Disrupting Defenses: Effects of Bisphenol A and Its Analogs on Human Antibody Production In Vitro
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Cells
2.3. Determination of the Range of Concentrations
2.4. Cell Treatment
2.5. Cell Viability Analysis
2.6. Ig Detection
2.7. Fate and Distribution In Vitro and Kinetic Models
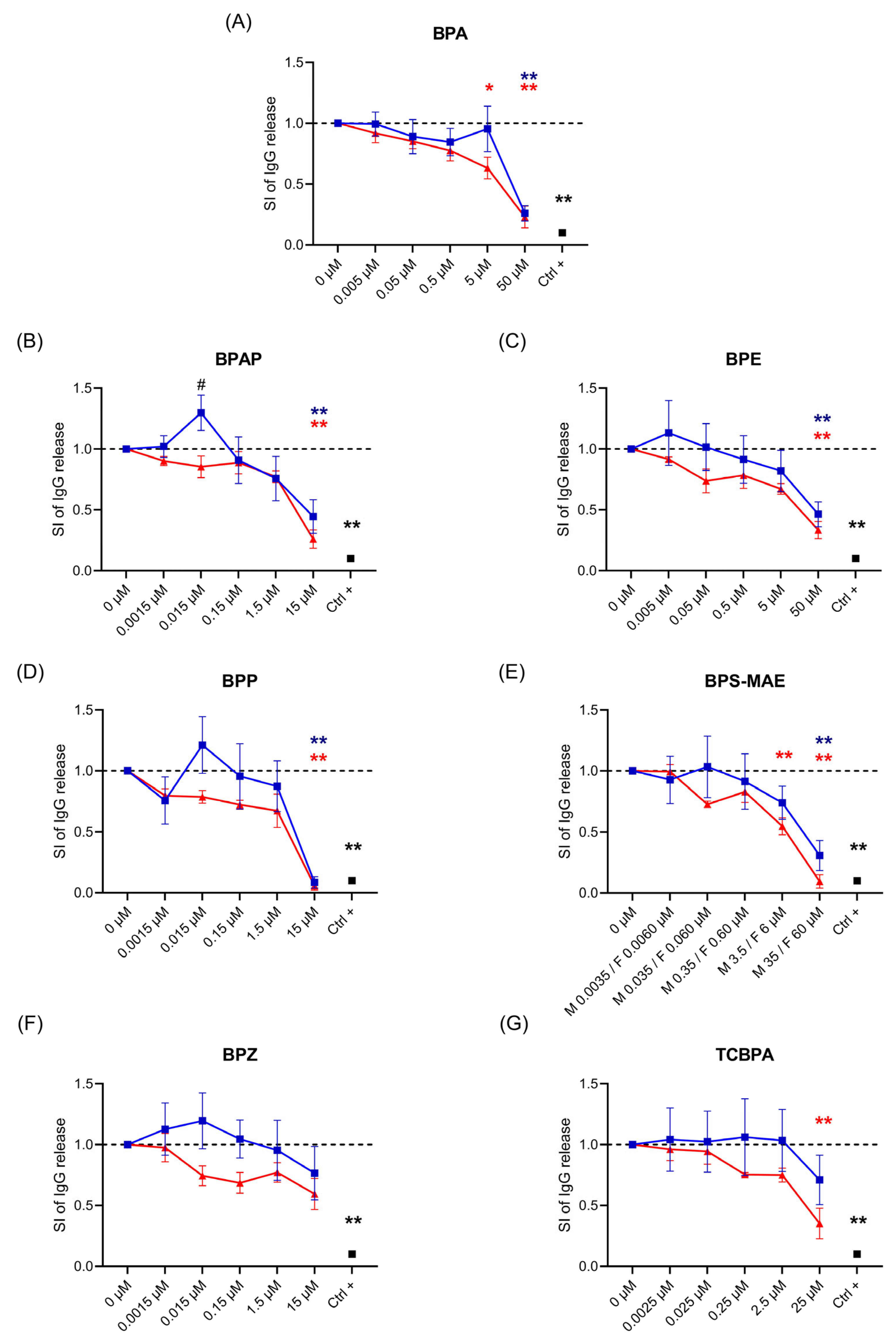
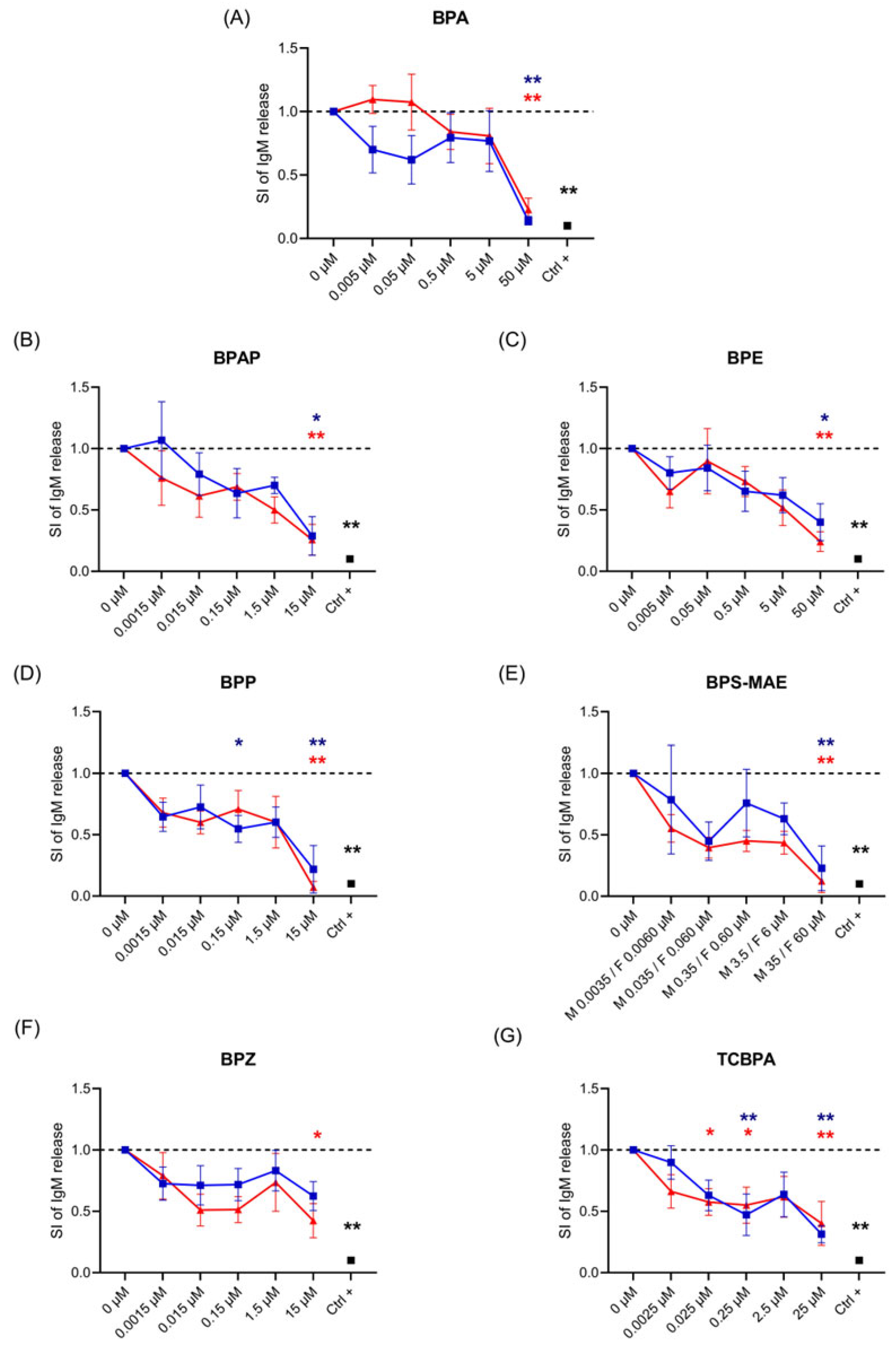
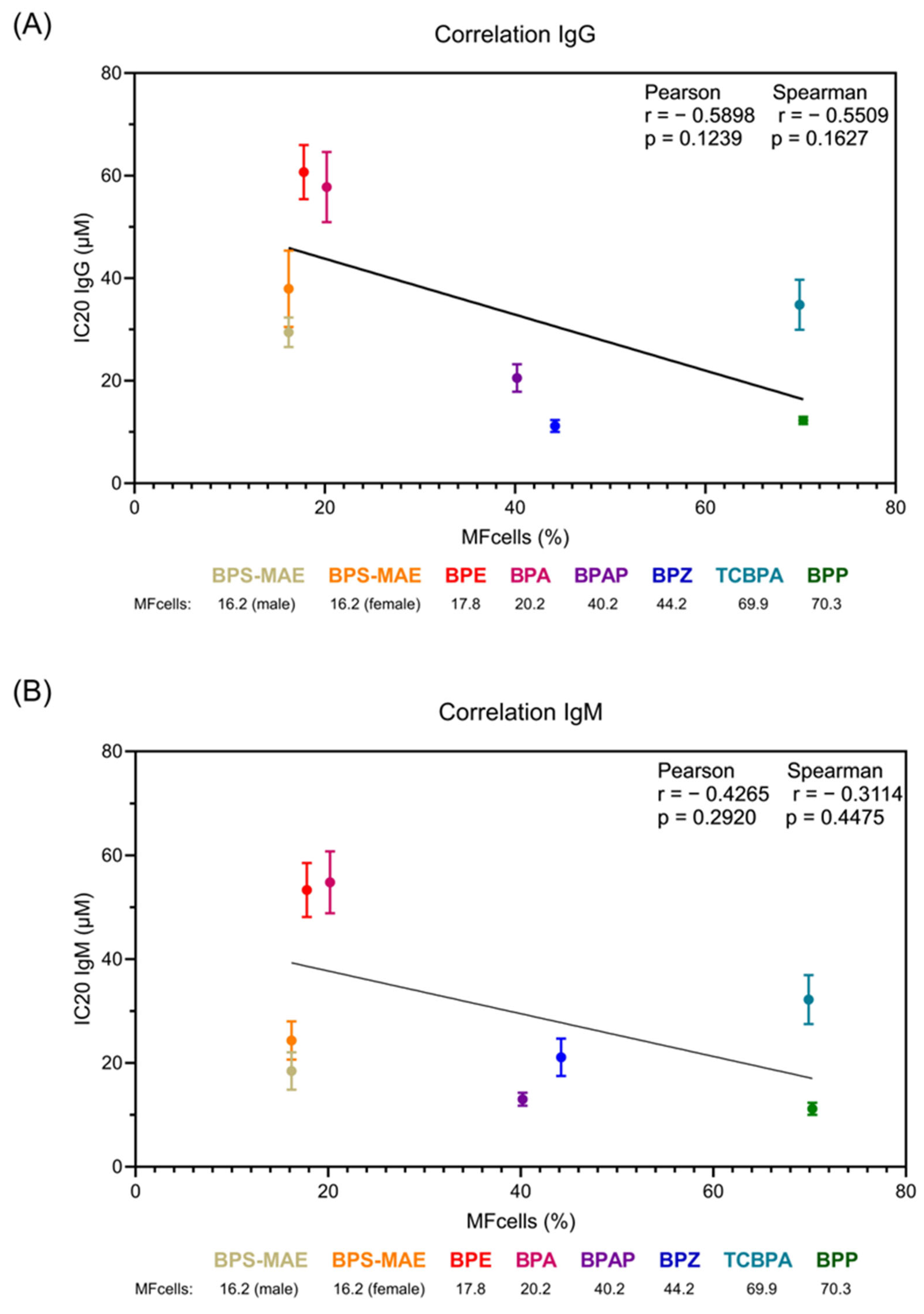
3. Results
3.1. Effects of BPA and BPA Analogs on Ig Release
3.2. In Vitro Distribution Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AhR | Aryl hydrocarbon receptor |
| AP buffer | Alkaline phosphatase buffer |
| AR | Androgen receptor |
| BPA | Bisphenol A |
| BPAP | Bisphenol AP |
| BPE | Bisphenol E |
| BPF | Bisphenol F |
| BPP | Bisphenol P |
| BPS | Bisphenol S |
| BPS-MAE | Bisphenol S 4-allyl ether |
| BPZ | Bisphenol Z |
| Bw | Body weight |
| Ctrl + | Positive control |
| CV | Cell viability |
| d-FBS | Dialyzed fetal bovine serum |
| DCs | Dendritic cells |
| DMSO | Dimethyl sulfoxide |
| EDs | Endocrine disruptors |
| ER | Estrogen receptor |
| EFSA | European Food Safety Authority |
| HBsAg | Hepatitis B surface antigen |
| HBV | Hepatits B virus |
| Ig | Immunoglobulin |
| IL | Interleukin |
| IV-MBM EQP v2.0 | In Vitro Mass Balance Equilibrium Partitioning Model version 2.0 |
| LDH | Lactate dehydrogenase |
| MDDC | Monocyte-derived dendritic cells |
| MW | Molecular weight |
| NAMs | New approach methodologies |
| NHANES | National Health and Nutrition Examination Survey |
| ODN | Class B CpG oligonucleotide |
| PARC | Partnership for the Assessment of Risk from Chemicals |
| PBMCs | Peripheral blood mononuclear cells |
| PBS | Phosphate-buffered saline |
| PI | Propidium iodide |
| PPAR | Peroxisome proliferator-activated receptor |
| rhIL-2 | Recombinant human IL-2 |
| SI | Stimulation index |
| TCBPA | 3,3′,5,5′-Tetrachlorobisphenol A |
| TDAR | T cell-dependent antibody response |
| Th | T helper |
| THR | Thyroid hormone receptor |
| TNF-α | Tumor necrosis factor-α |
References
- Holmer, M.L.; Holmberg, R.D.; Despicht, C.; Bouftas, N.; Axelstad, M.; Beronius, A.; Zilliacus, J.; Van Duursen, M.; Svingen, T. Assessment of endocrine disruptors in the European Union: Current regulatory framework, use of new approach methodologies (NAMs) and recommendations for improvements. Regul. Toxicol. Pharmacol. 2025, 162, 105883. [Google Scholar] [CrossRef]
- Raysyan, A.; Zwigart, S.D.; Eremin, S.A.; Schneider, R.J. BPA Endocrine Disruptor Detection at the Cutting Edge: FPIA and ELISA Immunoassays. Biosensors 2023, 13, 664. [Google Scholar] [CrossRef]
- Hong, X.; Zhou, Y.; Zhu, Z.; Li, Y.; Li, Z.; Zhang, Y.; Hu, X.; Zhu, F.; Wang, Y.; Fang, M.; et al. Environmental endocrine disruptor Bisphenol A induces metabolic derailment and obesity via upregulating IL-17A in adipocytes. Environ. Int. 2023, 172, 107759. [Google Scholar] [CrossRef]
- Buoso, E.; Masi, M.; Limosani, R.V.; Oliviero, C.; Saeed, S.; Iulini, M.; Passoni, F.C.; Racchi, M.; Corsini, E. Endocrine Disrupting Toxicity of Bisphenol A and Its Analogues: Implications in the Neuro-Immune Milieu. J. Xenobiotics 2025, 15, 13. [Google Scholar] [CrossRef]
- Kodila, A.; Franko, N.; Sollner Dolenc, M. A review on immunomodulatory effects of BPA analogues. Arch. Toxicol. 2023, 97, 1831–1846. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Liu, H.; Wu, J.; Yuan, L.; Wang, Y.; Du, X.; Wang, R.; Marwa, P.W.; Petlulu, P.; Chen, X.; et al. The adverse health effects of bisphenol A and related toxicity mechanisms. Environ. Res. 2019, 176, 108575. [Google Scholar] [CrossRef]
- Thayer, K.A.; Doerge, D.R.; Hunt, D.; Schurman, S.H.; Twaddle, N.C.; Churchwell, M.I.; Garantziotis, S.; Kissling, G.E.; Easterling, M.R.; Bucher, J.R.; et al. Pharmacokinetics of bisphenol A in humans following a single oral administration. Environ. Int. 2015, 83, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Kuklenyik, Z.; Needham, L.L.; Calafat, A.M. Automated on-line column-switching HPLC-MS/MS method with peak focusing for the determination of nine environmental phenols in urine. Anal. Chem. 2005, 77, 5407–5413. [Google Scholar] [CrossRef] [PubMed]
- Viñas, R.; Goldblum, R.M.; Watson, C.S. Rapid estrogenic signaling activities of the modified (chlorinated, sulfonated, and glucuronidated) endocrine disruptor bisphenol A. Endocr. Disruptors 2013, 1, e25411. [Google Scholar] [CrossRef]
- Cobellis, L.; Colacurci, N.; Trabucco, E.; Carpentiero, C.; Grumetto, L. Measurement of bisphenol A and bisphenol B levels in human blood sera from healthy and endometriotic women. Biomed. Chromatogr. BMC 2009, 23, 1186–1190. [Google Scholar] [CrossRef]
- Yang, Y.; Guan, J.; Yin, J.; Shao, B.; Li, H. Urinary levels of bisphenol analogues in residents living near a manufacturing plant in south China. Chemosphere 2014, 112, 481–486. [Google Scholar] [CrossRef]
- Zhang, H.; Feng, S.; Song, S.; Zhao, Q.; Gao, Y.; Zhang, T. First evidence in the association of phenolic endocrine-disrupting chemicals with secondary non-alcoholic fatty liver disease: A case-control study in South China. Environ. Pollut. 2025, 373, 126086. [Google Scholar] [CrossRef]
- Wang, H.; Gao, R.; Liang, W.; Wei, S.; Zhou, Y.; Wang, Z.; Lan, L.; Chen, J.; Zeng, F. Large-scale biomonitoring of bisphenol analogues and their metabolites in human urine from Guangzhou, China: Implications for health risk assessment. Chemosphere 2023, 338, 139601. [Google Scholar] [CrossRef]
- Jialin, S.; Qun, G.; Hong, L.; Yixing, F.; Runhui, Y.; Yuehan, L.; Jiale, R.; Chenhui, S.; Bingli, Z.; Yumin, N.; et al. Urinary profiles of bisphenol S derivatives and their exposure pathway analysis in maternal and infant populations of Beijing. Environ. Int. 2024, 194, 109169. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Food Contact Materials, Enzymes and Processing Aids (CEP); Lambré, C.; Baviera, J.M.B.; Bolognesi, C.; Chesson, A.; Cocconcelli, P.S.; Crebelli, R.; Gott, D.M.; Grob, K.; Lampi, E.; et al. Re-evaluation of the risks to public health related to the presence of bisphenol A (BPA) in foodstuffs. Eur. Food Saf. Auth. 2023, 21, e06857. [Google Scholar] [CrossRef]
- Wu, M.; Wang, S.; Weng, Q.; Chen, H.; Shen, J.; Li, Z.; Wu, Y.; Zhao, Y.; Li, M.; Wu, Y.; et al. Prenatal and postnatal exposure to Bisphenol A and Asthma: A systemic review and meta-analysis. J. Thorac. Dis. 2021, 13, 1684–1696. [Google Scholar] [CrossRef] [PubMed]
- Spanier, A.J.; Kahn, R.S.; Kunselman, A.R.; Hornung, R.; Xu, Y.; Calafat, A.M.; Lanphear, B.P. Prenatal exposure to bisphenol A and child wheeze from birth to 3 years of age. Environ. Health Perspect. 2012, 120, 916–920. [Google Scholar] [CrossRef]
- Abulehia, H.F.S.; Mohd Nor, N.S.; Sheikh Abdul Kadir, S.H. The Current Findings on the Impact of Prenatal BPA Exposure on Metabolic Parameters: In Vivo and Epidemiological Evidence. Nutrients 2022, 14, 2766. [Google Scholar] [CrossRef]
- D’Amico, R.; Gugliandolo, E.; Cordaro, M.; Fusco, R.; Genovese, T.; Peritore, A.F.; Crupi, R.; Interdonato, L.; Di Paola, D.; Cuzzocrea, S.; et al. Toxic Effects of Endocrine Disruptor Exposure on Collagen-Induced Arthritis. Biomolecules 2022, 12, 564. [Google Scholar] [CrossRef]
- Jain, R.; Jain, A.; Jain, S.; Thakur, S.S.; Jain, S.K. Linking bisphenol potential with deleterious effect on immune system: A review. Nucleus 2022, 65, 269–281. [Google Scholar] [CrossRef]
- Liu, Y.; Mei, C.; Liu, H.; Wang, H.; Zeng, G.; Lin, J.; Xu, M. Modulation of cytokine expression in human macrophages by endocrine-disrupting chemical Bisphenol-A. Biochem. Biophys. Res. Commun. 2014, 451, 592–598. [Google Scholar] [CrossRef]
- Švajger, U.; Dolenc, M.S.; Jeras, M. In vitro impact of bisphenols BPA, BPF, BPAF and 17β-estradiol (E2) on human monocyte-derived dendritic cell generation, maturation and function. Int. Immunopharmacol. 2016, 34, 146–154. [Google Scholar] [CrossRef]
- Yanagisawa, R.; Koike, E.; Win-Shwe, T.T.; Takano, H. Effects of Oral Exposure to Low-Dose Bisphenol S on Allergic Asthma in Mice. Int. J. Mol. Sci. 2022, 23, 10790. [Google Scholar] [CrossRef] [PubMed]
- Yanagisawa, R.; Koike, E.; Win-Shwe, T.T.; Takano, H. Oral exposure to low dose bisphenol A aggravates allergic airway inflammation in mice. Toxicol. Rep. 2019, 6, 1253–1262. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Cang, X.; Liu, J. Molecular mechanism of Bisphenol A on androgen receptor antagonism. Toxicol. Vitr. Int. J. Publ. Assoc. BIBRA 2019, 61, 104621. [Google Scholar] [CrossRef]
- Hernández Avila, R.; Palacios-Arreola, M.I.; Nava-Castro, K.E.; Morales-Montor, J.; Ostoa-Saloma, P. Neonatal Bisphenol A Exposure Affects the IgM Humoral Immune Response to 4T1 Breast Carcinoma Cells in Mice. Int. J. Environ. Res. Public Health 2019, 16, 1784. [Google Scholar] [CrossRef] [PubMed]
- Wang, I.J.; Chen, C.Y.; Bornehag, C.G. Bisphenol A exposure may increase the risk of development of atopic disorders in children. Int. J. Hyg. Environ. Health 2016, 219, 311–316. [Google Scholar] [CrossRef]
- Jang, J.W.; Lee, J.W.; Yoon, Y.D.; Kang, J.S.; Moon, E.Y. Bisphenol A and its substitutes regulate human B cell survival via Nrf2 expression. Environ. Pollut. 2020, 259, 113907. [Google Scholar] [CrossRef]
- Sugita-Konishi, Y.; Shimura, S.; Nishikawa, T.; Sunaga, F.; Naito, H.; Suzuki, Y. Effect of Bisphenol A on non-specific immunodefenses against non-pathogenic Escherichia coli. Toxicol. Lett. 2003, 136, 217–227. [Google Scholar] [CrossRef]
- Yan, H.; Takamoto, M.; Sugane, K. Exposure to Bisphenol A prenatally or in adulthood promotes T(H)2 cytokine production associated with reduction of CD4CD25 regulatory T cells. Environ. Health Perspect. 2008, 116, 514–519. [Google Scholar] [CrossRef]
- Del Río-Araiza, V.H.; Palacios-Arreola, M.I.; Nava-Castro, K.E.; Pérez-Sánchez, N.Y.; Ruíz-Manzano, R.; Segovia-Mendoza, M.; Girón-Pérez, M.I.; Navidad-Murrieta, M.S.; Morales-Montor, J. Perinatal exposure to bisphenol A increases in the adulthood of the offspring the susceptibility to the human parasite Toxocara canis. Environ. Res. 2020, 184, 109381. [Google Scholar] [CrossRef]
- Tian, X.; Takamoto, M.; Sugane, K. Bisphenol A promotes IL-4 production by Th2 cells. Int. Arch. Allergy Immunol. 2003, 132, 240–247. [Google Scholar] [CrossRef]
- Uhm, J.Y.; Kim, H.R. Cross-Sectional Association of Urinary Bisphenol A and Vaccine-Induced Immunity against Hepatitis B Virus: Data from the 2003-2014 National Health and Nutrition Examination Survey. Int. J. Environ. Res. Public Health 2022, 19, 1103. [Google Scholar] [CrossRef]
- Mishra, A.; Goel, D.; Shankar, S. Bisphenol A contamination in aquatic environments: A review of sources, environmental concerns, and microbial remediation. Environ. Monit. Assess. 2023, 195, 1352. [Google Scholar] [CrossRef]
- Ao, J.; Huo, X.; Zhang, J.; Mao, Y.; Li, G.; Ye, J.; Shi, Y.; Jin, F.; Bao, S.; Zhang, J. Environmental exposure to bisphenol analogues and unexplained recurrent miscarriage: A case-control study. Environ. Res. 2021, 204 Pt C, 112293. [Google Scholar] [CrossRef] [PubMed]
- Siracusa, J.S.; Yin, L.; Measel, E.; Liang, S.; Yu, X. Effects of bisphenol A and its analogues on reproductive health: A mini review. Reprod. Toxicol. 2018, 79, 96–123. [Google Scholar] [CrossRef] [PubMed]
- Tuijnenburg, P.; Aan de Kerk, D.J.; Jansen, M.H.; Morris, B.; Lieftink, C.; Beijersbergen, R.L.; van Leeuwen, E.M.M.; Kuijpers, T.W. High-throughput compound screen reveals mTOR inhibitors as potential therapeutics to reduce (auto)antibody production by human plasma cells. Eur. J. Immunol. 2020, 50, 73–85. [Google Scholar] [CrossRef] [PubMed]
- Corsini, E.; Iulini, M.; Galbiati, V.; Maddalon, A.; Pappalardo, F.; Russo, G.; Hoogenboom, R.; Beekmann, K.; Janssen, A.; Louisse, J.; et al. EFSA Project on the use of NAMs to explore the immunotoxicity of PFAS. EFSA Support. Publ. 2024, 21, EN-8926. [Google Scholar] [CrossRef]
- Iulini, M.; Bettinsoli, V.; Maddalon, A.; Galbiati, V.; Janssen, A.W.F.; Beekmann, K.; Russo, G.; Pappalardo, F.; Fragki, S.; Paini, A.; et al. In vitro approaches to investigate the effect of chemicals on antibody production: The case study of PFASs. Arch. Toxicol. 2025, 99, 2075–2086. [Google Scholar] [CrossRef]
- Dallio, M.; Ventriglia, L.; Romeo, M.; Scognamiglio, F.; Diano, N.; Moggio, M.; Cipullo, M.; Coppola, A.; Ziogas, A.; Netea, M.G.; et al. Environmental bisphenol A exposure triggers trained immunity-related pathways in monocytes. Front. Immunol. 2023, 14, 1270391. [Google Scholar] [CrossRef]
- Cimmino, I.; Oriente, F.; D’Esposito, V.; Liguoro, D.; Liguoro, P.; Ambrosio, M.R.; Cabaro, S.; D’Andrea, F.; Beguinot, F.; Formisano, P.; et al. Low-dose Bisphenol-A regulates inflammatory cytokines through GPR30 in mammary adipose cells. J. Mol. Endocrinol. 2019, 63, 273–283. [Google Scholar] [CrossRef]
- Mhaouty-Kodja, S.; Zalko, D.; Tait, S.; Testai, E.; Viguié, C.; Corsini, E.; Grova, N.; Buratti, F.M.; Cabaton, N.J.; Coppola, L.; et al. A critical review to identify data gaps and improve risk assessment of bisphenol A alternatives for human health. Crit. Rev. Toxicol. 2024, 54, 696–753. [Google Scholar] [CrossRef]
- Partnership for the Assessment of Risks from Chemicals (PARC)—Additional Deliverable AD5.1 List of Prioritized BPA Alternatives for WP5 Hazard Assessment Activities Workshop Report WP5. Available online: https://www.eu-parc.eu/sites/default/files/2023-08/PARC_AD5.1.pdf (accessed on 21 June 2025).
- Proença, S.; Escher, B.I.; Fischer, F.C.; Fisher, C.; Grégoire, S.; Hewitt, N.J.; Nicol, B.; Paini, A.; Kramer, N.I. Effective exposure of chemicals in in vitro cell systems: A review of chemical distribution models. Toxicol. Vitr. Int. J. Publ. Assoc. BIBRA 2021, 73, 105133. [Google Scholar] [CrossRef]
- Armitage, J.M.; Wania, F.; Arnot, J.A. Application of mass balance models and the chemical activity concept to facilitate the use of in vitro toxicity data for risk assessment. Environ. Sci. Technol. 2014, 48, 9770–9779. [Google Scholar] [CrossRef]
- Armitage, J.M.; Sangion, A.; Parmar, R.; Looky, A.B.; Arnot, J.A. Update and Evaluation of a High-Throughput In Vitro Mass Balance Distribution Model: IV-MBM EQP v2.0. Toxics 2021, 9, 315. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 6623, Bisphenol A. 2025. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Bisphenol-A (accessed on 22 July 2025).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 623849, 4,4′-(1-Phenylethylidene)bisphenol. 2025. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/4_4_-_1-Phenylethylidene_bisphenol (accessed on 20 May 2025).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 608116, 4,4′-Ethylidenebisphenol. 2025. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Bisphenol_E (accessed on 20 May 2025).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 630355, Bisphenol P. 2025. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Bisphenol-P (accessed on 20 May 2025).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 232446, 4,4′-Cyclohexylidenebisphenol. 2025. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/232446 (accessed on 20 May 2025).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 6619, 2,2′,6,6′-Tetrachlorobisphenol A. 2025. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Tetrachlorobisphenol-A (accessed on 20 May 2025).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 2054598, 4-(4-Allyloxy-benzenesulfonyl)-phenol. 2025. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/4-_4-Allyloxy-benzenesulfonyl_-phenol (accessed on 20 May 2025).
- Kharrazian, D. The Potential Roles of Bisphenol A (BPA) Pathogenesis in Autoimmunity. Autoimmune Dis. 2014, 2014, 743616. [Google Scholar] [CrossRef] [PubMed]
- Pahović, P.Š.; Iulini, M.; Maddalon, A.; Galbiati, V.; Buoso, E.; Dolenc, M.S.; Corsini, E. In Vitro Effects of Bisphenol Analogs on Immune Cells Activation and Th Differentiation. Endocr. Metab. Immune Disord. Drug Targets 2023, 23, 1750–1761. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, M.; Wang, P.; Lin, X.; Lai, K.P.; Ding, Z. Integrated analysis reveals the immunotoxicity mechanism of BPs on human lymphocytes. Chem.-Biol. Interact. 2024, 399, 111148. [Google Scholar] [CrossRef] [PubMed]
- Kovats, S. Estrogen receptors regulate innate immune cells and signaling pathways. Cell Immunol. 2015, 294, 63–69. [Google Scholar] [CrossRef]
- Lai, J.J.; Lai, K.P.; Zeng, W.; Chuang, K.H.; Altuwaijri, S.; Chang, C. Androgen receptor influences on body defense system via modulation of innate and adaptive immune systems: Lessons from conditional AR knockout mice. Am. J. Pathol. 2012, 181, 1504–1512. [Google Scholar] [CrossRef]
- Matsushima, A.; Teramoto, T.; Kakuta, Y. Crystal structure of endocrine-disrupting chemical bisphenol A and estrogen-related receptor γ. J. Biochem. 2022, 171, 23–25. [Google Scholar] [CrossRef]
- Della Rocca, Y.; Traini, E.M.; Diomede, F.; Fonticoli, L.; Trubiani, O.; Paganelli, A.; Pizzicannella, J.; Marconi, G.D. Current Evidence on Bisphenol A Exposure and the Molecular Mechanism Involved in Related Pathological Conditions. Pharmaceutics 2023, 15, 908. [Google Scholar] [CrossRef]
- Buoso, E.; Kenda, M.; Masi, M.; Linciano, P.; Galbiati, V.; Racchi, M.; Dolenc, M.S.; Corsini, E. Effects of Bisphenols on RACK1 Expression and Their Immunological Implications in THP-1 Cells. Front. Pharmacol. 2021, 12, 743991. [Google Scholar] [CrossRef]
- Rogers, J.A.; Metz, L.; Yong, V.W. Review: Endocrine disrupting chemicals and immune responses: A focus on bisphenol-A and its potential mechanisms. Mol. Immunol. 2013, 53, 421–430. [Google Scholar] [CrossRef]
- Frericks, M.; Temchura, V.V.; Majora, M.; Stutte, S.; Esser, C. Transcriptional signatures of immune cells in aryl hydrocarbon receptor (AHR)-proficient and AHR-deficient mice. Biol. Chem. 2006, 387, 1219–1226. [Google Scholar] [CrossRef] [PubMed]
- Bonefeld-Jørgensen, E.C.; Long, M.; Hofmeister, M.V.; Vinggaard, A.M. Endocrine-disrupting potential of bisphenol A, bisphenol A dimethacrylate, 4-n-nonylphenol, and 4-n-octylphenol in vitro: New data and a brief review. Environ. Health Perspect. 2007, 115 (Suppl. S1), 69–76. [Google Scholar] [CrossRef] [PubMed]
- Chinetti, G.; Griglio, S.; Antonucci, M.; Torra, I.P.; Delerive, P.; Majd, Z.; Fruchart, J.C.; Chapman, J.; Najib, J.; Staels, B. Activation of proliferator-activated receptors alpha and gamma induces apoptosis of human monocyte-derived macrophages. J. Biol. Chem. 1998, 273, 25573–25580. [Google Scholar] [CrossRef] [PubMed]
- Gosset, P.; Charbonnier, A.S.; Delerive, P.; Fontaine, J.; Staels, B.; Pestel, J.; Tonnel, A.B.; Trottein, F. Peroxisome proliferator-activated receptor gamma activators affect the maturation of human monocyte-derived dendritic cells. Eur. J. Immunol. 2001, 31, 2857–2865. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.C.; Ding, X.; Daynes, R.A. Nuclear receptor peroxisome proliferator-activated receptor alpha (PPARalpha) is expressed in resting murine lymphocytes. The PPARalpha in T and B lymphocytes is both transactivation and transrepression competent. J. Biol. Chem. 2002, 277, 6838–6845. [Google Scholar] [CrossRef]
- Setoguchi, K.; Misaki, Y.; Terauchi, Y.; Yamauchi, T.; Kawahata, K.; Kadowaki, T.; Yamamoto, K. Peroxisome proliferator-activated receptor-gamma haploinsufficiency enhances B cell proliferative responses and exacerbates experimentally induced arthritis. J. Clin. Investig. 2001, 108, 1667–1675. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Qiu, W.; Chen, B.; Chen, J.; Liu, S.; Wu, M.; Wang, K.J. The in vitro immune modulatory effect of bisphenol A on fish macrophages via estrogen receptor α and nuclear factor-κB signaling. Environ. Sci. Technol. 2015, 49, 1888–1895. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Bauer, S.M.; Lawrence, B.P. Developmental exposure to bisphenol A modulates innate but not adaptive immune responses to influenza A virus infection. PLoS ONE 2012, 7, e38448. [Google Scholar] [CrossRef]
- Sonavane, M.; Gassman, N.R. Bisphenol A co-exposure effects: A key factor in understanding BPA’s complex mechanism and health outcomes. Crit. Rev. Toxicol. 2019, 49, 371–386. [Google Scholar] [CrossRef]
- Liang, L.; Pan, Y.; Bin, L.; Liu, Y.; Huang, W.; Li, R.; Lai, K.P. Immunotoxicity mechanisms of perfluorinated compounds PFOA and PFOS. Chemosphere 2022, 291 Pt 2, 132892. [Google Scholar] [CrossRef]
| Name | Acronym | CAS N° | Chemical Structure |
|---|---|---|---|
| Bisphenol A | BPA | 80-05-7 | 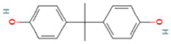 |
| Bisphenol AP | BPAP | 1571-75-1 | 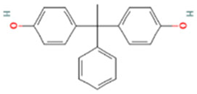 |
| Bisphenol E | BPE | 2081-08-5 | 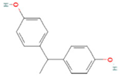 |
| Bisphenol P | BPP | 2167-51-3 | 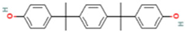 |
| Bisphenol S 4-allyl ether | BPS-MAE | 97042-18-7 | 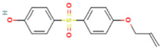 |
| Bisphenol Z | BPZ | 843-55-0 | 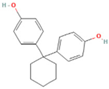 |
| 3,3′,5,5′-Tetrachlorobisphenol A | TCBPA | 79-95-8 | 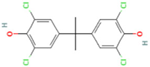 |
| Name | MW (g/mol) | MP (°C) | IOC Type | pKa | log KOW,N | log KAW,N | CSAT,W,N (mg/L) | ECx in μM | Reference |
|---|---|---|---|---|---|---|---|---|---|
| BPA | 228.3 | 158.0 | A | 9.60 | 3.32 | −9.43 | 3.80 × 102 | 50.00 | [47] |
| BPAP | 290.4 | 189.0 | A | 10.22 | 4.86 | −10.64 | 1.20 × 102 | 15.00 | [48] |
| BPE | 214.3 | 125.0 | A | 10.10 | 3.19 | −9.55 | 2.50 × 103 | 50.00 | [49] |
| BPP | 346.5 | 195.0 | A | 10.08 | 6.25 | −10.23 | 5.00 × 10 | 15.00 | [50] |
| BPZ | 268.4 | 189.0 | A | 9.91 | 5.00 | −9.41 | 1.00 × 102 | 15.00 | [51] |
| TCBPA | 366.1 | 136.0 | A | 6.91 | 6.22 | −8.50 | 4.00 × 10 | 25.00 | [52] |
| BPS-MAE (M) | 290.3 | 172.0 | A | 8.20 | 3.10 | −9.00 | 1.50 × 103 | 35.00 | [53] |
| BPS-MAE (F) | 290.3 | 172.0 | A | 8.20 | 3.10 | −9.00 | 1.50 × 103 | 60.00 | [53] |
| Name | CV80 Male (µM) | CV80 Female (µM) | Selected Highest Concentration (µM) | Concentrations Tested (µM) |
|---|---|---|---|---|
| BPA | 50.84 ± 4.3 | 54.37 ± 6.1 | 50 | 0.005–0.05–0.5–5–50 |
| BPAP | 12.90 ± 3.9 | 15.74 ± 1.2 | 15 | 0.0015–0.015–0.15–1.5–15 |
| BPE | 51.87 ± 9.1 | 59.33 ± 2.7 | 50 | 0.005–0.05–0.5–5–50 |
| BPP | 13.13 ± 16.0 | 20.44 ± 2.0 | 15 | 0.0015–0.015–0.15–1.5–15 |
| BPS-MAE * | 35.80 ± 3.4 | 60.65 ± 2.9 | 35 (M#) 60 (F##) | 0.0035–0.035–0.35–3.5–35 (M) 0.0060–0.060–0.60–6–60 (F) |
| BPZ | 13.81 ± 3.6 | 17.82 ± 1.2 | 15 | 0.0015–0.015–0.15–1.5–15 |
| TCBPA | 17.60 ± 8.5 | 30.23 ± 0.1 | 25 | 0.0025–0.025–0.25–2.5–25 |
| IC50 (µM) | ||||
| IgG | IgM | |||
| Male | Female | Male | Female | |
| BPA | 33.1 ± 6.1 | 29.2 ± 6.8 | 25.0 ± 4.3 | 32.0 ± 6.9 |
| BPAP | 14.9 ± 3.4 * | 9.8 ± 1.4 * | 7.2 ± 0.7 * | 13.8 ± 6.7 |
| BPE | 52.3 ± 14.7 | 34.9 ± 6.5 | 23.2 ± 5.0 | 35.9 ± 12.0 |
| BPP | 8.5 ± 1.4 * | 5.8 ± 0.7 * | 4.9 ± 0.3 ** | 5.80 ± 1.1 * |
| BPS-MAE | 27.5 ± 6.7 | 14.8 ± 2.3 | 16.9 ± 3.4 | 22.0 ± 9.6 |
| BPZ | 15.3 ± 4.7 | 12.6 ± 3.0 | 7.9 ± 6.4 | 15.6 ± 9.5 |
| TCBPA | 27.5 ± 9.8 | 15.6 ± 2.2 | 20.0 ± 4.5 | 14.3 ± 11.7 |
| IC20 (µM) | ||||
| IgG | IgM | |||
| Male | Female | Male | Female | |
| BPA | 59.7 ± 10.1 | 55.9 ± 10.3 | 53.5 ± 7.4 | 56.2 ± 10.3 |
| BPAP | 23.1 ± 4.5 ** | 18.0 ± 3.0 * | 13.7 ± 0.7 ** | 12.4 ± 2.5 * |
| BPE | 60.4 ± 5.6 | 60.9 ± 9.1 | 55.4 ± 10.1 | 51.8 ± 6.5 |
| BPP | 12.5 ± 1.3 ** | 12.1 ± 0.9 * | 11.1 ± 0.6 ** | 11.2 ± 2.0 * |
| BPS-MAE | 37.9 ± 7.4 | 29.5 ± 2.9 | 24.4 ± 3.7 * | 18.5 ± 3.6 * |
| BPZ | 11.1 ± 1.3 ** | 11.2 ± 2.0 * | 20.0 ± 4.3 ** | 21.8 ± 9.8 * |
| TCBPA | 42.3 ± 8.1 | 29.2 ± 5.1 | 35.5 ± 6.0 | 26.8 ± 8.0 |
| Name | MFBULK WAT | MFALB | MFS-LIP | MFWAT | MFDOM | MFCells | MFPlastic |
|---|---|---|---|---|---|---|---|
| BPA | 63.6% | 24.7% | 5.3% | 33.6% | 0.0% | 20.2% | 16.2% |
| BPAP | 53.2% | 41.0% | 10.3% | 1.8% | 0.0% | 40.2% | 6.6% |
| BPE | 65.8% | 21.2% | 4.7% | 39.9% | 0.0% | 17.8% | 16.4% |
| BPP | 27.0% | 9.3% | 17.5% | 0.1% | 0.0% | 70.3% | 2.8% |
| BPZ | 49.5% | 36.7% | 11.3% | 1.5% | 0.0% | 44.2% | 6.3% |
| BPS-MAE (M) | 67.6% | 18.9% | 4.2% | 44.5% | 0.0% | 16.2% | 16.2% |
| BPS-MAE (F) | 67.6% | 18.9% | 4.2% | 44.5% | 0.0% | 16.2% | 16.2% |
| TCBPA | 27.3% | 9.7% | 17.5% | 0.1% | 0.0% | 69.9% | 2.8% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Passoni, F.C.; Iulini, M.; Galbiati, V.; Marinovich, M.; Corsini, E. Disrupting Defenses: Effects of Bisphenol A and Its Analogs on Human Antibody Production In Vitro. Life 2025, 15, 1203. https://doi.org/10.3390/life15081203
Passoni FC, Iulini M, Galbiati V, Marinovich M, Corsini E. Disrupting Defenses: Effects of Bisphenol A and Its Analogs on Human Antibody Production In Vitro. Life. 2025; 15(8):1203. https://doi.org/10.3390/life15081203
Chicago/Turabian StylePassoni, Francesca Carlotta, Martina Iulini, Valentina Galbiati, Marina Marinovich, and Emanuela Corsini. 2025. "Disrupting Defenses: Effects of Bisphenol A and Its Analogs on Human Antibody Production In Vitro" Life 15, no. 8: 1203. https://doi.org/10.3390/life15081203
APA StylePassoni, F. C., Iulini, M., Galbiati, V., Marinovich, M., & Corsini, E. (2025). Disrupting Defenses: Effects of Bisphenol A and Its Analogs on Human Antibody Production In Vitro. Life, 15(8), 1203. https://doi.org/10.3390/life15081203







