Abstract
The genera Allium (Liliaceae) and Anchusa (Boraginaceae) are flowering plant genera with a rich diversity, also including the Allium kharputense Freyn & Sint. and Anchusa azurea Mill. var. azurea species. The antioxidant, anti-Alzheimer’s disease (AD), antidiabetic, and antiglaucoma effects of the Allium kharputense Freyn & Sint. and Anchusa azurea Mill. var. azurea species, which are commonly eaten foods in the Southeast of Türkiye in the treatment of several diseases, were studied. To interpret the antioxidant capacities of ethanol extract of two plant species, aerial parts were analyzed by ABTS and DPPH assays. The IC50 values of A. kharputense and A. azurea ethanol and water extracts for ABTS•+ activities were recorded in the range of 30.93 to 33.94 µg/mL and 33.45 to 33.78 µg/mL, respectively. Also, DPPH• activities were measured at 30.78 to 36.87 µg/mL and 31.67 to 32.45 µg/mL, respectively. The best of the IC50 values was measured in the ethanol extract of A. kharputense as 30.78 µg/mL for DPPH scavenging activity. The total phenolic and flavonoid quantities in A. kharputense and A. azurea plants were measured. The highest phenolic and flavonoid contents of A. kharputense and A. azurea species were recorded in amounts of 445.52 and 327.35 mg GAE/g in ethanol extracts, respectively, and 332.88 and 234.03 mg QE/g in ethanol extracts, respectively. The effects of A. kharputense and A. azurea on diabetes, AD, and glaucoma were studied on the target enzymes of diseases. The most efficient IC50 values were recorded at 10.72 μg/mL against α-glycosidase, 35.01 μg/mL against AChE, 38.05 μg/mL against BChE, 9.21 μg/mL towards hCA I, and 81.02 μg/mL towards hCA II isoenzymes. The kinds and amounts of phenolic compounds in A. kharputense and A. azurea were determined using LC-MS/MS against 53 standards. A. kharputense and A. azurea plants have prospective use in enhancing glaucoma, diabetes, AD, Parkinson’s disease, epilepsy, and cancerous disorders.
1. Introduction
Allium L. is one of the largest monocot genera, comprising more than 900 species. It is distributed almost exclusively in the northern hemisphere, especially in the eastern Mediterranean and southwest and central Asia. The Allium genus is found in Türkiye with 241 taxa, 40% of which are endemic [1]. Because of their nutritional value and possible health advantages, most people on the planet eat Allium species, particularly garlic and onions [2]. Garlic’s bioactive properties, which include antibacterial, antioxidant, anticancer, antidiabetic, and antiallergic activities, have been used medicinally throughout history [3]. Commonly called Italian bugloss, Anchusa azurea is a species of flowering plant belonging to the Boraginaceae family [4]. Little investigation has been conducted on the wild plant Anchusa officinalis (family Boraginaceae), which is endemic to Europe. A recent investigation verified that A. officinalis contains polyphenols, pyrrolysine alkaloids, and triterpenoids. Since the Anchusa genus has antibacterial, antitumor, antiviral, anti-inflammatory, and antidiabetic properties, additional species of the genus Anchusa, including Anchusa italica and Anchusa strigosa, are widely used in traditional medicine [5]. The Anchusa genus has been shown to contain polyphenols, including anthocyanins and flavonoids, fatty acids, phenolic acids, alkaloids, tannins, saponins, and triterpenes [6]. This plant is native to Europe but is not found in the far north, much of the west, and parts of the Mediterranean region. It is popularly known as blusher, duck’s nest, and medicinal common insecticide [7]. It is also popularly known as honey weed or mullein. Leaf, flower, and root parts of the plants are used in the folk medicine. The whole plant is used as a urine enhancer and cleanser. It has been reported that red dye is obtained from the roots and leaves, and flowers are used in the treatment of eczema [8]. People have utilized A. officinalis’s aboveground aerial portions as a diuretic and to cure wounds [9]. The leaves and bulbs of the Soyraz (A. kharputense) species are often consumed raw or used as a medicinal infusion [10]. A. kharputense is consumed fresh or dried in local dishes. A. azurea is used in the region as a diabetic and kidney stone reducer and for healing of wounds and cracks. The stem of the plant is peeled and consumed fresh, as well as cooked [11]. Guriz (A. azurea) is curative for rheumatism and stomach pain. It is useful for intestines and has healing properties for open wounds. Therefore, it is boiled in butter until it turns into cream for open wounds. The obtained cream is applied to the open wound. It is a plant generally used in gynecology [12].
A. kharputense is a plant that is mostly used in Siirt, Şırnak and east provinces of Türkiye in the winter months by being fried or stored in brine. It is a plant that grows spontaneously in nature, emerges with the melting of snow, and is collected for two months to make different dishes. A. azurea is a plant whose leaves and flowers are used in the treatment of eczema in Şırnak province and eaten fresh in case of poisoning. It is also traditionally eaten in the region by roasting with onion and egg in oil.
Excessive production of reactive oxygen species (ROS) during metabolism may lead to oxidative damage, which is linked to various human disorders and interferes with genetic machinery [13]. Antioxidants, made up of phenols and polyphenols, can slow down or prevent the oxidation of biomolecules and delay or reduce oxidative damage and ROS [14,15]. Antioxidants can be categorized into enzymatic and non-enzymatic groups and can be taken in through foods rich in vitamins, minerals, and biologically active substances [16,17]. Antioxidants like flavonoids, phenolic acids, tannins, and phenolic diterpenes scavenge free radicals, suppressing oxidative pathways that contribute to degenerative diseases [18]. Prevention of chronic diseases such as cancer, Parkinson’s disease (PD), cataracts, type 2 diabetes mellitus (T2DM), cardiovascular diseases, and Alzheimer’s disease (AD) is possible thanks to antioxidants [19]. Dietary practices, a critical lifestyle factor, have a significant impact on the risk of AD, and several studies have linked the illness’s preventative potential to bioactive substances found in different foods. One of the most important lifestyle variables that can reduce the risk of AD is an adequate diet, according to a wealth of studies. Neuroprotection can be achieved by a balanced diet, indicating that bioactive substances may have an impact on the main pathogenic pathways of AD [20].
Both cholinergic enzymes (AChE and BChE) share some structural resemblances, containing a catalytic center. Research into novel cholinesterase inhibitors appears to be an essential endeavor to optimize the development of new drug candidates against AD and related dementias [21]. As the fourth leading cause of mortality in wealthy nations, diabetes mellitus is regarded as an epidemic. There are several factors that contribute to the etiology of T2DM, such as genetic vulnerability, lifestyle decisions, and dyslipidemia. Treatment for T2DM focuses on inhibiting α-glycosidase, one of the most crucial digestive enzymes that catalyzes the digestion of dietary polysaccharide [22]. Diabetes can be treated in its early stages by lowering postprandial hyperglycemia. To do this, the digestive system’s α-glycosidase and α-amylase enzymes, which hydrolyze carbohydrates, are suppressed, preventing the absorption of glucose. Therefore, by slowing down glucose absorption, inhibitors of these enzymes reduce the postprandial plasma glucose increase [23].
Carbonic anhydrases (CAs) fulfill a wide range of metabolic and biochemical tasks, as well as ureagenesis, gluconeogenesis, and lipogenesis. Infection, convulsions, glaucoma, and cancer can all be therapeutically treated by CA inhibition [24]. Although hemolytic anemia is connected to both CA I and CA II, glaucoma, epilepsy, edema, and altitude sickness are also linked to the CA II isoenzyme [25]. From diabetes to cancer, various CA isoforms have been connected to a number of illnesses [26]. The vascular endothelium functions better when plant-based foods are consumed, which lowers the risk of high blood pressure, diabetes, AD, and other cardiovascular conditions [27]. Secondary metabolites from medicinal, aromatic, indigenous, endemic, or dietary plants, rich in phenolic and flavonoid contents, can be effective in the treatment of common diseases when used by determining the pharmacological doses of the extracts with environmentally friendly extraction methods.
Enzyme inhibition is a strategy that aims to eliminate disease-causing agents or symptoms by reducing or modulating the activities of enzymes in biological processes used in modern drug development. Nowadays, enzyme inhibition technology is utilized in the treatment of various chronic or acute diseases such as cancer, diabetes, and AD, as well as infections. The objective of this study was to analyze the inhibition effects of A. kharputense and A. azurea plant extracts on human carbonic anhydrases I and II (hCAs I and II), acetylcholinesterase (AChE), butyrylcholinesterase (BChE), and α-glycosidase enzymes. AChE and BChE are enzymes that are used in the development of symptomatic treatment in the middle phases of AD, and their inhibitors are used in the treatment of AD at this stage. The hCA I and hCA II isoenzyme inhibitors are used in the treatment of Parkinson’s disease and glaucoma. On the other hand, α-glycosidase enzyme inhibitors are used in the treatment of diabetes. In this research, the antioxidant, antidiabetic, anti-AD, and antiglaucoma properties of ethanol and water extracts of A. kharputense and A. azurea plants at different concentrations were investigated by Fe3+ reduction, Cu2+ reduction, and ABTS•+ and DPPH• radical scavenging effects. The results obtained from the antioxidant and enzyme inhibition analyses indicated the correlations between the phytochemical composition of the extracts and the bioactivities observed. The phytochemical composition of the both plant extracts was determined by LC-MS/MS analysis and spectrophotometrically by total phenol/flavonoid determinations.
2. Materials and Methods
2.1. Chemicals
Commercially available α-tocopherol, butylated hydroxyanisole (BHA), butylated hydroxytoluene (BHT), Trolox, 1,1-diphenyl-2-picrylhydrazyl (DPPH), 2,2′-azino-bis-3-ethylbenzthiazoline-6-sulphonic acid (ABTS), and other chemicals were purchased from Sigma-Aldrich GmbH (Steinheim, Germany).
2.2. Plant Materials
A. kharputense and A. azurea used in this study were collected from the Southeast region of Türkiye, Siirt and Şırnak provinces, at an altitude of 1150–1700 m. Plants were collected and identified by ensuring that the distribution of the sample collected for fieldwork in the area determined in the adjacent geography of the two provinces was representative of the universe of the plants. Plant taxonomist Prof. Dr. Mehmet Fidan registered the species to the Siirt University Herbarium with numbers SUFAF 1729 and SUFAF 1730 for A. kharputense and A. azurea plants, respectively [27]. The identified plants are given below in Figure 1.

Figure 1.
The Allium kharputense Freyn & Sint. and Anchusa azurea Mill. var. azurea species collected from Siirt and Şırnak provinces, Türkiye.
2.3. Preparation of Plant Extracts
Based on previous studies, the extraction procedure was carried out as follows [28]. First, the plant material was dried, crushed into little bits, and then water was added. This mixture was boiled with a magnetic stirrer for 20 min. The filtrates of the extracts were frozen and lyophilized at −50 °C under 5 mmHg pressure in a lyophilizer (Labconco, Freezone). For ethanol extracts of the samples, 25 g of dried A. kharputense and A. azurea were ground and combined with 100 mL of ethanol and stirred in a magnetic stirrer for 1 h. After the extracts were filtered, the ethanol was evaporated at 50 °C in a rotary evaporator (RE 100 Bibby, Stone Staffordshire, UK). Before use in experimental studies, all extracts were stored in a dark plastic bottle at 2 °C [28]. The % extraction yield was determined by the difference between the initial and final weights. Samples were labelled as A. azurea Mill. ethanol extract (EEAA), A. kharputense ethanol extract (EEAK), A. azurea water extract (WEAA), and A. kharputense water extract (WEAK) [29,30]. The studied plants are shown in Figure 1.
2.4. Total Phenolic Content
The method of Singleton and Rossi [31], with a few minor modifications [28], was used to determine the phenolic content of extracts of A. kharputense and A. azurea. Folin–Ciocalteu reagent (1.0 mL) was added to 0.5 mL of each extracted sample at three different concentrations (15–45 μg/mL) and allowed to react for 5 min. To complete the reaction, 0.5 mL of 1% sodium carbonate solution was added, the volume was made up to 2.0 mL with deionized water, and the solution was neutralized with thorough stirring. After incubation for two hours in the dark at room temperature, the absorbance at 760 nm was measured in comparison with a blank sample containing water. Phenolic content per gram of extract of A. kharputense and A. azurea plants was expressed as milligrams of gallic acid equivalent (GAE).
2.5. Total Flavonoid Content
Based on the method described previously [32], a colorimetric assay was used to estimate the total flavonoid content in ethanol and aqueous extracts of plants. First, 0.5 mL ethanol extract or aqueous extract sample was combined with 1.5 mL 95% methanol. Then, 0.5 mL CH3COOK (1 M) and 2.3 mL distilled and deionized water were combined with 1.5 mL 10% Al(NO3)3, and the samples were vortexed. Following this, the vortexed samples were incubated at room temperature for 40 min in the dark. Absorbance measurements were taken at 415 nm wavelength. Quercetin equivalents (QE) were reported as mg per gram of extract.
2.6. LC-MS/MS Analysis
2.6.1. Sample Preparation
Each 100 mg A. kharputense and A. azurea extract was dissolved in 5 mL ethanol–water (50:50 v/v) in a volumetric flask, and 1 mL of this solution was transferred to another volumetric flask of 5 mL capacity. Then, 100 μL of A. kharputense and A. azurea extracts were added and diluted to volume with ethanol–water (50:50 v/v). A 1.5 mL aliquot of the final solution was transferred into a capped vial, and 10 μL of sample was injected into the LC-MS/MS. Throughout the experiment, samples in the autosampler were kept at 15 °C [33].
2.6.2. LC-MS/MS Measurements and Method Validation Parameters
The method is based on targeted metabolomics to identify and quantify 53 phytochemicals (phenolic and flavonoid compounds) commonly found in plant extracts.
The analytical approach used in this study was designed by Yilmaz [34] and adapted for extracts of A. kharputense and A. azurea. A Shimadzu-Nexera model ultrahigh-performance liquid chromatograph (UHPLC) in connection with a tandem mass spectrometer was used to quantify 53 phytochemicals. An autosampler (SIL-30AC model), a column oven (CTO-10ASvp type), binary pumps (LC-30AD model), and a degasser were all installed on the reversed-phase UHPLC (DGU-20A3R model). Internal standard solutions were used to increase the reliability of the results by compensating for matrix effects and analyte losses during sample preparation and analyses. For flavonoid glycosides, flavonoids and non-flavonoid substances, rutin D3, quercetin D3, and ferulic acid D3 were used as deuterated internal standards, respectively. Relative standard uncertainty (95% confidence level (k = 2)), linearity, accuracy (recovery), limits of detection and quantification (LOD/LOQ), intra- and inter-day precision (repeatability), and other detailed methods of technique validation have already been detailed in the literature [34].
2.7. Reduction Capacity Assays
2.7.1. Fe3+ Reducing
The Fe3+ reducing activities of water and ethanol extracts of A. kharputense and A. azurea were measured [35]. At 700 nm, absorbance values of A. kharputense and A. azurea and references were recorded [36].
2.7.2. Cu2+ Reducing Ability
Equal volumes of 1.0 mM CuCl2, 7.5 mM neocuprine solutions, and 1.0 M ammonium acetate buffer (pH 6.5) were prepared according to the technique of Apak et al. [37]. The plants were mixed with three different concentrations of the samples (15–45 µg/mL in ethanol), and the total reaction volume was adjusted to 2 mL. After the tubes were kept at room temperature for 30 min, the absorbance values at 450 nm were read spectrophotometrically.
2.7.3. Fe3+-TPTZ Reducing
The ferric reducing capacity (FRAP) of plasma is measured using the technique outlined. At low pH, a strong blue hue with a maximum absorption at 593 nm is produced when a ferric–tripyridyltriazine (Fe3+-TPTZ) complex is reduced to the Fe2+ form [38].
2.8. Radical Scavenging Assays
2.8.1. DPPH Scavenging Activity
The Blois technique [39] was used to test the DPPH• scavenging capacity of water and ethanol extracts of A. kharputense and A. azurea. DPPH solution was prepared one day before the experimental measurement. The solution bottle was kept in the dark and stirred at 4 °C for 16 h. Aluminum foil was used to cover the bottle. Shortly after the preparation of 0.1 mM DPPH solution in ethanol, 0.5 mL of this solution was added to 2 mL A. kharputense and A. azurea extracts in ethanol at various concentrations (10–30 µg/mL). A. kharputense and A. azurea samples were vortexed and incubated at 30 °C for 30 min in the dark. The DPPH• absorbances were measured and evaluated at 517 nm in comparison with the blank sample [40].
2.8.2. ABTS Scavenging Activity
In addition, the ABTS•+ scavenging capacity of A. kharputense and A. azurea was determined [25]. The mixture comprised 2 mM ABTS in water combined with 2.45 mM potassium persulfate (K2S2O8) to form ABTS•+. This mixture was then kept for six hours at room temperature and in the dark. The study was performed by first diluting the solution in phosphate buffer (pH 7.4) to achieve an absorption of 0.800 ± 0.05 at 734 nm in a 1 mL cuvette. The solution was then equilibrated at 30 °C, the temperature at which all subsequent tests were conducted. Subsequently, 3 mL of each of A. kharputense and A. azurea in ethanol at various concentrations (10–30 µg/mL) were combined with 1 mL each of ABTS•+ solution. Following a 30 min period of agitation, the absorbances were measured at 734 nm, and the radical scavenging activities were calculated for each concentration [41].
2.9. Anti-Alzheimer’s Disease Studies
According to Ellman’s method, the study of the inhibitory effect of water and ethanol extracts of A. kharputense and A. azurea on AChE/BChE was carried out using AChE enzyme obtained from electric eel (Electrophorus electricus)/horse serum [42]. In short, the plant extracts were added to the enzyme solution (50 μL, 5.32 × 10−3 EU) at a specific concentration (10–30 µg/mL) in buffer (1.0 M Tris/HCl, 100 μL, pH 8.0). The solutions were held for 10 min at 20 °C. After that, 50 μL of solutions comprising acetylthiocholine iodide (AChI) and 5,5′-dithio-bis(2-nitro-benzoic acid) (DTNB) (0.5 mM) were administered. The absorbances were evaluated spectrophotometrically at 412 nm once the reaction medium was initiated [43]. Donepezil was used as a positive control for AChE and BChE inhibition.
2.10. Antidiabetic Assay
The effect of A. kharputense and A. azurea on α-glycosidase inhibition was evaluated using p-nitrophenyl-D-glycopyranoside (p-NPG) substrate following the procedure elucidated by Tao et al. [44]. Plants’ potential is to be used in the treatment of diabetes. α-glycosidase enzyme activity is based on the measurement of 4-nitrophenol, which gives a yellow absorbance at 405 nm as a result of the enzymatic activity of α-glycosidase on p-NPG [45,46]. Acarbose was used as a positive control for α-glycosidase.
2.11. Antiglaucoma Assay
Erythrocytes obtained from human blood used for laboratory testing were studied as a source of hCA I and II isoenzymes. For 30 min, erythrocytes were rotated at 10,000× g. After the serum had been isolated, solid Tris was used to adjust the pH down to 8.7 [47]. Purification of the isoenzymes was accomplished via Sepharose-4B-L-Tyrozyne sulfanilamide affinity column chromatography [48]. Tris-Na2SO4/HCl (22 mM/25 mM, pH: 8.7) was used to equilibrate the sample after it was placed on the affinity column. The hCA II isozyme was then eluted using sodium acetate/NaClO4 (0.5 M, pH 5.6, 25 °C). The Bradford technique [49] was utilized to evaluate the protein concentration throughout the purifying process. Bovine serum albumin was served as a reference protein. According to previous studies, SDS-PAGE was performed to determine the purity of the hCA I and hCA II isoforms [41]. In this study, the inhibition effects of ethanol and water extracts of A. kharputense and A. azurea on hCA II enzyme were carried out using the method based on the activity of CA isoenzyme as esterase. In this method, p-nitrophenylacetate (PNA) was used as a substrate. CA enzyme hydrolyzes PNA to p-nitrophenol and acetate, and the method is based on the absorbance of p-nitrophenol at 348 nm [50,51]. Esterase activity was carried out throughout the purification and inhibition processes of hCAs I and II isoforms [52]. Acetazolamide was used as a positive control for both hCAs.
2.12. Determination of IC50 Values
IC50 values were calculated as the percentage of decreasing enzyme inhibition for increasing concentrations of the samples. Graphpad Prism 8.4.0 was used to plot % inhibition versus concentration. IC50 values were determined by non-linear regression.
2.13. Statistical Analysis
Every test was conducted in triplicate. The data were presented as mean ± SD. In two-way ANOVA, significant differences were considered to have a value of p < 0.05.
3. Results
3.1. Analysis of Total Phenolics and Flavonoids
According to the physical state classification statement based on USP General Chapter <565> botanical extracts, dry extracts were prepared, and liquid extracts were obtained for further biological activity tests performed in this study. In addition to botanical classification, plant–extract ratios have been defined by a simple calculation of the extract production yield of dried plants. This ratio can be used to partially determine the amount of active substance extracted from plant biomass relative to the initial amount of biomass and is therefore useful for defining ‘standardized extracts’ for herbal formulations and dietary supplements. The plant–extract ratio of ethanol extracts was calculated to be 10:2.2 and 10:3.5 for EEAA and EEAK, while this ratio was 10:1.8 and 10:3.3 for water extracts WEAA and WEAK, respectively. Accordingly, the yields and plant–extract ratios of the plants obtained are as shown in Table 1.

Table 1.
Aerial parts of the plants were extracted in different solvents, and yields were obtained for EEAK and EEAA (A. kharputense and A. azurea ethanol extracts), and WEAK and WEAA (A. kharputense and A. azurea water extracts).
The obtained extracts were analyzed for total phenols and flavonoids. Accordingly, the highest phenolic content was determined in the ethanol extract of EEAK as 445.52 mg GAE/g extract. The following order was determined: WEAK > EEAA > WEAA (Table 1).
3.2. Chromatographic (LC-MS/MS) Phytochemical Analysis Results
The chromatographic analysis of phytochemical compounds was performed by LC-MS/MS for ethanol and water extracts of A. kharputense and A. azurea. For the qualitative and quantitative assessment of phytochemicals, a previously created and approved LC-MS/MS technique was used in this investigation [34]. This approach was chosen because the created method may be used for a large variety of plant species, not just a few chosen ones. Using the recently developed LC/MS/MS technique, 53 phytochemical molecules, including 14 flavonoid aglycones, 13 flavonoid glycosides, 20 phenolic acids, 3 phenolic aldehydes, 1 benzopyrone, 1 stilbenoid glycoside, and 1 biflavonoid, were detected and quantified in the investigated species.
In this study, it was shown that A. kharputense and A. azurea species’ methanol and water extracts have high phenolic and flavonoid contents. Both plant species’ ethanol and water extracts have shown in this study to have a comparable effective number of polyphenolics. Using fifty-three phenolic compounds as standards, the LC-MS/MS method was utilized to identify the major organic components in A. kharputense and A. azurea species extracts. A total of 21 compounds were measured (Table 2, Figure 2 and Figure 3), with 6 compounds in A. azurea species and 17 compounds in A. kharputense species. The average quantities for each substance as determined by the LC-MS/MS tests are displayed in Table 2. The major components detected in ethanol extracts of these species were Astragalin (20.045 mg/g), isoquercitrin (13.256 mg/g, kaempferol (7.263 mg/g), quercetin (6.637 mg/g), quinic acid (5.094 mg/g), p-coumaric acid (2.237 mg/g), fumaric acid (1.415 mg/g), and protocatechuic acid (1.053 mg/g); the other detected compounds were aconitic acid, 4-hydroxy-bezoic acid, caffeic acid, cynaroside, rutin, naringenin, and apigenin, which were in lesser amounts.

Table 2.
Quantitative LC-MS/MS results of ethanol and water extracts of A. kharputense and A. azurea: EEAK, EEAA, WEAK, and WEAA.
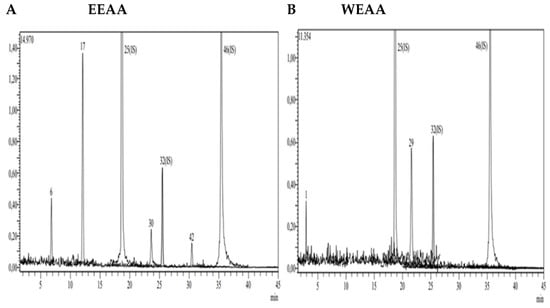
Figure 2.
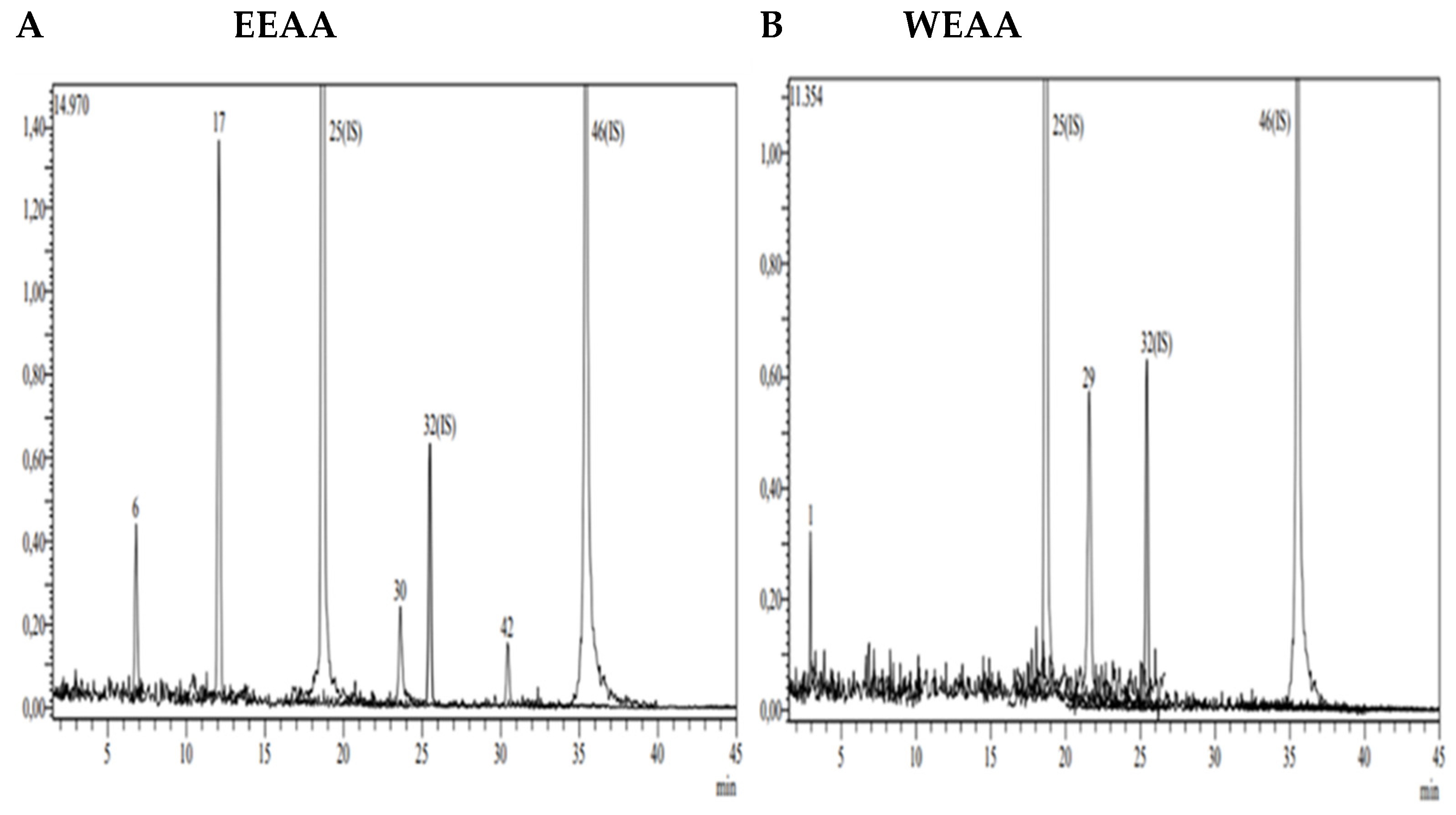
LC-MS/MS chromatogram of A. azurea. (A) Ethanol extract; (B) water extract (ESI neg).
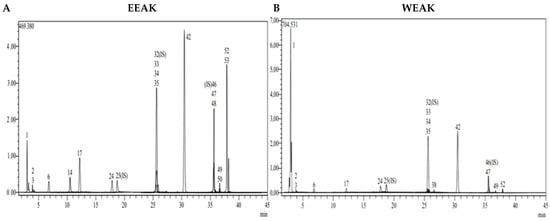
Figure 3.
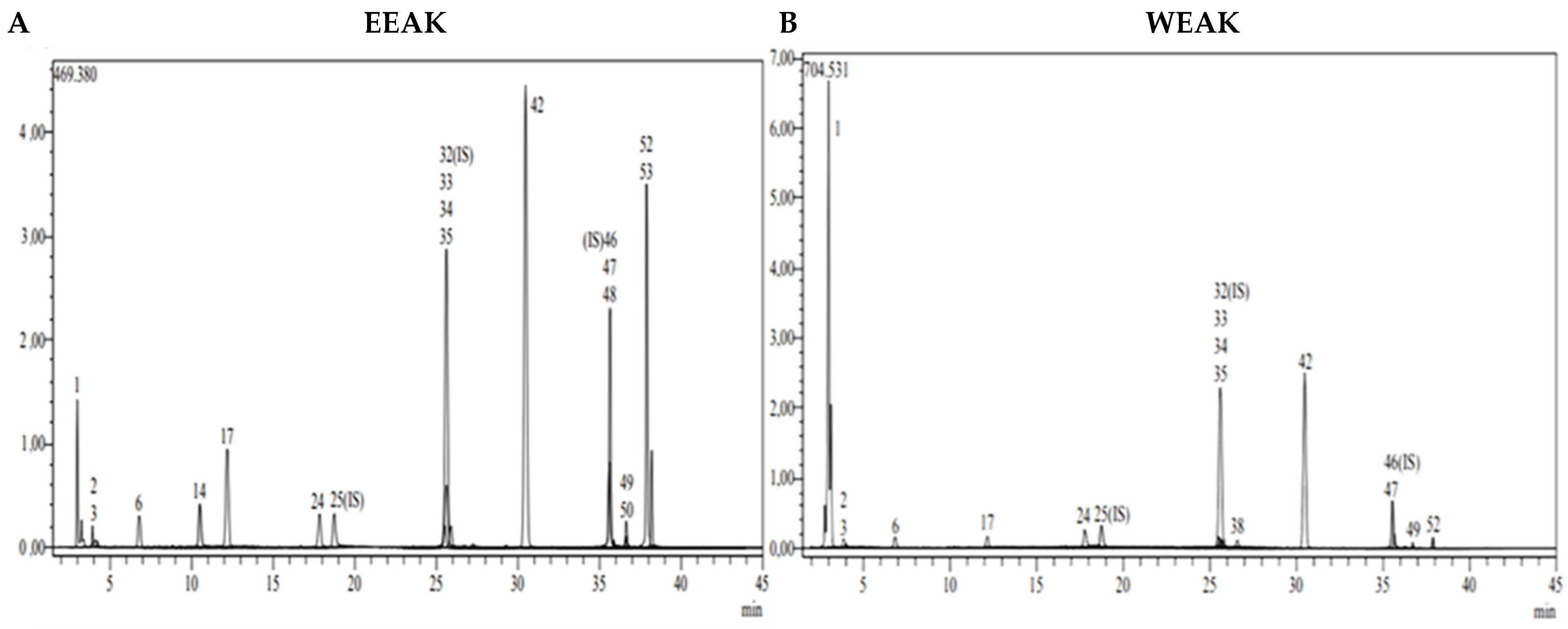
LC-MS/MS chromatogram of A. kharputense. (A) Ethanol extract and (B) water extract.
According to the data obtained from LC-MS/MS, EEAA extract contains protocatechuic acid, caffeic acid, cynaroside, and Astragalin (Figure 3A). A total of two secondary metabolites, quinic acid and salicylic acid (Figure 3B), were detected in WEAA content. The contents of these extracts were considered to be low. A total of 17 phytochemicals, including quinic acid, fumaric acid, aconitic acid, protocatechuic acid, caffeic acid, 4-OH benzoic acid, p-coumaric acid, rutin, isoquercetin, hesperidin, Astragalin, quercetin, naringenin, hesperetin, luteolin, kaempferol, and apigenin, were detected in very high amounts in EEAK. The highest amount of metabolite in this extract was determined to be Astragalin at the level of 20.045 mg in a one-gram extract. In addition, the extracts were found to contain isoquercitrin and kaempferol in very high amounts (13.256 mg and 7.263 mg) (Table 2).
A total of 14 phytochemicals, including quinic acid, fumaric acid, aconitic acid, protocatechuic acid, caffeic acid, p-coumaric acid, rutin, isoquercetin, hesperidin, rosmarinic acid, Astragalin, quercetin, hesperetin, and kaempferol, were detected in WEAK extract. As in the ethanol extract, Astragalin, isoquercetin, and quinic acid were the dominant metabolites (11.212 mg, 10.642 mg, and 5.094 mg). Detailed quantitative LC-MS/MS analysis results of the extracts are given in Table 2.
3.3. Determination of Reducing and Scavenging Abilities of Extracts
3.3.1. Reducing Ability Results
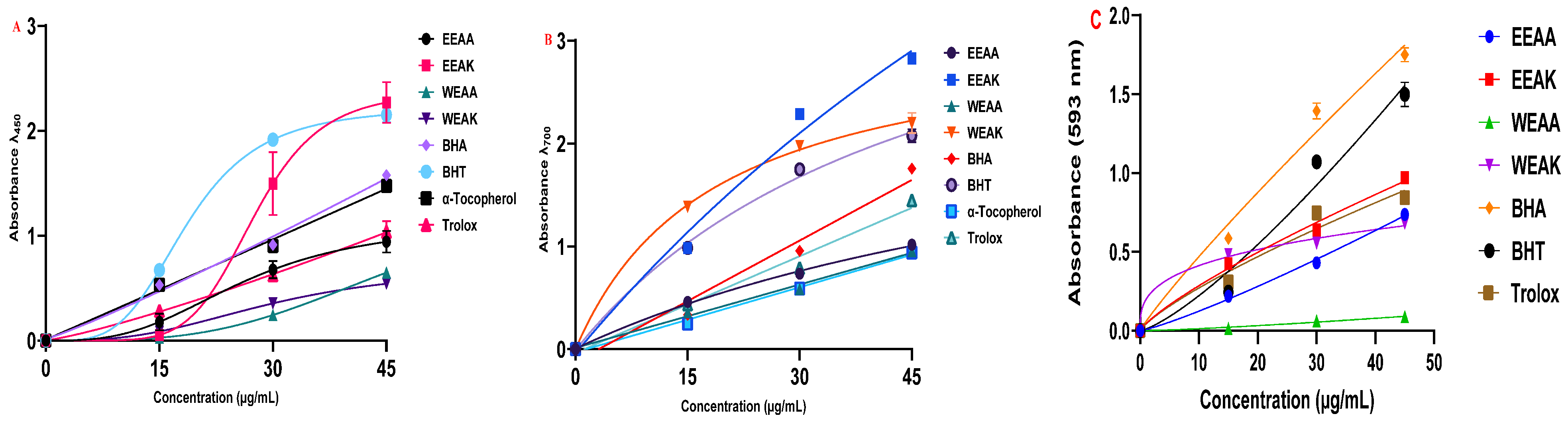
The antioxidant capacities of ethanol and water extracts of A. kharputense and A. azurea plants were tested by five different methods in two different extraction solutions. Cu2+, Fe3+, and Fe3+-TPTZ metal reduction tests were performed with three different reduction methods (Table 3). According to the results obtained from the CUPRAC test, the order of reduction of copper–neocuprine complex was determined as EEAK > BHT > BHA > α-Tocopherol > Trolox > EEAA > WEAA > WEAK (Figure 4A). According to the results obtained, it was determined that A. kharputense ethanol extract was superior to both other plant extracts and standard antioxidants.

Table 3.
Reduction antioxidant test results of A. kharputense and A. azurea ethanol and water extracts at 30 µg/mL: EEAK, EEAA, WEAK, and WEAA.
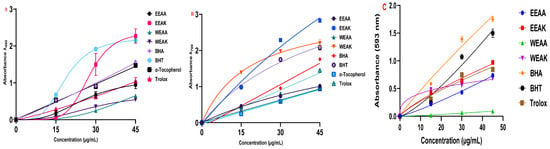
Figure 4.
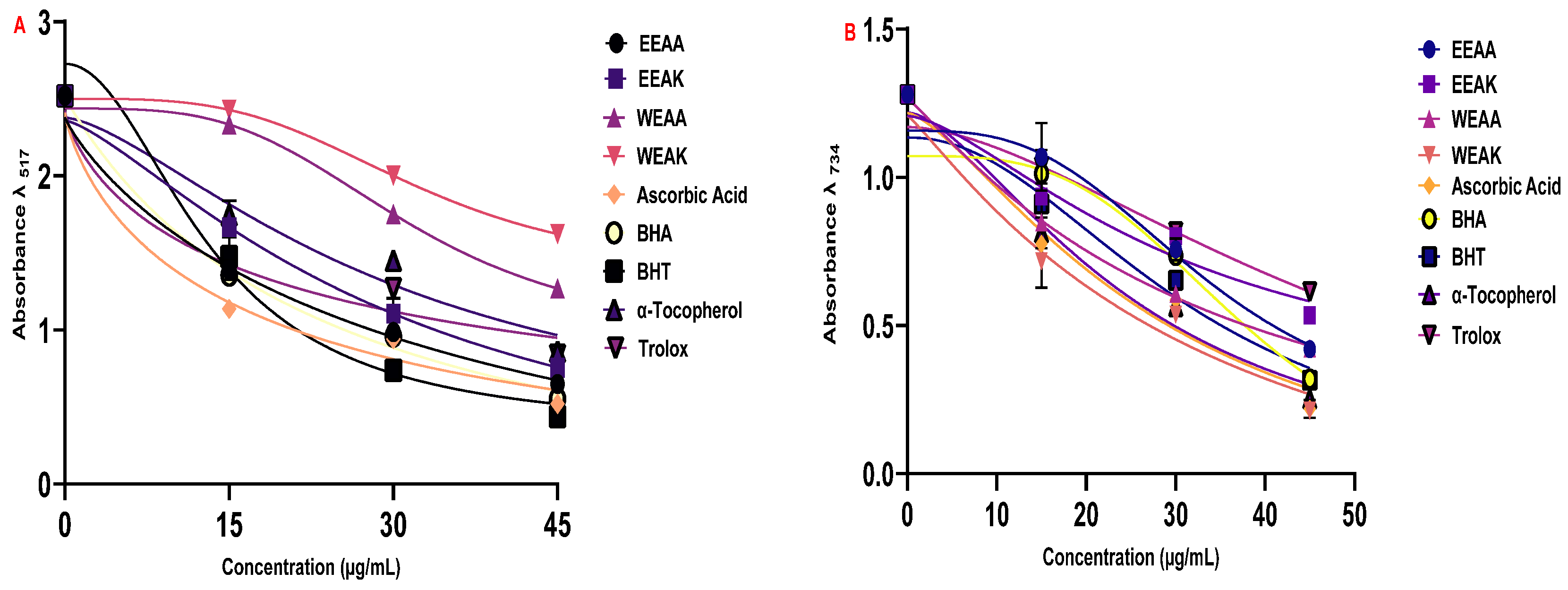
Reducing ability test results of A. kharputense and A. azurea ethanol and water extracts, including EEAK, EEAA, WEAK, and WEAA. (A) CUPRAC reducing assay; (B) Fe3+-reduction test; (C) FRAP reducing assay.
In the results obtained from the Fe3+-reduction test, the order was determined as EEAK > WEAK > BHT > BHA > Trolox > EEAA > WEAA > α-Tocopherol (Figure 4B). These results showed that both ethanol and water extracts of A. kharputense expressed more Fe3+-reducing features compared to all other extracts and standard antioxidants. Furthermore, A. azurea extracts (water and ethanol) were also recognized to be superior to the natural antioxidant α-tocopherol.
According to the results obtained from the FRAP test, the order of reduction of Fe3+-TPTZ complex was determined as BHA > BHT > EEAK > Trolox > EEAA > WEAK > WEAA (Figure 4C). These results showed that EEAK extracts were more potent Fe3+-TPTZ reductants compared to all other extracts. These extracts also showed better metal reducing properties than the synthetic antioxidant Trolox.
According to Table 4 given below, in this study, positive significant correlations were found between plant species’ ethanol and water extract phenolic and flavonoid contents and these extracts’ Fe3+-TPTZ and Cu2+ ion reducing activities. A strong positive correlation was found between phenolic and flavonoid contents and Fe3+-TPTZ (rs = 0.959 *, p: 0.04). An increase in the phytochemicals of species also contributes to plant species’ reducing capabilities and antioxidant activities, too.

Table 4.
Given test parameters and Pearson’s correlation analysis.
3.3.2. Radical Scavenging Abilities
The radical scavenging properties of the plant extracts were determined by DPPH radical scavenging and ABTS radical scavenging methods (Table 5). According to the results obtained from the ABTS radical scavenging test, another radical scavenging test, the order of radical scavenging was determined as α-Tocopherol > Ascorbic acid > EEAK > BHT > WEAK > WEAA > EEAA > BHA > Trolox.

Table 5.
Radical removal results of reducing assay ethanol and water extracts at 30 µg/mL: EEAK, EEAA, WEAK, and WEAA.
According to the results obtained from the ABTS test, it can be said that plant extracts have a better ABTS radical scavenging capacity compared to standard synthetic antioxidants (Figure 5B). In addition, it was found that A. kharputense ethanol extract was superior to the other extracts in both radical scavenging tests.
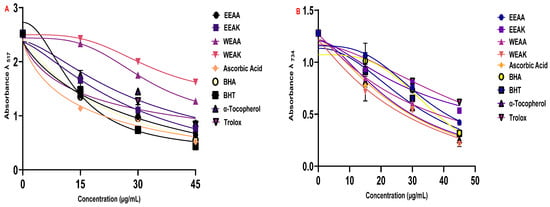
Figure 5.
Radical scavenging abilities of ethanol and water extracts (EEAK, EEAA, WEAK, and WEAA) of A. kharputense and A. azurea against radicals: (A) DPPH radical and (B) ABTS radical.
3.4. Enzyme Inhibition Results
3.4.1. Carbonic Anhydrase Inhibition Effects
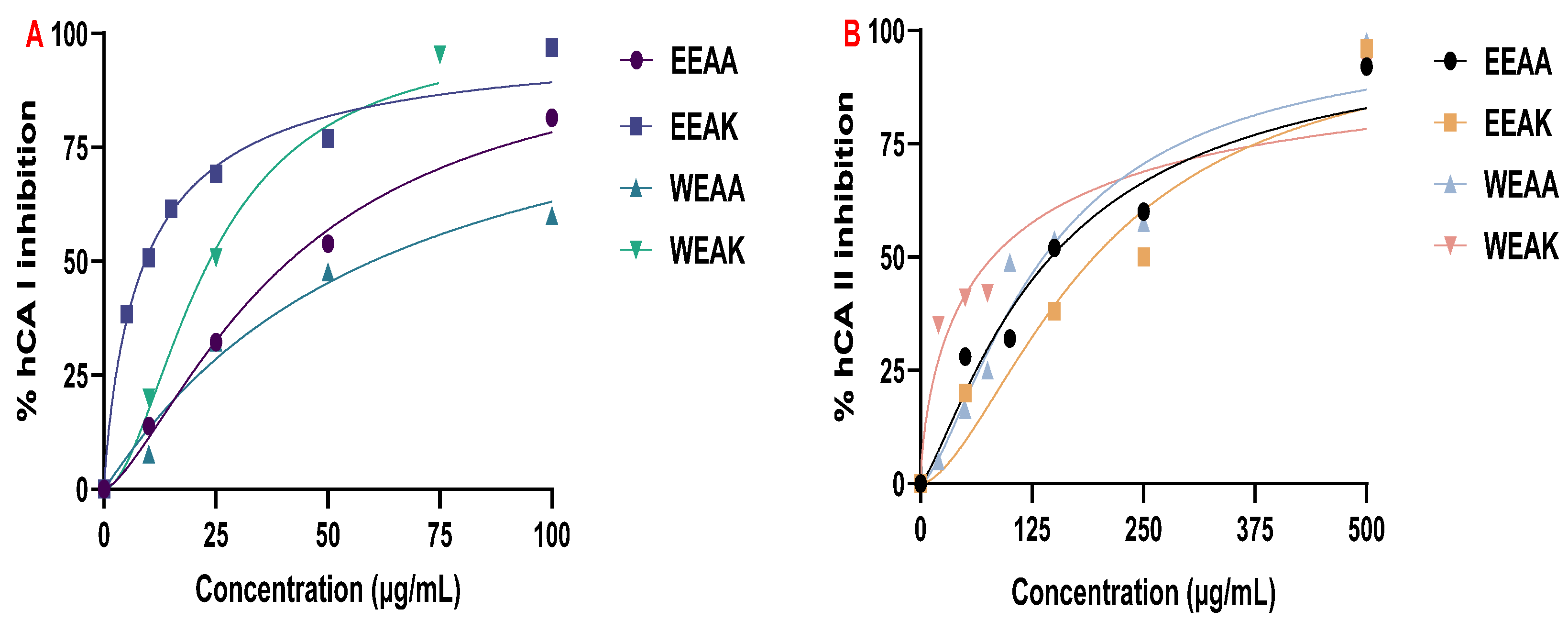
The inhibition effect of A. kharputense and A. azurea ethanol and water extracts on hCA I and II isoenzymes were determined by integrating increasing concentrations of plant extracts into the enzyme activity assay reaction. The percentage inhibition values (%) were determined by comparing the decrease in CA activity against increasing concentration to the control reaction (Figure 6).
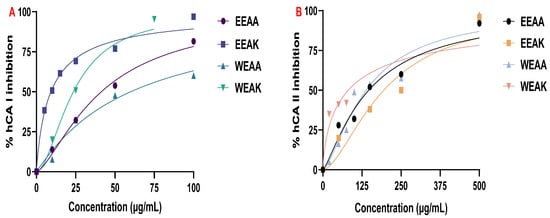
Figure 6.
Inhibition test results of A. kharputense and A. azurea ethanol and water extracts on enzymes: (A) hCA I isozyme and (B) hCA II isozyme.
3.4.2. AChE and BChE Inhibition Effects
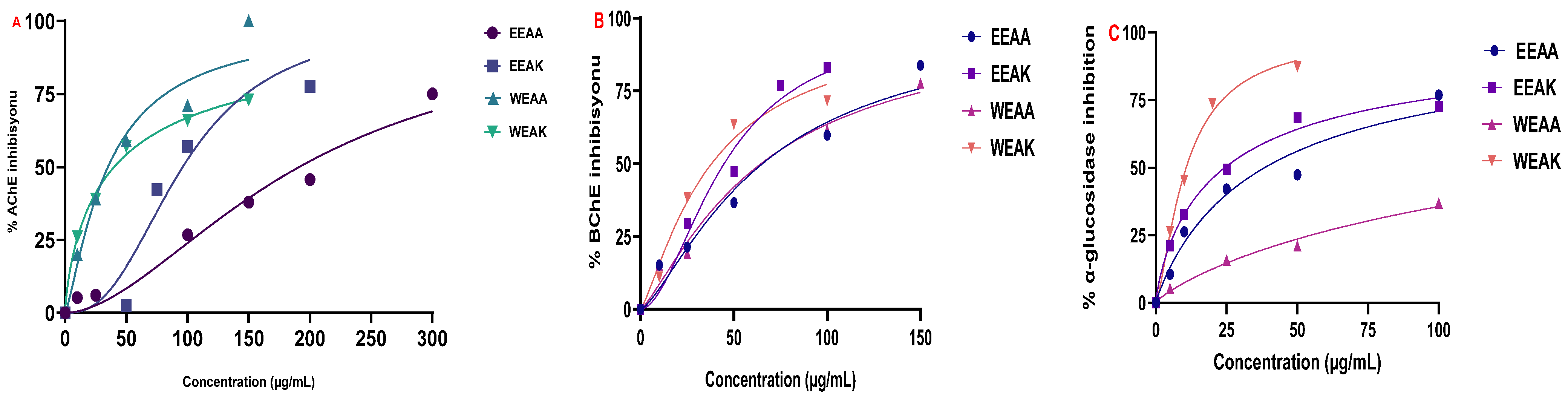
The results of enzyme inhibition with cholinergic enzymes revealed the cholinergic potential of all extracts on both enzymes. According to the IC50 values obtained, the most superior inhibition effect on AChE enzyme was determined as WEAA with an IC50 value of 35.01 µg/mL, followed by WEAK (IC50: 40.08 µg/mL) > EEAK (IC50: 96.14 µg/mL) > EEAA (IC50: 191.3 µg/mL) (Figure 7A). This value was determined as 12.22 µg/mL for donepezil, which is known as the standard inhibitor of AChE. Furthermore, according to the data obtained, WEAK had the most superior inhibition effect on BChE enzyme, with an IC50 value of 38.05 µg/mL, followed by EEAK (IC50: 44.77 µg/mL) > WEAA (IC50: 64.54 µg/mL) > EEAA (IC50: 65.27 µg/mL) (Figure 7B).
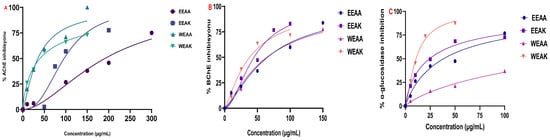
Figure 7.
Test results of inhibition of A. kharputense and A. azurea ethanol and water extracts, including EEAK, EEAA, WEAK, and WEAA, on (A) AChE, (B) BChE, and (C) α-glycosidase.
3.4.3. α-Glycosidase Inhibition Effects
The results obtained from the α-glycosidase inhibition test showed that the extract with the highest inhibition potential was WEAK, and its IC50 value was 10.72 μg/mL, followed by EEAK (IC50: 24.36 μg/mL) > EEAA (IC50: 38.46 μg/mL) > WEAA (IC50: 78.90 μg/mL). This value was 25.43 μg/mL for the standard inhibitor Acarbose. Detailed enzyme inhibition results and IC50 values of the extracts and standard inhibitors can be found in Table 6 and Figure 7.

Table 6.
The enzyme inhibition results of α-glycosidase inhibition effects of ethanol and water extracts of A. kharputense and A. azurea.
4. Discussion
One of the well-known methods for assessing the antioxidant capacity of a variety of biological samples, such as plant extracts, food, beverages, and medications, is the Fe3+ reducing assay, which is based on the reduction of ferric ions (Fe3+) to ferrous ions (Fe2+) in the presence of antioxidants acting as reducing agents [53]. Moreover, phenolics are the most extensively dispersed secondary metabolite within the kingdom of plants. These different chemical spectrums have received much interest as possible natural antioxidants due to their effectiveness as radical scavengers and metal chelators. Regarding reports, phenol’s redox properties, hydrogen donors, and singlet oxygen quenchers are important contributors to the compound’s antioxidant action [54,55,56]. Phenolic compounds in the plant kingdom are an essential and significant part of the human diet. Their biological properties include antioxidant properties, which is why they are receiving a lot of consideration [57]. Isolated from a spectrum of plants, flavonoids are phenolic compounds with multifunctional health benefits, including antibacterial and antioxidant capabilities. It has been well noted that flavonoids exhibit strong antioxidant activity due to their ability to both scavenge and inhibit the production of free radicals and ROS [58,59]. Furthermore, since flavonoids are a group of polyphenolic compounds and can be separated from polyphenols by their C-skeleton number, the total flavonoid content of the extracts was also determined [60]. While most flavonoids contain only 15 C-skeletons, this number may differ in polyphenols. Although flavonoids have the same field of application as phenolic compounds, such as cosmetics and the food industry, the most prominent applications of these compounds are in the medical field. Therefore, the determination of the total flavonoid content significantly expresses the medicinal value of the plant extract and plant-derived products [61]. The principle of the colorimetric aluminum chloride method is based on the formation of stable complexes of AlCl3 with C-4 keto groups and C-3 or C-5 hydroxyl groups of flavones and flavonols in acidic medium and the absorbance of these complexes in the visible region. According to the results obtained from this study, total phenolic and total flavonoid contents were determined in parallel in terms of the order of the samples. The highest phenolic content was 445.52 ± 13.50 mg GAE/g in EEAK extract, and the highest flavonoid content was 332.88 ± 2.76 mg QE/g in EEAK extract. The amount of phenolic and flavonoid compounds was higher in the ethanol extract of both plant species.
Ethanol extract showed the maximum content of flavonoids (30.26 ± 0.40 mgRU/g) and phenols (104.03 ± 0.63 mgGA/g) in Anchusa officinalis, according to an analysis of comparison conducted in a different study [7]. The greatest overall concentration of phenolic and flavonoid quantity was observed in A. officinalis (24,577.05 ± 15.06 μg/g) and (5906.07± 27.12 μg/g), according to measurements of phenolic compounds in the most common Boraginaceae species from Macedonia [9]. The greatest amount of total phenolics (5836 ± 373 mg GAE/100 g dw), total flavonoids (2301 ± 158 CE/100 g, dw), and total antioxidant compounds (1347 mg TE/100 g dw) were found in A. azurea species, based on the results of an investigation on the phenolic composition of herbs gathered from Eastern Anatolia [62]. The lyophilized water extract of Italian bugloss (A. azurea Mill.) aerial parts had total phenolic and flavonoid amounts of 18.18 ± 0.3 and 12.42 ± 0.5 µg/mL, respectively [63]. Aerial parts of Allium nigrum and Allium subhirsutum had total phenolic levels of 29.1 ± 2.3 and 13.3 ± 1.7 mg GAE/g extract, respectively, and total flavonoids of 5.4 ± 1.8 and 3.6 ± 0.3 mg QE/g extract [64]. Moreover, Allium scabrifolium (42.31 mg/g) had the highest accumulated phenolic quantity, followed by Allium goekyigiti (33.15 mg/g) and Allium atroviolaceum (28.35 mg/g), owing to maceration in methanol [65].
Following investigation, phenolics identified as substantial constituents mainly in A. kharputanse and partially A. azurea species were shown to contain flavonoid derivatives with significant bioactivities. Numerous traditional medicinal herbs have been found to contain the natural flavonoid Astragalin. Additionally, Astragalin can prevent endotoxin-induced oxidative damage and reduce ROS generation [66]. Astragalin and isoquercitrin are biologically active, important flavonoid glycosides with diverse pharmacological properties. Astragalin, a kaempferol glycoside, exhibits potent antioxidant, anti-inflammatory, and neuroprotective effects, making Astragalin valuable in combating oxidative stress-related diseases. It has also been reported to show potential for regulating immune responses and protecting against cardiovascular disorders [66]. The anti-inflammatory, antioxidant, neuroprotective, cardioprotective, antidiabetic, and anticancer effects of Astragalin are among its pharmacological properties [67]. Isoquercitrin, a quercetin glycoside, is known for its potent antioxidant activity and contributes to its anticancer, antidiabetic, and hepatoprotective effects. It improves vascular health by improving endothelial function and reducing inflammation [68]. Previous studies have demonstrated that the α-glycosidase inhibitory effect of isoquercitrin is stronger than that of Acarbose [44]. Natural flavanols like kaempferol can lower the cancer risk. In order to combat cancer-causing free radicals and ROS, it boosts the body’s antioxidants [69]. Through its ability to modulate gastrointestinal carbohydrate and fat digestion and absorption, quercetin and its derivatives are a promising option for phytotherapy and combinatorial obesity–T2DM prevention as functional foods and nutraceuticals [70]. Quercetin has been shown in studies to have antidepressant, anti-inflammatory, antiviral, anti-obesity, and antioxidant activities. It also has the ability to protect against cardiovascular disease, diabetes, cancer, and asthma [71]. It has been shown that quinic acid has anticancer properties by causing apoptosis-mediated cytotoxicity in breast cancer cells [72]. The results of a study also highlight that p-coumaric acid is an efficient compound with antioxidant properties and improves the diabetes-induced change in lipid peroxidation and activities of antioxidant enzymes, including catalase, glutathione-S-transferase, and superoxide dismutase [73]. A previous study reported on the anti-inflammatory and antioxidant profile of fumaric acid esters in neurodegenerative diseases [74]. It was reported that enhanced pancreatic, cerebral, and cerebellar functioning were associated with the avoidance of diabetes-mediated increases in AChE activity, indicators of oxidative and inflammatory stress, and caspase-3 activity by protocatechuic acid administration [75]. The presence of high amounts of phenolic and flavonoid contents in the aerial parts of both plants increases the antioxidant capacity of the plants and directly affects the inhibition of enzymes at the control points in the metabolic pathways of diseases.
In the human body, ROS, which are produced as a by-product of metabolic processes occurring in cells in daily life, play important physiological roles in immune system activation and cell signaling and must be present at a certain level in metabolism. However, high levels of ROS produced in the body can be neutralized due to different internal (e.g., metabolism, mitochondrial dysfunction and reactions, electron transport chain and inflammatory responses) and external factors (e.g., radiation, pollutants, radiation, certain foods and drugs, environmental carcinogens, tobacco smoke, toxins, alcohol, drugs, synthetic solvents, and dietary sources), and formations above a certain amount are very important factors, being stimulators of some inflammatory diseases affecting the soft tissues of the body [61]. For example, neurodegeneration, liver dysfunction, ischemic heart disease, AD, infertility, and kidney disease can be listed among these adverse effects. Oxidative stress is triggered by the production of ROS and free radicals, highly reactive compounds containing one or more unpaired electrons that can oxidize many substrates, especially lipids, carbohydrates, proteins, and DNA. Under normal physiological conditions, there is a balance between ROS production and endogenous antioxidant defense mechanisms. However, any disruption in this balance results in oxidative stress, which causes cellular damage. As a result, oxidative stress causes many diseases such as cancer, atherosclerosis, cardiovascular diseases, diabetes, and inflammatory disorders, as well as the diseases mentioned above. This is where antioxidants come into play, protecting the metabolism from oxidative damage by preventing these unwanted metabolic processes caused by ROS and oxidative stress [60]. From this point, antioxidants are known to neutralize ROS, which are known to cause hundreds of diseases. Phenolic compounds and their derivatives, known as secondary metabolites in plants, are the compounds with the highest antioxidant activity. It has been reported that phenolic compounds, especially flavonoids, constitute the most powerful antioxidant effects and mechanisms of medicinal plants. These are various chemicals that carry an aromatic ring and include phenolic acids, flavonoids, coumarins, tannins, and stilbenes. These components are widely and differentially distributed in the plant kingdom. However, their presence in excess in any plant increases the potential antioxidant power and other biological activities of the plant [76,77].
It was discovered that the phytochemicals in the aerial parts extract of A. orientale showed a strong antioxidant profile in a positive association with the total phenolic content, and that the extract had a high capacity to scavenge DPPH and hydroxyl radicals [2]. An evaluation of the antioxidant activity by the DPPH radical scavenging assay determined that for all extracts, the crude extract of A. officinalis had 0.141 ± 0.002 mg/mL scavenging activity, the nanofiltration retentate was more effective, and the A. officinalis nanofiltrate retentate had the highest scavenging activity (IC50: 0.0032 mg/mL), comparable with ascorbic acid used as the reference compound (IC50: 0.0036 mg/mL) [5]. With an antioxidant activity of 55.57 ± 0.45 μg/mL, the ethanolic extract of A. officinalis exhibited the highest level in the DPPH scavenging test [7]. The determination of antioxidant activity of lyophilized water extract of aerial parts of A azurea showed that IC50 (μg/mL) values for DPPH• and ABTS•+ scavenging activity were found to be 231.0 ± 0.059 and 16.500 ± 0.005, accordingly [63]. On the other hand, Allium akaka and A. kharputense indicated abundant sources of phenolics, according to the phytochemical composition and in vitro biological activities of wild-edible Allium species. The majority of phenolic chemicals were flavonols, with quercetin and its derivatives predominating. Ethanol extracts of the leaf samples from A. akaka and A. kharputense exhibited superior antioxidant activity in the ORAC experiment, which demonstrated the hydrogen atom transfer mechanism [10]. DPPH and ABTS radical scavenging tests were used to measure the antioxidant activity of ethanol extracts from the roots and aerial portions of several Allium species. The roots of A. subakaka from Hakkari showed the greatest radical scavenging activity among the others in the DPPH radical scavenging assay (IC50: 302.2 μg/mL). The Allium samples generally demonstrated poor DPPH radical scavenging activity. A helpful technique for figuring out the antioxidant activity of large, water-soluble compounds is the ABTS radical scavenging test, which performs better than the DPPH assay. The aerial parts and roots of A. subakaka (AA.H.1) from Hakkari (IC50: 83.55 μg/mL and 80.81 μg/mL) showed the greatest activity in the ABTS assay, followed by the roots of A. scabriscapum extract (ASca.V.2) from Van (IC50: 79.03 μg/mL). The A. kharputense aerial parts’ DPPH and ABTS scavenging activity IC50 values were measured as 726 ± 21 and 260 ± 10, and A. kharputense roots’ DPPH and ABTS IC50 values were found as ≥ 1000 253 ± 3.71, respectively [78]. The IC50 values for DPPH radical scavenging activity of the ethanol extracts from A. lazikkiyense were 0.54 ± 0.017 mg/mL [79]. The methanolic extract of Allium undulata was shown to have a DPPH radical scavenging activity of 2.086 mmol TEs/g extract. To measure radical scavenging activity, another often used test is the ABTS radical cation decolorization assay. The colored (blue) radical is transformed back into colorless ABTS using this approach when antioxidants are present. A Trolox equivalent value of 0.112 mmol TEs/g extract was achieved for the ABTS radical scavenging test [80]. Remarkably, diethyl ether extracts from Allium galanthum bulbs exhibited the highest level of antioxidant activity in the DPPH radical scavenging experiment, but diethyl ether extracts from A. turkestanicum bulbs had the highest level of activity in the ABTS radical scavenging assay. It was discovered that the antioxidant potential of ethanol and water extracts was lower [81]. The extract from the aerial portions of Allium ilgazense showed the maximum radical scavenging activity in DPPH (55.91 ± 0.42 μg/mL) and ABTS (11.07 ± 0.09 μg/mL), whereas the biological activity of indigenous Allium species revealed that Allium olympicum had the best ABTS•+ scavenging ability (10.19 ± 0.09 μg/mL) [82].
According to the common findings obtained as a result of the antioxidant tests, it was concluded that A. kharputense ethanol extract has a high antioxidant activity when compared to standard natural and synthetic antioxidants. Plants of the genus Allium belong to the Amaryllidaceae family and consist of at least 918 listed species worldwide. In the bulbs, flowers, stems, and leaves of these species, several secondary metabolites with numerous biological properties have been isolated and identified, including anthocyanins, flavonoids, organo-sulfur compounds, sterols, saponins, phenolic acids, amino acids, vitamins, and minerals.
From the results obtained from hCA I enzyme inhibition, the extracts showed strong hCA I enzyme inhibitory properties. According to the results obtained, hCA I inhibition potentials were determined as follows: EEAK > WEAK > EEAA > WEAA, respectively. The IC50 values obtained are given in Table 5. Among these values, only WEAA showed less enzyme inhibition potential than the standard CA I (IC50: 55.10 μg/mL), while the other extracts showed higher inhibition of hCA I. According to the findings obtained from hCA II enzyme inhibition studies, it was determined that the water extract of A. kharputense inhibited the hCA II isoenzyme with the lowest IC50 value—in other words, the highest activity. According to the data obtained, this value was determined as 81.02 μg/mL. The IC50 values of the extracts were determined as EEAK < EEAA < WEAA< WEAK, respectively. The reference inhibitor value of hCA II isoenzyme and standard are given in Table 5. Although the findings obtained from the hCA II inhibition results show that the plant extracts have an inhibitory effect on the hCA II isoenzyme, it is understood that the IC50 values determined are quite low compared to the reference inhibitor. So far, a CA isoenzyme inhibition study involving A. kharputense and A. azurea species and other species in Allium and Anchusa genera is not available in the literature. This will be the first study in the literature.
Alzheimer’s disease (AD) is a neurodegenerative disease that is more common in the elderly, clinically characterized by memory and cognitive impairment and caused by the loss of neurons, which are nerve cells, and a decrease in cholinergic systems. The cholinergic hypothesis is the only hypothesis that explains the cause of AD and is still the only hypothesis that continues to persist [83]. The cholinergic hypothesis suggests that the decrease in the amount of acetylcholine, an important neurotransmitter that increases learning and cholinergic activity in the nervous system, is the cause. Cholinesterase inhibitors are first-line drugs in the treatment of AD. The most prescribed drugs containing cholinesterase inhibitors are donepezil, rivastigmine, and galantamine. Donepezil is the most commonly prescribed drug for the treatment of cognitive symptoms of AD dementia. None of these medications affect neurodegeneration, yet they all reduce the symptoms of AD dementia. In addition, side effects such as widespread vomiting, diarrhea, dizziness, and gastrointestinal disturbances necessitate the discovery of alternatives to these drugs [84]. The use of medicinal plants as an alternative form of treatment and as a source of new medications with potential therapeutic benefits has long been acknowledged. Because plants have a vast number of biosynthetic intermediates that exhibit specificities for diverse targets, they have been the primary focus of pharmacological discovery due to the need for a multitarget treatment for AD [85]. Both main enzymes associated with AD, AChE, and BChE were also used to assess the anticholinesterase activity of Allium species. AChE and BChE inhibitory activities were either absent from all extracts or extremely weak, as the 200 μg/mL extracts showed inhibitions ranging from 5 to 20% [78]. Like another study conducted with Allium lazikkiyense species, all extracts exhibited low inhibitory effect on AChE and BChE [79]. The methanolic extract of Allium undulata was tested for cholinesterase inhibitory activity using spectrophotometric Ellman’s techniques; the findings were reported as galantamine equivalents. AChE’s and BChE’s respective findings were found to be 2.238 and 1.239 µmol GALAEs/g extract [80]. The inhibition effects of Allium proponticum IC50 values of enzymes were measured, and inhibitory activities were 258.47 ± 3.28 for AChE and 522.81 ± 1.29 for BChE [86]. Aerial parts of A. nigrum were the most effective (IC50: 6.1, 3.27 µg/mL), while samples of A. nigrum and A. subhirsutum demonstrated anti-AChE and anti-BChE actions [64]. Enzyme inhibition effects of the different Allium species’ aerial parts extracts indicated that the best results were taken from Allium goekyigiti as 1.93 ± 0.07 mg/GALAE to AChE enzyme and 0.45 ± 0.01 mg/GALAE towards BChE enzyme [65]. For both AChE (IC50: 0.335 ± 0.04 mg/mL) and BChE (IC50: 0.375 ± 0.04 mg/mL), the extract of Allium eldivanense demonstrated encouraging inhibitory effects [82]. The strongest inhibition against AChE and BChE was shown by the leaf sample of Allium stylosum, which had IC50 values of 23.95 ± 0.08 and 8.95 ± 1.24 mg/mL, respectively [87]. The methanol extract of Allium tuncelianum had an IC50 value of 11.25 μg/mL (r2: 0.9952) for AChE. The results clearly showed that methanol extracts of A. tuncelianum had effective inhibition against AChE. The ACh levels decrease with the ageing process, which results in the progression of neurological disorders, such as AD. The AChE inhibition increases the levels of Ach; thus, AChE inhibition is considered a useful therapeutic approach to treat neurological disorders like AD [88]. The content of the species may also vary and be enriched due to factors such as growth, altitude, climate, geography, humidity, temperature, and time during production.
Diabetes is one of the eight of the top ten causes of death reported by the WHO, affecting more than 400 million people worldwide. Many pharmacological and non-pharmacological strategies have been developed to combat the disease, but without the necessary success. Pharmacological approaches include the introduction of blood glucose level-lowering agents into the body and lifestyle improvements. These lowering agents include insulin, insulin-like growth factors, insulin analogs, insulin secretagogues, antihyperglycemics, insulin sensitizers, glucose reabsorption inhibitors, and α-glycosidase inhibitors [89]. α-glycosidase inhibitors (AGIs) are considered a reasonable option as first-line drugs in the treatment of patients with T2DM, as they specifically target postprandial hyperglycemia, a possible independent risk factor for cardiovascular complications [90]. AGIs utilized in therapeutic settings, such as Acarbose, Miglitol, and Voglibose, work by delaying the breakdown of complex carbs. BGL and osmotic effects are reduced because of this mode of action. Bloating, diarrhea, and stomach discomfort are some of the adverse consequences of ingested disaccharides [22]. Therefore, research is ongoing on new AGIs with fewer side effects. The inhibitory activities of the A. kharputense ethanol extracts against α-glycosidase were recorded as 0.6 ± 0.1 mg/mL [10]. The α-glycosidase inhibitory effects of crude and concentrated extracts of Anchusa officinalis were found to be 151.76 ± 4.30 and 99.15 ± 2.81, respectively [5]. The IC50 values for α-glycosidase inhibitory effects of the extracts from A. lazikkiyense were 0.98 ± 0.04 mg/mL [79]. The extract of Allium eldivanense exhibited a promising inhibitory effect against α-glycosidase (IC50: 0.148 ± 0.01 mg/mL) enzyme [83]. In another study, the methanol extract of A. tuncelianum exhibited IC50 values of 9.85 µM (r2: 0.9577) for α-glycosidase. The obtained results showed that the methanol extract of A. tuncelianum had a high affinity for α-glycosidase enzyme [88]. According to the results obtained in this study, A. kharputanse species has a higher α-glycosidase inhibition activity than different species of the Allium genus.
Based on the findings obtained, it was determined that the ethanol and water extracts of A. kharputense showed a higher inhibitory property on hCA I isoenzyme and α-glycosidase enzymes than standard inhibitors. In addition, ethanol and water extracts of A. azurea also showed a better inhibitory profile on hCA I isoenzyme with a better IC50 value than the standard inhibitor.
5. Conclusions
A. kharputense and A. azurea are edible plants, traditionally. The results of LC-MC/MS analysis gave results parallel to total phenol and flavonoid analyses; 17 metabolites were detected in A. kharputense plant ethanol extract, 14 metabolites in A. kharputense plant water extract, 4 metabolites in A. azurea plant ethanol extract, and 2 metabolites in A. azurea plant water extract. Seventeen phenolics and flavonoids and their quantities recorded in the ethanol extract of A. kharputense species were Astragalin, isoquercitrin, kaempferol, quercetin, quinic acid, p-coumaric acid, fumaric acid, and protocatechuic acid, respectively, in this herb. Isoquercitrin and Astragalin, as secondary metabolites, were detected at microgram levels as the two dominant metabolites in both ethanol and water extracts of A. kharputense. Both metabolites are important indicators that these two extracts have strong antioxidant properties.
From the ethanol and water extraction yield of these plants, whose biological activity was evaluated and compared, it was determined that A. kharputense extracts were extracted with higher yields by both methods. According to the determination of total phenolic and flavonoid contents, the richest extract content was obtained from the ethanol extract of A. kharputense. When compared in terms of free radical scavenging capacity, it was found that the ethanol extract of A. kharputense was superior to other extracts in both radical scavenging tests (DPPH and ABTS). In addition, this extract was found to have higher DPPH free radical scavenging potential than the standard antioxidants Trolox and BHA. When the reducing capacities were compared, it was determined that the ethanol extract of A. kharputense was a stronger reductant compared to all other extracts in all three tests. It was shown by enzyme inhibition tests that all the obtained extracts showed antidiabetic, anticholinergic, antiepileptic, and antiglaucoma properties. In addition, the ethanol and water extracts of A. kharputense showed higher inhibitory properties on hCA I and α-glycosidase enzymes than standard inhibitors. In addition, ethanol and water extracts of A. azurea also showed a better inhibition property on hCA I isoenzyme, with a better IC50 value than the standard inhibitor.
As a result, A. kharputense and A. azurea plants, which are widely consumed as food by the local people within the scope of this research, were found to have similar antioxidant, antidiabetic, anticholinergic, and antiglaucoma effects by in vitro bioanalytical tests and their phytochemical contents. The determination of the use of extracts and combinations of compounds obtained from plants that have been traditionally consumed as food and medicine for many years among the people at pharmacological doses will be a sustainable, ecofriendly approach when the effectiveness, safety, suitability, and cost calculation is made in the prevention and non-severe, stable course and recovery of the specified common diseases.
Author Contributions
Conceptualization, V.T., H.K., K.A. and İ.G.; methodology and investigation, V.T., H.K., K.A. and İ.G.; software, validation, and visualization, V.T., H.K., K.A. and İ.G.; data curation, writing—original draft preparation, writing—review and editing, supervision, funding, and acquisition, V.T., H.K., K.A. and İ.G. All authors have read and agreed to the published version of the manuscript.
Funding
Scientific Research Projects Coordination at Şırnak University (2024.FNAP.18.03.01) provided funding for this study. İlhami Gulcin is a member of the Turkish Academy of Sciences (TÜBA). He would like to extend his sincere appreciation to the TÜBA for their financial support.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are publicly available in an accessible repository.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Kiran, Y.; Arikboga, G.; Dogan, G.; Eroğlu, H.; Pınar, S.M. Karyomorphological analysis of eight Allium L. (Amaryllidaceae) species from Turkey. Cytologia 2022, 87, 271–275. [Google Scholar] [CrossRef]
- Ceylan, O.; Alic, H. Antibiofilm, antioxidant, antimutagenic activities and phenolic compounds of Allium orientale BOISS. Brazilian Arch. Biol. Technol. 2015, 58, 935–943. [Google Scholar] [CrossRef]
- Kilic-Buyukkurt, O.; Kelebek, H.; Bordiga, M.; Keskin, M.; Selli, S. Changes in the aroma and key odorants from white garlic to black garlic using approaches of molecular sensory science: A review. Heliyon 2023, 9, e19056. [Google Scholar] [CrossRef] [PubMed]
- Aysu, T.; Durak, H. Catalytic effects of borax and iron (III) chloride on supercritical liquefaction of Anchusa azurea with methanol and isopropanol. Energy Sources Part A Recover. Util. Environ. Eff. 2016, 38, 1739–1749. [Google Scholar] [CrossRef]
- Paun, G.; Neagu, E.; Albu, C.; Savin, S.; Radu, G.L. In vitro evaluation of antidiabetic and anti-inflammatory activities of polyphenolic-rich extracts from Anchusa officinalis and Melilotus officinalis. ACS Omega 2020, 5, 13014–13022. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, Y.; Song, J.; Wang, R.; Gao, L.; Zhang, L.; Fang, L.; Lu, Y.; Du, G. Total flavonoids from Anchusa italica Retz. improve cardiac function and attenuate cardiac remodeling post myocardial infarction in mice. J. Ethnopharmacol. 2020, 257, 112887. [Google Scholar] [CrossRef]
- Boskovic, I.; Đukić, D.A.; Maskovic, P.; Mandić, L.; Perovic, S. Phytochemical composition and antimicrobial, antioxidant and cytotoxic activities of Anchusa officinalis L. extracts. Biologia. 2018, 73, 1035–1041. [Google Scholar] [CrossRef]
- Karaaslan, Ö.; Çöteli, E.; Karataş, F. Kenger (Gundelia tournefortii) bitkisindeki A, E, C Vitaminleri ile malondialdehit ve glutatyon miktarlarının araştırılması. EÜFBED-Fen Bilim. Enstitüsü Derg. 2014, 7, 159–168. [Google Scholar]
- Petreska Stanoeva, J.; Stefova, M.; Matevski, V. Extraction, Distribution and diversity of phenolic compounds in most widespread boraginaceae species from Macedonia. Chem. Biodivers. 2023, 20, e202201149. [Google Scholar] [CrossRef]
- Mukemre, M. Wild-edible allium species from highlands of eastern anatolia: Phytochemical composition and in vitro biological activities. Plants 2024, 13, 1949. [Google Scholar] [CrossRef]
- Sirri, M.; Özaslan, C.; Fidan, M. Eruh (Siirt) ilçesinde gıda ve halk tababetinde kullanılan bazı doğal ve yabancı otlar 1. MAS J. Appl. Sci. 2021, 6, 1118–1129. [Google Scholar] [CrossRef]
- Kardaş, C. U sage of savage plants in the folk medicine in Muş. Lokman Hekim J. 2019, 9, 85–96. [Google Scholar] [CrossRef]
- Martínez Medina, J.J.; Naso, L.G.; Pérez, A.L.; Rizzi, A.; Ferrer, E.G.; Williams, P.A.M. Antioxidant and anticancer effects and bioavailability studies of the flavonoid baicalin and its oxidovanadium (IV) complex. J. Inorg. Biochem. 2017, 166, 150–161. [Google Scholar] [CrossRef]
- Gülçin, İ.; Gören, A.C.; Taslimi, P.; Alwasel, S.H.; Kılıc, O.; Bursal, E. Anticholinergic, antidiabetic and antioxidant activities of anatolian pennyroyal (Mentha pulegium)-Analysis of its polyphenol contents by LC-MS/MS. Biocatal. Agric. Biotechnol. 2020, 23, 101441. [Google Scholar] [CrossRef]
- Atalar, M.N.; Köktürk, M.; Altındağ, F.; Ozhan, G.; Özen, T.; Demirtas, İ.; Gülçin, İ. LC-ESI-MS/MS Analysis of secondary metabolites of different St. John’s Wort (Hypericum perforatum) extracts used as food supplements and evaluation of developmental toxicity on zebrafish (Danio rerio) embryos and larvae. S. Afr. J. Bot. 2023, 159, 580–587. [Google Scholar] [CrossRef]
- Li, K.; Fan, H.; Yin, P.; Yang, L.; Xue, Q.; Li, X.; Sun, L.; Liu, Y. Structure-activity relationship of eight high content flavonoids analyzed with a preliminary assign-score method and their contribution to antioxidant ability of flavonoids-rich extract from Scutellaria baicalensis shoots. Arab. J. Chem. 2018, 11, 159–170. [Google Scholar] [CrossRef]
- Mota, J.C.; Almeida, P.P.; Freitas, M.Q.; Stockler-Pinto, M.B.; Guimarães, J.T. Far from being a simple question: The complexity between in vitro and in vivo responses from nutrients and bioactive compounds with antioxidant potential. Food Chem. 2023, 402, 134351. [Google Scholar] [CrossRef]
- Hatamnia, A.A.; Abbaspour, N.; Darvishzadeh, R. Antioxidant activity and phenolic profile of different parts of bene (Pistacia atlantica Subsp. Kurdica) fruits. Food Chem. 2014, 145, 306–311. [Google Scholar] [CrossRef]
- Bingol, Z.; Kızıltaş, H.; Gören, A.C.; Kose, L.P.; Topal, M.; Durmaz, L.; Alwasel, S.H.; Gulcin, İ. Antidiabetic, anticholinergic and antioxidant activities of aerial parts of shaggy bindweed (Convulvulus betonicifolia Miller Subsp.)-Profiling of phenolic compounds by LC-HRMS. Heliyon 2021, 7, e06986. [Google Scholar] [CrossRef]
- Bordoloi, S.; Pathak, K.; Devi, M.; Saikia, R.; Das, J.; Kashyap, V.H.; Das, D.; Ahmad, M.Z.; Abdel-Wahab, B.A. Some promising medicinal plants used in Alzheimer’s disease: An ethnopharmacological perspective. Discov. Appl. Sci. 2024, 6, 215. [Google Scholar] [CrossRef]
- Gülçin, İ.; Trofimov, B.; Kaya, R.; Taslimi, P.; Sobenina, L.; Schmidt, E.; Petrova, O.; Malysheva, S.; Gusarova, N.; Farzaliyev, V.; et al. Synthesis of nitrogen, phosphorus, selenium and sulfur-containing heterocyclic compounds—Determination of their carbonic anhydrase, acetylcholinesterase, butyrylcholinesterase and α-glycosidase inhibition properties. Bioorg. Chem. 2020, 103, 104171. [Google Scholar] [CrossRef]
- Durmaz, L.; Karagecili, H.; Gulcin, İ. Evaluation of carbonic anhydrase, acetylcholinesterase, butyrylcholinesterase, and α-glycosidase inhibition effects and antioxidant activity of baicalin hydrate. Life 2023, 13, 2136. [Google Scholar] [CrossRef] [PubMed]
- Kashtoh, H.; Baek, K.H. Recent updates on phytoconstituent alpha-glucosidase inhibitors: An approach towards the treatment of type two diabetes. Plants 2022, 11, 2722. [Google Scholar] [CrossRef] [PubMed]
- Hisar, O.; Beydemir, S.; Gülçin, I.; Küfrevioglu, O.I.; Supuran, C.T. Effects of low molecular weight plasma inhibitors of rainbow trout (Oncorhynchus mykiss) on human erythrocyte carbonic anhydrase-II isozyme activity in vitro and rat erythrocytes in vivo. J. Enzyme Inhib. Med. Chem. 2005, 20, 35–39. [Google Scholar] [CrossRef]
- Karagecili, H.; İzol, E.; Kirecci, E.; Gulcin, İ. Determination of antioxidant, anti-alzheimer, antidiabetic, antiglaucoma and antimicrobial effects of Zivzik pomegranate (Punica granatum)—A chemical profiling by LC-MS/MS). Life 2023, 13, 735. [Google Scholar] [CrossRef]
- Aslan, K.; Kiziltas, H.; Guven, L.; Karagecili, H.; Arslan, D.; Gulcin, İ. Enzyme inhibition property of different daisies from Asteraceae family; Calendula officinalis, Matricaria chamomilla, and Anthemis pseudocotula: Kinetics and molecular docking studies, Rec. Nat. Prod. 2025, 19, 247–262. [Google Scholar] [CrossRef]
- Guan, R.; Van Le, Q.; Yang, H.; Zhang, D.; Gu, H.; Yang, Y.; Sonne, C.; Lam, S.S.; Zhong, J.; Jianguang, Z.; et al. A review of dietary phytochemicals and their relation to oxidative stress and human diseases. Chemosphere 2021, 271, 129499. [Google Scholar] [CrossRef]
- Gulcin, I.; Tel, A.Z.; Kirecci, E. Antioxidant, antimicrobial, antifungal, and antiradical activities of Cyclotrichium niveum (BOISS.) Manden and Scheng. Int. J. Food Prop. 2008, 11, 450–471. [Google Scholar] [CrossRef]
- Karageçili, H.; Polat, T.; Yılmaz, M.A.; Fidan, M.; Karaismailoğlu, M.C.; Gülçin, İ. Evaluation of the antioxidant, antidiabetic and anti-Alzheimer effects of Capsella bursa-pastoris-Polyphenolic profiling by LC-MS/MS. Rec. Nat. Prod. 2024, 18, 643–662. [Google Scholar] [CrossRef]
- Aslan, K.; Kopar, E.E.; Kelle, K.; Karageçili, H.; Yilmaz, M.A.; Cakir, O.; Alwasel, S.; Gulcin, I. Phytochemical profile and bioactive properties of sage (Salvia fruticosa) and thyme (Thymus vulgaris) extracts. Int. J. Food Prop. 2025, 28, 2481148. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Gülçin, I.; Topal, F.; Çakmakçi, R.; Bilsel, M.; Gören, A.C.; Erdogan, U. Pomological features, nutritional quality, polyphenol content analysis, and antioxidant properties of domesticated and 3 wild ecotype forms of raspberries (Rubus idaeus L.). J. Food Sci. 2011, 76, 585–593. [Google Scholar] [CrossRef]
- Gülçin, I.; Bursal, E.; Şehitoĝlu, M.H.; Bilsel, M.; Gören, A.C. Polyphenol contents and antioxidant activity of lyophilized aqueous extract of propolis from Erzurum, Turkey. Food Chem. Toxicol. 2010, 48, 2227–2238. [Google Scholar] [CrossRef]
- Yilmaz, M.A. Simultaneous quantitative screening of 53 phytochemicals in 33 species of medicinal and aromatic plants: A detailed, robust and comprehensive LC–MS/MS method validation. Ind. Crops Prod. 2020, 149, 112347. [Google Scholar] [CrossRef]
- Karagecili, H.; Yılmaz, M.A.; Ertürk, A.; Kiziltas, H.; Güven, L.; Alwasel, S.H.; Gulcin, İ. Comprehensive metabolite profiling of berdav propolis using LC-MS/MS: Determination of antioxidant, anticholinergic, antiglaucoma, and antidiabetic effects. Molecules 2023, 28, 1739. [Google Scholar] [CrossRef] [PubMed]
- Tohma, H.; Gülçin, İ.; Bursal, E.; Gören, A.C.; Alwasel, S.H.; Köksal, E. Antioxidant activity and phenolic compounds of ginger (Zingiber officinale Rosc.) determined by HPLC-MS/MS. J. Food Meas. Charact. 2017, 11, 556–566. [Google Scholar] [CrossRef]
- Apak, R.; Calokerinos, A.; Gorinstein, S.; Segundo, M.A.; Hibbert, D.B.; Gülçin, İ.; Demirci Çekiç, S.; Güçlü, K.; Özyürek, M.; Esin Çelik, S.; et al. Methods to evaluate the scavenging activity of antioxidants toward reactive oxygen and nitrogen species. Pure Appl. Chem. 2022, 94, 87–144. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “Antioxidant Power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Blois, M.S. Antioxidant determinations by the use of a stable free radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Gülçin, I. Antioxidant activity of caffeic acid (3,4-dihydroxycinnamic acid). Toxicology 2006, 217, 213–220. [Google Scholar] [CrossRef]
- Karageçili, H.; Izol, E.; Kireçci, E.; Gülçin, I. Antioxidant, antidiabetic, antiglaucoma, and anticholinergic effects of Tayfi grape (Vitis vinifera): A phytochemical screening by LC-MS/MS analysis. Open Chem. 2023, 21, 20230120. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Šavikin, K.; Živković, J.; Alimpić, A.; Zdunić, G.; Janković, T.; Duletić-Laušević, S.; Menković, N. Activity guided fractionation of pomegranate extract and its antioxidant, antidiabetic and antineurodegenerative properties. Ind. Crops Prod. 2018, 113, 142–149. [Google Scholar] [CrossRef]
- Tao, Y.; Zhang, Y.; Cheng, Y.; Wang, Y. Rapid screening and identification of α-glucosidase inhibitors from mulberry leaves using enzyme-immobilized magnetic beads coupled with HPLC/MS and NMR. Biomed. Chromatogr. 2013, 27, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Durmaz, L.; Karageçili, H.; Erturk, A.; Ozden, E.M.; Taslimi, P.; Alwasel, S.; Gülçin, İ. Hamamelitannin’s antioxidant effect and its inhibition capability on α-glycosidase, carbonic anhydrase, acetylcholinesterase, and butyrylcholinesterase enzymes. Processes 2024, 12, 2341. [Google Scholar] [CrossRef]
- Durmaz, L.; Kiziltas, H.; Karagecili, H.; Alwasel, S.; Gulcin, İ. Potential antioxidant, anticholinergic, antidiabetic and antiglaucoma activities and molecular docking of spiraeoside as a secondary metabolite of onion (Allium cepa). Saudi Pharm. J. 2023, 31, 101760. [Google Scholar] [CrossRef]
- Burmaoglu, S.; Kazancioglu, E.A.; Kazancioglu, M.Z.; Sağlamtaş, R.; Yalcin, G.; Gulcin, I.; Algul, O. Synthesis, molecular docking and some metabolic enzyme inhibition properties of biphenyl-substituted chalcone derivatives. J. Mol. Struct. 2022, 1254, 132358. [Google Scholar] [CrossRef]
- Ozden, E.M.; Bingol, Z.; Mutlu, M.; Karagecili, H.; Köksal, E.; Goren, A.C.; Alwasel, S.H.; Gulcin, İ. Antioxidant, antiglaucoma, anticholinergic, and antidiabetic effects of kiwifruit (Actinidia deliciosa) oil: Metabolite profile analysis using LC-HR/MS, GC/MS and GC-FID. Life 2023, 13, 1939. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 67, 248–254. [Google Scholar] [CrossRef]
- Akbaba, Y.; Akincioglu, A.; Göçer, H.; Göksu, S.; Gülçin, I.; Supuran, C.T. Carbonic anhydrase inhibitory properties of novel sulfonamide derivatives of aminoindanes and aminotetralins. J. Enzyme Inhib. Med. Chem. 2014, 29, 35–42. [Google Scholar] [CrossRef]
- Tugrak, M.; Gul, H.I.; Bandow, K.; Sakagami, H.; Gulcin, I.; Ozkay, Y.; Supuran, C.T. Synthesis and biological evaluation of some new mono mannich bases with piperazines as possible anticancer agents and carbonic anhydrase inhibitors. Bioorg. Chem. 2019, 90, 103095. [Google Scholar] [CrossRef]
- Çetinkaya, Y.; Göçer, H.; Menzek, A.; Gülçin, I. Synthesis and antioxidant properties of (3,4-dihydroxyphenyl)(2,3,4-trihydroxyphenyl)methanone and its derivatives. Arch. Pharm. 2012, 345, 323–334. [Google Scholar] [CrossRef]
- Gulcin, İ.; Alwasel, S.H. Fe3+ reducing power as the most common assay for understanding the biological functions of antioxidants. Processes 2025, 13, 1296. [Google Scholar] [CrossRef]
- Kumar, N.; Goel, N. Phenolic acids: Natural versatile molecules with promising therapeutic applications. Biotechnol. Rep. 2019, 24, e00370. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.K.; Singh, A.P. In vitro antioxidant and free radical scavenging activity of Nardostachys jatamansi DC. JAMS J. Acupunct. Meridian Stud. 2012, 5, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Taslimi, P.; Köksal, E.; Gören, A.C.; Bursal, E.; Aras, A.; Kılıç, Ö.; Alwasel, S.; Gülçin, İ. Anti-Alzheimer, antidiabetic and antioxidant potential of Satureja cuneifolia and analysis of its phenolic contents by LC-MS/MS. Arab. J. Chem. 2020, 13, 4528–4537. [Google Scholar] [CrossRef]
- Kalın, P.; Gülçin, İ.; Gören, A.C. Antioxidant activity and polyphenol content of cranberries (Vaccinium macrocarpon). Rec. Nat. Prod. 2015, 9, 496–502. [Google Scholar]
- Eruygur, N.; Koçyiğit, U.M.; Taslimi, P.; Ataş, M.; Tekin, M.; Gülçin, I. Screening the in vitro antioxidant, antimicrobial, anticholinesterase, antidiabetic activities of endemic Achillea cucullata (Asteraceae) ethanol extract. S. Afr. J. Bot. 2019, 120, 141–145. [Google Scholar] [CrossRef]
- Artunc, T.; Menzek, A.; Taslimi, P.; Gulcin, I.; Kazaz, C.; Sahin, E. Synthesis and antioxidant activities of phenol derivatives from 1,6-bis(dimethoxyphenyl)hexane-1,6-dione. Bioorg. Chem. 2020, 100, 103884. [Google Scholar] [CrossRef]
- Gulcin, İ. Antioxidants-A comprehensive review. Arch. Toxicol. 2025, 99, 1893–1997. [Google Scholar] [CrossRef]
- Gulcin, İ. Antioxidants and antioxidant methods-An updated overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef]
- Ozkan, G.; Sakarya, F.B.; Tas, D.; Yurt, B.; Ercisli, S.; Capanoglu, E. Effect of in vitro digestion on the phenolic content of herbs collected from Eastern Anatolia. ACS Omega 2023, 8, 12730–12738. [Google Scholar] [CrossRef]
- Kiziltaş, H. Antioxidant activity of lyophilized water extract of aerial parts of Italian bugloss (Anchusa azurea Mill.). J. Agric. Nat. 2022, 25, 1225–1233. [Google Scholar] [CrossRef]
- Emir, A.; Emir, C.; Yıldırım, H. Characterization of phenolic profile by LC-ESI-MS/MS and enzyme inhibitory activities of two wild edible garlic: Allium nigrum L. and Allium subhirsutum L. J. Food Biochem. 2020, 44, e13165. [Google Scholar] [CrossRef] [PubMed]
- Rocchetti, G.; Zhang, L.; Bocchi, S.; Giuberti, G.; Ak, G.; Elbasan, F.; Yıldıztugay, E.; Ceylan, R.; Picot-Allain, M.C.N.; Mahomoodally, M.F.; et al. The functional potential of nine allium species related to their untargeted phytochemical characterization, antioxidant capacity and enzyme inhibitory ability. Food Chem. 2022, 368, 130782. [Google Scholar] [CrossRef] [PubMed]
- Riaz, A.; Rasul, A.; Hussain, G.; Zahoor, M.K.; Jabeen, F.; Subhani, Z.; Younis, T.; Ali, M.; Sarfraz, I.; Selamoglu, Z. Astragalin: A bioactive phytochemical with potential therapeutic activities. Adv. Pharmacol. Sci. 2018, 2018, 9794625. [Google Scholar] [CrossRef] [PubMed]
- Araujo-Padilla, X.; Ramón-Gallegos, E.; Díaz-Cedillo, F.; Silva-Torres, R. Astragalin identification in graviola pericarp indicates a possible participation in the anticancer activity of pericarp crude extracts: In vitro and in silico approaches. Arab. J. Chem. 2022, 15, 103720. [Google Scholar] [CrossRef]
- Buckner, C.A.; Lafrenie, R.M.; Dénommée, J.A.; Caswell, J.M.; Want, D.A.; Gan, G.G.; Leong, Y.C.; Bee, P.C.; Chin, E.; Teh, A.K.H.; et al. We are intechopen, the world’s leading publisher of open access books built by scientists, for scientists TOP 1%. Intech 2016, 11, 13. [Google Scholar]
- Ullah, A.; Munir, S.; Badshah, S.L.; Khan, N.; Ghani, L.; Poulson, B.G.; Emwas, A.H.; Jaremko, M. Important flavonoids and their role as a therapeutic agent. Molecules 2020, 25, 5243. [Google Scholar] [CrossRef]
- Sargin, S.A. Plants used against obesity in Turkish folk medicine: A review. J. Ethnopharmacol. 2021, 270, 113841. [Google Scholar] [CrossRef]
- Shabbir, U.; Rubab, M.; Daliri, E.B.M.; Chelliah, R.; Javed, A.; Oh, D.H. Curcumin, quercetin, catechins and metabolic diseases: The role of gut microbiota. Nutrients 2021, 13, 206. [Google Scholar] [CrossRef]
- Samimi, S.; Ardestani, M.S.; Dorkoosh, F.A. Preparation of carbon quantum dots- quinic acid for drug delivery of gemcitabine to breast cancer cells. J. Drug Deliv. Sci. Technol. 2021, 61, 102287. [Google Scholar] [CrossRef]
- Mani, A.; Kushwaha, K.; Khurana, N.; Gupta, J. P-Coumaric acid attenuates high-fat diet-induced oxidative stress and nephropathy in diabetic rats. J. Anim. Physiol. Anim. Nutr. 2022, 106, 872–880. [Google Scholar] [CrossRef] [PubMed]
- Xaviera, A.; Saleem, A.; Akhtar, M.F.; Alshammari, A.; Albekairi, N.A. Fumaric acid per Se and in combination with methotrexate arrests inflammation via moderating inflammatory and oxidative stress biomarkers in arthritic rats. Immunopharmacol. Immunotoxicol. 2024, 46, 793–804. [Google Scholar] [CrossRef] [PubMed]
- Adedara, I.A.; Fasina, O.B.; Ayeni, M.F.; Ajayi, O.M.; Farombi, E.O. Protocatechuic acid ameliorates neurobehavioral deficits via suppression of oxidative damage, inflammation, caspase-3 and acetylcholinesterase activities in diabetic rats. Food Chem. Toxicol. 2019, 125, 170–181. [Google Scholar] [CrossRef]
- Hamid, A.A.; Aiyelaagbe, O.; Usman, L.A.; Oloduowo Ameen, M. Antioxidants: Its medicinal and pharmacological applications composition and bioactivities of essential oils view project. Afr. J. Pure Appl. 2010, 4, 142–151. [Google Scholar]
- Augustyniak, A.; Bartosz, G.; Čipak, A.; Duburs, G.; Horáková, L.; Łuczaj, W.; Majekova, M.; Odysseos, A.D.; Rackova, L.; Skrzydlewska, E.; et al. Natural and synthetic antioxidants: An updated overview. Free Radical Res. 2010, 44, 1216–1262. [Google Scholar] [CrossRef]
- Izol, E.; Temel, H.; Yilmaz, M.A.; Yener, I.; Olmez, O.T.; Kaplaner, E.; Fırat, M.; Hasimi, N.; Ozturk, M.; Ertas, A. A Detailed chemical and biological investigation of twelve allium species from eastern anatolia with chemometric studies. Chem. Biodivers. 2021, 18, e2000560. [Google Scholar] [CrossRef]
- Hasbal-Celikok, G.; Azami, F.T.; Celikok, Y.; Duranay, S.; Kocyigit, M.; Yilmaz-Ozden, T. Determination of the biological activities of endemic allium lazikkiyense and its phytochemical profile by LC-MS/MS analysis. Food Chem. 2025, 464, 141930. [Google Scholar] [CrossRef]
- Sarikurkcu, C.; Zengin, G.; Aktumsek, A.; Ceylan, O.; Uysal, S. Screening of possible in vitro neuroprotective, skin care, antihyperglycemic, and antioxidative effects of Anchusa undulata L. Subsp. Hybrida (Ten.) coutinho from Turkey and its fatty acid profile. Int. J. Food Prop. 2015, 18, 1491–1504. [Google Scholar] [CrossRef]
- Kadyrbayeva, G.; Zagórska, J.; Grzegorczyk, A.; Gaweł-Bęben, K.; Strzępek-Gomółka, M.; Ludwiczuk, A.; Czech, K.; Kumar, M.; Koch, W.; Malm, A.; et al. The phenolic compounds profile and cosmeceutical significance of two kazakh species of onions: Allium galanthum and a. Turkestanicum. Molecules 2021, 26, 5491. [Google Scholar] [CrossRef]
- Özel, H.B.; Baş Topcu, K.S.; Dere, S.; Genç, N.; Kisa, D. In vitro and in silico based assessment of biological activity of endemic allium species: LC-MS/MS analysis of onions. Food Biosci. 2024, 59, 104209. [Google Scholar] [CrossRef]
- Durmaz, L.; Erturk, A.; Akyüz, M.; Kose, L.P.; Uc, E.M.; Bingol, Z.; Saglamtas, R.; Alwasel, S.; Gulcin, İ. Screening of carbonic anhydrase, acetylcholinesterase, butyrylcholinesterase, and α-glycosidase enzyme inhibition effects and antioxidant activity of coumestrol. Molecules 2022, 27, 3091. [Google Scholar] [CrossRef] [PubMed]
- Imtaiyaz Hassan, M.; Shajee, B.; Waheed, A.; Ahmad, F.; Sly, W.S. Structure, function and applications of carbonic anhydrase isozymes. Bioorg. Med. Chem. 2013, 21, 1570–1582. [Google Scholar] [CrossRef]
- Kundo, N.K.; Manik, M.I.N.; Biswas, K.; Khatun, R.; Al-Amin, M.Y.; Alam, A.H.M.K.; Tanaka, T.; Sadik, G. Identification of polyphenolics from Loranthus globosus as Potential inhibitors of cholinesterase and oxidative stress for Alzheimer’s disease treatment. Biomed Res. Int. 2021, 2021, 9154406. [Google Scholar] [CrossRef]
- Emir, C.; Emir, A. Chemical analysis and enzyme inhibitory activities of essential oil obtained from Allium proponticum Subsp. Proponticum, an endemic species. J. Res. Pharm. 2022, 26, 574–580. [Google Scholar] [CrossRef]
- Emir, C.; Emir, A. Phytochemical analyses with LC-MS/MS and in vitro enzyme inhibitory activities of an endemic species “Allium stylosum O. Schwarz” (Amaryllidaceae). S. Afr. J. Bot. 2021, 136, 70–75. [Google Scholar] [CrossRef]
- Takim, K.; Yigin, A.; Koyuncu, I.; Kaya, R.; Gülçin, İ. Anticancer, anticholinesterase and antidiabetic activities ofTunceli garlic (Allium tuncelianum): Determining its phytochemical content by LC–MS/MS analysis. J. Food Meas. Charact. 2021, 15, 3323–3335. [Google Scholar] [CrossRef]
- Khursheed, R.; Singh, S.K.; Wadhwa, S.; Kapoor, B.; Gulati, M.; Kumar, R.; Ramanunny, A.K.; Awasthi, A.; Dua, K. Treatment strategies against diabetes: Success so far and challenges ahead. Eur. J. Pharmacol. 2019, 862, 172625. [Google Scholar] [CrossRef]
- van de Laar, F.A. Alpha-glucosidase inhibitors in the early treatment of type 2 diabetes. Vasc. Health Risk Manag. 2008, 4, 1189–1195. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).