. Int. J. Biochem. Cell Biol. 2013, 45, 2580–2584. [Google Scholar] [CrossRef]
- Lepetsos, P.; Papavassiliou, K.A.; Papavassiliou, A.G. Redox and NF-κB signaling in osteoarthritis. Free Radic. Biol. Med. 2019, 132, 90–100. [Google Scholar] [CrossRef]
- Oeckinghaus, A.; Ghosh, S. The NF-κB family of transcription factors and its regulation. Cold Spring Harb. Perspect. Biol. 2009, 1, a000034. [Google Scholar] [CrossRef] [PubMed]
- Herrington, F.D.; Carmody, R.J.; Goodyear, C.S. Modulation of NF-κB signaling as a therapeutic target in autoimmunity. J. Biomol. Screen. 2016, 21, 223–242. [Google Scholar] [CrossRef]
- Sun, S.-C. The non-canonical NF-κB pathway in immunity and inflammation. Nat. Rev. Immunol. 2017, 17, 545–558. [Google Scholar] [CrossRef]
- Saito, T.; Tanaka, S. Molecular mechanisms underlying osteoarthritis development: Notch and NF-κB. Arthritis Res. Ther. 2017, 19, 94. [Google Scholar] [CrossRef]
- Noort, A.R.; Tak, P.P.; Tas, S.W. Non-canonical NF-κB signaling in rheumatoid arthritis: Dr Jekyll and Mr Hyde? Arthritis Res. Ther. 2015, 17, 15. [Google Scholar] [CrossRef]
- Kapoor, M.; Martel-Pelletier, J.; Lajeunesse, D.; Pelletier, J.-P.; Fahmi, H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat. Rev. Rheumatol. 2011, 7, 33–42. [Google Scholar] [CrossRef]
- Li, Z.; Dai, A.; Yang, M.; Chen, S.; Deng, Z.; Li, L. p38MAPK signaling pathway in osteoarthritis: Pathological and therapeutic aspects. J. Inflamm. Res. 2022, 723–734. [Google Scholar] [CrossRef]
- Gao, T.; Hu, Q.; Hu, X.; Lei, Q.; Feng, Z.; Yu, X.; Peng, C.; Song, X.; He, H.; Xu, Y. Novel selective TOPK inhibitor SKLB-C05 inhibits colorectal carcinoma growth and metastasis. Cancer Lett. 2019, 445, 11–23. [Google Scholar] [CrossRef]
- Chen, Y.; Shou, K.; Gong, C.; Yang, H.; Yang, Y.; Bao, T. Anti-inflammatory effect of geniposide on osteoarthritis by suppressing the activation of p38 MAPK signaling pathway. BioMed Res. Int. 2018, 2018. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Ye, J.; Xia, Z.; Cheng, B. Effect of luteolin on apoptosis, MAPK and JNK signaling pathways in guinea pig chondrocyte with osteoarthritis. Cell. Mol. Biol. 2019, 65, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Ge, Q.; Wang, H.; Xu, X.; Xu, L.; Zhai, L.; Tao, R. PDK1 promotes apoptosis of chondrocytes via modulating MAPK pathway in osteoarthritis. Tissue Cell 2017, 49, 719–725. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Liu, L.; Jiang, H.; Zhou, J.; Tang, Y. Inhibition of interleukin-6 function attenuates the central sensitization and pain behavior induced by osteoarthritis. Eur. J. Pharmacol. 2017, 811, 260–267. [Google Scholar] [CrossRef]
- Miller, R.J.; Malfait, A.-M.; Miller, R.E. The innate immune response as a mediator of osteoarthritis pain. Osteoarthr. Cartil. 2020, 28, 562–571. [Google Scholar] [CrossRef]
- Yao, Q.; Wu, X.; Tao, C.; Gong, W.; Chen, M.; Qu, M.; Zhong, Y.; He, T.; Chen, S.; Xiao, G. Osteoarthritis: Pathogenic signaling pathways and therapeutic targets. Signal Transduct. Target. Ther. 2023, 8, 56. [Google Scholar] [CrossRef]
- Sun, K.; Luo, J.; Guo, J.; Yao, X.; Jing, X.; Guo, F. The PI3K/AKT/mTOR signaling pathway in osteoarthritis: A narrative review. Osteoarthr. Cartil. 2020, 28, 400–409. [Google Scholar] [CrossRef]
- Fisch, K.M.; Gamini, R.; Alvarez-Garcia, O.; Akagi, R.; Saito, M.; Muramatsu, Y.; Sasho, T.; Koziol, J.A.; Su, A.I.; Lotz, M.K. Identification of transcription factors responsible for dysregulated networks in human osteoarthritis cartilage by global gene expression analysis. Osteoarthr. Cartil. 2018, 26, 1531–1538. [Google Scholar] [CrossRef]
- Rosa, S.; Rufino, A.; Judas, F.; Tenreiro, C.; Lopes, M.; Mendes, A. Expression and function of the insulin receptor in normal and osteoarthritic human chondrocytes: Modulation of anabolic gene expression, glucose transport and GLUT-1 content by insulin. Osteoarthr. Cartil. 2011, 19, 719–727. [Google Scholar] [CrossRef]
- Yao, X.; Jing, X.; Ye, Y.; Guo, J.; Sun, K.; Guo, F. Fibroblast growth factor 18 exerts anti-osteoarthritic effects through PI3K-AKT signaling and mitochondrial fusion and fission. Pharmacol. Res. 2019, 139, 314–324. [Google Scholar] [CrossRef]
- Hu, Z.C.; Gong, L.F.; Li, X.B.; Fu, X.; Xuan, J.W.; Feng, Z.H.; Ni, W.F. Inhibition of PI3K/Akt/NF-κB signaling with leonurine for ameliorating the progression of osteoarthritis: In vitro and in vivo studies. J. Cell. Physiol. 2019, 234, 6940–6950. [Google Scholar] [CrossRef]
- Lu, C.; Li, Y.; Hu, S.; Cai, Y.; Yang, Z.; Peng, K. Scoparone prevents IL-1β-induced inflammatory response in human osteoarthritis chondrocytes through the PI3K/Akt/NF-κB pathway. Biomed. Pharmacother. 2018, 106, 1169–1174. [Google Scholar] [CrossRef]
- Zhou, Q.; Ren, Q.; Jiao, L.; Huang, J.; Yi, J.; Chen, J.; Lai, J.; Ji, G.; Zheng, T. The potential roles of JAK/STAT signaling in the progression of osteoarthritis. Front. Endocrinol. 2022, 13. [Google Scholar] [CrossRef] [PubMed]
- O’Shea, J.J.; Schwartz, D.M.; Villarino, A.V.; Gadina, M.; McInnes, I.B.; Laurence, A. The JAK-STAT pathway: Impact on human disease and therapeutic intervention. Annu. Rev. Med. 2015, 66, 311–328. [Google Scholar] [CrossRef] [PubMed]
- Xin, P.; Xu, X.; Deng, C.; Liu, S.; Wang, Y.; Zhou, X.; Ma, H.; Wei, D.; Sun, S. The role of JAK/STAT signaling pathway and its inhibitors in diseases. Int. Immunopharmacol. 2020, 80, 106210. [Google Scholar] [CrossRef]
- Li, B.; Yu, J.; Liu, P.; Zeng, T.; Zeng, X. Astragaloside IV protects cardiomyocytes against hypoxia injury via HIF-1α and the JAK2/STAT3 pathway. Ann. Transl. Med. 2021, 9, 1435. [Google Scholar] [CrossRef]
- Yan, Z.; Ji, L. Hck promotes IL-1β-induced extracellular matrix degradation, inflammation, and apoptosis in osteoarthritis via activation of the JAK-STAT3 signaling pathway. Adv. Rheumatol. 2024, 64, 88. [Google Scholar] [CrossRef] [PubMed]
- Zeng, R.; Lu, X.; Lin, J.; Ron, Z.; Fang, J.; Liu, Z.; Zeng, W. FOXM1 activates JAK1/STAT3 pathway in human osteoarthritis cartilage cell inflammatory reaction. Exp. Biol. Med. 2021, 246, 644–653. [Google Scholar] [CrossRef]
- Chiu, Y.-S.; Bamodu, O.A.; Fong, I.-H.; Lee, W.-H.; Lin, C.-C.; Lu, C.-H.; Yeh, C.-T. The JAK inhibitor Tofacitinib inhibits structural damage in osteoarthritis by modulating JAK1/TNF-alpha/IL-6 signaling through Mir-149-5p. Bone 2021, 151, 116024. [Google Scholar] [CrossRef]
- Visser, A.; De Mutsert, R.; Le Cessie, S.; Den Heijer, M.; Rosendaal, F.; Kloppenburg, M.; Rabelink, T.J.; Smit, J.W.; Jukema, J.W.; de Roos, A. The relative contribution of mechanical stress and systemic processes in different types of osteoarthritis: The NEO study. Ann. Rheum. Dis. 2015, 74, 1842–1847. [Google Scholar] [CrossRef] [PubMed]
- Hodgkinson, T.; Kelly, D.C.; Curtin, C.M.; O’Brien, F.J. Mechanosignalling in cartilage: An emerging target for the treatment of osteoarthritis. Nat. Rev. Rheumatol. 2022, 18, 67–84. [Google Scholar] [CrossRef]
- Egloff, C.; Hügle, T.; Valderrabano, V. Biomechanics and pathomechanisms of osteoarthritis. Swiss Med. Wkly. 2012, 142, w13583. [Google Scholar] [CrossRef]
- Jia, Y.; Le, H.; Wang, X.; Zhang, J.; Liu, Y.; Ding, J.; Zheng, C.; Chang, F. Double-edged role of mechanical stimuli and underlying mechanisms in cartilage tissue engineering. Front. Bioeng. Biotechnol. 2023, 11, 1271762. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Li, W.; Zhang, P.; Wang, Z.; Ma, X.; Liu, C.; Vasilev, K.; Zhang, L.; Zhou, X.; Liu, L. Mechanical overloading induces GPX4-regulated chondrocyte ferroptosis in osteoarthritis via Piezo1 channel facilitated calcium influx. J. Adv. Res. 2022, 41, 63–75. [Google Scholar] [CrossRef]
- Jiang, W.; Chen, H.; Lin, Y.; Cheng, K.; Zhou, D.; Chen, R.; Song, C.; Zeng, L.; Yu, H. Mechanical stress abnormalities promote chondrocyte senescence-The pathogenesis of knee osteoarthritis. Biomed. Pharmacother. 2023, 167, 115552. [Google Scholar] [CrossRef]
- Zhang, H.; Shao, Y.; Yao, Z.; Liu, L.; Zhang, H.; Yin, J.; Xie, H.; Li, K.; Lai, P.; Zeng, H. Mechanical overloading promotes chondrocyte senescence and osteoarthritis development through downregulating FBXW7. Ann. Rheum. Dis. 2022, 81, 676–686. [Google Scholar] [CrossRef]
- Huang, P.; Yan, R.; Zhang, X.; Wang, L.; Ke, X.; Qu, Y. Activating Wnt/β-catenin signaling pathway for disease therapy: Challenges and opportunities. Pharmacol. Ther. 2019, 196, 79–90. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, T.; Hamilton, J.L.; Chen, D. Wnt/β-catenin signaling in osteoarthritis and in other forms of arthritis. Curr. Rheumatol. Rep. 2017, 19, 53. [Google Scholar] [CrossRef]
- Liu, J.; Xiao, Q.; Xiao, J.; Niu, C.; Li, Y.; Zhang, X.; Zhou, Z.; Shu, G.; Yin, G. Wnt/β-catenin signalling: Function, biological mechanisms, and therapeutic opportunities. Signal Transduct. Target. Ther. 2022, 7, 3. [Google Scholar] [CrossRef]
- Lorzadeh, S.; Kohan, L.; Ghavami, S.; Azarpira, N. Autophagy and the Wnt signaling pathway: A focus on Wnt/β-catenin signaling. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2021, 1868, 118926. [Google Scholar] [CrossRef] [PubMed]
- Jeong, W.-J.; Ro, E.J.; Choi, K.-Y. Interaction between Wnt/β-catenin and RAS-ERK pathways and an anti-cancer strategy via degradations of β-catenin and RAS by targeting the Wnt/β-catenin pathway. NPJ Precis. Oncol. 2018, 2, 5. [Google Scholar] [CrossRef]
- Li, X.; Han, Y.; Li, G.; Zhang, Y.; Wang, J.; Feng, C. Role of Wnt signaling pathway in joint development and cartilage degeneration. Front. Cell Dev. Biol. 2023, 11, 1181619. [Google Scholar] [CrossRef] [PubMed]
- Shang, X.; Böker, K.O.; Taheri, S.; Hawellek, T.; Lehmann, W.; Schilling, A.F. The interaction between microRNAs and the wnt/β-catenin signaling pathway in osteoarthritis. Int. J. Mol. Sci. 2021, 22, 9887. [Google Scholar] [CrossRef]
- Xu, K.; Ma, C.; Xu, L.; Ran, J.; Jiang, L.; He, Y.; Moqbel, S.A.A.; Wang, Z.; Wu, L. Polygalacic acid inhibits MMPs expression and osteoarthritis via Wnt/β-catenin and MAPK signal pathways suppression. Int. Immunopharmacol. 2018, 63, 246–252. [Google Scholar] [CrossRef]
- Geoghegan, I.P.; Hoey, D.A.; McNamara, L.M. Integrins in osteocyte biology and mechanotransduction. Curr. Osteoporos. Rep. 2019, 17, 195–206. [Google Scholar] [CrossRef]
- Jin, H.; Jiang, S.; Wang, R.; Zhang, Y.; Dong, J.; Li, Y. Mechanistic insight into the roles of integrins in osteoarthritis. Front. Cell Dev. Biol. 2021, 9, 693484. [Google Scholar] [CrossRef]
- Song, F.; Mao, X.; Dai, J.; Shan, B.; Zhou, Z.; Kang, Y. Integrin αVβ3 signaling in the progression of osteoarthritis induced by excessive mechanical stress. Inflammation 2023, 46, 739–751. [Google Scholar] [CrossRef]
- Urciuoli, E.; Peruzzi, B. Involvement of the FAK network in pathologies related to altered mechanotransduction. Int. J. Mol. Sci. 2020, 21, 9426. [Google Scholar] [CrossRef]
- Huang, Y.; Liao, J.; Vlashi, R.; Chen, G. Focal adhesion kinase (FAK): Its structure, characteristics, and signaling in skeletal system. Cell Signal. 2023, 111, 110852. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; He, T.; Zhong, Y.; Chen, M.; Yao, Q.; Chen, D.; Shao, Z.; Xiao, G. Roles of focal adhesion proteins in skeleton and diseases. Acta Pharm. Sin. 2023, 13, 998–1013. [Google Scholar] [CrossRef] [PubMed]
- Hansen, U. Analysis of collagen-binding integrin interactions with supramolecular aggregates of the extracellular matrix. In Collagen: Methods and Protocols; Springer: New York, NY, USA, 2019; pp. 157–166. [Google Scholar]
- Kadry, Y.A.; Calderwood, D.A. Structural and signaling functions of integrins. Biochim. Biophys. Acta (BBA)-Biomembr. 2020, 1862, 183206. [Google Scholar] [CrossRef]
- Wang, Q.; Onuma, K.; Liu, C.; Wong, H.; Bloom, M.S.; Elliott, E.E.; Cao, R.R.; Hu, N.; Lingampalli, N.; Sharpe, O. Dysregulated integrin αVβ3 and CD47 signaling promotes joint inflammation, cartilage breakdown, and progression of osteoarthritis. JCI Insight 2019, 4. [Google Scholar] [CrossRef]
- Hirose, N.; Okamoto, Y.; Yanoshita, M.; Asakawa, Y.; Sumi, C.; Takano, M.; Nishiyama, S.; Su, S.C.; Mitsuyoshi, T.; Kunimatsu, R. Protective effects of cilengitide on inflammation in chondrocytes under excessive mechanical stress. Cell Biol. Int. 2020, 44, 966–974. [Google Scholar] [CrossRef]
- Takano, M.; Hirose, N.; Sumi, C.; Yanoshita, M.; Nishiyama, S.; Onishi, A.; Asakawa, Y.; Tanimoto, K. ANGPTL2 promotes inflammation via integrin α5β1 in chondrocytes. Cartilage 2021, 13, 885S–897S. [Google Scholar] [CrossRef]
- Michael, M.; Parsons, M. New perspectives on integrin-dependent adhesions. Curr. Opin. Cell Biol. 2020, 63, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhang, F.-J.; Bai, R.-J. The Hippo-YAP Signaling Pathway in Osteoarthritis and Rheumatoid Arthritis. J. Inflamm. Res. 2024, 17, 1105–1120. [Google Scholar] [CrossRef] [PubMed]
- Moya, I.M.; Halder, G. The Hippo pathway in cellular reprogramming and regeneration of different organs. Curr. Opin. Cell Biol. 2016, 43, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Samji, P.; Rajendran, M.K.; Warrier, V.P.; Ganesh, A.; Devarajan, K. Regulation of Hippo signaling pathway in cancer: A MicroRNA perspective. Cell Signal. 2021, 78, 109858. [Google Scholar] [CrossRef]
- Han, J.; Zhang, J.; Zhang, X.; Luo, W.; Liu, L.; Zhu, Y.; Liu, Q.; Zhang, X.-a. Emerging role and function of Hippo-YAP/TAZ signaling pathway in musculoskeletal disorders. Stem Cell Res. Ther. 2024, 15, 386. [Google Scholar] [CrossRef]
- Lu, M.; Zhu, M.; Wu, Z.; Liu, W.; Cao, C.; Shi, J. The role of YAP/TAZ on joint and arthritis. Fed. Am. Soc. Exp. Biol. J. 2024, 38, e23636. [Google Scholar] [CrossRef] [PubMed]
- Meng, Z.; Moroishi, T.; Guan, K.-L. Mechanisms of Hippo pathway regulation. Genes. Dev. 2016, 30, 1–17. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, X.; Song, G. The Hippo pathway: A master regulatory network important in cancer. Cells 2021, 10, 1416. [Google Scholar] [CrossRef]
- Cunningham, R.; Hansen, C.G. The Hippo pathway in cancer: YAP/TAZ and TEAD as therapeutic targets in cancer. Clin. Sci. 2022, 136, 197–222. [Google Scholar] [CrossRef]
- Sun, K.; Guo, J.; Guo, Z.; Hou, L.; Liu, H.; Hou, Y.; He, J.; Guo, F.; Ye, Y. The roles of the Hippo-YAP signalling pathway in Cartilage and Osteoarthritis. Ageing Res. Rev. 2023, 90, 102015. [Google Scholar] [CrossRef]
- Li, Z.; Lin, J.; Wu, J.; Suo, J.; Wang, Z. The Hippo signalling pathway in bone homeostasis: Under the regulation of mechanics and aging. Cell Prolif. 2024, 57, e13652. [Google Scholar] [CrossRef]
- Fang, T.; Zhou, X.; Jin, M.; Nie, J.; Li, X. Molecular mechanisms of mechanical load-induced osteoarthritis. Int. Orthop. 2021, 45, 1125–1136. [Google Scholar] [CrossRef]
- Vincent, T.L. Mechanoflammation in Osteoarthritis Pathogenesis. Semin. Arthritis Rheum. 2019, 49, S36–S38. [Google Scholar] [CrossRef]
- Guilak, F.; Nims, R.J.; Dicks, A.; Wu, C.-L.; Meulenbelt, I. Osteoarthritis as a disease of the cartilage pericellular matrix. Matrix Biol. 2018, 71, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Song, M.; Song, T.; Jia, X.; Song, Y. Osteoimmunology in Osteoarthritis: Unraveling the Interplay of Immunity, Inflammation, and Joint Degeneration. J. Inflamm. Res. 2025, 18, 4121–4142. [Google Scholar] [CrossRef] [PubMed]
- Segarra-Queralt, M.; Piella, G.; Noailly, J. Network-based modelling of mechano-inflammatory chondrocyte regulation in early osteoarthritis. Front. Bioeng. Biotechnol. 2023, 11, 1006066. [Google Scholar] [CrossRef]
- Yeap, S.S.; Abu Amin, S.R.; Baharuddin, H.; Koh, K.C.; Lee, J.K.; Lee, V.K.M.; Mohamad Yahaya, N.H.; Tai, C.C.; Tan, M.P. A Malaysian Delphi consensus on managing knee osteoarthritis. BMC Musculoskelet. Disord. 2021, 22, 514. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Nie, P.; Lu, J.; Ling, Y.; Guo, J.; Zhang, B.; Hu, J.; Liao, J.; Gu, J.; Dai, B. Less mechanical loading attenuates osteoarthritis by reducing cartilage degeneration, subchondral bone remodelling, secondary inflammation, and activation of NLRP3 inflammasome. Bone Jt. Res. 2020, 9, 731–741. [Google Scholar] [CrossRef]
- Lin, J.; Jia, S.; Zhang, W.; Nian, M.; Liu, P.; Yang, L.; Zuo, J.; Li, W.; Zeng, H.; Zhang, X. Recent advances in small molecule inhibitors for the treatment of osteoarthritis. J. Clin. Med. 2023, 12, 1986. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Lee, S.Y. A review of osteoarthritis signaling intervention using small-molecule inhibitors. Medicine 2022, 101, e29501. [Google Scholar] [CrossRef]
- Southey, M.W.Y.; Brunavs, M. Introduction to small molecule drug discovery and preclinical development. Front. Drug Discov. 2023, 3. [Google Scholar] [CrossRef]
- Kloppenburg, M.; Peterfy, C.; Haugen, I.K.; Kroon, F.; Chen, S.; Wang, L.; Liu, W.; Levy, G.; Fleischmann, R.M.; Berenbaum, F. Phase IIa, placebo-controlled, randomised study of lutikizumab, an anti-interleukin-1α and anti-interleukin-1β dual variable domain immunoglobulin, in patients with erosive hand osteoarthritis. Ann. Rheum. Dis. 2019, 78, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Zhong, G.; Long, H.; Chen, F.; Yu, Y. Oxoglaucine mediates Ca2+ influx and activates autophagy to alleviate osteoarthritis through the TRPV5/calmodulin/CAMK-II pathway. Br. J. Pharmacol. 2021, 178, 2931–2947. [Google Scholar] [CrossRef]
- Kloppenburg, M.; Ramonda, R.; Bobacz, K.; Kwok, W.-Y.; Elewaut, D.; Huizinga, T.W.; Kroon, F.P.; Punzi, L.; Smolen, J.S.; Vander Cruyssen, B. Etanercept in patients with inflammatory hand osteoarthritis (EHOA): A multicentre, randomised, double-blind, placebo-controlled trial. Ann. Rheum. Dis. 2018, 77, 1757–1764. [Google Scholar] [CrossRef]
- Liu, D.; Li, X.; Zhang, L.; Hu, B.; Hu, S.; Zhang, X.; Hu, J. Small molecule inhibitors of osteoarthritis: Current development and future perspective. Front. Physiol. 2023, 14, 1156913. [Google Scholar] [CrossRef]
- Karlapudi, V.; Sunkara, K.B.; Konda, P.R.; Sarma, K.V.; Rokkam, M.P. Efficacy and safety of Aflapin®, a novel boswellia serrata extract, in the treatment of osteoarthritis of the knee: A short-term 30-day randomized, double-blind, placebo-controlled clinical study. J. Am. Nutr. Assoc. 2023, 42, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Howes, L. Is this a golden age of small-molecule drug discovery? C&EN Global Enterp. 2023, 101, 28–32. [Google Scholar]
- Delanois, R.E.; Sax, O.C.; Chen, Z.; Cohen, J.M.; Callahan, D.M.; Mont, M.A. Biologic therapies for the treatment of knee osteoarthritis: An updated systematic review. J. Arthroplast. 2022, 37, 2480–2506. [Google Scholar] [CrossRef]
- Butala, S.; Castelo-Soccio, L.; Seshadri, R.; Simpson, E.L.; O’Shea, J.J.; Bieber, T.; Paller, A.S. Biologic Versus Small Molecule Therapy for Treating Moderate to Severe Atopic Dermatitis: Clinical Considerations. J. Allergy Clin. Immunol. Pr. 2023, 11, 1361–1373. [Google Scholar] [CrossRef]
- Thornton, W.; Glyn-Jones, S. The Use of Biologic Treatments for Osteoarthritis: A Review. Open J. Regen. Med. 2024, 13, 21–40. [Google Scholar] [CrossRef]
- Weber, A.E.; Bolia, I.K.; Trasolini, N.A. Biological strategies for osteoarthritis: From early diagnosis to treatment. Int. Orthop. 2021, 45, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Krüger, J.P.; Hondke, S.; Endres, M.; Pruss, A.; Siclari, A.; Kaps, C. Human platelet-rich plasma stimulates migration and chondrogenic differentiation of human subchondral progenitor cells. J. Orthop. Res. 2012, 30, 845–852. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.A.; Adithan, A.; Alam, M.J.; Kopalli, S.R.; Kim, B.; Kang, C.-W.; Hwang, K.-C.; Kim, J.-H. IGF-1 facilitates cartilage reconstruction by regulating PI3K/AKT, MAPK, and NF-kB signaling in rabbit osteoarthritis. J. Inflamm. Res. 2021, 14, 3555–3568. [Google Scholar] [CrossRef] [PubMed]
- Wen, C.; Xu, L.; Xu, X.; Wang, D.; Liang, Y.; Duan, L. Insulin-like growth factor-1 in articular cartilage repair for osteoarthritis treatment. Arthritis Res. Ther. 2021, 23, 1–9. [Google Scholar] [CrossRef]
- Rider, P.; Carmi, Y.; Cohen, I. Biologics for targeting inflammatory cytokines, clinical uses, and limitations. Int. J. Cell Biol. 2016, 2016, 9259646. [Google Scholar] [CrossRef]
- Lana, J.F.; Purita, J.; Jeyaraman, M.; de Souza, B.F.; Rodrigues, B.L.; Huber, S.C.; Caliari, C.; Santos, G.S.; da Fonseca, L.F.; Dallo, I.; et al. Innovative Approaches in Knee Osteoarthritis Treatment: A Comprehensive Review of Bone Marrow-Derived Products. Biomedicines 2024, 12, 2812. [Google Scholar] [CrossRef]
- Escribano-Núñez, A.; Cornelis, F.M.; De Roover, A.; Sermon, A.; Cailotto, F.; Lories, R.J.; Monteagudo, S. IGF1 drives Wnt-induced joint damage and is a potential therapeutic target for osteoarthritis. Nat. Commun. 2024, 15, 9170. [Google Scholar] [CrossRef] [PubMed]
- Jeyaraman, M.; Jeyaraman, N.; Ramasubramanian, S.; Ranjan, R.; Jha, S.K.; Gupta, A. Bone Marrow Aspirate Concentrate for Treatment of Primary Knee Osteoarthritis: A Prospective, Single-Center, Non-randomized Study with 2-Year Follow-Up. Indian J. Orthop. 2024, 58, 1–11. [Google Scholar] [CrossRef]
- Henrickson, S.E.; Ruffner, M.A.; Kwan, M. Unintended Immunological Consequences of Biologic Therapy. Curr. Allergy Asthma Rep. 2016, 16, 46. [Google Scholar] [CrossRef]
- Cossu, G.; Fears, R.; Griffin, G.; Ter Meulen, V. Regenerative medicine: Challenges and opportunities. Lancet 2020, 395, 1746–1747. [Google Scholar] [CrossRef]
- Im, G.-I. The concept of early osteoarthritis and its significance in regenerative medicine. Tissue Eng. Regen. Med. 2022, 19, 431–436. [Google Scholar] [CrossRef]
- Richards, M.M.; Maxwell, J.S.; Weng, L.; Angelos, M.G.; Golzarian, J. Intra-articular treatment of knee osteoarthritis: From anti-inflammatories to products of regenerative medicine. Physician Sportsmed. 2016, 44, 101–108. [Google Scholar] [CrossRef]
- Goudarzi, R.; Dehpour, A.R.; Partoazar, A. Nanomedicine and regenerative medicine approaches in osteoarthritis therapy. Aging Clin. Exp. Res. 2022, 34, 2305–2315. [Google Scholar] [CrossRef] [PubMed]
- Angele, P.; Docheva, D.; Pattappa, G.; Zellner, J. Cell-based treatment options facilitate regeneration of cartilage, ligaments and meniscus in demanding conditions of the knee by a whole joint approach. Knee Surg. Sports Traumatol. Arthrosc. 2022, 30, 1138–1150. [Google Scholar] [CrossRef]
- Freitag, J.; Kenihan, M.A. Mesenchymal stem cell therapy in osteoarthritis and regenerative medicine. Curr. Sports Med. Rep. 2018, 17, 441–443. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Wu, W.; Qu, X. Mesenchymal stem cells in osteoarthritis therapy: A review. Am. J. Transl. Res. 2021, 13, 448. [Google Scholar] [PubMed]
- Lv, Z.; Cai, X.; Bian, Y.; Wei, Z.; Zhu, W.; Zhao, X.; Weng, X. Advances in mesenchymal stem cell therapy for osteoarthritis: From preclinical and clinical perspectives. Bioengineering 2023, 10, 195. [Google Scholar] [CrossRef]
- Colombini, A.; Libonati, F.; Lopa, S.; Peretti, G.; Moretti, M.; de Girolamo, L. Autologous chondrocyte implantation provides good long-term clinical results in the treatment of knee osteoarthritis: A systematic review. Knee Surg. Sports Traumatol. Arthrosc. 2023, 31, 2338–2348. [Google Scholar] [CrossRef]
- Dhillon, J.; Decilveo, A.P.; Kraeutler, M.J.; Belk, J.W.; McCulloch, P.C.; Scillia, A.J. Third-generation autologous chondrocyte implantation (cells cultured within collagen membrane) is superior to microfracture for focal chondral defects of the knee joint: Systematic review and meta-analysis. Arthrosc. J. Arthrosc. Relat. Surg. 2022, 38, 2579–2586. [Google Scholar] [CrossRef] [PubMed]
- Yue, L.; Lim, R.; Owens, B.D. Latest Advances in Chondrocyte-Based Cartilage Repair. Biomedicines 2024, 12, 1367. [Google Scholar] [CrossRef]
- Qin, S.; Zhu, J.; Zhang, G.; Sui, Q.; Niu, Y.; Ye, W.; Ma, G.; Liu, H. Research progress of functional motifs based on growth factors in cartilage tissue engineering: A review. Front. Bioeng. Biotechnol. 2023, 11. [Google Scholar] [CrossRef]
- Liu, S.; Deng, Z.; Chen, K.; Jian, S.; Zhou, F.; Yang, Y.; Fu, Z.; Xie, H.; Xiong, J.; Zhu, W. Cartilage tissue engineering: From proinflammatory and anti-inflammatory cytokines to osteoarthritis treatments. Mol. Med. Rep. 2022, 25, 1–15. [Google Scholar] [CrossRef]
- Ivirico, J.L.E.; Bhattacharjee, M.; Kuyinu, E.; Nair, L.S.; Laurencin, C.T. Regenerative engineering for knee osteoarthritis treatment: Biomaterials and cell-based technologies. Engineering 2017, 3, 16–27. [Google Scholar] [CrossRef]
- Maihemuti, A.; Zhang, H.; Lin, X.; Wang, Y.; Xu, Z.; Zhang, D.; Jiang, Q. 3D-printed fish gelatin scaffolds for cartilage tissue engineering. Bioact. Mater. 2023, 26, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Dou, H.; Wang, S.; Hu, J.; Song, J.; Zhang, C.; Wang, J.; Xiao, L. Osteoarthritis models: From animals to tissue engineering. J. Tissue Eng. 2023, 14, 20417314231172584. [Google Scholar] [CrossRef]
- Vinatier, C.; Guicheux, J. Cartilage tissue engineering: From biomaterials and stem cells to osteoarthritis treatments. Ann. Phys. Rehabil. Med. 2016, 59, 139–144. [Google Scholar] [CrossRef]
- O’Shea, D.G.; Curtin, C.M.; O’Brien, F.J. Articulation inspired by nature: A review of biomimetic and biologically active 3D printed scaffolds for cartilage tissue engineering. Biomater. Sci. 2022, 10, 2462–2483. [Google Scholar] [CrossRef] [PubMed]
- Asensio, G.; Benito-Garzón, L.; Ramírez-Jiménez, R.A.; Guadilla, Y.; Gonzalez-Rubio, J.; Abradelo, C.; Parra, J.; Martín-López, M.R.; Aguilar, M.R.; Vázquez-Lasa, B.; et al. Biomimetic Gradient Scaffolds Containing Hyaluronic Acid and Sr/Zn Folates for Osteochondral Tissue Engineering. Polymers 2021, 14, 12. [Google Scholar] [CrossRef]
- Evans, C.H.; Ghivizzani, S.C.; Robbins, P.D. Gene delivery to joints by intra-articular injection. Hum. Gene Ther. 2018, 29, 2–14. [Google Scholar] [CrossRef]
- Evans, C.H.; Ghivizzani, S.C.; Robbins, P.D. Osteoarthritis gene therapy in 2022. Curr. Opin. Rheumatol. 2023, 35, 37–43. [Google Scholar] [CrossRef]
- Zhao, L.; Huang, J.; Fan, Y.; Li, J.; You, T.; He, S.; Xiao, G.; Chen, D. Exploration of CRISPR/Cas9-based gene editing as therapy for osteoarthritis. Ann. Rheum. Dis. 2019, 78, 676–682. [Google Scholar] [CrossRef]
- Wu, Y.; Lu, X.; Shen, B.; Zeng, Y. The therapeutic potential and role of miRNA, lncRNA, and circRNA in osteoarthritis. Curr. Gene Ther. 2019, 19, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Shestovskaya, M.V.; Bozhkova, S.A.; Sopova, J.V.; Khotin, M.G.; Bozhokin, M.S. Methods of Modification of Mesenchymal Stem Cells and Conditions of Their Culturing for Hyaline Cartilage Tissue Engineering. Biomedicines 2021, 9, 1666. [Google Scholar] [CrossRef]
- Holden, M.A.; Nicolson, P.J.; Thomas, M.J.; Corp, N.; Hinman, R.S.; Bennell, K.L. Osteoarthritis year in review 2022: Rehabilitation. Osteoarthr. Cartil. 2023, 31, 177–186. [Google Scholar] [CrossRef]
- Florjančič, K.; Vauhnik, R. Effects of Standard Physiotherapy with the Addition of Mechanical Traction on Pain, Physical Activity and Quality of Life in Patients with Knee Osteoarthritis. Medicina 2025, 61, 507. [Google Scholar] [CrossRef] [PubMed]
- Verhagen, A.; Ferreira, M.; Reijneveld-van de Vendel, E.; Teirlinck, C.; Runhaar, J.; van Middelkoop, M.; Hermsen, L.; de Groot, I.; Bierma-Zeinstra, S. Do we need another trial on exercise in patients with knee osteoarthritis?: No new trials on exercise in knee OA. Osteoarthr. Cartil. 2019, 27, 1266–1269. [Google Scholar] [CrossRef]
- Block, J.A.; Cherny, D. Management of knee osteoarthritis: What internists need to know. Med. Clin. 2021, 105, 367–385. [Google Scholar]
- Messier, S.P.; Callahan, L.F.; Losina, E.; Mihalko, S.L.; Guermazi, A.; Ip, E.; Miller, G.D.; Katz, J.N.; Loeser, R.F.; Pietrosimone, B.G. The osteoarthritis prevention study (TOPS)-A randomized controlled trial of diet and exercise to prevent Knee Osteoarthritis: Design and rationale. Osteoarthr. Cartil. Open 2024, 6, 100418. [Google Scholar] [CrossRef]
- Zhang, S.; Li, T.; Feng, Y.; Zhang, K.; Zou, J.; Weng, X.; Yuan, Y.; Zhang, L. Exercise improves subchondral bone microenvironment through regulating bone-cartilage crosstalk. Front. Endocrinol. 2023, 14, 1159393. [Google Scholar] [CrossRef] [PubMed]
- Shishira, P.; Nageswran, S. Knee Braces for Prevention of Unilateral Knee Osteoarthritis-KOA. ICAICTSEE–2020 2020, 138. [Google Scholar]
- Barber, T.; Jahanbani, S.S. Physiotherapy and knee osteoarthritis. Br. Columbia Med. J. 2024, 66, 165–170. [Google Scholar]
- Bishop, E.L.; Bonhomme, J.; Joffe, M.; Cowper-Smith, C.; Ronsky, J.L.; Clark, M.L. A feasibility randomised trial evaluating the levitation tri-compartment offloader knee Brace for Multicompartment knee osteoarthritis. Pilot. Feasibility Stud. 2025, 11, 81. [Google Scholar] [CrossRef]
- Mistry, D.A.; Chandratreya, A.; Lee, P.Y. An update on unloading knee braces in the treatment of unicompartmental knee osteoarthritis from the last 10 years: A literature review. Surg. J. 2018, 4, e110–e118. [Google Scholar] [CrossRef]
- Imboden, M.; Séguin, É.; Doumit, M. Design and evaluation of an offloading orthosis for medial knee osteoarthritis. Med. Eng. Phys. 2023, 121, 104063. [Google Scholar] [CrossRef]
- Mahmoodi, M.; Arazpour, M.; Mousavi, M.E. Evaluation of the effect of knee unloader orthoses, lateral wedge insoles, and ankle foot orthoses on pain, function, and knee adduction moment in subjects with medial compartment knee osteoarthritis: A literature review. J. Prosthet. Orthot. 2023, 35, e48–e61. [Google Scholar] [CrossRef]
- Lin, K.-W.; Chou, L.-W.; Su, Y.-T.; Wei, S.-H.; Chen, C.-S. Biomechanical Effect of 3D-Printed Foot Orthoses in Patients with Knee Osteoarthritis. Appl. Sci. 2021, 11, 4200. [Google Scholar] [CrossRef]
- Clark, N.C.; Glaister, M.; Cannon, L.M.; Perrem, N. The physiology of disuse, immobilization and low-load environments. In A Comprehensive Guide to Sports Physiology and Injury Management: An Interdisciplinary Approach; Elsevier: Amsterdam, The Netherlands, 2020; pp. 15–24. [Google Scholar]
- Gilbert, S.J.; Bonnet, C.S.; Blain, E.J. Mechanical cues: Bidirectional reciprocity in the extracellular matrix drives mechano-signalling in articular cartilage. Int. J. Mol. Sci. 2021, 22, 13595. [Google Scholar] [CrossRef]
- Statham, P.; Jones, E.; Jennings, L.M.; Fermor, H.L. Reproducing the biomechanical environment of the chondrocyte for cartilage tissue engineering. Tissue Eng. Part. B Rev. 2022, 28, 405–420. [Google Scholar] [CrossRef]
- Long, J.; You, J.; Yang, Y. Effect of Digital Exercise Therapy on the Pain and Physical Function of Patients With Osteoarthritis: Systematic Review and Meta-Analysis. J. Med. Internet Res. 2025, 27, e66037. [Google Scholar] [CrossRef] [PubMed]
- Allen, K.D.; Ambrose, K.R.; Booker, S.Q.; Buck, A.N.; Huffman, K.F. Non-pharmacological pain management for osteoarthritis: Review update. Curr. Rheumatol. Rep. 2025, 27, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.-J.; Lu, J.-W.; Ho, L.-J.; Lai, J.-H.; Huang, H.-S.; Lee, C.-C.; Lin, T.-Y.; Lien, S.-B.; Lin, L.-C.; Chen, L.W. Anti-inflammatory and anti-osteoarthritis effects of Cm-02 and Ck-02. Biochem. Biophys. Res. Commun. 2019, 517, 155–163. [Google Scholar] [CrossRef]
- Brandt, M.D.; Malone, J.B.; Kean, T.J. Advances and Challenges in the Pursuit of Disease-Modifying Osteoarthritis Drugs: A Review of 2010–2024 Clinical Trials. Biomedicines 2025, 13, 355. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, L.; Feather, K.; Sofat, N. New Developments in Clinical Trials for Osteoarthritis: Are We Closer to Improving Pain Management and Disease Modification? EMJ 2024. [Google Scholar] [CrossRef]
- Jakaba, M.; Kiesslicha, T.; van der Zee-Neuena, A.; Wirthe, W.; Rittera, M. Sprifermin for Treatment of Osteoarthritis: Recombinant Fibroblast Growth Factor 18 as a Possible Disease-Modifying Knee Osteoarthritis Drug. Cell Physiol. Biochem. 2023, 18. [Google Scholar] [CrossRef]
- Carneiro, D.d.C.; Araújo, L.T.d.; Santos, G.C.; Damasceno, P.K.F.; Vieira, J.L.; Santos, R.R.d.; Barbosa, J.D.V.; Soares, M.B.P. Clinical trials with mesenchymal stem cell therapies for osteoarthritis: Challenges in the regeneration of articular cartilage. Int. J. Mol. Sci. 2023, 24, 9939. [Google Scholar] [CrossRef] [PubMed]
- Gil, T.-H.; Zheng, H.; Lee, H.G.; Shin, J.-W.; Hwang, S.W.; Jang, K.-M.; Jeon, O.H. Senolytic drugs relieve pain by reducing peripheral nociceptive signaling without modifying joint tissue damage in spontaneous osteoarthritis. Aging 2022, 14, 6006. [Google Scholar] [CrossRef] [PubMed]
- Berteau, J.-P. Knee pain from osteoarthritis: Pathogenesis, risk factors, and recent evidence on physical therapy interventions. J. Clin. Med. 2022, 11, 3252. [Google Scholar] [CrossRef] [PubMed]






| Pathway | Key Activators | Major Downstream Effects in OA |
|---|---|---|
| NF-κB | IL-1β, TNF-α, TLRs, mechanical stress | -Upregulates MMPs (1, 3, 13), ADAMTS (4, 5), COX-2, NOS, PGE2. -Promotes catabolic cytokine production (IL-6, IL-8). -Induces chondrocyte apoptosis. |
| MAPK | IL-1β, TNF-α, mechanical stress, TGF-β | -Activates ERK, JNK, p38 → upregulates MMPs, IL-1, TNF-α. -Induces chondrocyte hypertrophy/death. -Mediates pain sensitization (NGF, prostaglandins). |
| PI3K/AKT/mTOR | IL-1β, TNF-α, growth factors | -Downregulated in OA cartilage. -Crosstalk with NF-κB to amplify inflammation. -Regulates chondrocyte survival/apoptosis. |
| JAK/STAT | IL-6, IL-1β, interferons, growth factors | -Upregulates MMPs, ADAMTS, NO, and PGE2. -Synovial fibroblast proliferation. -Sustains inflammatory feedback loops. |
| Category | Strategy | Target/Pathway | Mechanism of Action | Example Interventions | Current Status (References) |
|---|---|---|---|---|---|
| Small-Molecule Inhibitors | MMP Inhibitors | MMP-13, ADAMTS-5 | Block cartilage-degrading enzymes. | Cm-02/Ck-02 | Preclinical [178] |
| NF-κB Pathway Inhibitors | IKKβ, NF-κB | Suppress inflammatory gene expression. | SAR113945 | Phase II trial [179] | |
| JAK Inhibitors | JAK1/2/3 | Attenuate cytokine signaling. | Tofacitinib | Preclinical [70] | |
| WNT/β-Catenin Inhibitors | WNT pathway | Prevent chondrocyte hypertrophy. | Lorecivivint (SM04690) | Phase III trial [179] | |
| Biologics | Anti-Cytokine Therapies | IL-1β, TNF-α, IL-6 | Neutralize pro-inflammatory cytokines. | Canakinumab (Anti-IL-1β) | Phase II trial [180] |
| Growth Factor Therapies | FGF-18, IGF-1 | Stimulate cartilage repair. | Sprifermin (FGF-18) | Phase II trial [181] | |
| Regenerative Medicine | Stem Cell Therapy | Mesenchymal stem cells (MSCs) | Promote cartilage regeneration via paracrine signaling. | Autologous MSC injections | Phase II trial [182] |
| Senolytics | Senescent cells (p16, p21) | Clear senescent chondrocytes to reduce inflammation. | ABT263 + Dasatinib + Quercetin | Preclinical [183] | |
| Non-Pharmacological | Mechanotherapy | Joint loading | Optimize biomechanics to reduce stress on cartilage. | Unloader knee braces | Clinical practice [184] |
| Physical Rehabilitation | Muscle/joint function | Improve stability and load distribution through exercise. | Structured physical therapy | Clinical practice [177] | |
| Weight Management | Systemic metabolic factors | Reduce obesity-associated inflammation and joint load. | Diet/exercise programs | Clinical practice [164] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Batarfi, W.A.; Yunus, M.H.M.; Hamid, A.A.; Maarof, M.; Abdul Rani, R. Breaking Down Osteoarthritis: Exploring Inflammatory and Mechanical Signaling Pathways. Life 2025, 15, 1238. https://doi.org/10.3390/life15081238
Batarfi WA, Yunus MHM, Hamid AA, Maarof M, Abdul Rani R. Breaking Down Osteoarthritis: Exploring Inflammatory and Mechanical Signaling Pathways. Life. 2025; 15(8):1238. https://doi.org/10.3390/life15081238
Chicago/Turabian StyleBatarfi, Wafa Ali, Mohd Heikal Mohd Yunus, Adila A. Hamid, Manira Maarof, and Rizal Abdul Rani. 2025. "Breaking Down Osteoarthritis: Exploring Inflammatory and Mechanical Signaling Pathways" Life 15, no. 8: 1238. https://doi.org/10.3390/life15081238
APA StyleBatarfi, W. A., Yunus, M. H. M., Hamid, A. A., Maarof, M., & Abdul Rani, R. (2025). Breaking Down Osteoarthritis: Exploring Inflammatory and Mechanical Signaling Pathways. Life, 15(8), 1238. https://doi.org/10.3390/life15081238






