1. Introduction
The adrenal medulla (AM), organs of Zuckerkandl (OZ), and other paraganglia (including the carotid body) collectively constitute the sympathoadrenal system. Despite more than a century of research on these organs, their morphogenesis and patterns of interaction during human antenatal development remain poorly understood. This knowledge gap can be attributed to the challenges associated with studying human tissue and the complex evolutionary trajectory of the chromaffin system, which varies significantly across species, thereby limiting the applicability of animal model data to human biology.
The term “OZ” typically refers to the largest collections of chromaffin tissue near the origin of the inferior mesenteric artery. However, the International Federation of Associations of Anatomists recommends the term “para-aortic paraganglia” to describe these structures (also known as the Bodies of Zuckerkandl), effectively eliminating the distinction between the OZ and other paraganglia in humans. Since these organs in humans demonstrate inconstancy in shape, precise location, and poorly defined boundaries between the main bodies and smaller paraganglia, we argue that differentiating the OZ from other paraganglia is an artificial and poorly reproducible categorization. Therefore, in this work, we use the abbreviation “OZ” to refer to all para-aortic paraganglia.
The OZ reaches peak development by the age of 3 years in humans [
1], followed by progressive degeneration through mechanisms that are not yet fully understood, potentially involving autophagy [
2]. The OZ are thought to be provisional organs, probably involved in the morphogenesis of the AM during the embryonic and fetal periods. Their potential function in human development may include serving as a source of cellular material for the developing AM. In rabbits, for instance, the AM forms through the U-shaped incorporation of the OZ into the cortical anlage, while in rats, the AM is described as developing through a migratory process from the OZ [
3].
At the same time, only a few studies on human material have described both extra-adrenal paraganglia and the AM, along with their interactions during embryonic development. One such study is by Hervonen, who identified an invasion route connecting extra-adrenal formaldehyde-induced fluorescing elements with the premedullary area [
4]. Molenaar et al. described two distinct cell types in the para-aortic region of a human embryo, referred to as “large” and “small” cells. In a specimen at 9 weeks of gestational age (g.w.), the “large” tyrosine hydroxylase-immunopositive cells were observed traversing the adrenal cortex, whereas the “small” cells were not [
5]. This observation raises questions about the ability of partially differentiated cells along the chromaffin pathway to migrate into the cortical anlage. It also remains unclear whether these cells migrate in association with nerves, freely between cortical cells, or through both mechanisms. Kameneva et al. conducted single-cell transcriptomic research on both the human adrenal gland and extra-adrenal paraganglia, revealing different cell lineages and their transitions [
6].
Additionally, the role of the OZ is not limited to its morphogenetic function. The OZ may also serve a provisional endocrine role. Their relatively large size compared to the immature fetal AM and their early development of chromaffin characteristics suggest that the OZ could function actively when the AM is not yet fully capable of performing endocrine functions. Specifically, the norepinephrine-synthesizing OZ may serve as effector organs during fetal distress—a role that likely has its roots in their evolutionary history. For instance, there is evidence that norepinephrine is the predominant amine released by chromaffin tissue during hypoxia in hagfishes [
7] and, importantly, during fetal distress in humans [
8]. This indicates that norepinephrine-storing chromaffin cells in hagfishes play a crucial role in responding to hypoxemia and anoxia. Similarly, it is highly probable that the norepinephrine-containing OZ in humans are key organs in the response to fetal distress.
Another unresolved issue concerning chromaffin tissue is the synthesis of epinephrine. While epinephrine is present in the AM, it is generally absent in extra-adrenal paraganglia, which also lack phenylethanolamine N-methyltransferase (PNMT)—the enzyme responsible for converting norepinephrine into epinephrine. PNMT is a key enzyme in this synthesis process, and numerous studies have shown that glucocorticoids stimulate PNMT expression in chromaffin cells [
9,
10,
11,
12]. This has led to the hypothesis that a portal system within the adrenal gland might exist, allowing higher concentrations of glucocorticoids to reach the AM [
13]. However, this idea remains controversial, as no such portal system has been observed in rats [
14]. In contrast, in hagfishes, chromaffin cells have been shown to be insensitive to glucocorticoids [
15] and have minimal contact with interrenal cells. Despite this, they are still capable of synthesizing PNMT and epinephrine. This raises questions about the evolutionary aspects of the morpho-functional integration of chromaffin tissue with the adrenal cortex, which remain unclear. Thus, considering the evolutionary development of the sympathoadrenal system and its reflection in human ontogenesis, we believe that studying the comparative morphological characteristics of the key organs in this system during antenatal development is most productive.
Although several studies have investigated molecular genetic aspects of human OZ and AM development [
6,
16], they lack comprehensive data on spatio-temporal distribution patterns of differentiating chromaffin cells. This gap hinders interpretation within the broader context of the para-aortic region and the mutual interactions between these structures. A critical outstanding question involves the organizational architecture of these cells, particularly their microstructural arrangements and emergent tissue patterns. For these reasons, classical histological methods combined with immunohistochemistry retain fundamental importance, even amidst advances in single-cell transcriptomics and other molecular methods. This is particularly relevant given that the presence of transcripts in cells does not necessarily correlate with the presence of functional protein product [
17]. Moreover, the presence of a particular protein in a cell does not define the cell type because cell type specification requires combined analysis of molecular signatures and morphological characteristics.
In this study, we address these knowledge gaps by systematically characterizing cytological, histological, and immunohistochemical features of the developing human sympathoadrenal system, with particular focus on spatiotemporal dynamics of chromaffin cell distribution in OZ and AM.
2. Materials and Methods
2.1. Antenatal Material
The investigation was carried out on human embryos, prefetuses, and fetuses (for details, see
Supplementary Table S1). During sample collection, we recorded the gender, gestational age, clinical diagnosis of the mother and fetus, and the causes of pregnancy termination or fetal death. When clinical data were insufficient or unavailable, fetal age was determined according to the criteria established by Milovanov and Saveliev [
18]. The study was conducted on autopsy samples from the Collection of the Laboratory of Nervous System Development at the Avtsyn Research Institute of Human Morphology, Petrovsky National Research Centre of Surgery, Moscow, Russian Federation. Tissue specimen collection and handling adhered to Russian legislation and the Declaration of Helsinki. Consent for the use of human tissue in research was obtained from the legal representatives of all subjects involved in the study. All protocols were approved by the local Ethics Committee of the Research Institute of Human Morphology (protocol No. 33 (9), 7 February 2022).
In fetuses and late prefetuses, the adrenal glands and aorta, along with surrounding tissues including the OZ, were taken separately. For embryos, the entire body was fixed and embedded, followed by serial sectioning. In early prefetuses, the adrenal glands were either taken as part of an organ complex with the aorta and surrounding tissues or, depending on the size of the organ complex, one adrenal gland was taken separately.
2.2. Fixation and Processing
All the material was fixed in 10% buffered formalin (BioVitrum, St. Petersburg, Russia), dehydrated using a standard tissue protocol with IsoPrep (BioVitrum, St. Petersburg, Russia), and embedded in Histomix (BioVitrum, St. Petersburg, Russia). Serial sections (thickness = 5 μm) were taken using a Leica RM2245 microtome (Leica Biosystems, Nussloch, Germany). Every 20th section was deparaffinized and routinely stained with hematoxylin and eosin. The sections were examined under Leica DM2500 and Leica DM2000 light microscopes (Leica Microsystems, Wetzlar, Germany).
2.3. Immunohistochemistry
The most representative sections were selected for immunohistochemical (IHC) analysis. These sections were deparaffinized, rehydrated, and subjected to antigen retrieval by boiling in citrate buffer (pH 6.0) for 5 min. For immunohistochemistry using antibodies against tyrosine hydroxylase and β-III tubulin, the antigen retrieval step was omitted. However, for PNMT antibodies, the sections were boiled in citrate buffer for an extended period (30 min). Next, the sections were treated with a 3% H2O2 peroxide solution for 10 min to block endogenous peroxidase activity. They were then treated with Ultra V Block (Thermo Fisher Scientific, Waltham, MA, USA) for 5 min. After blocking, the specimens were incubated with primary antibodies for 60 min at room temperature (see
Table 1 for details). We also utilized 200 kDa neurofilaments (murine monoclonal, Merck, Darmstadt, Germany). However, we were unable to obtain stable results with the antibodies for neurofilaments, likely due to the instability of the target antigen [
19]. The UltraVision Quanto Detection System kit (Thermo Fisher Scientific, Waltham, MA, USA) was used as the detection system.
The sections were photographed with Leica Flexacam C3 (Leica Microsystems, Wetzlar, Germany). The digital images were saved in TIFF format and matched for brightness and contrast using Adobe Photoshop CC 2019 (Adobe Systems, Inc., San Jose, CA, USA).
2.4. Morphometry and Statistics
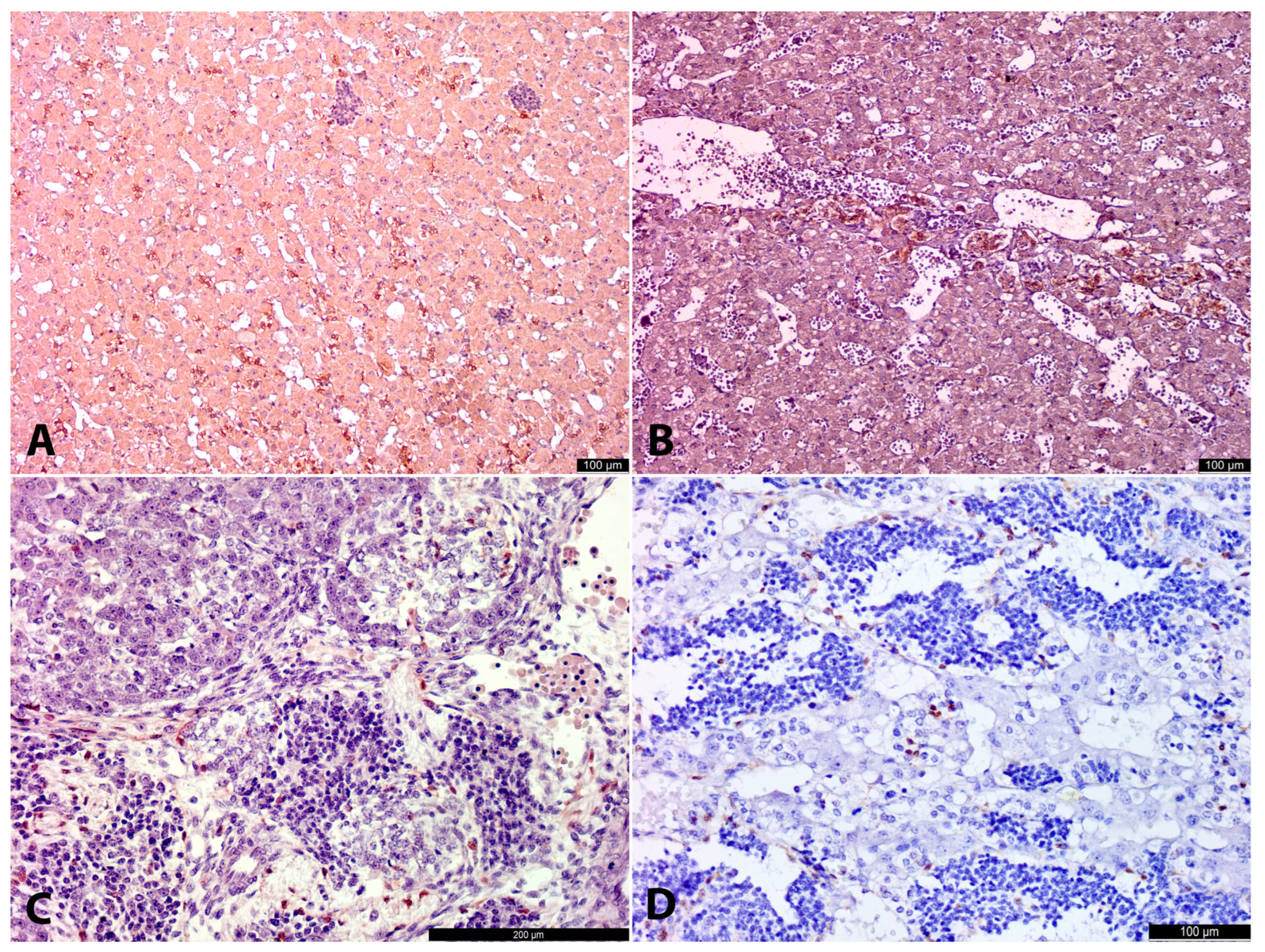
In the developing AM two cell types were detected morphologically: large cells and small cells (for details, see Results). The most representative AM-containing sections in terms of volume were stained with antibodies for TH and photographed in their entirety using Leica Flexacam C3 (Leica Microsystems, Wetzlar, Germany) with a 10× objective. The digital images were saved in TIFF format.
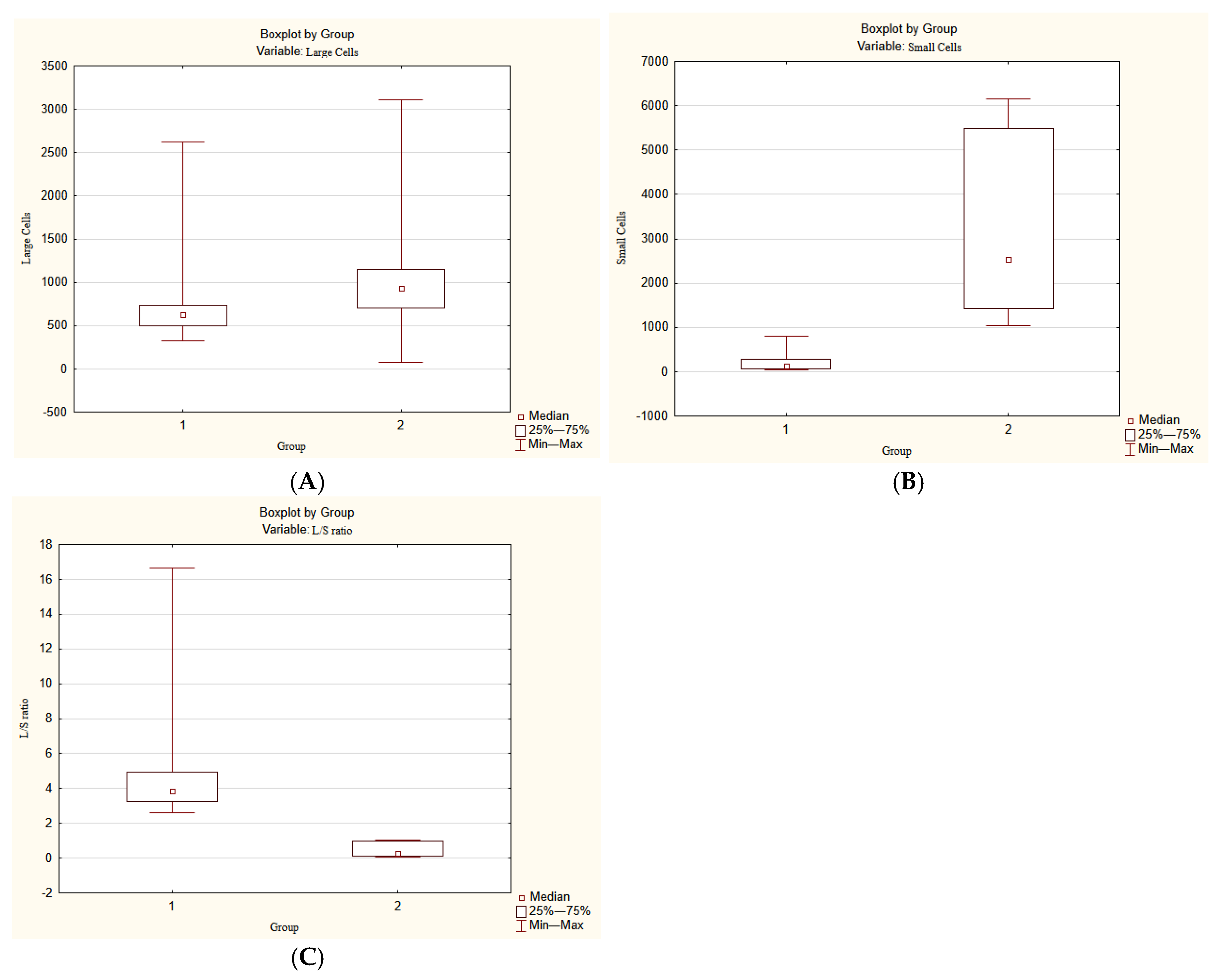
Morphometry was performed using the program ImageJ 1.54p. The number of large cells and small cells was counted. The ratio of large cells to small cells was also calculated.
Three distinct age groups were identified by morphological and morphometrical patterns. The first age group contained 6 embryos and prefetuses from 8 to 12 g.w. (No. 1–6). The second age group consisted of 6 fetuses from 16 to 21–22 g.w. (No. 7–12). The third age group contained 2 fetuses from 22–23 g.w. to 25–26 g.w. (No. 13–14).
Since there were only two cases in the third age group, statistical analysis was performed only for the first and the second age groups. The statistical significance of the results was assessed using Statistica 10.0 software (StatSoft, Tulsa, OK, USA). Three parameters of these two groups were analyzed: number of large cells, number of small cells, and the ratio of large cells to small cells.
The significance of differences was assessed using the nonparametric Mann–Whitney test. A stronger Bonferroni-corrected
p-value test was used. It was obtained by following formula:
Given that p = 0.05, the differences were considered significant at .
4. Discussion
Chromaffin tissue has undergone significant evolutionary changes from cyclostomes to placental mammals. In cyclostomes, this tissue is represented by scattered stellate cells within the walls of the cardinal vein, aorta, and its branches, and in the endocardium of the heart. Cyclostomes possess a poorly developed autonomic nervous system, and in this group, chromaffin tissue generally operates independently of nervous control [
3]. For example, in hagfishes, chromaffin tissue is known to lack preganglionic innervation [
24,
25]. Over the course of evolution, chromaffin cells have lost their independence from the nervous system and acquired innervation. Simultaneously, these cells consolidated into a distinct organ. This development improved the regulatory mechanisms of chromaffin cell endocrine function. As a result, placental mammals now possess a well-developed, richly innervated AM as part of the adrenal gland, a complex endocrine organ. The AM became an integral component of the mammalian sympathoadrenal system, while most paraganglia have become nearly non-functional rudiments. However, some paraganglia are believed to have acquired specific functions during phylogenesis. For instance, the carotid body developed chemosensitive function [
26]. As demonstrated in our study, other paraganglia, such as the OZ, play an important role in human embryological and fetal development. Thus, the OZ function not only as endocrine provisional organs but also as a morphogenic factor for the AM.
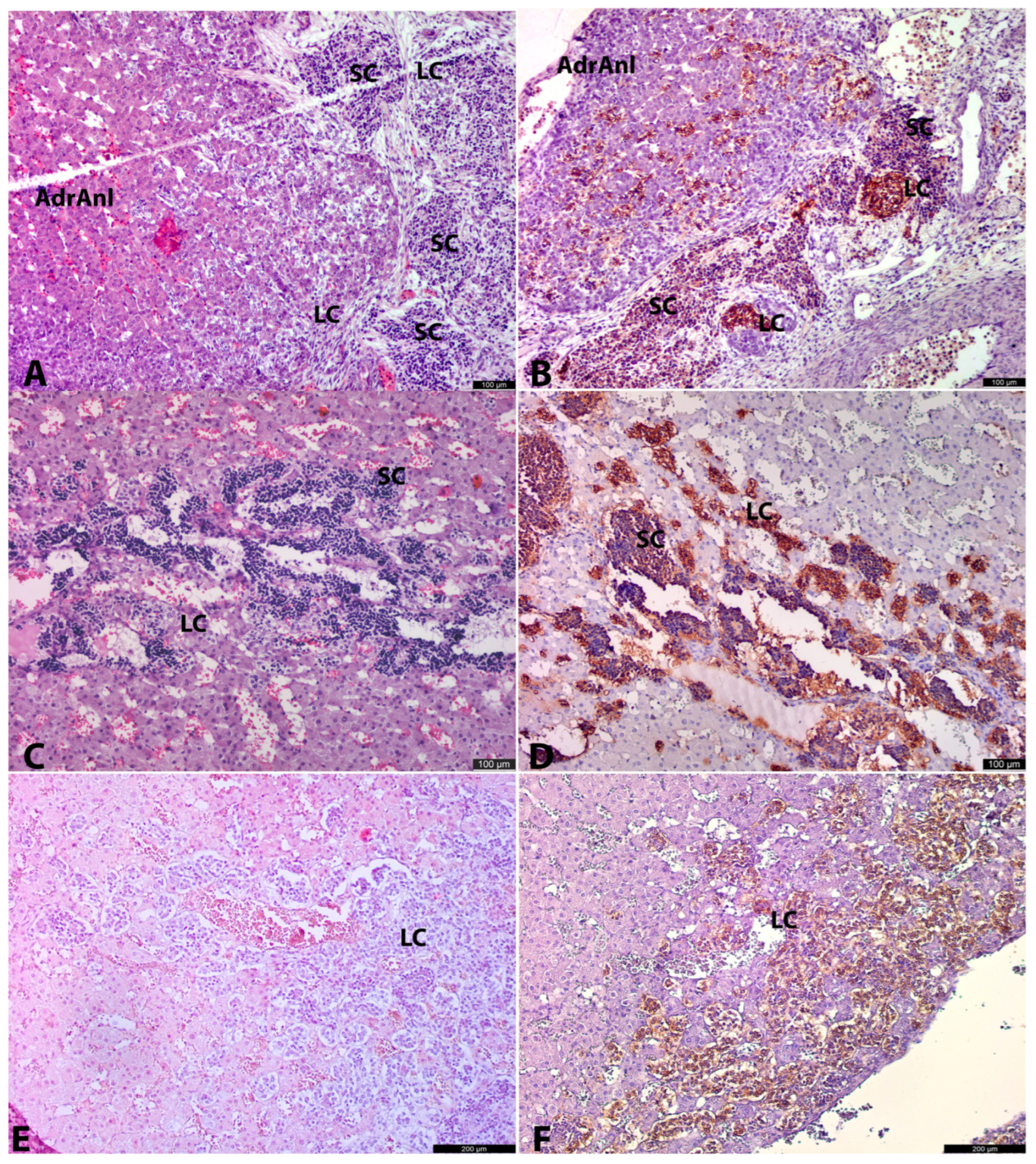
Our results suggest two potential pathways for AM cell population recruitment during embryogenesis. The first and most prevalent pathway involves LC migration from OZ anlagen. The second, less histologically apparent pathway is associated with the migration of SCs from extra-adrenal conglomerates.
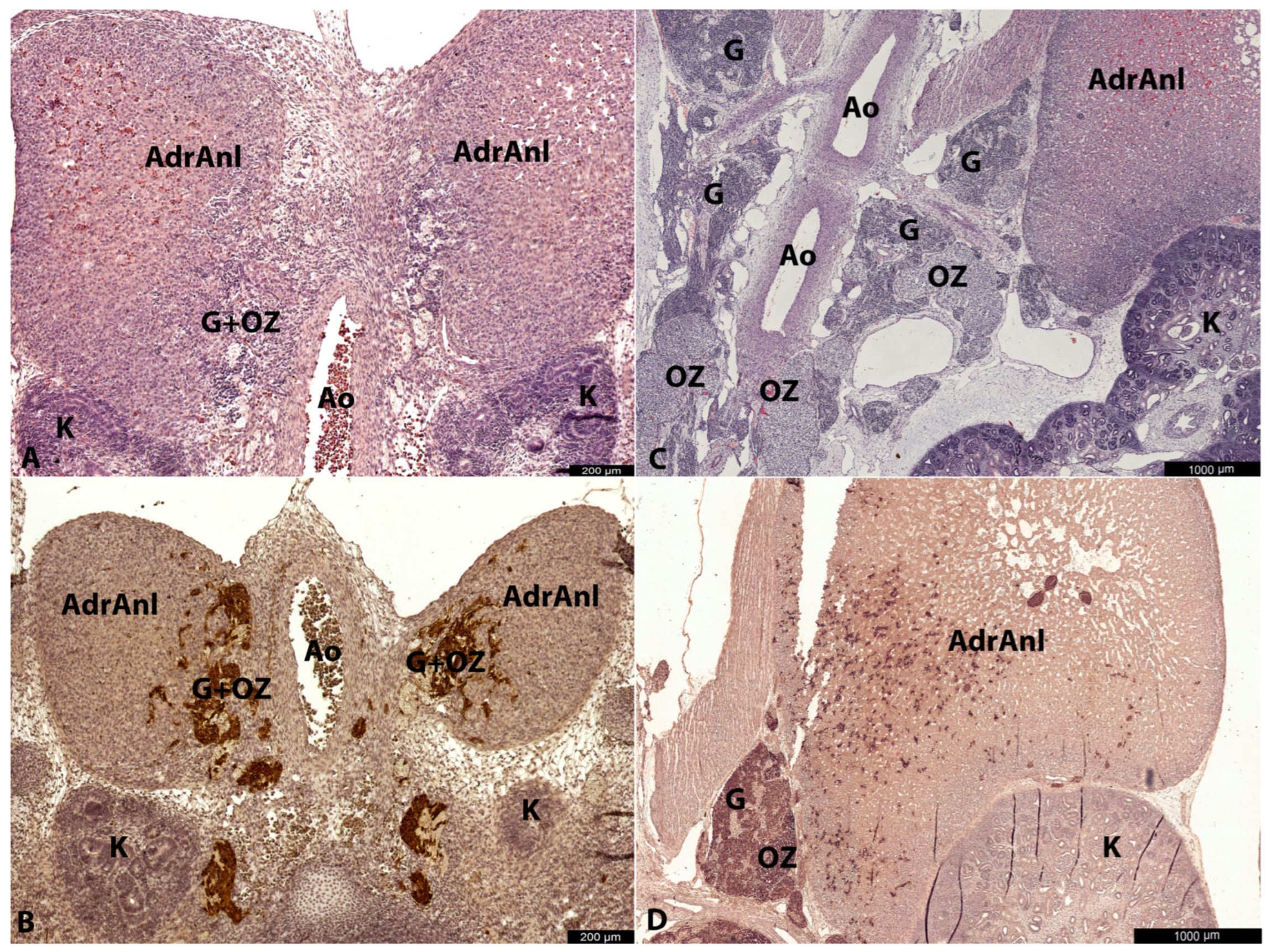
At the earliest investigated stage (8–9 gestational weeks), chromaffin cells (indicated here by LC morphology and marked cytoplasmic TH+ and DBH+ immunoreactivity) were already present in the extra-adrenal para-aortic region. These developing chromaffin cells were also observed in the medial part of the cortical anlage, either freely interspersed among cortical cells or in connection with nerves, resembling a migratory pattern. This suggests that the OZ anlagen may play a crucial role in the distribution of partially differentiated (TH+, DBH+) chromaffin lineage cells, contributing to the formation of the AM. Alternatively, it is possible that even at the earliest investigated stage (8–9 g.w.), we captured not the ongoing migration, but the result of previous migration transitionally through the para-aortic region with subsequent differentiation of cells along the chromaffin pathway.
At the earliest investigated stages, SCs were primarily located extra-adrenally in close association with nerve bundles, with only occasional SCs observed among cortical cells. The weak SCs positivity for antibodies could have contributed to difficulties in SCs detection. As a result, their migration and presence in cortical anlage were less apparent compared to the more evident migratory pattern of LCs.
At later stages, the intra-adrenal LC migratory pattern became more obvious. Alongside LCs, small groups of SCs were also observed intra-adrenally. These SCs were typically found in close contact with nerves, whereas LCs were often seen within the cortical anlage, independent of nerve structures. This suggests that LCs may migrate not only along nerves but also freely between cortical cells, while SCs primarily migrate to the adrenal anlage along nerve pathways.
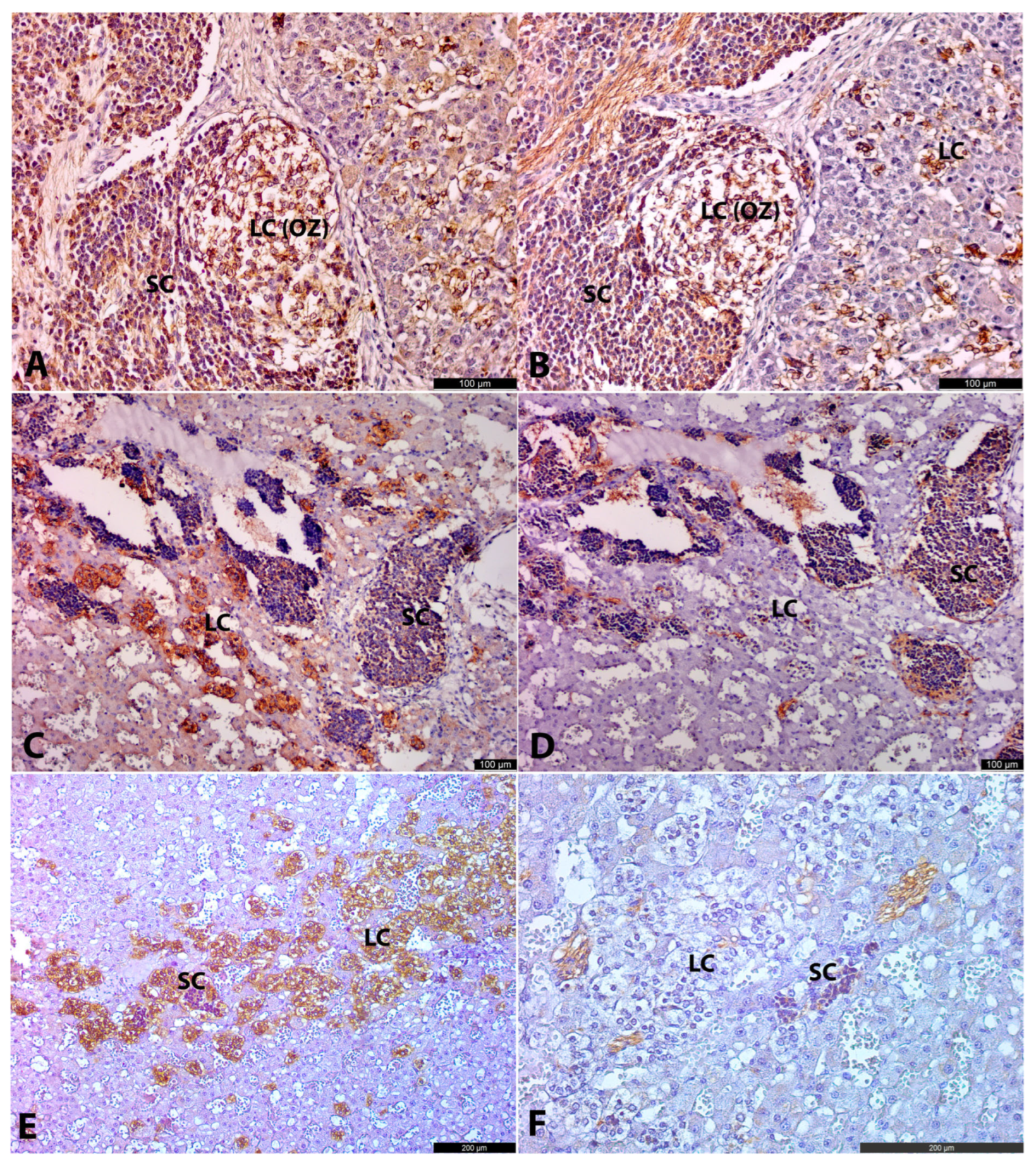
At the same time, some LCs were located at the periphery of SC clusters, closely associated with them. This suggests that a portion of SCs may differentiate into LCs. Moreover, at the age of 22.5–26 g.w., the ratio of LCs to SCs visually increased, likely indicating that the majority of SCs had differentiated into LCs and, to a lesser extent, into neurons. However, more samples are needed to confirm this.
Placing our findings in the context of recent single-cell transcriptomics work [
6], we identified some correlations with their data. According to Kameneva’s work, there are two ways of chromaffin cell differentiation. The first one is a direct transition from Schwann cell precursors (SCPs) to chromaffin cells. These chromaffin cells correspond to the primarily migrated LCs we observed. The second one is a two-step transition from SCPs via intra-adrenal sympathoblasts (IASs) to chromaffin cells. These IASs correspond precisely to the SC populations identified in our study. Kameneva et al. classified the clusters of SCs with LCs at the periphery as ganglia-like structures (GLS) following the idea that these structures predominantly give rise to intra-adrenal ganglia. They justify this by the discovery of intra-adrenal ganglia in the postnatal period.
However, our analysis of later fetal stages demonstrates that only a minority of these so-called ganglia-like structures differentiate into ganglia. First of all, it is proved by non-ganglion morphology obtained by these clusters at later stages, which is characterized by poorly defined borders and vessel-like spaces. Secondly, we observed the discrepancy between abundant SCs in our second age group and the scarcity of ganglia in the third age group and mature adrenal glands, which was demonstrated by other researchers [
20]. In addition, the postnatal ganglia described by Kameneva et al. were positive only for chromogranin A and vasoactive intestinal peptide, but not for tyrosine hydroxylase, although cells associated with GLS in the antenatal period were strongly positive for it.
This raises questions about the role of the initially migrating LCs, which are not connected to the differentiation of intra-adrenal SC clusters. One possibility is that these primarily migrating LCs play a provisional role in producing catecholamines and facilitating the migration of SCs into the cortical anlage. Following this, the primarily migrated LCs may undergo apoptosis. Another possibility is that both the initially migrating LCs and those differentiated from SCs collectively contribute to the formation of the AM (
Figure 7). Additionally, the specific roles of these two cell types in AM morphogenesis remain uncertain. At the early stages, the developing AM was primarily composed of migrating LCs, with only occasional SCs present in the cortical anlage. However, at later stages, large clusters of SCs were observed within the adrenal gland. This may be attributed to the high proliferative activity of SCs, which likely contributes to the significant increase in the overall cell population.
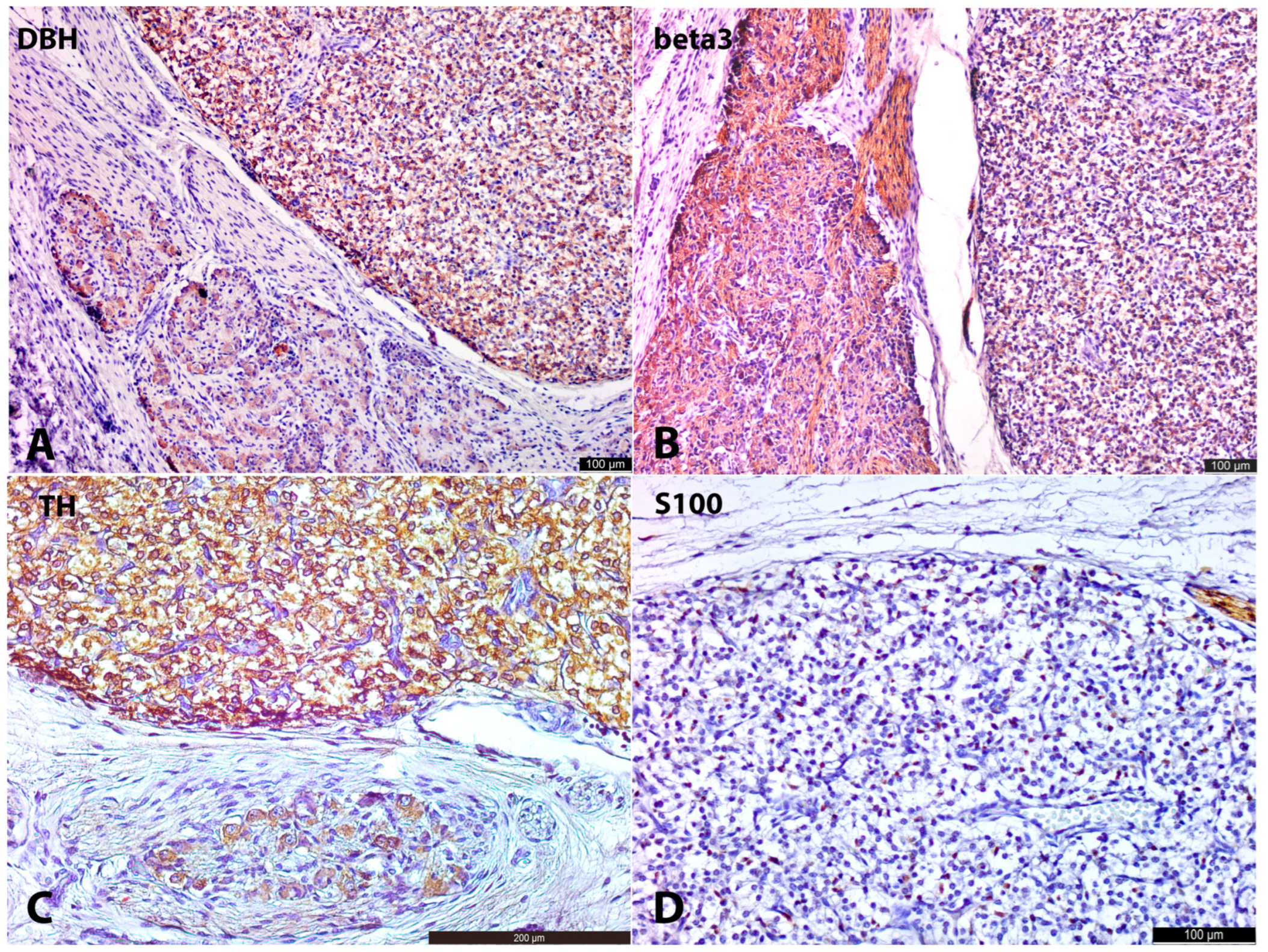
The last interesting observation is related to the peculiarities of PNMT synthesis. At the age of 12 g.w., we observed strong cytoplasmic positivity for PNMT in intra-adrenal LCs, both those associated with SCs and those lying freely. This finding is intriguing in light of ultrastructural results reported by Hervonen [
4], who identified the first ultrastructural features of epinephrine-containing granules at 16 g.w. in the human fetus AM, approximately four weeks after the appearance of PNMT in our study. Hervonen also noted that typical synaptic profiles were present on medullary chromaffin cells in a 12-week-old fetus but stated that mature synapses on these cells did not appear until 14 weeks. In contrast to intra-adrenal chromaffin cells, Hervonen demonstrated that extra-adrenal paraganglia remained non-innervated, as no synapses were found on their chromaffin cells, and they lacked epinephrine-containing granules.
These observations, along with our results, suggest a correlation between the presence of synapses on chromaffin cells and the presence of epinephrine-containing granules, and consequently, the presence of PNMT, the enzyme responsible for epinephrine synthesis. Therefore, it is possible that PNMT synthesis in humans is connected to the process of synaptogenesis. Moreover, it is probable that the establishment of synapses triggers the further differentiation of chromaffin cells from norepinephrine-synthesizing to epinephrine-synthesizing cells. This idea, that synaptogenesis can induce differentiation, dates back to an early study by Golub [
21]. This hypothesis is also in accordance with widely known facts about the influence of synaptic activity on the expression of some genes. The most well-known example is neurotrophic effects on muscles [
27] and some effects on neurons in the brain [
28,
29]. Once a synaptic terminal appears on a cell, it begins to function, since there is no structure without function [
21]. This functioning synapse can cause a shift in the cellular program, after which the expression of PNMT begins.
However, validating this hypothesis through existing literature presents significant challenges. For example, the temporal relationship between synapse formation and PNMT expression remains unclear in rats. Ultrastructural features of mature synapses on chromaffin cells in rats appear at 15.5 days of gestation, suggesting that neural control over medullary chromaffin cells could be established from this point [
30]. However, functional studies show that in 1-day-old newborn rats, neural regulation of catecholamine release is absent until about 8 days of postnatal life [
31], even though epinephrine is already present in medullary chromaffin cells. Critically, this developmental dissociation between structural synaptogenesis (day 15.5) and functional neural control (day 8) does not preclude synaptic influence on PNMT induction. Nevertheless, these apparent discrepancies highlight the need for further investigation.
The appearance of PNMT at 12 gestational weeks (g.w.) in a human prefetus raises questions about the role of glucocorticoids in the expression of PNMT. The adrenal cortex in human fetuses is distinct from that in other species, and cortisol is believed to be produced transiently early in gestation (around 7–10 g.w.). However, due to the lack of hydroxy-delta-5-steroid dehydrogenase, 3 beta- and steroid delta-isomerase 2 activity—the enzyme essential for de novo cortisol synthesis—cortisol biosynthesis appears to be suppressed until 23–24 g.w. [
32]. Consequently, at 12 g.w., the hypothetical level of cortisol would be low, making it unlikely to stimulate PNMT expression. This suggests that glucocorticoid induction of PNMT synthesis in humans may not be the primary mechanism. However, additional human samples are needed to prove that our result is not connected with some individual variability, and further investigation into the adrenal cortex, adrenal medulla, and extra-adrenal paraganglia as a complex is necessary to understand this issue in humans.