Severe Malaria Due to Plasmodium falciparum in an Immunocompetent Young Adult: Rapid Progression to Multiorgan Failure
Abstract
1. Introduction
2. Case Report
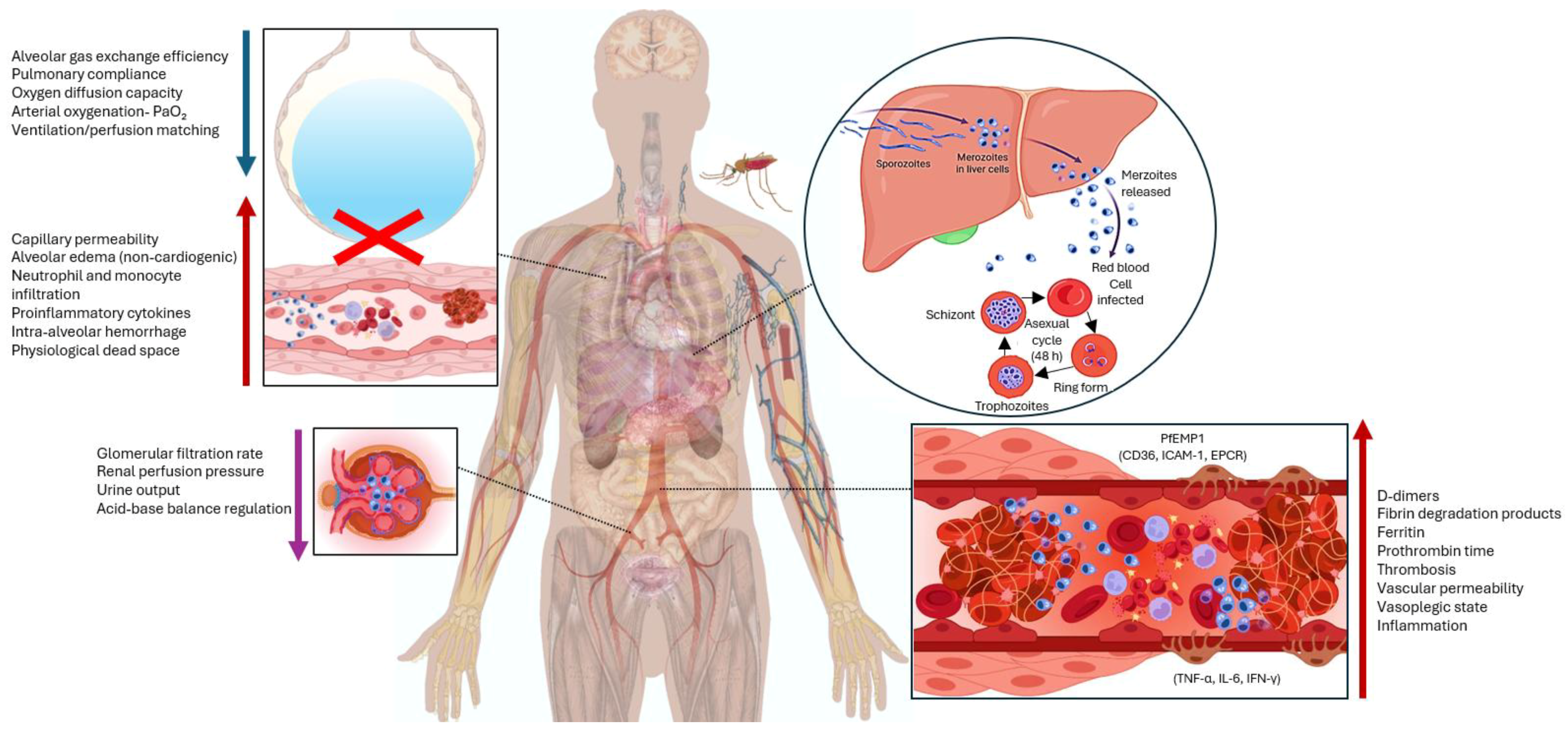
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Olliaro, P. Editorial commentary: Mortality associated with severe Plasmodium falciparum malaria increases with age. Clin. Infect. Dis. 2008, 47, 158–160. [Google Scholar] [CrossRef] [PubMed]
- Organización Mundial de la Salud (OMS). Malaria. 2024. Available online: https://www.who.int/news-room/fact-sheets/detail/malaria (accessed on 9 June 2025).
- Instituto Nacional de Salud (INS). Boletín Epidemiológico Malaria Semana 17-SIVIGILA; Instituto Nacional de Salud (INS): Bogotá, Colombia, 2025. [Google Scholar]
- Trampuz, A.; Jereb, M.; Muzlovic, I.; Prabhu, R.M. Clinical review: Severe malaria. Crit. Care 2003, 7, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.D.; Rowe, J.A.; Higgins, M.K.; Lavstsen, T. Malaria’s deadly grip: Cytoadhesion of Plasmodium falciparum-infected erythrocytes. Cell. Microbiol. 2013, 15, 1976–1983. [Google Scholar] [CrossRef] [PubMed]
- Clark, I.A.; Cowden, W.B. The pathophysiology of falciparum malaria. Pharmacol. Ther. 2003, 99, 221–260. [Google Scholar] [CrossRef] [PubMed]
- Wooldridge, G.; Nandi, D.; Chimalizeni, Y.; O’Brien, N. Cardiovascular Findings in Severe Malaria: A Review. Glob. Heart 2020, 15, 75. [Google Scholar] [CrossRef] [PubMed]
- Wassmer, S.C.; Taylor, T.E.; Rathod, P.K.; Mishra, S.K.; Mohanty, S.; Arevalo-Herrera, M.; Duraisingh, M.T.; Smith, J.D. Investigating the Pathogenesis of Severe Malaria: A Multidisciplinary and Cross-Geographical Approach. Am. J. Trop. Med. Hyg. 2015, 93 (Suppl. S3), 42–56. [Google Scholar] [CrossRef] [PubMed]
- Organización Panamericana de la Salud. Malaria; Organización Panamericana de la Salud: Washington, DC, USA, 2025. Available online: https://www.paho.org/es/temas/malaria (accessed on 9 June 2025).
- Moxon, C.A.; Gibbins, M.P.; McGuinness, D.; Milner, D.A., Jr.; Marti, M. New Insights into Malaria Pathogenesis. Annu. Rev. Pathol. 2020, 2415, 315–343. [Google Scholar] [CrossRef] [PubMed]
- Abdi, A.I.; Achcar, F.; Sollelis, L.; Silva-Filho, J.L.; Mwikali, K.; Muthui, M.; Mwangi, S.; Kimingi, H.W.; Orindi, B.; Kivisi, C.A. Plasmodium falciparum adapts its investment into replication versus transmission according to the host environment. eLife 2023, 12, e85140. [Google Scholar] [CrossRef] [PubMed]
- Yam, X.Y.; Niang, M.; Madnani, K.G.; Preiser, P.R. Three Is a Crowd—New Insights into Rosetting in Plasmodium falciparum. Trends Parasitol. 2017, 33, 309–320. [Google Scholar] [CrossRef] [PubMed]
- Clark, I.A.; Alleva, L.M.; Budd, A.C.; Cowden, W.B. Understanding the role of inflammatory cytokines in malaria and related diseases. Travel. Med. Infect. Dis. 2008, 6, 67–81. [Google Scholar] [CrossRef] [PubMed]
- Koopmans, L.C.; van Wolfswinkel, M.E.; Hesselink, D.A.; Hoorn, E.J.; Koelewijn, R.; van Hellemond, J.J.; van Genderen, P.J. Acute kidney injury in imported Plasmodium falciparum malaria. Malar. J. 2015, 14, 523. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.K.; Das, B.S. Malaria and acute kidney injury. Semin. Nephrol. 2008, 28, 395–408. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Kaushik, R.; Kaushik, R.M. Malarial hepatopathy: Clinical profile and association with other malarial complications. Acta Trop. 2016, 159, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Fazil, A.; Vernekar, P.V.; Geriani, D.; Pant, S.; Senthilkumaran, S.; Anwar, N.; Prabhu, A.; Menezes, R.G. Clinical profile and complication of malaria hepatopathy. J. Infect. Public Health 2013, 6, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Hoffmeister, B. Respiratory Distress Complicating Falciparum Malaria Imported to Berlin, Germany: Incidence, Burden, and Risk Factors. Microorganisms 2023, 11, 1579. [Google Scholar] [CrossRef] [PubMed]
- Sanclemente-Cardoza, V.; Torres Heredia, L.Y.; Payan Salcedo, H.A.; Estela Zape, J.L. Activación De Muerte Celular En Sepsis Y Síndrome De Dificultad Respiratoria Aguda (SDRA). Medicina 2025, 46, 788–798. [Google Scholar] [CrossRef]
- Redditt, V.; Bogoch, I.; Rashid, M. A 38-year-old man with fever and a history of malaria. Can. Med. Assoc. J. 2018, 190, E1081–E1082. [Google Scholar] [CrossRef] [PubMed]
- Al Farsi, F.; Chandwani, J.; Mahdi, A.S.; Petersen, E. Severe imported malaria in an intensive care unit: A case series. IDCases 2019, 17, e00544. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, J.A.; Roa, A.A.; Leonso-Bravo, A.A.; Khatiwada, P.; Eckardt, P.; Lemos-Ramirez, J. A Case of Plasmodium falciparum Malaria Treated with Artesunate in a 55-Year-Old Woman on Return to Florida from a Visit to Ghana. Am. J. Case Rep. 2020, 21, e926097. [Google Scholar] [CrossRef] [PubMed]
- Teressa, M.; Purnama, A.; Henrina, J.; Wiraatmadja, A.; Boro, A.M.B.; Sam, C.I.L.; Dedang, T.A.; Cahyadi, A. Severe Malaria in an Adult Patient from Low-Endemic Area in Flores Island, East Nusa Tenggara. Case Rep. Med. 2023, 2023, 1239318. [Google Scholar] [CrossRef] [PubMed]

| Patient’s Values | ||||
|---|---|---|---|---|
| Parameter | Reference Range | Emergencies, Date of Entry 6 June 2024 | ICU | |
| 6 June 2024 | 7 June 2024 | |||
| Blood Biochemistry | ||||
| Hemoglobin (g/dL) | 13.5–17.5 | 16.4 | 17.2 | |
| Platelets (×103/µL) | 150,000–450,000 | 33,000 | 42,000 | |
| Leukocytes (×103/µL) | 4.5–11.0 | 8.23 | 20.37 | |
| Coagulation | ||||
| Prothrombin time (seconds) | 11.7–15.5 | 25.7 | ||
| Partial thromboplastin time (seconds) | 24–45 | 35.6 | 40.2 | |
| Electrolytes | ||||
| Sodium (mmol/L) | 135–145 | 136 | 133 | |
| Potassium (mmol/L) | 3.5–4.5 | 6.24 | 6.14 | |
| Arterial Blood Gases (mmHg) | Supplemental Oxygen Support | Non-rebreathing Mask | Invasive mechanical ventilation | |
| FiO2 | 60% | 100% | 100% | |
| pH | 7.35–7.45 | 7.33 | 6.96 | 7.12 |
| pCO2 (mmHg) | 35–45 | 41 | 74 | 77 |
| PaO2 (mmHg) | 75–100 | 58 | 77 | 41 |
| HCO3− (mEq/L) | 22–26 | 21.3 | 16.4 | 24.8 |
| Base excess (BE) | −17.2 | −6.5 | ||
| PaO2/FiO2 | >400 | 96 | 77 | 41 |
| Lactic acid (mmol/L) | 0.5–2.2 | 1.5 | 9.0 | 2.5 |
| Kidney Function | ||||
| Creatinine (mg/dL) | 0.7–1.3 | 1.68 | 1.10 | 3.24 |
| Blood urea nitrogen (BUN) (mg/dL) | 7–20 | 23.3 | 34.3 | 34.2 |
| Liver Function | ||||
| Glutamic-pyruvic transaminase (U/L) | 0–45 | 309 | 402 | |
| Glutamic-oxalacetic transaminase (U/L) | 11–45 | 795 | 1190 | |
| Extended Diagnostic Tests | ||||
| Thick blood smear for hemoparasites | Plasmodium falciparum trophozoites (rings): Positive | |||
| Dengue combo antigen/antibody (NS1Ag/IgM/IgG) | Dengue NS1Ag: Negative Dengue IgM: Negative Dengue IgG: Positive | |||
| Additional Findings | ||||
| Thoracic ultrasound (pericardium and pleura) | Bilateral pleural effusion, predominantly on the right side, not susceptible to drainage. | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanclemente-Cardoza, V.; Payán-Salcedo, H.A.; Estela-Zape, J.L. Severe Malaria Due to Plasmodium falciparum in an Immunocompetent Young Adult: Rapid Progression to Multiorgan Failure. Life 2025, 15, 1201. https://doi.org/10.3390/life15081201
Sanclemente-Cardoza V, Payán-Salcedo HA, Estela-Zape JL. Severe Malaria Due to Plasmodium falciparum in an Immunocompetent Young Adult: Rapid Progression to Multiorgan Failure. Life. 2025; 15(8):1201. https://doi.org/10.3390/life15081201
Chicago/Turabian StyleSanclemente-Cardoza, Valeria, Harold Andrés Payán-Salcedo, and Jose Luis Estela-Zape. 2025. "Severe Malaria Due to Plasmodium falciparum in an Immunocompetent Young Adult: Rapid Progression to Multiorgan Failure" Life 15, no. 8: 1201. https://doi.org/10.3390/life15081201
APA StyleSanclemente-Cardoza, V., Payán-Salcedo, H. A., & Estela-Zape, J. L. (2025). Severe Malaria Due to Plasmodium falciparum in an Immunocompetent Young Adult: Rapid Progression to Multiorgan Failure. Life, 15(8), 1201. https://doi.org/10.3390/life15081201





