Histopathological and Molecular Insights into Chronic Nasopharyngeal and Otic Disorders in Children: Structural and Immune Mechanisms Underlying Disease Chronicity
Abstract
1. Introduction
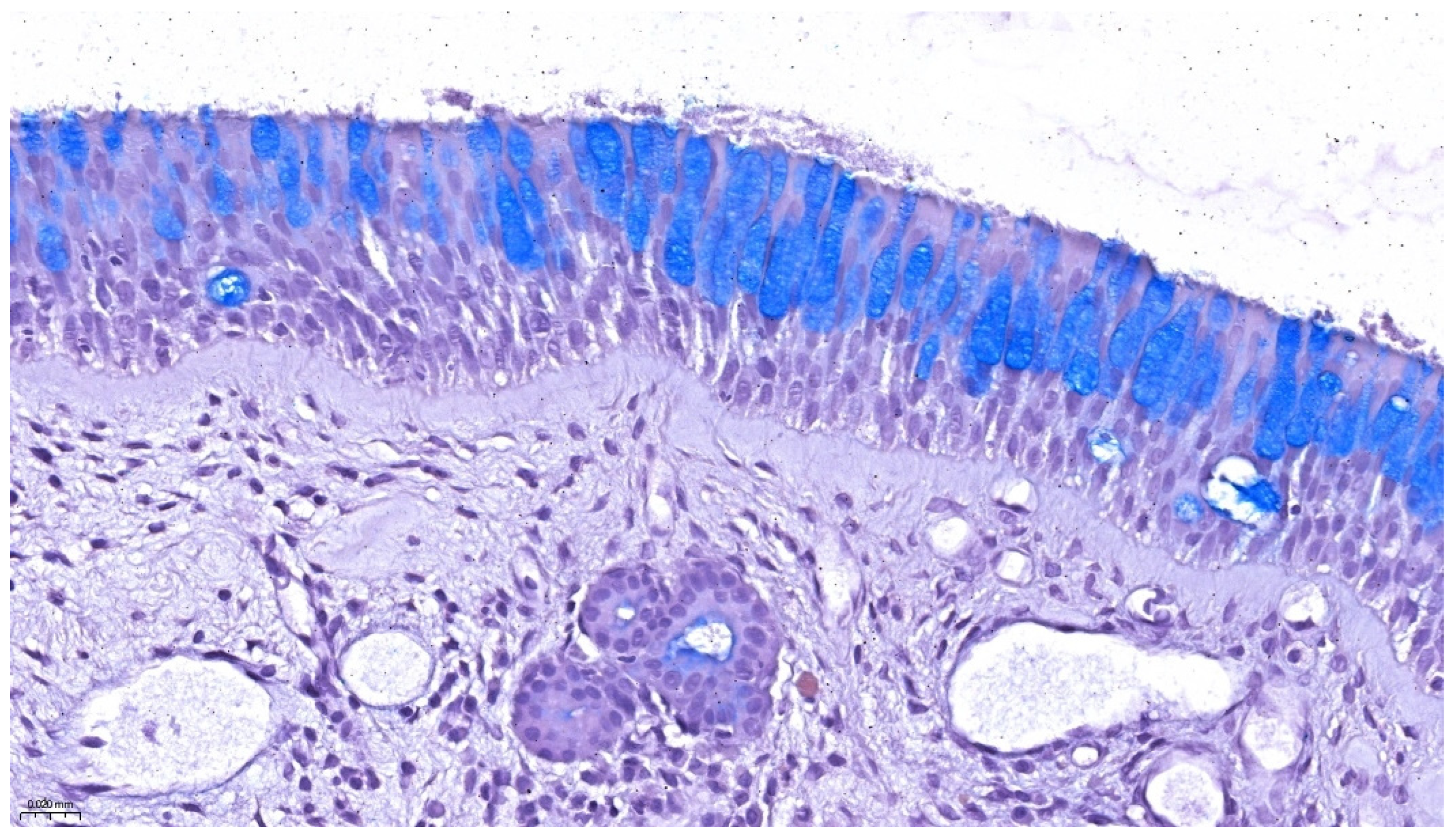
2. Normal Histology of the Nasopharynx, Eustachian Tube, and Middle Ear
2.1. Nasopharyngeal Epithelium and Mucosal Immunity
2.2. Eustachian Tube: Segmental Architecture and Functional Morphology
2.3. Middle Ear Mucosa and Cellular Composition
3. Histopathological Features in Chronic Disease
3.1. Adenoidal Hypertrophy and Nasopharyngeal Inflammation
3.2. Middle Ear Mucosal Remodeling in Otitis Media
3.3. Epithelial Barrier Dysfunction and Metaplastic Changes
3.4. Immune Cell Infiltrates and Chronicity Markers
4. Molecular Mechanisms of Inflammation and Remodeling
4.1. Cytokine Profiles and Inflammatory Signaling Pathways
4.2. Pattern Recognition Receptors and Epithelial Activation
4.3. Tissue Remodeling Mediators: MMPs, TGF-β, and Fibrosis-Associated Pathways
4.4. Barrier Dysfunction and Epithelial Plasticity
4.5. Epigenetic and Microbiome-Immune Interactions
5. Immunohistochemistry and Molecular Markers
6. Diagnostic and Clinical Implications of Histopathologic and Molecular Insights
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Licari, A.; Magri, P.; De Silvestri, A.; Giannetti, A.; Indolfi, C.; Mori, F.; Marseglia, G.L.; Peroni, D. Epidemiology of Allergic Rhinitis in Children: A Systematic Review and Meta-Analysis. J. Allergy Clin. Immunol. Pract. 2023, 11, 2547–2556. [Google Scholar] [CrossRef]
- Brandtzaeg, P. Potential of Nasopharynx-Associated Lymphoid Tissue for Vaccine Responses in the Airways. Am. J. Respir. Crit. Care Med. 2011, 183, 1595–1604. [Google Scholar] [CrossRef]
- Tang, T.; Ni, X.; Song, X. Mucosa-Associated Lymphoid Tissue of Nasopharynx: A Case Report and Literature Review. Radiol. Case Rep. 2022, 17, 2991–2995. [Google Scholar] [CrossRef]
- Alobid, I.; Armengot-Carceller, M.; Urraca, M.P.; Maza-Solano, J.; Guijarro, I.G.; Jiménez, S.U.; Fraile, P.S.M.; Mullol, J. When the Nose Meets the Lab: Histopathological Analysis in Chronic Rhinosinusitis with Nasal Polyps for Routine Clinical Practice. Curr. Allergy Asthma Rep. 2024, 24, 657–665. [Google Scholar] [CrossRef]
- Cassano, M.; De Corso, E.; Fiore, V.; Giancaspro, R.; Moffa, A.; Casale, M.; Trecca, E.M.C.; Mele, D.A.; Cassano, P.; Gelardi, M. Update of Endoscopic Classification System of Adenoid Hypertrophy Based on Clinical Experience on 7621 Children. Acta Otorhinolaryngol. Ital. 2022, 42, 257–264. [Google Scholar] [CrossRef]
- Luers, J.C.; Hüttenbrink, K.B. Surgical Anatomy and Pathology of the Middle Ear. J. Anat. 2016, 228, 338–353. [Google Scholar] [CrossRef] [PubMed]
- Cohen, D.; Raveh, D.; Peleg, U.; Nazarian, Y.; Perez, R. Ventilation and Clearance of the Middle Ear. J. Laryngol. Otol. 2009, 123, 1314–1320. [Google Scholar] [CrossRef] [PubMed]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W.; et al. Chronic Inflammation in the Etiology of Disease across the Life Span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef]
- Cabral-Pacheco, G.A.; Garza-Veloz, I.; Castruita-De la Rosa, C.; Ramirez-Acuña, J.M.; Perez-Romero, B.A.; Guerrero-Rodriguez, J.F.; Martinez-Avila, N.; Martinez-Fierro, M.L. The Roles of Matrix Metalloproteinases and Their Inhibitors in Human Diseases. Int. J. Mol. Sci. 2020, 21, 9739. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Liu, J.; Gu, Y.; Ji, X.; Nan, G. Intermittent Hypoxia Conditioning as a Potential Prevention and Treatment Strategy for Ischemic Stroke: Current Evidence and Future Directions. Front. Neurosci. 2022, 16, 1067411. [Google Scholar] [CrossRef]
- Massa, H.M.; Lim, D.J.; Kurono, Y.; Cripps, A.W. Middle Ear and Eustachian Tube Mucosal Immunology. In Mucosal Immunology, 4th ed.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 1923–1942. [Google Scholar] [CrossRef]
- Torretta, S.; Drago, L.; Marchisio, P.; Ibba, T.; Pignataro, L. Role of Biofilms in Children with Chronic Adenoiditis and Middle Ear Disease. J. Clin. Med. 2019, 8, 671. [Google Scholar] [CrossRef]
- Mendhe, S.; Badge, A.; Ugemuge, S.; Chandi, D. Impact of Biofilms on Chronic Infections and Medical Challenges. Cureus 2023, 15, e48204. [Google Scholar] [CrossRef]
- Sahni, D.; Verma, P.; Bhagat, S.; Sharma, V. Hearing Assessment in Patients of Allergic Rhinitis: A Study on 200 Subjects. Indian J. Otolaryngol. Head Neck Surg. 2022, 74 (Suppl. S1), 125–131. [Google Scholar] [CrossRef]
- Damar, M.; Dinç, A.E.; Erdem, D.; Bişkin, S.; Eliçora, Ş.Ş.; Kumbul, Y.Ç. The Role of the Nasal and Paranasal Sinus Pathologies on the Development of Chronic Otitis Media and Its Subtypes: A Computed Tomography Study. Niger. J. Clin. Pract. 2017, 20, 1156–1160. [Google Scholar] [CrossRef]
- Leick, M.; Azcutia, V.; Newton, G.; Luscinskas, F.W. Leukocyte Recruitment in Inflammation: Basic Concepts and New Mechanistic Insights Based on New Models and Microscopic Imaging Technologies. Cell Tissue Res. 2014, 355, 647–656. [Google Scholar] [CrossRef]
- Mahapatro, M.; Erkert, L.; Becker, C. Cytokine-Mediated Crosstalk between Immune Cells and Epithelial Cells in the Gut. Cells 2021, 10, 111. [Google Scholar] [CrossRef] [PubMed]
- Lewis, K.L.; Del Cid, N.; Traver, D. Perspectives on Antigen Presenting Cells in Zebrafish. Dev. Comp. Immunol. 2014, 46, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Kimura, S. Molecular Insights into the Mechanisms of M-Cell Differentiation and Transcytosis in the Mucosa-Associated Lymphoid Tissues. Anat. Sci. Int. 2018, 93, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Casale, J.; Shumway, K.R.; Hatcher, J.D. Physiology, Eustachian Tube Function. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/sites/books/NBK532284/ (accessed on 9 July 2025).
- Chauhan, G.; Tadi, P. Physiology, Postpartum Changes. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK555918/ (accessed on 9 July 2025).
- Sundar, P.S.; Chowdhury, C.; Kamarthi, S. Evaluation of Human Ear Anatomy and Functionality by Axiomatic Design. Biomimetics 2021, 6, 31. [Google Scholar] [CrossRef]
- Mittal, R.; Lisi, C.V.; Gerring, R.; Mittal, J.; Mathee, K.; Narasimhan, G.; Azad, R.K.; Yao, Q.; Grati, M.; Yan, D.; et al. Current Concepts in the Pathogenesis and Treatment of Chronic Suppurative Otitis Media. J. Med. Microbiol. 2015, 64, 1103–1116. [Google Scholar] [CrossRef]
- Nistico, L.; Kreft, R.; Gieseke, A.; Coticchia, J.M.; Burrows, A.; Khampang, P.; Liu, Y.; Kerschner, J.E.; Post, J.C.; Lonergan, S.; et al. Adenoid Reservoir for Pathogenic Biofilm Bacteria. J. Clin. Microbiol. 2011, 49, 1411–1420. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, S.; Meng, L.; Lin, M.; Zhai, X.; Zhang, Q.; Wang, W. The Function and Mechanism of Human Nasal Mucosa-Derived Mesenchymal Stem Cells in Allergic Rhinitis in Mice. Inflamm. Res. 2024, 73, 1819–1832. [Google Scholar] [CrossRef]
- Mesuraca, M.; Nisticò, C.; Lombardo, N.; Piazzetta, G.L.; Lobello, N.; Chiarella, E. Cellular and Biochemical Characterization of Mesenchymal Stem Cells from Killian Nasal Polyp. Int. J. Mol. Sci. 2022, 23, 13214. [Google Scholar] [CrossRef]
- Bhutta, M.F.; Thornton, R.B.; Kirkham, L.S.; Kerschner, J.E.; Cheeseman, M.T. Understanding the Aetiology and Resolution of Chronic Otitis Media from Animal and Human Studies. Dis. Model. Mech. 2017, 10, 1289–1300. [Google Scholar] [CrossRef]
- Vanneste, P.; Page, C. Otitis Media with Effusion in Children: Pathophysiology, Diagnosis, and Treatment. A Review. J. Otol. 2019, 14, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Brasier, A.R. Interactions between Epithelial Mesenchymal Plasticity, Barrier Dysfunction and Innate Immune Pathways Shape the Genesis of Allergic Airway Disease. Expert Rev. Respir. Med. 2025, 19, 29–41. [Google Scholar] [CrossRef]
- Marzoog, B.A. Cytokines and Regulating Epithelial Cell Division. Curr. Drug Targets 2024, 25, 190–200. [Google Scholar] [CrossRef]
- Painter, J.T.; Clayton, N.P.; Herbert, R.A. Useful Immunohistochemical Markers of Tumor Differentiation. Toxicol. Pathol. 2009, 38, 131–141. [Google Scholar] [CrossRef]
- Kuo, W.T.; Odenwald, M.A.; Turner, J.R.; Zuo, L. Tight Junction Proteins Occludin and ZO-1 as Regulators of Epithelial Proliferation and Survival. Ann. N. Y. Acad. Sci. 2022, 1514, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Fujieda, S.; Imoto, Y.; Kato, Y.; Ninomiya, T.; Tokunaga, T.; Tsutsumiuchi, T.; Yoshida, K.; Kidoguchi, M.; Takabayashi, T. Eosinophilic Chronic Rhinosinusitis. Allergol. Int. 2019, 68, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Alharbi, T.A.F.; Rababa, M.; Alsuwayl, H.; Alsubail, A.; Alenizi, W.S. Diagnostic Challenges and Patient Safety: The Critical Role of Accuracy—A Systematic Review. J. Multidiscip. Healthc. 2025, 18, 3051–3064. [Google Scholar] [CrossRef]
- Malkov, M.I.; Lee, C.T.; Taylor, C.T. Regulation of the Hypoxia-Inducible Factor (HIF) by Pro-Inflammatory Cytokines. Cells 2021, 10, 2340. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Müller, D.; Clark, A.G. Mechanosensory Feedback Loops during Chronic Inflammation. Front. Cell Dev. Biol. 2023, 11, 1225677. [Google Scholar] [CrossRef]
- Deshmane, S.L.; Kremlev, S.; Amini, S.; Sawaya, B.E. Monocyte Chemoattractant Protein-1 (MCP-1): An Overview. J. Interf. Cytokine Res. 2009, 29, 313–326. [Google Scholar] [CrossRef] [PubMed]
- Patel, G.B.; Kern, R.C.; Bernstein, J.A.; Hae-Sim, P.; Peters, A.T. Current and Future Treatments of Rhinitis and Sinusitis. J. Allergy Clin. Immunol. Pract. 2020, 8, 1522–1531. [Google Scholar] [CrossRef]
- Ross, K.F.; Herzberg, M.C. Autonomous Immunity in Mucosal Epithelial Cells: Fortifying the Barrier against Infection. Microbes Infect. 2016, 18, 387–398. [Google Scholar] [CrossRef]
- Rather, M.A.; Gupta, K.; Mandal, M. Microbial Biofilm: Formation, Architecture, Antibiotic Resistance, and Control Strategies. Braz. J. Microbiol. 2021, 52, 1701–1718. [Google Scholar] [CrossRef] [PubMed]
- Pat, Y.; Yazici, D.; D’Avino, P.; Li, M.; Ardicli, S.; Ardicli, O.; Mitamura, Y.; Akdis, M.; Dhir, R.; Nadeau, K.; et al. Recent Advances in the Epithelial Barrier Theory. Int. Immunol. 2024, 36, 211–222. [Google Scholar] [CrossRef]
- Lee, H.S.; Kim, W.J. The Role of Matrix Metalloproteinase in Inflammation with a Focus on Infectious Diseases. Int. J. Mol. Sci. 2022, 23, 10546. [Google Scholar] [CrossRef]
- Budi, E.H.; Schaub, J.R.; Decaris, M.; Turner, S.; Derynck, R. TGF-β as a Driver of Fibrosis: Physiological Roles and Therapeutic Opportunities. J. Pathol. 2021, 254, 358–373. [Google Scholar] [CrossRef]
- Cross, M.J.; Claesson-Welsh, L. FGF and VEGF Function in Angiogenesis: Signalling Pathways, Biological Responses and Therapeutic Inhibition. Trends Pharmacol. Sci. 2001, 22, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Klibaner-Schiff, E.; Simonin, E.M.; Akdis, C.A.; Cheong, A.; Johnson, M.M.; Karagas, M.R.; Kirsh, S.; Kline, O.; Mazumdar, M.; Oken, E.; et al. Environmental Exposures Influence Multigenerational Epigenetic Transmission. Clin. Epigenetics 2024, 16, 145. [Google Scholar] [CrossRef]
- Yu, K.; Tenaglia, V.; Chua, E.G.; Haines, R.; Bahal, G.; Nicol, M.P.; Bahal, R.K. Interactions between Bacteria in the Human Nasopharynx: A Scoping Review. Lancet Microbe 2025, 6, 101062. [Google Scholar] [CrossRef]
- Chiner, E.; Murcia, M.; Boira, I.; Bernabeu, M.Á.; Esteban, V.; Martínez-Moragón, E. Real-Life Clinical Outcomes of Benralizumab Treatment in Patients with Uncontrolled Severe Asthma and Coexisting Chronic Rhinosinusitis with Nasal Polyposis. J. Clin. Med. 2024, 13, 4247. [Google Scholar] [CrossRef]
- Gevaert, P.; Omachi, T.A.; Corren, J.; Mullol, J.; Han, J.; Lee, S.E.; Kaufman, D.; Ligueros-Saylan, M.; Howard, M.; Zhu, R.; et al. Efficacy and Safety of Omalizumab in Nasal Polyposis: Two Randomized Phase 3 Trials. J. Allergy Clin. Immunol. 2020, 146, 595–605. [Google Scholar] [CrossRef]
- Bachert, C.; Han, J.K.; Desrosiers, M.; Hellings, P.W.; Amin, N.; Lee, S.E.; Mullol, J.; Greos, L.S.; Bosso, J.V.; Laidlaw, T.M.; et al. Efficacy and Safety of Dupilumab in Patients with Severe Chronic Rhinosinusitis with Nasal Polyps (LIBERTY NP SINUS-24 and LIBERTY NP SINUS-52): Results from Two Multicentre, Randomised, Double-Blind, Placebo-Controlled, Parallel-Group Phase 3 Trials. Lancet 2019, 394, 1638–1650. [Google Scholar] [CrossRef]
- Subramanian, D.; Cruz, C.V.; Garcia-Bournissen, F. Systematic Review of Early Phase Pediatric Clinical Pharmacology Trials. J. Pediatr. Pharmacol. Ther. 2022, 27, 609–617. [Google Scholar] [CrossRef] [PubMed]
- Wedner, H.J.; Fujisawa, T.; Guilbert, T.W.; Ikeda, M.; Mehta, V.; Tam, J.S.; Lukka, P.B.; Asimus, S.; Durżyński, T.; Johnston, J.; et al. Benralizumab in Children with Severe Eosinophilic Asthma: Pharmacokinetics and Long-Term Safety (TATE Study). Pediatr. Allergy Immunol. 2024, 35, e14092. [Google Scholar] [CrossRef] [PubMed]
- Hillson, K.; Saglani, S.; Bush, A. The New Biologic Drugs: Which Children with Asthma Should Get What? Pediatr. Pulmonol. 2024, 59, 3057–3074. [Google Scholar] [CrossRef]
- Kim, S.W.; Roh, J.; Park, C.S. Immunohistochemistry for Pathologists: Protocols, Pitfalls, and Tips. J. Pathol. Transl. Med. 2016, 50, 411–418. [Google Scholar] [CrossRef]
- Pahwa, R.; Goyal, A.; Jialal, I. Chronic Inflammation. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK493173/ (accessed on 11 July 2025).
- Pavlasova, G.; Mraz, M. The Regulation and Function of CD20: An “Enigma” of B-Cell Biology and Targeted Therapy. Haematologica 2020, 105, 1494–1506. [Google Scholar] [CrossRef]
- Kuang, F.L. Approach to Patients with Eosinophilia. Med. Clin. N. Am. 2020, 104, 1–14. [Google Scholar] [CrossRef]
- Li, A.; Yang, D.H. Application of Immunohistochemistry in Basic and Clinical Studies. In Methods in Molecular Biology; Humana Press: Clifton, NJ, USA, 2020; Volume 2108, pp. 43–55. [Google Scholar] [CrossRef]
- Eyerich, K.; Dimartino, V.; Cavani, A. IL-17 and IL-22 in Immunity: Driving Protection and Pathology. Eur. J. Immunol. 2017, 47, 607–614. [Google Scholar] [CrossRef]
- Alam, M.S.; Otsuka, S.; Wong, N.; Abbasi, A.; Gaida, M.M.; Fan, Y.; Meerzaman, D.; Ashwell, J.D. TNF Plays a Crucial Role in Inflammation by Signaling via T Cell TNFR2. Proc. Natl. Acad. Sci. USA 2021, 118, e2109972118. [Google Scholar] [CrossRef]
- Hamed, M.A.; Nakata, S.; Sayed, R.H.; Ueda, H.; Badawy, B.S.; Nishimura, Y.; Kojima, T.; Iwata, N.; Ahmed, A.R.; Dahy, K.; et al. Pathogenesis and Bone Resorption in Acquired Cholesteatoma: Current Knowledge and Future Prospectives. Clin. Exp. Otorhinolaryngol. 2016, 9, 298–308. [Google Scholar] [CrossRef]
- Ott, L.W.; Resing, K.A.; Sizemore, A.W.; Heyen, J.W.; Cocklin, R.R.; Pedrick, N.M.; Woods, H.C.; Chen, J.Y.; Goebl, M.G.; Witzmann, F.A.; et al. Tumor Necrosis Factor-Alpha- and Interleukin-1-Induced Cellular Responses: Coupling Proteomic and Genomic Information. J. Proteome Res. 2007, 6, 2176–2185. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.; Takai, K.; Weaver, V.M.; Werb, Z. Extracellular Matrix Degradation and Remodeling in Development and Disease. Cold Spring Harb. Perspect. Biol. 2011, 3, a005058. [Google Scholar] [CrossRef]
- Ahmad, Z.; Krüger, K.; Lautermann, J.; Lippert, B.; Tenenbaum, T.; Tigges, M.; Tisch, M. Adenoid Hypertrophy—Diagnosis and Treatment: The New S2k Guideline. HNO 2023, 71 (Suppl. S1), 67–72. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Bai, J.; Zhang, J.; Yang, W.; Zuo, K.; Li, H. IL-6 Promotes the Expression of Vascular Endothelial Growth Factor through the p38 Signalling Pathway in Hypertrophied Adenoids in Children. Int. J. Pediatr. Otorhinolaryngol. 2013, 77, 205–209. [Google Scholar] [CrossRef]
- Kayama, H.; Takeda, K. Manipulation of Epithelial Integrity and Mucosal Immunity by Host and Microbiota-Derived Metabolites. Eur. J. Immunol. 2020, 50, 921–931. [Google Scholar] [CrossRef] [PubMed]
- Coopman, P.; Djiane, A. Adherens Junction and E-Cadherin Complex Regulation by Epithelial Polarity. Cell Mol. Life Sci. 2016, 73, 3535–3553. [Google Scholar] [CrossRef] [PubMed]
- Doran, E.; Cai, F.; Holweg, C.T.J.; Wong, K.; Brumm, J.; Arron, J.R. Interleukin-13 in Asthma and Other Eosinophilic Disorders. Front. Med. 2017, 4, 139. [Google Scholar] [CrossRef] [PubMed]
- Pant, H.; Hughes, A.; Schembri, M.; Miljkovic, D.; Krumbiegel, D. CD4(+) and CD8(+) Regulatory T Cells in Chronic Rhinosinusitis Mucosa. Am. J. Rhinol. Allergy 2014, 28, e83–e89. [Google Scholar] [CrossRef]
- Ryu, G.; Bae, J.S.; Yoo, S.H.; Kim, D.K.; Han, D.H.; Mo, J.H.; Cho, S.H.; Jin, H.R. Elevated IL-17A-Secreting Regulatory T Cells in Sinonasal Tissues of Chronic Rhinosinusitis with Nasal Polyps. Inflammation, 2025; in press. [Google Scholar] [CrossRef]
- Poposki, J.A.; Klingler, A.I.; Stevens, W.W.; Suh, L.A.; Tan, B.K.; Peters, A.T.; Abdala-Valencia, H.; Grammer, L.C.; Welch, K.C.; Smith, S.S.; et al. Elevation of Activated Neutrophils in Chronic Rhinosinusitis with Nasal Polyps. J. Allergy Clin. Immunol. 2022, 149, 1666–1674. [Google Scholar] [CrossRef]
- Caruso, C.; Giancaspro, R.; Guida, G.; Macchi, A.; Landi, M.; Heffler, E.; Gelardi, M. Nasal Cytology: A Easy Diagnostic Tool in Precision Medicine for Inflammation in Epithelial Barrier Damage in the Nose. A Perspective Mini Review. Front. Allergy 2022, 3, 768408. [Google Scholar] [CrossRef]
- Danisman, Z.; Linxweiler, M.; Kühn, J.P.; Linxweiler, B.; Solomayer, E.F.; Wagner, M.; Wagenpfeil, G.; Schick, B.; Berndt, S. Differential Nasal Swab Cytology Represents a Valuable Tool for Therapy Monitoring but Not Prediction of Therapy Response in Chronic Rhinosinusitis with Nasal Polyps Treated with Dupilumab. Front. Immunol. 2023, 14, 1127576. [Google Scholar] [CrossRef]
- Artono, A.; Purnami, N.; Handoko, E.; Widodo, A.D.W.; Juniastuti, J. Pseudomonas aeruginosa in Chronic Suppurative Otitis Media. Infect. Chemother. 2025, 57, 63–71. [Google Scholar] [CrossRef]
- Endo, L.H.; Vassallo, J.; Sakano, E.; Brousset, P. Detection of Epstein-Barr Virus and Subsets of Lymphoid Cells in Adenoid Tissue of Children under 2 Years of Age. Int. J. Pediatr. Otorhinolaryngol. 2002, 66, 223–226. [Google Scholar] [CrossRef]
- Hsueh, C.Y.; Yang, C.F.; Gau, J.P.; Kuan, E.C.; Ho, C.Y.; Chiou, T.J.; Hsiao, L.T.; Lin, T.A.; Lan, M.Y. Nasopharyngeal Lymphoma: A 22-Year Review of 35 Cases. J. Clin. Med. 2019, 8, 1604. [Google Scholar] [CrossRef]
- Abdel Aziz, A.A.R.; Youssef, A.M.; Mostafa, M.M.; Talaat, M.; Abdelzaher, K.M.; Sadeq, A.A. Cartilage Tympanoplasty in the Treatment of Adhesive Otitis Media with and without Eustachian Tube Balloon Dilatation. J. Otol. 2022, 17, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.L.; Yang, H.L.; Ding, W.W.; Li, H.K.; Zhou, Y.Q.; Zhang, T.T. Down-Expression of Foxj1 on Airway Epithelium with Impaired Cilia Architecture in Non-Cystic Fibrosis Bronchiectasis Implies Disease Severity. Clin. Respir. J. 2023, 17, 405–413. [Google Scholar] [CrossRef] [PubMed]

| Feature | Normal Nasopharynx/Middle Ear | Chronic Inflammatory State |
|---|---|---|
| Epithelial type [22,27] | Pseudostratified ciliated columnar | Squamous/metaplastic or hyperplastic |
| Goblet cells [2,11] | Sparse, functionally balanced | Hyperplasia, excessive mucus production |
| Subepithelial stroma [28,33] | Loose connective tissue | Fibrosis, neovascularization, glandular hypertrophy |
| Immune infiltrate [4,33] | Scattered lymphocytes | Dense T/B cells, plasma cells, eosinophils |
| Barrier integrity (tight junctions) [29,30] | Preserved | Compromised: reduced claudin/occludin expression |
| Marker | Cell Type/Target | Associated Pathology | Clinical Implication |
|---|---|---|---|
| CD3 [55] | T lymphocytes | Chronic cellular immune response | Intensity reflects Th1/Th17 activity |
| CD20 [56] | B lymphocytes | Follicular hyperplasia in adenoids | Active humoral response |
| CD138 [56] | Plasma cells | Longstanding inflammation | Supports chronicity |
| IL-5, IL-13 [58] | Th2 cytokines | Eosinophilic inflammation | Predicts steroid/biologic responsiveness |
| IL-17A [59] | Th17 cytokine | Neutrophilic, biofilm-associated pathology | Marker of recalcitrant disease |
| MMP-9 [63] | Matrix degradation enzyme | Tissue remodeling and tympanic retraction | Linked to recurrence |
| TGF-β [64] | Fibrosis and repair | Adhesive otitis, subepithelial scarring | Indicator of irreversible remodeling |
| VEGF [65] | Angiogenesis | Adenoidal hypertrophy, mucosal edema | May predict surgical outcome |
| FOXJ1 [65] | Ciliogenesis marker | Loss of cilia in metaplastic epithelium | Indicates impaired mucociliary clearance |
| E-cadherin [66] | Adherens junctions | Epithelial dedifferentiation | Barrier integrity assessment |
| Occludin, Claudin-1 [29] | Tight junction proteins | Epithelial barrier dysfunction | Reflects mucosal permeability and immune activation |
| Mechanism | Molecular Drivers | Histologic Correlate | Clinical Relevance |
|---|---|---|---|
| Epithelial barrier dysfunction [29] | ↓ occludin, ↓ claudin, ROS | Metaplasia, loss of cilia | Increased pathogen susceptibility |
| Immune polarization (Th2) [62] | ↑ IL-5, ↑ IL-13 | Eosinophilic infiltrate, goblet cell ↑ | Responsive to corticosteroids/anti-IL-5 therapy |
| Fibrosis/remodeling [62,63] | ↑ TGF-β, ↑ MMP-9 | Lamina propria thickening, loss of glands | May predict recurrence post-surgery |
| Biofilm persistence [1,2] | TLR stimulation, EPS | Chronic low-grade inflammation | Poor antibiotic response, surgical indication |
| Hypoxia-induced angiogenesis [35,63] | ↑ VEGF, ↑ HIF-1α | Vascular congestion, edema | Surgical outcomes may vary |
| Inflammatory Phenotype | Histological Features | Key Markers | Preferred Therapy | Biologic Therapy Options |
|---|---|---|---|---|
| Eosinophilic | Goblet cell hyperplasia, eosinophil infiltration | IL-5↑, IL-13↑, MBP+, EPO+, Siglec-8+ [56,58] | Intranasal corticosteroids, leukotriene antagonists [58] | Anti-IL-5 (benralizumab) [47], anti-IgE (omalizumab) [48], anti-IL-4Rα (dupilumab) [49] |
| Neutrophilic | Subepithelial neutrophils, biofilm presence | IL-8↑, MMP-9↑, TLR4↑ [34,38] | Topical steroids, antibiotics, surgery for biofilm [12,24] | Experimental (e.g., anti-TNF, anti-IL-17) [59] |
| Mixed eosinophilic–mast cell | Edema, eosinophils + mast cells | IL-5↑, CCR3+, tryptase+ [56] | Corticosteroids, antihistamines [58] | Dupilumab [49] |
| Fibrotic/refractory | Basement membrane thickening, fibrosis, loss of cilia | TGF-β↑, MMP-9↑, FOXJ1↓ [63,64,66] | Surgery (e.g., adenoidectomy, tympanoplasty) [9,76] | Biologic therapy if eosinophilic features persist [49,52] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szekely, D.; Zara, F.; Patrascu, R.; Dumitru, C.S.; Novacescu, D.; Manole, A.; Mogoanta, C.A.; Iovanescu, D.; Iovanescu, G. Histopathological and Molecular Insights into Chronic Nasopharyngeal and Otic Disorders in Children: Structural and Immune Mechanisms Underlying Disease Chronicity. Life 2025, 15, 1228. https://doi.org/10.3390/life15081228
Szekely D, Zara F, Patrascu R, Dumitru CS, Novacescu D, Manole A, Mogoanta CA, Iovanescu D, Iovanescu G. Histopathological and Molecular Insights into Chronic Nasopharyngeal and Otic Disorders in Children: Structural and Immune Mechanisms Underlying Disease Chronicity. Life. 2025; 15(8):1228. https://doi.org/10.3390/life15081228
Chicago/Turabian StyleSzekely, Diana, Flavia Zara, Raul Patrascu, Cristina Stefania Dumitru, Dorin Novacescu, Alexia Manole, Carmen Aurelia Mogoanta, Dan Iovanescu, and Gheorghe Iovanescu. 2025. "Histopathological and Molecular Insights into Chronic Nasopharyngeal and Otic Disorders in Children: Structural and Immune Mechanisms Underlying Disease Chronicity" Life 15, no. 8: 1228. https://doi.org/10.3390/life15081228
APA StyleSzekely, D., Zara, F., Patrascu, R., Dumitru, C. S., Novacescu, D., Manole, A., Mogoanta, C. A., Iovanescu, D., & Iovanescu, G. (2025). Histopathological and Molecular Insights into Chronic Nasopharyngeal and Otic Disorders in Children: Structural and Immune Mechanisms Underlying Disease Chronicity. Life, 15(8), 1228. https://doi.org/10.3390/life15081228








