Coronary Artery Disease and Atherosclerosis in Other Vascular Districts: Epidemiology, Risk Factors and Atherosclerotic Plaque Features
Abstract
1. Introduction
2. Coronary Artery Disease and Atherosclerotic Carotid Artery Disease
2.1. Prevalence of Concomitant CAD and Atherosclerotic Carotid Artery Disease
2.2. Risk Factors
2.3. Plaque Features and Mechanisms of Plaque Destabilization
2.4. Characteristics of Plaques in the Case of Concomitant Atherosclerosis in the Two Arterial Beds
2.5. Therapeutic Implications
3. Coronary Artery Disease and Atherosclerotic Lower Extremity Arterial Disease
3.1. Prevalence of Concomitant CAD and LEAD
3.2. Risk Factors
3.3. Plaque Features and Mechanisms of Plaque Destabilization
3.4. Characteristics of Plaques in the Case of Concomitant Atherosclerosis in the Two Arterial Beds
3.5. Therapeutic Implications
4. Coronary Artery Disease and Mesenteric Artery Atherosclerosis
4.1. Prevalence of Concomitant CAD and Gastrointestinal Artery Diseases
4.2. Risk Factors
4.3. Plaque Features and Mechanisms of Plaque Destabilization
4.4. Characteristics of Plaques in the Case of Concomitant Atherosclerosis in the Two Arterial Beds
4.5. Therapeutic Implications
5. Coronary Artery Disease and Renal Artery Atherosclerosis
5.1. Prevalence of Concomitant CAD and Renal Artery Stenosis
5.2. Risk Factors
5.3. Plaque Features and Mechanisms of Plaque Destabilization
5.4. Characteristics of Plaques in the Case of Concomitant Atherosclerosis in the Two Arterial Beds
5.5. Therapeutic Implications
6. Coronary Artery Disease and Aortic Atherosclerosis
6.1. Prevalence of Concomitant CAD and Aortic Disease
6.2. Risk Factors
6.3. Plaque Features and Mechanisms of Plaque Destabilization
6.4. Characteristics of Plaques in the Case of Concomitant Atherosclerosis in the Two Arterial Beds
6.5. Therapeutic Implications
7. Clinical Perspectives and Future Directions
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AAA | abdominal aortic aneurysms |
| ACS | acute coronary syndrome |
| ASCVD | atherosclerotic vascular disease |
| CAD | coronary artery disease |
| CACs | coronary artery calcium score |
| CKD | chronic kidney disease |
| CTA | computed tomography angiography |
| IHD | ischemic heart disease |
| LEAD | lower extremity arterial disease |
| LRNC | lipid-rich necrotic core |
| MRI | magnetic resonance imaging |
| OCT | optical coherence tomography |
| PAD | peripheral arterial disease |
| VH-IVUS | virtual histology intravascular ultrasound |
References
- Mensah, G.A.; Fuster, V.; Murray, C.J.; Roth, G.A.; Abate, Y.H.; Abbasian, M.; Abd-Allah, F.; Abdollahi, A.; Abdollahi, M.; Abdulah, D.M.; et al. Global Burden of Cardiovascular Diseases and Risks Collaborators. Global Burden of Cardiovascular Diseases and Risks, 1990–2022. J. Am. Coll. Cardiol. 2023, 82, 2350–2473. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- GBD 2021 Forecasting Collaborators. Burden of disease scenarios for 204 countries and territories, 2022–2050: A forecastingxf analysis for the Global Burden of Disease Study 2021. Lancet 2024, 403, 2204–2256. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gurgoglione, F.L.; Denegri, A.; Russo, M.; Calvieri, C.; Benatti, G.; Niccoli, G. Intracoronary Imaging of Coronary Atherosclerotic Plaque: From Assessment of Pathophysiological Mechanisms to Therapeutic Implication. Int. J. Mol. Sci. 2023, 24, 5155. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Madaudo, C.; Coppola, G.; Parlati, A.L.M.; Corrado, E. Discovering Inflammation in Atherosclerosis: Insights from Pathogenic Pathways to Clinical Practice. Int. J. Mol. Sci. 2024, 25, 6016. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Achim, A.; Péter, O.Á.; Cocoi, M.; Serban, A.; Mot, S.; Dadarlat-Pop, A.; Nemes, A.; Ruzsa, Z. Correlation between Coronary Artery Disease with Other Arterial Systems: Similar, Albeit Separate, Underlying Pathophysiologic Mechanisms. J. Cardiovasc. Dev. Dis. 2023, 10, 210. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mitchell, J.R.A.; Schwartz, C.J. Relationship between arterial disease in different sites. A study of the aorta and coronary, carotid, and iliac arteries. Br. Med. J. 1962, 1, 1293–1301. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bytyçi, I.; Shenouda, R.; Wester, P.; Henein, M.Y. Carotid Atherosclerosis in Predicting Coronary Artery Disease: A Systematic Review and Meta-Analysis. Arterioscler. Thromb. Vasc. Biol. 2021, 41, e224–e237. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, J.A.; Aday, A.W.; Patel, M.R.; Jones, W.S. Polyvascular Disease: Reappraisal of the Current Clinical Landscape. Circ. Cardiovasc. Interv. 2019, 12, e007385. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Esin, F.; İnce, H.S.; Kırış, T.; Çelik, A.; Karaca, M. Impact of Atherosclerotic Burden on Long-term Major Adverse Cardiovascular and Cerebrovascular Events. Int. J. Cardiovasc. Acad. 2024, 10, 123–131. [Google Scholar] [CrossRef]
- Jovin, D.G.; Sumpio, B.E.; Greif, D.M. Manifestations of human atherosclerosis across vascular beds. JVS Vasc. Insights 2024, 2, 100089. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Acosta, S.; Du, Y.; Borné, Y.; Gottsäter, A. Differences in risk factor profiles for peripheral artery disease compared to coronary, cerebral and carotid artery. Sci. Rep. 2025, 15, 3864. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Garcia-Garcia, H.M.; Sanz-Sanchez, J.; Pinilla-Echeverri, N.; Blanco, P.J.; Bourantas, C.; Alfonso, F. Advances in coronary imaging of atherosclerotic plaques. EuroIntervention 2025, 21, e778–e795. [Google Scholar] [CrossRef] [PubMed]
- Pabon, M.; Cheng, S.; Altin, S.E.; Sethi, S.S.; Nelson, M.D.; Moreau, K.L.; Hamburg, N.; Hess, C.N. Sex Differences in Peripheral Artery Disease. Circ. Res. 2022, 130, 496–511. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gurgoglione, F.L.; Solinas, E.; Pfleiderer, B.; Vezzani, A.; Niccoli, G. Coronary atherosclerotic plaque phenotype and physiopathologic mechanisms: Is there an influence of sex? Insights from intracoronary imaging. Atherosclerosis 2023, 384, 117273. [Google Scholar] [CrossRef] [PubMed]
- White, C.J. Chronic mesenteric ischemia: Diagnosis and management. Prog. Cardiovasc. Dis. 2011, 54, 36–40. [Google Scholar] [CrossRef] [PubMed]
- Wilkins, L.R.; Stone, J.R. Chronic mesenteric ischemia. Tech. Vasc. Interv. Radiol. 2015, 18, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Przewlocki, T.; Kablak-Ziembicka, A.; Tracz, W.; Kopec, G.; Rubis, P.; Pasowicz, M.; Musialek, P.; Kostkiewicz, M.; Kozanecki, A.; Stompor, T.; et al. Prevalence and Prediction of Renal Artery Stenosis in Patients with Coronary and Supraaortic Artery Atherosclerotic Disease. Nephrol. Dial. Transpl. Transplant. 2008, 23, 580–585. [Google Scholar] [CrossRef] [PubMed]
- Jebari-Benslaiman, S.; Galicia-García, U.; Larrea-Sebal, A.; Olaetxea, J.R.; Alloza, I.; Vandenbroeck, K.; Benito-Vicente, A.; Martín, C. Pathophysiology of Atherosclerosis. Int. J. Mol. Sci. 2022, 23, 3346. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ji, X.; Leng, X.Y.; Dong, Y.; Ma, Y.H.; Xu, W.; Cao, X.P.; Hou, X.H.; Dong, Q.; Tan, L.; Yu, J.T. Modifiable risk factors for carotid atherosclerosis: A meta-analysis and systematic review. Ann. Transl. Med. 2019, 7, 632. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Montone, R.A.; Camilli, M.; Calvieri, C.; Magnani, G.; Bonanni, A.; Bhatt, D.L.; Rajagopalan, S.; Crea, F.; Niccoli, G. Exposome in ischaemic heart disease: Beyond traditional risk factors. Eur. Heart J. 2024, 45, 419–438. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kannel, W.B.; Wolf, P.A. Peripheral and cerebral atherothrombosis and cardiovascular events in different vascular territories: Insights from the Framingham Study. Curr. Atheroscler. Rep. 2006, 8, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Zimarino, M.; Cappelletti, L.; Venarucci, V.; Gallina, S.; Scarpignato, M.; Acciai, N.; Calafiore, A.M.; Barsotti, A.; De Caterina, R. Age-dependence of risk factors for carotid stenosis: An observational study among candidates for coronary arteriography. Atherosclerosis 2001, 159, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.; Vega, G.L. Coronary and peripheral artery plaques: Do differences in plaque characteristics translate to differences in lipid management? J. Investig. Med. 2020, 68, 1141–1151. [Google Scholar] [CrossRef] [PubMed]
- Ding, N.; Sang, Y.; Chen, J.; Ballew, S.H.; Kalbaugh, C.A.; Salameh, M.J.; Blaha, M.J.; Allison, M.; Heiss, G.; Selvin, E.; et al. Cigarette Smoking, Smoking Cessation, and Long-Term Risk of 3 Major Atherosclerotic Diseases. J. Am. Coll. Cardiol. 2019, 74, 498–507. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Giménez-Pérez, G.; Viñals, C.; Mata-Cases, M.; Vlacho, B.; Real, J.; Franch-Nadal, J.; Ortega, E.; Mauricio, D. Epidemiology of the first-ever cardiovascular event in people with type 1 diabetes: A retrospective cohort population-based study in Catalonia. Cardiovasc. Diabetol. 2023, 22, 179. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kou, M.; Ding, N.; Ballew, S.H.; Salameh, M.J.; Martin, S.S.; Selvin, E.; Heiss, G.; Ballantyne, C.M.; Matsushita, K.; Hoogeveen, R.C. Conventional and Novel Lipid Measures and Risk of Peripheral Artery Disease. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 1229–1238. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, Q.; Smith, C.Y.; Bailey, K.R.; Wennberg, P.W.; Kullo, I.J. Disease location is associated with survival in patients with peripheral arterial disease. J. Am. Heart Assoc. 2013, 2, e000304. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Alahdab, F.; Arwani, R.; Pasha, A.K.; Razouki, Z.A.; Prokop, L.J.; Huber, T.S.; Murad, M.H. A systematic review and meta-analysis of endovascular versus open surgical revascularization for chronic mesenteric ischemia. J. Vasc. Surg. 2018, 67, 1598–1605. [Google Scholar] [CrossRef]
- Veenstra, R.P.; ter Steege, R.W.; Geelkerken, R.H.; Huisman, A.B.; Kolkman, J.J. The cardiovascular risk profile of atherosclerotic gastrointestinal ischemia is different from other vascular beds. Am. J. Med. 2012, 125, 394–398. [Google Scholar] [CrossRef] [PubMed]
- Sana, A.; van Noord, D.; Mensink, P.B.; Kooij, S.; van Dijk, K.; Bravenboer, B.; Lieverse, A.G.; Sijbrands, E.J.; Langendonk, J.G.; Kuipers, E.J. Patients with chronic gastrointestinal ischemia have a higher cardiovascular disease risk and mortality. Atherosclerosis 2012, 224, 235–241. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lin, T.C.; Wright, C.M.; Criqui, M.H.; Allison, M.A. Superior mesenteric artery calcification is associated with cardiovascular risk factors, systemic calcified atherosclerosis, and increased mortality. J. Vasc. Surg. 2018, 67, 1484–1490. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Takagi, H.; Umemoto, T.; ALICE (All-Literature Investigation of Cardiovascular Evidence) Group. Association of Hypertension with Abdominal Aortic Aneurysm Expansion. Ann. Vasc. Surg. 2017, 39, 74–89. [Google Scholar] [CrossRef] [PubMed]
- Climent, E.; Benaiges, D.; Chillarón, J.J.; Flores-Le Roux, J.A.; Pedro-Botet, J. Diabetes mellitus as a protective factor of abdominal aortic aneurysm: Possible mechanisms. Clin. Investig. Arterioscler. 2018, 30, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Borén, J.; Chapman, M.J.; Krauss, R.M.; Packard, C.J.; Bentzon, J.F.; Binder, C.J.; Daemen, M.J.; Demer, L.L.; Hegele, R.A.; Nicholls, S.J.; et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease: Pathophysiological, genetic, and therapeutic insights: A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur. Heart J. 2020, 41, 2313–2330. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Song, B.; Bie, Y.; Feng, H.; Xie, B.; Liu, M.; Zhao, F. Inflammatory Factors Driving Atherosclerotic Plaque Progression New Insights. J. Transl. Int. Med. 2022, 10, 36–47. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Virmani, R.; Ladich, E.R.; Burke, A.P.; Kolodgie, F.D. Histopathology of carotid atherosclerotic disease. Neurosurgery 2006, 59, S3-219–S3-227. [Google Scholar] [CrossRef] [PubMed]
- Sondore, D.; Trušinskis, K.; Linde, M.; Briede, I.; Narbute, I.; Jēgere, S.; Griķis, K.; Štrenge, K.; Ērglis, A. Association between carotid and coronary atherosclerotic plaque morphology: A virtual histology intravascular ultrasound study. J. Clin. Transl. Res. 2023, 9, 253–260. [Google Scholar] [PubMed] [PubMed Central]
- Kawai, K.; Finn, A.V.; Virmani, R.; Garg, P.; Bhatia, H.; Allen, T.; Pouncey, A.-L.; Dichek, D.; Golledge, J.; Allison, M.; et al. Subclinical Atherosclerosis: Part 1: What Is it? Can it Be Defined at the Histological Level? Arterioscler. Thromb. Vasc. Biol. 2024, 44, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Russo, M.; Coccato, M.; Preti, G.; Cinquetti, M.; Macor, F.; Sitta, N.; Carchesio, F.; Cattarin, S.; Piccoli, G.; Mantovan, R. Coronary computed tomography angiography and optical coherence tomography imaging of an intraplaque hemorrhage. J. Cardiovasc. Med. 2023, 24, 850–851. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Hom, J.; Li, Y.; Jiang, B.; Rodriguez, F.; Fleischmann, D.; Saloner, D.; Porcu, M.; Zhang, Y.; Saba, L.; et al. Carotid plaque imaging and the risk of atherosclerotic cardiovascular disease. Cardiovasc. Diagn. Ther. 2020, 10, 1048–1067. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Russo, M.; Fracassi, F.; Kurihara, O.; Kim, H.O.; Thondapu, V.; Araki, M.; Shinohara, H.; Sugiyama, T.; Yamamoto, E.; Lee, H.; et al. Healed Plaques in Patients with Stable Angina Pectoris. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 1587–1597. [Google Scholar] [CrossRef] [PubMed]
- Soor, G.S.; Vukin, I.; Leong, S.W.; Oreopoulos, G.; Butany, J. Peripheral vascular disease: Who gets it and why? A histomorphological analysis of 261 arterial segments from 58 cases. Pathology 2008, 40, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Torii, S.; Mustapha, J.A.; Narula, J.; Mori, H.; Saab, F.; Jinnouchi, H.; Yahagi, K.; Sakamoto, A.; Romero, M.E.; Narula, N.; et al. Histopathologic Characterization of Peripheral Arteries in Subjects with Abundant Risk Factors: Correlating Imaging with Pathology. JACC Cardiovasc. Imaging 2019, 12, 1501–1513. [Google Scholar] [CrossRef] [PubMed]
- Abbas, A.E.; Zacharias, S.K.; Goldstein, J.A.; Hanson, I.D.; Safian, R.D. Invasive characterization of atherosclerotic plaque in patients with peripheral arterial disease using near-infrared spectroscopy intravascular ultrasound. Catheter. Cardiovasc. Interv. 2017, 90, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, T.; Kikumori, A.; Kimura, N.; Shiomi, M. Distribution of atherosclerotic lesions in various arteries of WHHLMI rabbits, an animal model of familial hypercholesterolemia. Exp. Anim. 2019, 68, 293–300. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schnee, S.; Sass, K.; Moellmer, H.; Hohenfellner, R.; Spanel-Borowski, K. Heterogeneity of atherosclerosis in mesenteric arteries and outgrowth remodeling. Cardiovasc. Pathol. 2010, 19, e195–e203. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, Y.; Takumi, T.; Mathew, V.; Chung, W.-Y.; Barsness, G.W.; Rihal, C.S.; Gulati, R.; McCue, E.T.; Holmes, D.R.; Eeckhout, E.; et al. Plaque characteristics and arterial remodeling in coronary and peripheral arterial systems. Atherosclerosis 2012, 223, 365–371. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hildebrandt, H.A.; Gossl, M.; Mannheim, D.; Versari, D.; Herrmann, J.; Spendlove, D.; Bajanowski, T.; Malyar, N.M.; Erbel, R.; Lerman, L.O.; et al. Differential distribution of vasa vasorum in different vascular beds in humans. Atherosclerosis 2008, 199, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Mackey, R.H.; Venkitachalam, L.; Sutton-Tyrrell, K. Calcifications, arterial stiffness and atherosclerosis. Adv. Cardiol. 2007, 44, 234–244. [Google Scholar] [CrossRef] [PubMed]
- Spagnoli, L.G.; Mauriello, A.; Sangiorgi, G.; Fratoni, S.; Bonanno, E.; Schwartz, R.S.; Piepgras, D.G.; Pistolese, R.; Ippoliti, A.; Holmes, D.R. Extracranial thrombotically active carotid plaque as a risk factor for ischemic stroke. JAMA 2004, 292, 1845–1852. [Google Scholar] [CrossRef] [PubMed]
- Russo, M.; Kim, H.O.; Thondapu, V.; Kurihara, O.; Araki, M.; Shinohara, H.; Yamamoto, E.; Lee, H.; Yonetsu, T.; Minami, Y.; et al. Ethnic Differences in the Pathobiology of Acute Coronary Syndromes Between Asians and Whites. Am. J. Cardiol. 2020, 125, 1757–1764. [Google Scholar] [CrossRef] [PubMed]
- Montone, R.A.; Cosentino, N.; Graziani, F.; Gorla, R.; Del Buono, M.G.; La Vecchia, G.; Rinaldi, R.; Marenzi, G.; Bartorelli, A.L.; De Marco, F.; et al. Precision medicine versus standard of care for patients with myocardial infarction with non-obstructive coronary arteries (MINOCA): Rationale and design of the multicentre, randomised PROMISE trial. EuroIntervention 2022, 18, e933–e939. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gerhardt, T.; Seppelt, C.; Abdelwahed, Y.S.; Meteva, D.; Wolfram, C.; Stapmanns, P.; Erbay, A.; Zanders, L.; Nelles, G.; Musfeld, J.; et al. Culprit plaque morphology determines inflammatory risk and clinical outcomes in acute coronary syndrome. Eur. Heart J. 2023, 44, 3911–3925. [Google Scholar] [CrossRef] [PubMed]
- Narula, N.; Olin, J.W.; Narula, N. Pathologic Disparities Between Peripheral Artery Disease and Coronary Artery Disease. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 1982–1989. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Cai, Z.; Cai, J.; Zhao, X.; Li, F.; Yuan, C. Correlation of coronary plaque phenotype and carotid atherosclerotic plaque composition. Am. J. Med. Sci. 2011, 342, 480–485. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Jin, H.; Cheng, W.; Rao, S.; Lu, X.; Zeng, M. Correlation of coronary atherosclerosis and subclinical plaque phenotype of carotid artery: A 320-row multidetector computed tomographic angiography study. J. Comput. Assist. Tomogr. 2013, 37, 701–706. [Google Scholar] [CrossRef] [PubMed]
- Hamirani, Y.S.; Larijani, V.; Isma’eel, H.; Pagali, S.R.; Bach, P.; Karlsberg, R.P.; Budoff, M.J. Association of plaque in the carotid and coronary arteries, using MDCT angiography. Atherosclerosis 2010, 211, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Arad, Y.; Spadaro, L.A.; Roth, M.; Scordo, J.; Goodman, K.; Sherman, S.; Lledo, A.; Lerner, G.; Guerci, A.D. Correlations between vascular calcification and atherosclerosis: A comparative electron beam CT study of the coronary and carotid arteries. J. Comput. Assist. Tomogr. 1998, 22, 207–211. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.B.; Budoff, M.J.; Zavodni, A.; Polak, J.F.; Carr, J.J.; Burke, G.L.; Herrington, D.M. Coronary artery calcium is associated with degree of stenosis and surface irregularity of carotid artery. Atherosclerosis 2012, 223, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Saito, D.; Shiraki, T.; Oka, T.; Kajiyama, A.; Doi, M.; Masaka, T. Morphologic correlation between atherosclerotic lesions of the carotid and coronary arteries in patients with angina pectoris. Jpn. Circ. J. 1999, 63, 522–526. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, A.; Biasucci, L.M.; Lanza, G.A.; Coli, S.; Silvestri, P.; Cianflone, D.; Liuzzo, G.; Burzotta, F.; Crea, F.; Maseri, A. Inflammation as a possible link between coronary and carotid plaque instability. Circulation 2004, 109, 3158–3163. [Google Scholar] [CrossRef] [PubMed]
- Synetos, A.; Bounas, P.; Karanasos, A.; Latsios, G.; Drakopoulou, M.; Papanikolaou, A.; Olympios, C.; Trantalis, G.; Tsioufis, K.; Toutouzas, K. In vivo correlation between morphological characteristics of coronary plaques and functional characteristics of carotid arteries in acute coronary syndrome. Am. J. Cardiovasc. Dis. 2021, 11, 360–367. [Google Scholar] [PubMed] [PubMed Central]
- Fracassi, F.; Niccoli, G.; Vetrugno, V.; Russo, M.; Rettura, F.; Vergni, F.; Scalone, G.; Montone, R.A.; Vergallo, R.; D’AMario, D.; et al. Optical coherence tomography and C-reactive protein in risk stratification of acute coronary syndromes. Int. J. Cardiol. 2019, 286, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Kato, A.; Minami, Y.; Asakura, K.; Katamine, M.; Katsura, A.; Muramatsu, Y.; Sato, T.; Kakizaki, R.; Hashimoto, T.; Meguro, K.; et al. Characteristics of carotid atherosclerosis in patients with plaque erosion. J. Thromb. Thrombolysis 2021, 52, 620–627. [Google Scholar] [CrossRef] [PubMed]
- Weng, Z.; Zhao, C.; Qin, Y.; Liu, C.; Pan, W.; Hu, S.; He, L.; Xu, Y.; Zeng, M.; Feng, X.; et al. Peripheral atherosclerosis in acute coronary syndrome patients with plaque rupture vs plaque erosion: A prospective coronary optical coherence tomography and peripheral ultrasound study. Am. Heart J. 2023, 263, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.J.; Chen, Q.; Cheng, Y. Noninvasive carotid ultrasound for predicting vulnerable plaques of the coronary artery based on optical coherence tomography images. Quant. Imaging Med. Surg. 2024, 14, 316–324. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Miszalski-Jamka, T.; Lichołai, S.; Karwat, K.; Laskowicz, B.; Okraska-Bylica, A.; Wilkosz, T.; Konieczyńska, M.; Trystuła, M.; Słowik, L.; Urbańczyk, M.; et al. Computed tomography characteristics of coronary artery atherosclerosis in subjects with lower extremity peripheral artery disease and no cardiac symptoms. Pol. Arch. Med. Wewn. 2013, 123, 657–663. [Google Scholar] [CrossRef] [PubMed]
- Hussein, A.A.; Uno, K.; Wolski, K.; Kapadia, S.; Schoenhagen, P.; Tuzcu, E.M.; Nissen, S.E.; Nicholls, S.J. Peripheral arterial disease and progression of coronary atherosclerosis. J. Am. Coll. Cardiol. 2011, 57, 1220–1225. [Google Scholar] [CrossRef] [PubMed]
- Bryniarski, K.L.; Yamamoto, E.; Takumi, H.; Xing, L.; Zanchin, T.; Sugiyama, T.; Lee, H.; Jang, I.-K. Differences in coronary plaque characteristics between patients with and those without peripheral arterial disease. Coron. Artery Dis. 2017, 28, 658–663. [Google Scholar] [CrossRef] [PubMed]
- Baber, U.; Stone, G.W.; Weisz, G.; Moreno, P.; Dangas, G.; Maehara, A.; Mintz, G.S.; Cristea, E.; Fahy, M.; Xu, K.; et al. Coronary plaque composition, morphology, and outcomes in patients with and without chronic kidney disease presenting with acute coronary syndromes. JACC Cardiovasc. Imaging 2012, 5, S53–S61. [Google Scholar] [CrossRef] [PubMed]
- Kato, K.; Yonetsu, T.; Jia, H.; Abtahian, F.; Vergallo, R.; Hu, S.; Tian, J.; Kim, S.-J.; Lee, H.; McNulty, I.; et al. Nonculprit coronary plaque characteristics of chronic kidney disease. Circ. Cardiovasc. Imaging 2013, 6, 448–456. [Google Scholar] [CrossRef] [PubMed]
- Chin, C.Y.; Matsumura, M.; Maehara, A.; Zhang, W.; Lee, C.T.; Yamamoto, M.H.; Song, L.; Parviz, Y.; Jhalani, N.B.; Mohan, S.; et al. Coronary Plaque Characteristics in Hemodialysis-Dependent Patients as Assessed by Optical Coherence Tomography. Am. J. Cardiol. 2017, 119, 1313–1319. [Google Scholar] [CrossRef] [PubMed]
- Yuki, H.; Isselbacher, E.; Niida, T.; Suzuki, K.; Kinoshita, D.; Fujimoto, D.; Lee, H.; McNulty, I.; Nakamura, S.; Kakuta, T.; et al. Protruding Aortic Plaque and Coronary Plaque Vulnerability. J. Am. Heart Assoc. 2024, 13, e032742. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mazzolai, L.; Teixido-Tura, G.; Lanzi, S.; Boc, V.; Bossone, E.; Brodmann, M.; Bura-Rivière, A.; De Backer, J.; Deglise, S.; Della Corte, A.; et al. 2024 ESC Guidelines for the management of peripheral arterial and aortic diseases. Eur. Heart J. 2024, 45, 3538–3700. [Google Scholar] [CrossRef] [PubMed]
- Naylor, R.; Rantner, B.; Ancetti, S.; de Borst, G.J.; De Carlo, M.; Halliday, A.; Kakkos, S.K.; Markus, H.S.; McCabe, D.J.; Sillesen, H.; et al. Editor’s Choice—European Society for Vascular Surgery (ESVS) 2023 Clinical Practice Guidelines on the Management of Atherosclerotic Carotid and Vertebral Artery Disease. Eur. J. Vasc. Endovasc. Surg. 2023, 65, 7–111. [Google Scholar] [CrossRef] [PubMed]
- Visseren, F.L.J.; Mach, F.; Smulders, Y.M.; Carballo, D.; Koskinas, K.C.; Bäck, M.; Benetos, A.; Biffi, A.; Boavida, J.-M.; Capodanno, D.; et al. ESC Guidelines on cardiovascular disease prevention in clinical practice. Eur. Heart J. 2021, 42, 3227–3337, Erratum in Eur. Heart J. 2022, 43, 4468. [Google Scholar] [CrossRef] [PubMed]
- Thondapu, V.; Kurihara, O.; Yonetsu, T.; Russo, M.; Kim, H.O.; Lee, H.; Soeda, T.; Minami, Y.; Jang, I.-K. Comparison of Rosuvastatin Versus Atorvastatin for Coronary Plaque Stabilization. Am. J. Cardiol. 2019, 123, 1565–1571. [Google Scholar] [CrossRef] [PubMed]
- Räber, L.; Ueki, Y.; Otsuka, T.; Losdat, S.; Häner, J.D.; Lonborg, J.; Fahrni, G.; Iglesias, J.F.; van Geuns, R.-J.; Ondracek, A.S.; et al. Effect of Alirocumab Added to High-Intensity Statin Therapy on Coronary Atherosclerosis in Patients with Acute Myocardial Infarction: The PACMAN-AMI Randomized Clinical Trial. JAMA 2022, 327, 1771–1781. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Han, Y.; Ren, L.; Fei, X.; Wang, J.; Chen, T.; Guo, J.; Wang, Q. Effect of moderate-intensity statin on carotid intraplaque neovascularization of coronary artery disease: A retrospective cohort study. Quant. Imaging Med. Surg. 2024, 14, 1660–1672. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mazer, C.D.; Moody, A.R.; Teoh, H.; Quan, A.; Elituv, R.; Vivekanandan, T.; Li, P.; Thorpe, K.E.; Lafreniere-Roula, M.; Verma, R.; et al. SLICE-CEA CardioLink-8: A Randomized Trial of Evolocumab on Carotid Artery Atherosclerotic Plaque Characteristics in Asymptomatic High-Risk Carotid Stenosis. Circulation 2025, 151, 351–355. [Google Scholar] [CrossRef] [PubMed]
- D’aMario, D.; Cappetta, D.; Cappannoli, L.; Princi, G.; Migliaro, S.; Diana, G.; Chouchane, K.; Borovac, J.A.; Restivo, A.; Arcudi, A.; et al. Colchicine in ischemic heart disease: The good, the bad and the ugly. Clin. Res. Cardiol. 2021, 110, 1531–1542. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nidorf, S.M.; Fiolet, A.T.L.; Mosterd, A.; Eikelboom, J.W.; Schut, A.; Opstal, T.S.J.; The, S.H.K.; Xu, X.-F.; Ireland, M.A.; Lenderink, T.; et al. Colchicine inpatients with chronic coronary disease. N. Engl. J. Med. 2020, 383, 1838–1847. [Google Scholar] [CrossRef]
- Fiolet, A.T.; Poorthuis, M.H.; Opstal, T.S.; Amarenco, P.; Boczar, K.E.; Buysschaert, I.; Budgeon, C.; Chan, N.C.; Cornel, J.H.; Jolly, S.S.; et al. Colchicine Cardiovascular Trialists Collaboration. Colchicine for secondary prevention of ischaemic stroke and atherosclerotic events: A meta-analysis of randomised trials. eClinicalMedicine 2024, 76, 102835. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Canonico, M.E.; Hess, C.N.; Rogers, R.K.; Bonaca, M.P. Medical Therapy for Peripheral Artery Disease. Curr. Cardiol. Rep. 2024, 26, 651–659. [Google Scholar] [CrossRef] [PubMed]
- Magnani, G.; Denegri, A.; Gurgoglione, F.L.; Barocelli, F.; Indrigo, E.; Catellani, D.; Signoretta, G.; Bettella, A.; Tuttolomondo, D.; Solinas, E.; et al. Dual Antiplatelet Therapy or Antiplatelet Plus Anticoagulant Therapy in Patients with Peripheral and Chronic Coronary Artery Disease: An Updated Review. J. Clin. Med. 2023, 12, 5284. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kaplovitch, E.; Eikelboom, J.W.; Dyal, L.; Aboyans, V.; Abola, M.T.; Verhamme, P.; Avezum, A.; Fox, K.A.A.; Berkowitz, S.D.; Bangdiwala, S.I.; et al. Rivaroxaban and Aspirin in Patients with Symptomatic Lower Extremity Peripheral Artery Disease: A Subanalysis of the COMPASS Randomized Clinical Trial. JAMA Cardiol. 2021, 6, 21–29, Erratum in JAMA Cardiol. 2021, 6, 246. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Connolly, S.J.; Eikelboom, J.W.; Bosch, J.; Dyal, L.; Lanas, F.; Metsarinne, K.; O’DOnnell, M.; Dans, A.L.; Ha, J.-W.; Parkhomenko, A.N.; et al. Rivaroxaban with or without aspirin in patients with stable coronary artery disease: An international, randomised, double-blind, placebo-controlled trial. Lancet 2018, 391, 205–218, Erratum in Lancet 2018, 391, 204. [Google Scholar] [CrossRef] [PubMed]
- Bonaca, M.P.; Nault, P.; Giugliano, R.P.; Keech, A.C.; Pineda, A.L.; Kanevsky, E.; Kuder, J.; Murphy, S.A.; Jukema, J.W.; Lewis, B.S.; et al. Low-Density Lipoprotein Cholesterol Lowering with Evolocumab and Outcomes in Patients with Peripheral Artery Disease: Insights From the FOURIER Trial (Further Cardiovascular Outcomes Research with PCSK9 Inhibition in Subjects with Elevated Risk). Circulation 2018, 137, 338–350. [Google Scholar] [CrossRef] [PubMed]
- Sabatine, M.S.; Giugliano, R.P.; Keech, A.C.; Honarpour, N.; Wiviott, S.D.; Murphy, S.A.; Kuder, J.F.; Wang, H.; Liu, T.; Wasserman, S.M.; et al. Evolocumab and Clinical Outcomes in Patients with Cardiovascular Disease. N. Engl. J. Med. 2017, 376, 1713–1722. [Google Scholar] [CrossRef] [PubMed]
- Jain, H.; Patel, N.; Erum, M.; Odat, R.M.; Passey, S.; Khan, R.; Jain, J.; Cheema, A.H.; Fox, S.; Ahmed, R. Efficacy of colchicine in lower extremity peripheral arterial disease: A meta-analysis. Heart Lung 2025, 73, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Russell, K.S.; Yates, D.P.; Kramer, C.M.; Feller, A.; Mahling, P.; Colin, L.; Clough, T.; Wang, T.; LaPerna, L.; Patel, A.; et al. A randomized, placebo-controlled trial of canakinumab in patients with peripheral artery disease. Vasc. Med. 2019, 24, 414–421. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Terlouw, L.G.; Moelker, A.; Abrahamsen, J.; Acosta, S.; Bakker, O.J.; Baumgartner, I.; Boyer, L.; Corcos, O.; van Dijk, L.J.; Duran, M.; et al. European guidelines on chronic mesenteric ischaemia—Joint United European Gastroenterology, European Association for Gastroenterology, Endoscopy and Nutrition, European Society of Gastrointestinal and Abdominal Radiology, Netherlands Association of Hepatogastroenterologists, Hellenic Society of Gastroenterology, Cardiovascular and Interventional Radiological Society of Europe, and Dutch Mesenteric Ischemia Study group clinical guidelines on the diagnosis and treatment of patients with chronic mesenteric ischaemia. United Eur. Gastroenterol. J. 2020, 8, 371–395. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Alnahhal, K.I.; Sorour, A.A.; Lyden, S.P.; Caputo, F.J.; Park, W.M.; Rowse, J.W.; Quatromoni, J.G.; Khalifeh, A.; Dehaini, H.; Bena, J.F.; et al. Management of patients with chronic mesenteric ischemia across three consecutive eras. J. Vasc. Surg. 2023, 78, 1228–1238.e1. [Google Scholar] [CrossRef] [PubMed]
- Safian, R.D. Renal artery stenosis. Prog. Cardiovasc. Dis. 2021, 65, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Salata, K.; Syed, M.; Hussain, M.A.; de Mestral, C.; Greco, E.; Mamdani, M.; Tu, J.V.; Forbes, T.L.; Bhatt, D.L.; Verma, S.; et al. Statins Reduce Abdominal Aortic Aneurysm Growth, Rupture, and Perioperative Mortality: A Systematic Review and Meta-Analysis. J. Am. Heart Assoc. 2018, 7, e008657. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bonati, L.H.; Jansen, O.; de Borst, G.J.; Brown, M.M. Management of atherosclerotic extracranial carotid artery stenosis. Lancet Neurol. 2022, 21, 273–283. [Google Scholar] [CrossRef] [PubMed]
- Song, P.; Fang, Z.; Wang, H.; Cai, Y.; Rahimi, K.; Zhu, Y.; Fowkes, F.G.R.; Fowkes, F.J.I.; Rudan, I. Global and regional prevalence, burden, and risk factors for carotid atherosclerosis: A systematic review, meta-analysis, and modelling study. Lancet Glob. Health 2020, 8, e721–e729. [Google Scholar] [CrossRef] [PubMed]
- Fisher, C.M.; Gore, I.; Okabe, N.; White, P.D. Atherosclerosis of the carotid and vertebral arteries—Extracranial and intracranial. J. Neuropathol. Exp. Neurol. 1965, 24, 455–476. [Google Scholar] [CrossRef]
- Aboyans, V.; Lacroix, P. Indications for carotid screening in patients with coronary artery disease. Presse Med. 2009, 38, 977–986. [Google Scholar] [CrossRef] [PubMed]
- Artyszuk, Ł.; Błażejowska, E.; Danielecka, Z.; Jurek, J.; Olek, E.; Abramczyk, P. Peripheral atherosclerosis evaluation through ultrasound: A promising diagnostic tool for coronary artery disease. Echocardiography 2023, 40, 841–851. [Google Scholar] [CrossRef] [PubMed]
- Kallikazaros, I.; Tsioufis, C.; Sideris, S.; Stefanadis, C.; Toutouzas, P. Carotid artery disease as a marker for the presence of severe coronary artery disease in patients evaluated for chest pain. Stroke 1999, 30, 1002–1007. [Google Scholar] [CrossRef] [PubMed]
- Ozcaglayan, O.; Ozcaglayan, T.I.K.; Gur, D.O.; Gumusel, H.K.; Topcu, B.; Unal, A. Carotid arteries and vertebrobasilary system intracranial stenosis correlates with multi vessel coronary artery disease. Bratisl. Lek. Listy 2019, 120, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Ogutu, P.; Werner, R.; Oertel, F.; Beyer, M. Should patients with asymptomatic significant carotid stenosis undergo simultaneous carotid and cardiac surgery? Interact. Cardiovasc. Thorac. Surg. 2014, 18, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Jia, L.; Li, J.; Hua, Y. Correlation between carotid and/or subclavian atherosclerotic plaque and coronary atherosclerotic disease. BMC Cardiovasc. Disord. 2024, 24, 678. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Komorovsky, R.; Desideri, A.; Coscarelli, S.; Cortigiani, L.; Tonello, D.; Visonà, A.; Celegon, L. Prognostic implications of sonographic characteristics of carotid plaques in patients with acute coronary syndromes. Heart 2005, 91, 819–820. [Google Scholar] [CrossRef] [PubMed] [PubMed Central][Green Version]
- Gao, J.; Cheng, Y. Ultrasound-based prevalence of polyvascular disease and its association with adverse outcome in patients undergoing coronary artery bypass grafting. Sci. Prog. 2024, 107, 368504241297206. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Park, H.W.; Kim, W.H.; Kim, K.H.; Yang, D.J.; Kim, J.H.; Song, I.G.; Kwon, T.G.; Bae, J.H. Carotid plaque is associated with increased cardiac mortality in patients with coronary artery disease. Int. J. Cardiol. 2013, 166, 658–663. [Google Scholar] [CrossRef] [PubMed]
- Steinvil, A.; Sadeh, B.; Bornstein, N.M.; Havakuk, O.; Greenberg, S.; Arbel, Y.; Konigstein, M.; Finkelstein, A.; Banai, S.; Halkin, A. Impact of carotid atherosclerosis on the risk of adverse cardiac events in patients with and without coronary disease. Stroke 2014, 45, 2311–2317. [Google Scholar] [CrossRef] [PubMed]
- Reis, P.F.F.D.; Linhares, P.V.; Pitta, F.G.; Lima, E.G. Approach to concurrent coronary and carotid artery disease: Epidemiology, screening and treatment. Rev. Assoc. Med. Bras. 2017, 63, 1012–1016. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Illuminati, G.; Schneider, F.; Greco, C.; Mangieri, E.; Schiariti, M.; Tanzilli, G.; Barillà, F.; Paravati, V.; Pizzardi, G.; Calio’, F.; et al. Long-term results of a randomized controlled trial analyzing the role of systematic pre-operative coronary angiography before elective carotid endarterectomy in patients with asymptomatic coronary artery disease. Eur. J. Vasc. Endovasc. Surg. 2015, 49, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Sulženko, J.; Paluszek, P.; Machnik, R.; Widimský, P.; Jarkovský, J.; Pieniazek, P. Prevalence and predictors of coronary artery disease in patients undergoing carotid artery stenting. Coron. Artery Dis. 2019, 30, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.-Y.; Shin, S.J.; Yoo, J.; Lee, K.; Song, D.; Kim, Y.D.; Nam, H.S.; Lee, K.Y.; Lee, H.S.; Kim, D.J.; et al. Coronary Calcium Score for the Prediction of Asymptomatic Coronary Artery Disease in Patients with Ischemic Stroke. Front. Neurol. 2020, 11, 206. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gongora-Rivera, F.; Labreuche, J.; Jaramillo, A.; Steg, P.G.; Hauw, J.J.; Amarenco, P. Autopsy prevalence of coronary atherosclerosis in patients with fatal stroke. Stroke 2007, 38, 1203–1210. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.; Zhang, C.; Liu, X.; Yang, S.; Ma, M.; Tang, J.; Yin, T.; Zhao, S.; Tu, W.; Hu, H. Prevalence and associated risk factors of carotid plaque and artery stenosis in China: A population-based study. Front. Med. 2025, 19, 64–78. [Google Scholar] [CrossRef] [PubMed]
- E Mantella, L.; Colledanchise, K.N.; Hétu, M.-F.; Feinstein, S.B.; Abunassar, J.; Johri, A.M. Carotid intraplaque neovascularization predicts coronary artery disease and cardiovascular events. Eur. Heart J. Cardiovasc. Imaging 2019, 20, 1239–1247. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Usman, A.; Sadat, U.; Teng, Z.; Graves, M.J.; Boyle, J.R.; Varty, K.; Hayes, P.D.; Gillard, J.H. Magnetic Resonance Imaging-Based Assessment of Carotid Atheroma: A Comparative Study of Patients with and without Coronary Artery Disease. J. Stroke Cerebrovasc. Dis. 2017, 26, 347–351. [Google Scholar] [CrossRef] [PubMed]
- Dzaye, O.; Razavi, A.C.; Dardari, Z.A.; Nasir, K.; Matsushita, K.; Mok, Y.; Santilli, F.; Cobo, A.M.L.; Johri, A.M.; Albrecht, G.; et al. Carotid Ultrasound-Based Plaque Score for the Allocation of Aspirin for the Primary Prevention of Cardiovascular Disease Events: The Multi-Ethnic Study of Atherosclerosis and the Atherosclerosis Risk in Communities Study. J. Am. Heart Assoc. 2024, 13, e034718. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zimarino, M.; Angiolillo, D.J.; Dangas, G.; Capodanno, D.; Barbato, E.; Hahn, J.-Y.; Giustino, G.; Watanabe, H.; Costa, F.; Cuisset, T.; et al. Antithrombotic therapy after percutaneous coronary intervention of bifurcation lesions. EuroIntervention 2021, 17, 59–66. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fowkes, F.G.R.; Rudan, D.; Rudan, I.; Aboyans, V.; Denenberg, J.O.; McDermott, M.M.; Norman, P.E.; Sampson, U.K.A.; Williams, L.J.; Mensah, G.A.; et al. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: A systematic review and analysis. Lancet 2013, 382, 1329–1340. [Google Scholar] [CrossRef] [PubMed]
- Bonaca, M.P.; Gutierrez, J.A.; Cannon, C.; Giugliano, R.; Blazing, M.; Park, J.G.; White, J.; Tershakovec, A.; Braunwald, E. Polyvascular disease, type 2 diabetes, and long-term vascular risk: A secondary analysis of the IMPROVE-IT trial. Lancet Diabetes Endocrinol. 2018, 6, 934–943. [Google Scholar] [CrossRef] [PubMed]
- Sigvant, B.; Kragsterman, B.; Falkenberg, M.; Hasvold, P.; Johansson, S.; Thuresson, M.; Nordanstig, J. Contemporary cardiovascular risk and secondary preventive drug treatment patterns in peripheral artery disease patients undergoing revascularization. J. Vasc. Surg. 2016, 64, 1009–1017. [Google Scholar] [CrossRef] [PubMed]
- Criqui, M.H.; Matsushita, K.; Aboyans, V.; Hess, C.N.; Hicks, C.W.; Kwan, T.W.; McDermott, M.M.; Misra, S.; Ujueta, F.; American Heart Association Council on Epidemiology and Prevention; et al. Lower Extremity Peripheral Artery Disease: Contemporary Epidemiology, Management Gaps, and Future Directions: A Scientific Statement From the American Heart Association. Circulation 2021, 144, e171–e191, Erratum in Circulation 2021, 144, e193. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fernández-Friera, L.; Peñalvo, J.L.; Fernández-Ortiz, A.; Ibañez, B.; López-Melgar, B.; Laclaustra, M.; Oliva, B.; Mocoroa, A.; Mendiguren, J.; de Vega, V.M.; et al. Prevalence, Vascular Distribution, and Multiterritorial Extent of Subclinical Atherosclerosis in a Middle-Aged Cohort: The PESA (Progression of Early Subclinical Atherosclerosis) Study. Circulation 2015, 131, 2104–2113. [Google Scholar] [CrossRef] [PubMed]
- Blin, P.; Philippe, F.; Bouée, S.; Laurendeau, C.; Torreton, E.; Gourmelin, J.; Leproust, S.; Levy-Bachelot, L.; Steg, P.G. Outcomes following acute hospitalised myocardial infarction in France: An insurance claims database analysis. Int. J. Cardiol. 2016, 219, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Pinxterhuis, T.H.; Ploumen, E.H.; van Vliet, D.; van Houwelingen, K.G.; Stoel, M.G.; de Man, F.H.; Hartmann, M.; Zocca, P.; Linssen, G.C.; Geelkerken, R.H.; et al. Ten-year mortality after treating obstructive coronary atherosclerosis with contemporary stents in patients with or without concomitant peripheral arterial disease. Atherosclerosis 2024, 392, 117488. [Google Scholar] [CrossRef] [PubMed]
- Fowkes, F.; Murray, G.; Butcher, I.; Heald, C.L.; Lee, R.J.; E Chambless, L.; Folsom, A.R.; Hirsch, A.T.; Dramaix, M.; Debacker, G.; et al. Ankle brachial index combined with Framingham Risk Score to predict cardiovascular events and mortality: A meta-analysis. JAMA 2008, 300, 197–208. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Huish, S.; Nawaz, S.; Bellasi, A.; Diaz-Tocados, J.M.; Haarhaus, M.; Sinha, S. Clinical management of peripheral arterial disease in chronic kidney disease-a comprehensive review from the European Renal Association CKD-MBD Working Group. Clin. Kidney J. 2025, 18, sfaf089. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, J.; Mohler, E.R.; Xie, D.; Shlipak, M.; Townsend, R.R.; Appel, L.J.; Ojo, A.; Schreiber, M.; Nessel, L.; Zhang, X.; et al. Traditional and non-traditional risk factors for incident peripheral arterial disease among patients with chronic kidney disease. Nephrol. Dial. Transpl. Transplant. 2016, 31, 1145–1151. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wu, S.P.; Ringgaard, S.; Oyre, S.; Hansen, M.S.; Rasmus, S.; Pedersen, E.M. Wall shear rates differ between the normal carotid, femoral, and brachial arteries: An in vivo MRI study. J. Magn. Reson. Imaging 2004, 19, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Newman, J.D.; Cornwell, M.G.; Zhou, H.; Rockman, C.; Heguy, A.; Suarez, Y.; Cheng, H.S.; Feinberg, M.W.; Hochman, J.S.; Ruggles, K.V.; et al. Gene Expression Signature in Patients with Symptomatic Peripheral Artery Disease. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 1521–1533. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wilson, D.B.; Mostafavi, K.; Craven, T.E.; Ayerdi, J.; Edwards, M.S.; Hansen, K.J. Clinical course of mesenteric artery stenosis in elderly americans. Arch. Intern. Med. 2006, 166, 2095–2100. [Google Scholar] [CrossRef] [PubMed]
- Kolkman, J.J.; Geelkerken, R.H. Diagnosis and treatment of chronic mesenteric ischemia: An update. Best Pract. Res. Clin. Gastroenterol. 2017, 31, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Järvinen, O.; Laurikka, J.; Sisto, T.; Salenius, J.-P.; Tarkka, M.R.; Lindholm, T.S. Atherosclerosis in the abdominal aorta and its visceral branches: Associations with other manifestations of atherosclerosis in an autopsy study. Int. J. Angiol. 1996, 5, 41–44. [Google Scholar] [CrossRef]
- Bron, K.M.; Redman, H.C. Splanchnic artery stenosis and occlusion. Incidence; arteriographic and clinical manifestations. Radiology 1969, 92, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Järvinen, O.; Laurikka, J.; Sisto, T.; Salenius, J.P.; Tarkka, M.R. Atherosclerosis of the visceral arteries. Vasa 1995, 24, 9–14. [Google Scholar] [PubMed]
- Günenç Beşer, C.; Karcaaltıncaba, M.; Çelik, H.H.; Başar, R. The prevalence and distribution of the atherosclerotic plaques in the abdominal aorta and its branches. Folia Morphol. 2016, 75, 364–375. [Google Scholar] [CrossRef] [PubMed]
- Mateo, R.B.; O’Hara, P.J.; Hertzer, N.R.; Mascha, E.J.; Beven, E.G.; Krajewski, L.P. Elective surgical treatment of symptomatic chronic mesenteric occlusive disease: Early results and late outcomes. J. Vasc. Surg. 1999, 29, 821–831; discussion 832. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Krishnamurthy, G.; Menon, A.; Kannan, K.; Prakash, S.; Rajendran, A.; Philips, D. Coronary artery disease and mesenteric artery stenosis—Two sides of the same coin?—Long term prospective analysis. Intractable Rare Dis. Res. 2019, 8, 245–251. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Acosta, S. Epidemiology of mesenteric vascular disease: Clinical implications. Semin. Vasc. Surg. 2010, 23, 4–8. [Google Scholar] [CrossRef] [PubMed]
- Yaari, S.; Hiller, N.; Samet, Y.; Heyman, S.N. Reversible ‘Unstable’ Abdominal Angina Caused by Ruptured Plaque of the Superior Mesenteric Artery: Clinical and Radiological Correlations. Eur. J. Case Rep. Intern. Med. 2023, 10, 003766. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Björck, M.; Koelemay, M.; Acosta, S.; Goncalves, F.B.; Kölbel, T.; Kolkman, J.; Lees, T.; Lefevre, J.; Menyhei, G.; Oderich, G.; et al. Editor’s Choice—Management of the Diseases of Mesenteric Arteries and Veins: Clinical Practice Guidelines of the European Society of Vascular Surgery (ESVS). Eur. J. Vasc. Endovasc. Surg. 2017, 53, 460–510. [Google Scholar] [CrossRef] [PubMed]
- Safian, R.D.; Textor, S.C. Renal-artery stenosis. N. Engl. J. Med. 2001, 344, 431–442. [Google Scholar] [CrossRef] [PubMed]
- Mast, Q.; Beutler, J.J. The prevalence of atherosclerotic renal artery stenosis in risk groups: A systematic literature review. J. Hypertens. 2009, 27, 1333–1340. [Google Scholar] [CrossRef] [PubMed]
- Przewłocki, T.; Kablak-Ziembicka, A.; Tracz, W.; Kozanecki, A.; Kopeć, G.; Rubiś, P.; Kostkiewicz, M.; Rosławiecka, A.; Rzeźnik, D.; Stompór, T. Renal artery stenosis in patients with coronary artery disease. Kardiol. Pol. 2008, 66, 856–862; discussion 863–864. [Google Scholar] [PubMed]
- E Buller, C.; Nogareda, J.G.; Ramanathan, K.; Ricci, D.R.; Djurdjev, O.; Tinckam, K.J.; Penn, I.M.; Fox, R.S.; A Stevens, L.; A Duncan, J.; et al. The profile of cardiac patients with renal artery stenosis. J. Am. Coll. Cardiol. 2004, 43, 1606–1613. [Google Scholar] [CrossRef] [PubMed]
- Tomaru, R. Renin-Angiotensin-Aldosterone System (RAS) Inhibitors May Suppress the Prevalence of Peripheral Arterial Disease (PAD) in Elderly, Chronic Hemodialysis Patients. Cureus 2022, 14, e25087. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Harding, M.B.; Smith, L.R.; I Himmelstein, S.; Harrison, K.; Phillips, H.R.; Schwab, S.J.; Hermiller, J.B.; Davidson, C.J.; Bashore, T.M. Renal artery stenosis: Prevalence and associated risk factors in patients undergoing routine cardiac catheterization. J. Am. Soc. Nephrol. 1992, 2, 1608–1616. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, T.; Mathew, V.; Rubinshtein, R.; Rihal, C.S.; Lennon, R.; Lerman, L.O.; Lerman, A. Association of plaque composition and vessel remodeling in atherosclerotic renal artery stenosis: A comparison with coronary artery disease. JACC Cardiovasc. Imaging 2009, 2, 327–338. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tyczyński, P.; Kądziela, J.; Michałowska, I.; Puciłowska, B.; Witkowski, A. Ruptured plaque in the renal artery by optical coherence tomography. Kardiol. Pol. 2016, 74, 193. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ozawa, T.; Kimura, M.; Takemoto, R.; Sawada, T.; Matoba, S. Acute Renal Infarction and Cholesterol Crystal Embolism Due to Plaque Rupture in a Renal Artery. Circ. J. 2022, 87, 151. [Google Scholar] [CrossRef] [PubMed]
- Iwai, T.; Kataoka, Y.; Otsuka, F.; Asaumi, Y.; Nicholls, S.J.; Noguchi, T.; Yasuda, S. Chronic kidney disease and coronary atherosclerosis: Evidences from intravascular imaging. Expert Rev. Cardiovasc. Ther. 2019, 17, 707–716. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, S.; Suarez-Martinez, A.D.; Bagher, P.; Gonzalez, A.; Liu, R.; Murfee, W.L.; Mohandas, R. Microvascular dysfunction and kidney disease: Challenges and opportunities? Microcirc. 2021, 28, e12661. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sugiyama, T.; Kimura, S.; Ohtani, H.; Yamakami, Y.; Kojima, K.; Sagawa, Y.; Hishikari, K.; Hikita, H.; Ashikaga, T.; Takahashi, A.; et al. Impact of chronic kidney disease stages on atherosclerotic plaque components on optical coherence tomography in patients with coronary artery disease. Cardiovasc. Interv. Ther. 2017, 32, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Nicholls, S.J.; Tuzcu, E.M.; Wolski, K.; Sipahi, I.; Schoenhagen, P.; Crowe, T.; Kapadia, S.R.; Hazen, S.L.; Nissen, S.E. Coronary artery calcification and changes in atheroma burden in response to established medical therapies. J. Am. Coll. Cardiol. 2007, 49, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Isselbacher, E.M.; Preventza, O.; Black, J.H., 3rd; Augoustides, J.G.; Beck, A.W.; Bolen, M.A.; Braverman, A.C.; Bray, B.E.; Brown-Zimmerman, M.M.; Chen, E.P.; et al. 2022 ACC/AHA Guideline for the Diagnosis and Management of Aortic Disease: A Report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. Circulation 2022, 146, e334–e482. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ohman, E.M.; Bhatt, D.L.; Steg, P.G.; Goto, S.; Hirsch, A.T.; Liau, C.-S.; Mas, J.-L.; Richard, A.-J.; Röther, J.; Wilson, P.W.; et al. The REduction of Atherothrombosis for Continued Health (REACH) Registry: An international, prospective, observational investigation in subjects at risk for atherothrombotic events-study design. Am. Heart J. 2006, 151, 786.e1–786.e10. [Google Scholar] [CrossRef] [PubMed]
- Hertzer, N.R.; Young, J.R.; Beven, E.G.; O’Hara, P.J.; Graor, R.A.; Ruschhaupt, W.F.; Maljovec, L.C. Late results of coronary bypass in patients with infrarenal aortic aneurysms. The Cleveland Clinic Study. Ann. Surg. 1987, 205, 360–367. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hołda, M.K.; Iwaszczuk, P.; Wszołek, K.; Chmiel, J.; Brzychczy, A.; Trystuła, M.; Misztal, M. Coexistence and management of abdominal aortic aneurysm and coronary artery disease. Cardiol. J. 2020, 27, 384–393. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tatasciore, A.; Zimarino, M.; Tommasi, R.; Renda, G.; Schillaci, G.; Parati, G.; De Caterina, R. Increased short-term blood pressure variability is associated with early left ventricular systolic dysfunction in newly diagnosed untreated hypertensive patients. J. Hypertens. 2013, 31, 1653–1661. [Google Scholar] [CrossRef] [PubMed]
- Galiè, N.; Guarnieri, C.; Ussia, G.P.; Zimarino, M.; Traini, A.M.; Parlangeli, R.; Vaona, I.; Branzi, A.; Magnani, B. Limitation of myocardial infarct size by nicorandil after sustained ischemia in pigs. J. Cardiovasc. Pharmacol. 1995, 26, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Montone, R.A.; Vetrugno, V.; Camilli, M.; Russo, M.; Fracassi, F.; Khan, S.Q.; Doshi, S.N.; Townend, J.N.; Ludman, P.F.; Trani, C.; et al. Macrophage infiltrates in coronary plaque erosion and cardiovascular outcome in patients with acute coronary syndrome. Atherosclerosis 2020, 311, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Faa, G.; Cau, R.; Ravarino, A.; Canino, A.; Van Eyken, P.; Fraschini, M.; Suri, J.S.; Saba, L. Lessons from autopsy: Topographical variability of atherosclerosis plaques. J. Public Health Res. 2024, 13, 22799036241249659. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bentzon, J.F.; Otsuka, F.; Virmani, R.; Falk, E. Mechanisms of plaque formation and rupture. Circ. Res. 2014, 114, 1852–1866. [Google Scholar] [CrossRef] [PubMed]
- Lanzer, P.; Hannan, F.M.; Lanzer, J.D.; Janzen, J.; Raggi, P.; Furniss, D.; Schuchardt, M.; Thakker, R.; Fok, P.-W.; Saez-Rodriguez, J.; et al. Medial Arterial Calcification: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2021, 78, 1145–1165. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yang, G.; Khan, A.; Liang, W.; Xiong, Z.; Stegbauer, J. Aortic aneurysm: Pathophysiology and therapeutic options. MedComm 2024, 5, e703. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Acartürk, E.; Demir, M.; Kanadaşi, M. Aortic atherosclerosis is a marker for significant coronary artery disease. Jpn. Heart J. 1999, 40, 775–781. [Google Scholar] [CrossRef] [PubMed]
- Momiyama, Y.; Kato, R.; Fayad, Z.A.; Tanaka, N.; Taniguchi, H.; Ohmori, R.; Kihara, T.; Kameyama, A.; Miyazaki, K.; Kimura, K.; et al. A possible association between coronary plaque instability and complex plaques in abdominal aorta. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 903–909. [Google Scholar] [CrossRef] [PubMed]
- Montone, R.A.; Rinaldi, R.; Niccoli, G.; Andò, G.; Gragnano, F.; Piccolo, R.; Pelliccia, F.; Moscarella, E.; Zimarino, M.; Fabris, E.; et al. Optimizing Management of Stable Angina: A Patient-Centered Approach Integrating Revascularization, Medical Therapy, and Lifestyle Interventions. J. Am. Coll. Cardiol. 2024, 84, 744–760. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, R.; Kunadian, V.; Crea, F.; Montone, R.A. Management of angina pectoris. Trends Cardiovasc. Med. 2025, 35, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Anand, S.S.; Bosch, J.; Eikelboom, J.W.; Connolly, S.J.; Diaz, R.; Widimsky, P.; Aboyans, V.; Alings, M.; Kakkar, A.K.; Keltai, K.; et al. Rivaroxaban with or without aspirin in patients with stable peripheral or carotid artery disease: An international, randomised, double-blind, placebo-controlled trial. Lancet 2018, 391, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Vrints, C.; Andreotti, F.; Koskinas, K.C.; Rossello, X.; Adamo, M.; Ainslie, J.; Banning, A.P.; Budaj, A.; Buechel, R.R.; Chiariello, G.A.; et al. 2024 ESC Guidelines for the management of chronic coronary syndromes. Eur. Heart J. 2024, 45, 3415–3537, Erratum in Eur. Heart J. 2025, 46, 1565. [Google Scholar] [CrossRef] [PubMed]
- AbuRahma, A.F.; Avgerinos, E.D.; Chang, R.W.; Darling, R.C.; Duncan, A.A.; Forbes, T.L.; Malas, M.B.; Murad, M.H.; Perler, B.A.; Powell, R.J.; et al. Society for Vascular Surgery clinical practice guidelines for management of extracranial cerebrovascular disease. J. Vasc. Surg. 2022, 75, 4S–22S. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Everett, B.M.; Thuren, T.; MacFadyen, J.G.; Chang, W.H.; Ballantyne, C.; Fonseca, F.; Nicolau, J.; Koenig, W.; Anker, S.D.; et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N. Engl. J. Med. 2017, 377, 1119–1131. [Google Scholar] [CrossRef] [PubMed]
- Cialdella, P.; Sergi, S.C.; Zimbardo, G.; Donahue, M.; Talarico, G.P.; Lombardi d’Aquino, U.M.; Di Fusco, P.; Calò, L. Calcified coronary lesions. Eur. Heart J. Suppl. 2023, 25, C68–C73. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lim, S.; Cha, J.J.; Hong, S.J.; Kim, J.H.; Joo, H.J.; Park, J.H.; Yu, C.W.; Ahn, T.H.; Lim, D.S. Association between High Lipid Burden of Target Lesion and Slow TIMI Flow in Coronary Interventions. J. Clin. Med. 2022, 11, 5401. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Aday, A.W.; Matsushita, K. Epidemiology of Peripheral Artery Disease and Polyvascular Disease. Circ. Res. 2021, 128, 1818–1832. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nagy, E.E.; Puskás, A.; Kelemen, P.; Makó, K.; Brassai, Z.; Hársfalvi, J.; Frigy, A. Elevated Serum Cystatin C and Decreased Cathepsin S/Cystatin C Ratio Are Associated with Severe Peripheral Arterial Disease and Polyvascular Involvement. Diagnostics 2022, 12, 833. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, A.; Tian, X.; Zuo, Y.; Zhang, X.; Wu, S.; Zhao, X. Association between the triglyceride-glucose index and carotid plaque stability in nondiabetic adults. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 2921–2928. [Google Scholar] [CrossRef] [PubMed]
- Bay, B.; Vogel, B.; Sharma, R.; Sartori, S.; Leone, P.P.; Nathani, M.; Oliva, A.; Smith, K.F.; Hooda, A.; Sweeny, J.; et al. Inflammatory risk and clinical outcomes according to polyvascular atherosclerotic disease status in patients undergoing PCI. Clin. Res. Cardiol. 2025, 114, 969–977. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, T.; Tanaka, M.; Sekioka, R.; Itoh, H. Serum total bilirubin concentration in patients with type 2 diabetes as a possible biomarker of polyvascular disease. Diabetol. Int. 2017, 9, 129–135. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
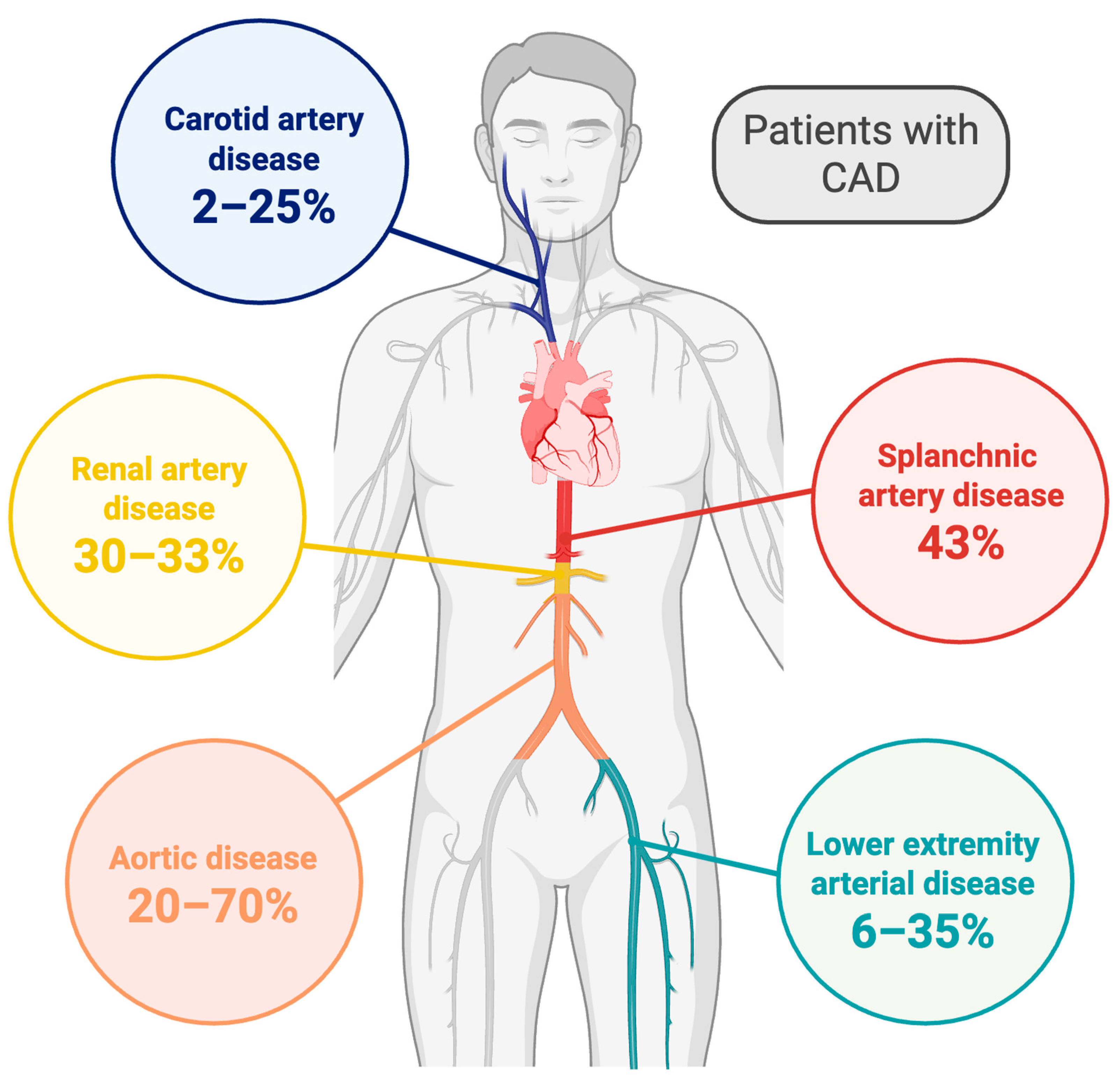

| Atherosclerotic District | Carotid Artery Disease | Lower Limb Artery Disease | Mesenteric Artery Disease | Renal Artery Disease | Aortic Disease |
|---|---|---|---|---|---|
| Most affected sex | Male [11] | Male = Female [13] | Female [14,15,16] | Female [17] | Male [18] |
| Main risk factors | Age, hypertension, smoking and diabetes. Lower weight of dyslipidemia than CAD [11,19,20,21,22] | Age. Hypertension, smoking and diabetes greater weight than CAD. Particular lipid profile (more triglycerides and lower HDL) [11,23,24,25,26,27] | Age, smoking and hypertension. Lower weight of hypercholesterolemia and diabetes than CAD [28,29,30,31] | Age, hypertension, diabetes and dyslipidemia (high LDL) [17] | Age, hypertension, smoking and dyslipidemia. Diabetes mellitus inversely related to abdominal aortic aneurysms [18,32,33,34,35] |
| Main plaque features (vs. CAD) | More lipid rich plaques, higher FCT, less macrophages and more IPH. Similar calcifications and healed lesions [10,36,37,38,39,40,41] | More fibrotic and calcified lesions in femoropopliteal arteries [42,43,44] | No dedicated studies. Mesenteric artery lesions were mainly fibrotic in a rabbit model. More severe atherosclerosis and vulnerable plaques in SMA than IMA [45,46] | More PIT, lower prevalence of fibroatheroma and lower density of vasa vasorum [47,48] | No dedicated studies. More fibrotic and heavily calcified plaque in the abdominal aorta [49] |
| Mechanisms of plaque destabilization (vs. CAD) | Less PE, more CN [36,50,51,52,53] | More CN [23,54] | No dedicated studies | No dedicated studies | No dedicated studies |
| CAD features in the presence of atherosclerosis on the indicated district | More calcified coronary plaque in the presence of carotid calcifications. Correlations between necrotic core, fibrotic and fibrofatty tissue in carotid and coronary arteries. Association between unstable and vulnerable carotid and coronary plaque features [37,55,56,57,58,59,60,61,62,63,64,65,66] | Greater percent atheroma volume, more coronary calcified plaques, higher vulnerability features (higher lipid burden, macrophage accumulation and CCs) [67,68,69] | More coronary calcification in the presence of SMA calcifications [31] | In CKD patients (higher prevalence in RAS) higher lipid burden and more calcifications in coronary plaques [70,71,72] | More features of coronary plaque vulnerability (more TCFA, lipid rich-plaque, layered plaque, macrophages, plaque rupture) in patients with protruding aortic plaques [73] |
| Medical therapy | Lifestyle intervention and risk factors control [74,75,76] Antiplatelet therapy is recommended in patients with symptomatic carotid artery stenosis; low-dose aspirin should be considered in asymptomatic carotid stenosis >50% if bleeding risk is low [74] Lipid lowering therapy (statins if tolerated as first step with or without ezetimibe; iPCSK9 showed clinical benefits) [74,77,78,79,80] Colchicine showed efficacy to prevent recurrent strokes [81,82,83] | Lifestyle intervention and risk factors control [74,84,85] Antiplatelet therapy is recommended in patients with symptomatic PAD; low-dose aspirin may be considered in patients with asymptomatic PAD and diabetes mellitus without contraindications; association of aspirin + rivaroxaban should be considered in patients with PAD, high ischemic risk and non-high bleeding risk [74,86,87] Lipid lowering therapy (statins if tolerated as first step with or without ezetimibe; iPCSK9 showed clinical benefits [74,88,89] Colchicine showed efficacy in lower extremity arterial disease; in a preliminary study, canakinumab showed clinical benefits in PAD. Future studies are warranted [90,91] | Lifestyle intervention and risk factors control [74,92] Antiplatelet therapy is recommended after arterial revascularization; lacking evidence about the use of antiplatelet therapy in asymptomatic patients [74] Lipid lowering therapy (statins if tolerated as first step with or without ezetimibe) [74,93] | Lifestyle intervention and risk factors control [74,94] Low-dose aspirin may be considered [74] Lipid lowering therapy (statins if tolerated as first step with or without ezetimibe) [74] | Lifestyle intervention and risk factors control [74] Antiplatelet therapy is recommended in secondary prevention after an embolic event related to aortic atherosclerosis; it should be considered in severe/complex aortic plaques [74] Lipid lowering therapy (statins if tolerated as first step with or without ezetimibe) [74,95] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Russo, M.; Gurgoglione, F.L.; Russo, A.; Rinaldi, R.; Torlai Triglia, L.; Foschi, M.; Vigna, C.; Vergallo, R.; Montone, R.A.; Benedetto, U.; et al. Coronary Artery Disease and Atherosclerosis in Other Vascular Districts: Epidemiology, Risk Factors and Atherosclerotic Plaque Features. Life 2025, 15, 1226. https://doi.org/10.3390/life15081226
Russo M, Gurgoglione FL, Russo A, Rinaldi R, Torlai Triglia L, Foschi M, Vigna C, Vergallo R, Montone RA, Benedetto U, et al. Coronary Artery Disease and Atherosclerosis in Other Vascular Districts: Epidemiology, Risk Factors and Atherosclerotic Plaque Features. Life. 2025; 15(8):1226. https://doi.org/10.3390/life15081226
Chicago/Turabian StyleRusso, Michele, Filippo Luca Gurgoglione, Alessandro Russo, Riccardo Rinaldi, Laura Torlai Triglia, Matteo Foschi, Carlo Vigna, Rocco Vergallo, Rocco Antonio Montone, Umberto Benedetto, and et al. 2025. "Coronary Artery Disease and Atherosclerosis in Other Vascular Districts: Epidemiology, Risk Factors and Atherosclerotic Plaque Features" Life 15, no. 8: 1226. https://doi.org/10.3390/life15081226
APA StyleRusso, M., Gurgoglione, F. L., Russo, A., Rinaldi, R., Torlai Triglia, L., Foschi, M., Vigna, C., Vergallo, R., Montone, R. A., Benedetto, U., Niccoli, G., & Zimarino, M. (2025). Coronary Artery Disease and Atherosclerosis in Other Vascular Districts: Epidemiology, Risk Factors and Atherosclerotic Plaque Features. Life, 15(8), 1226. https://doi.org/10.3390/life15081226








