Cometabolic Biodegradation of Hydrazine by Chlorella vulgaris–Bacillus Extremophilic Consortia: Synergistic Potential for Space and Industry
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Growth with Hydrazine
2.2. Identification of Extremophile Bacterial Isolates
2.3. Algae Growth and Hydrazine Tolerance
2.4. Analysis of Hydrazine Concentration
2.5. Co-Culture of Extremophiles and C. vulgaris
2.5.1. Assessment of Bacterial Symbiosis
2.5.2. Growth Dynamics in Broth Media
2.5.3. Flow Cytometry Analysis of Sub-Populations
2.6. Growth with Titanium Plates
3. Results
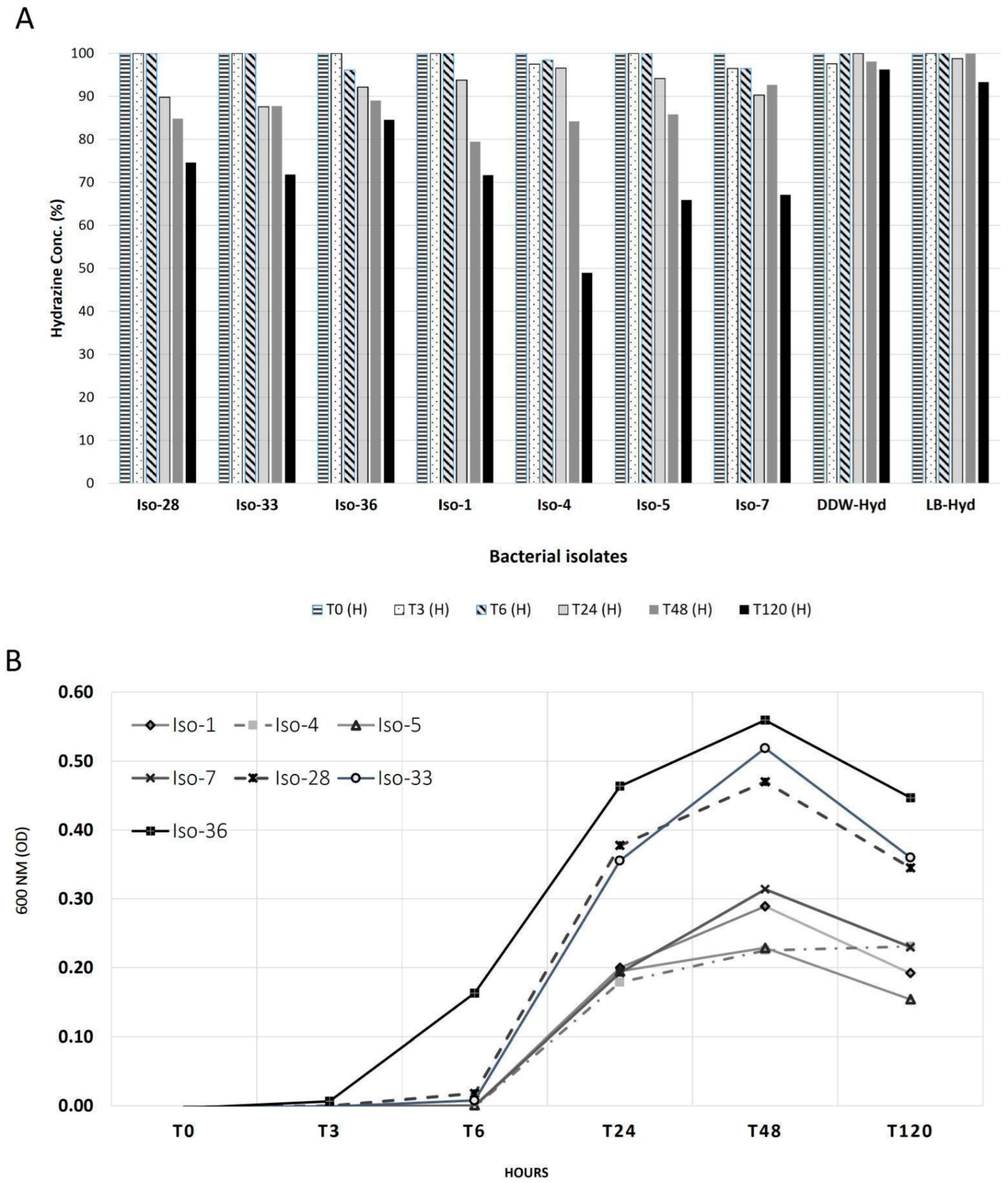
3.1. Growth of Bacterial Isolates with Hydrazine
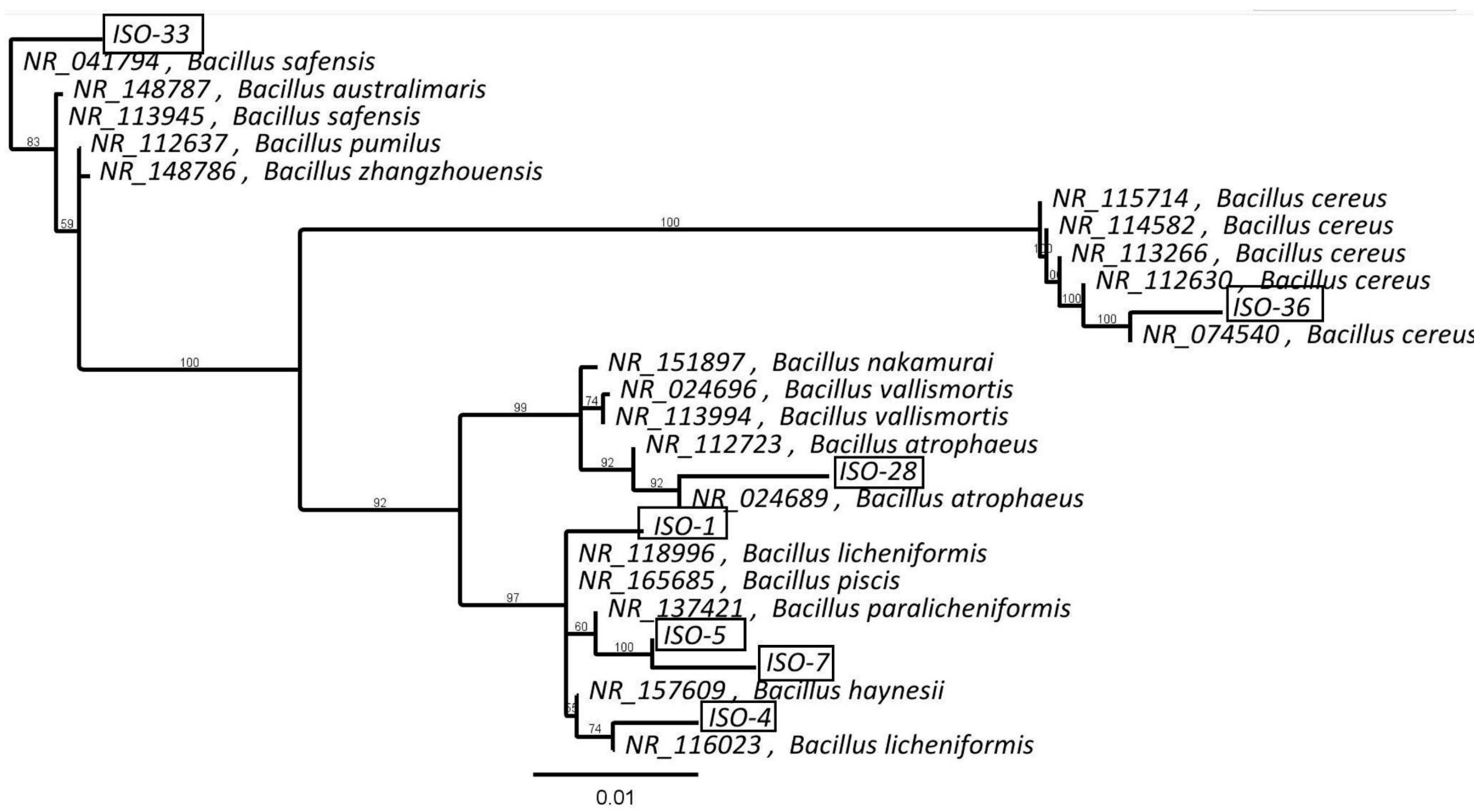
3.2. Identification of Extremophilic Bacterial Isolates
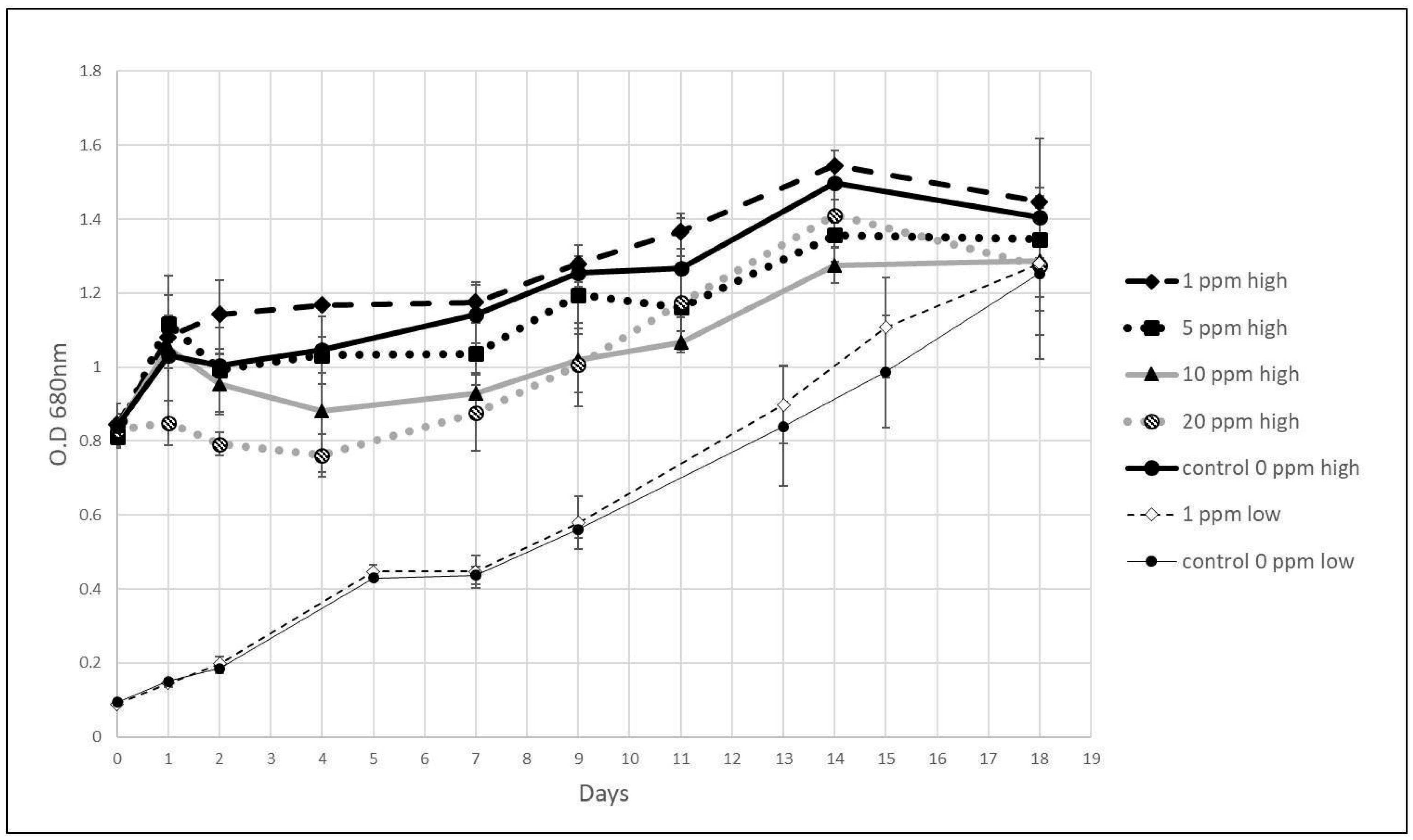
3.3. Growth of Algae with Hydrazine
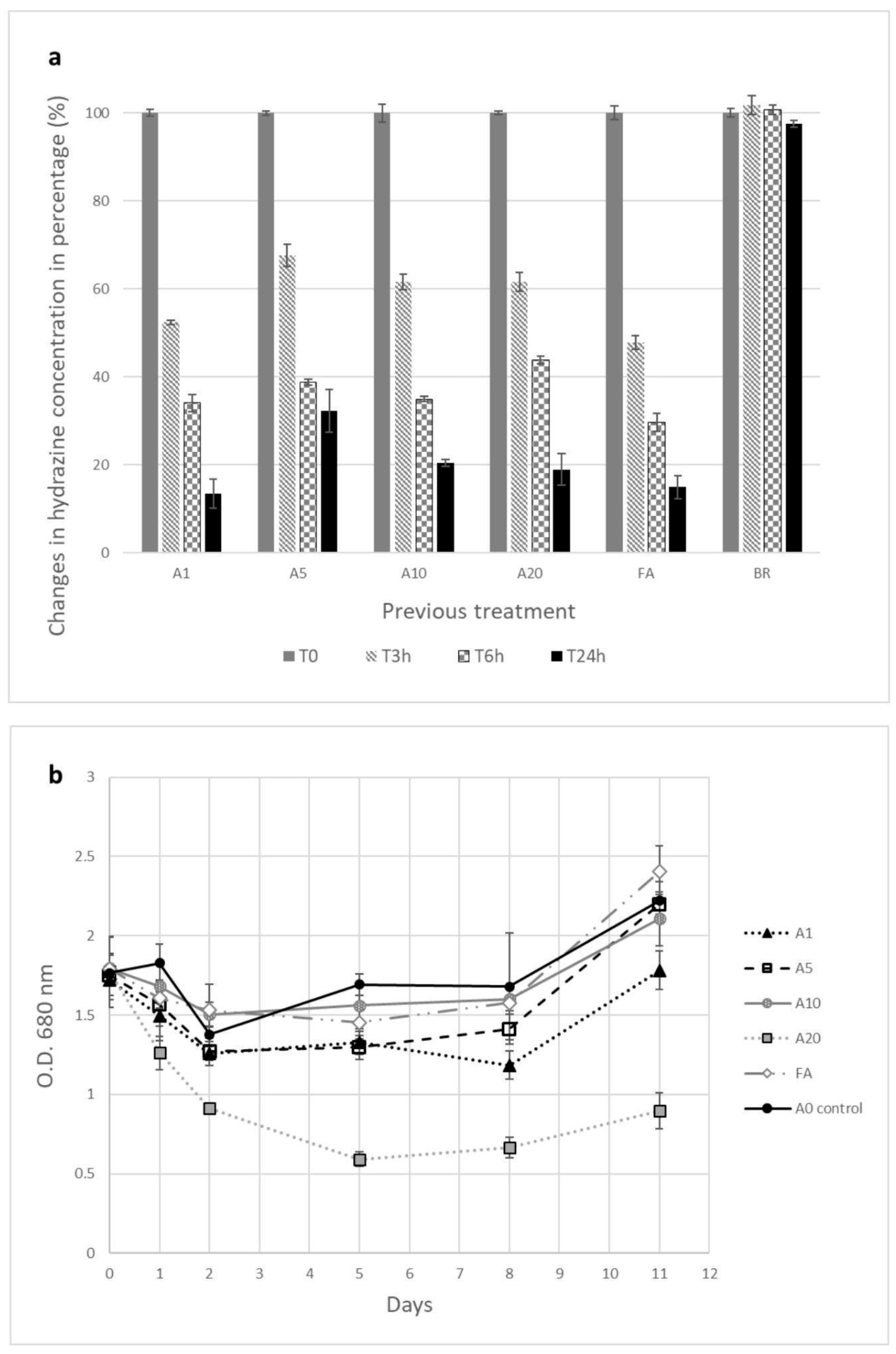
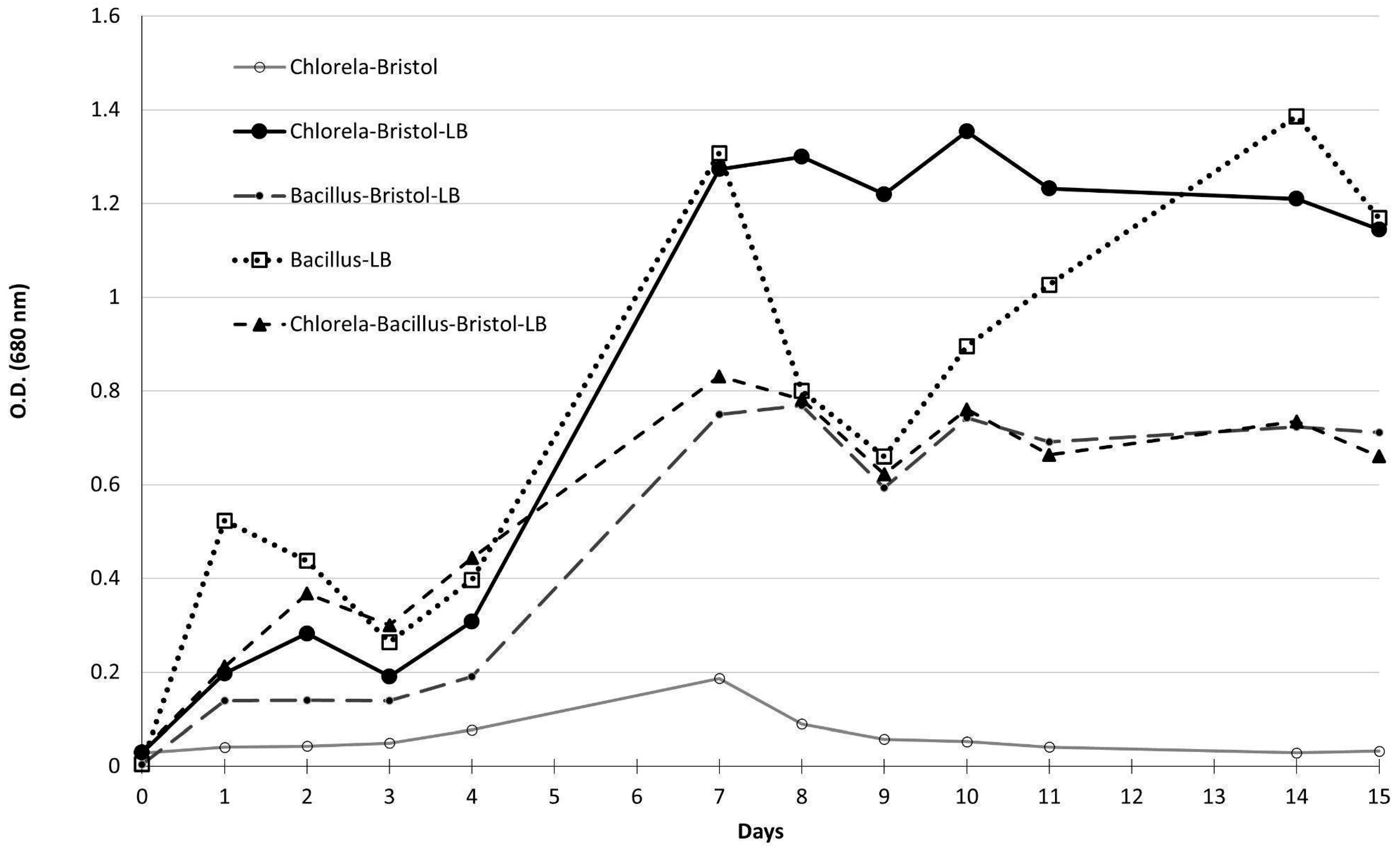
3.4. Co-Culture of Bacillus spp. Isolates and C. vulgaris
3.5. Impact of Titanium on Growth Patterns
4. Discussion
4.1. Growth and Reduction in Hydrazine Hydrate
4.2. Effects of Hydrazine on Algae and Photosynthesis
4.3. Co-Culture Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Seedhouse, E. Life Support Systems for Humans in Space; Springer International Publishing: Cham, Switzerland, 2020. [Google Scholar]
- Alexandrov, S. Algal research in space: History, current status and future prospects. Inenovare J. Life Sci. 2016, 4, 1–4. [Google Scholar]
- Niederwieser, T.; Kociolek, P.; Klaus, D. A review of algal research in space. Acta Astronaut. 2018, 146, 359–367. [Google Scholar] [CrossRef]
- Helisch, H.; Keppler, J.; Detrell, G.; Belz, S.; Ewald, R.; Fasoulas, S.; Heyer, A.G. High density long-term cultivation of Chlorella vulgaris SAG 211-12 in a novel microgravity-capable membrane raceway photobioreactor for future bioregenerative life support in SPACE. Life Sci. Space Res. 2020, 24, 91–107. [Google Scholar] [CrossRef]
- Keppler, J.; Helisch, H.; Belz, S.; Bretschneider, J.; Detrell, G.; Henn, N.; Fasoulas, S.; Ewald, R.; Angerer, O.; Adrian, A. From breadboard to protoflight model—The ongoing development of the algae-Based ISS Experiment PBR@LRS. In Proceedings of the 47th International Conference on Environmental Systems, Charleston, SC, USA, 16–20 July 2017. [Google Scholar]
- Kolodziejczyk, A.M.; Summerer, L. Bioreactors and biomaterials in space architecture. In Proceedings of the 46th International Conference on Environmental Systems, Vienna, Austria, 10–14 July 2016; pp. 1–12. [Google Scholar]
- Ru, I.T.K.; Sung, Y.Y.; Jusoh, M.; Wahid, M.E.A.; Nagappan, T. Chlorella vulgaris: A perspective on its potential for combining high biomass with high value bioproducts. Appl. Phycol. 2020, 1, 2–11. [Google Scholar] [CrossRef]
- Schmidt, E.W. Hydrazine and Its Derivatives: Preparation, Properties, Applications; John Wiley & Sons: Hoboken, NJ, USA, 2001; Volume 2. [Google Scholar]
- Scherfig, J.; Dixon, P.S.; Justice, C.A. Environmental Quality Research, Use of Unicellular Algae for Evaluation of Potential Aquatic Contaminants; California Univ Irvine Water Resources Lab: Irvine, CA, USA, 1978. [Google Scholar]
- Christensen, W.D.; Hudson, R.F.; Lewis, S.; Kane, D.A. The Second Conference on the Environmental Chemistry of Hydrazine Fuels: 15 February 1979; The Air Force Engineering and Services Center Engineering and Services Lab: Tyndall AFB, FL, USA, 1979. [Google Scholar]
- World Health Organization (WHO) (Ed.) Hydrazine (Environmental Health Criteria); World Health Organisation (WHO): Geneva, Switzerland, 1987. [Google Scholar]
- Niehaus, F.; Bertoldo, C.; Kähler, M.; Antranikian, G. Extremophiles as a source of novel enzymes for industrial application. Appl. Microbiol. Biotechnol. 1999, 51, 711–729. [Google Scholar] [CrossRef]
- Morozkina, E.V.; Slutskaya, E.S.; Fedorova, T.V.; Tugay, T.I.; Golubeva, L.I.; Koroleva, O.V. Extremophilic microorganisms: Biochemical adaptation and biotechnological application (review). Appl. Biochem. Microbiol. 2010, 46, 1–14. [Google Scholar] [CrossRef]
- Gupta, G.N.; Srivastava, S.; Khare, S.K.; Prakash, V. Extremophiles: An overview of microorganism from extreme environment. Intern J. Agric. Environ. Biotech. 2014, 7, 371. [Google Scholar] [CrossRef]
- Bonch-Osmolovskaya, E.; Atomi, H. Editorial overview: Extremophiles: From extreme environments to highly stable biocatalysts. Curr. Opin. Microbiol. 2015, 25, vii–viii. [Google Scholar] [CrossRef]
- LaRue, T.A.; Child, J.J. Bacterial utilization of a hydrazine derivative as nitrogen source for growth. Can. J. Microbiol. 1979, 25, 822–825. [Google Scholar] [CrossRef] [PubMed]
- DAKane Williamson, K.J. Bacterial toxicity and metabolism of hydrazine fuels. Arch. Environ. Contimination 1983, 12, 447–453. [Google Scholar]
- Al Ashhab, A.; Meshner, S.; Alexander-Shani, R.; Dimerets, H.; Brandwein, M.; Bar-Lavan, Y.; Winters, G. Temporal and spatial changes in phyllosphere microbiome of acacia trees growing in arid environments. Front. Microbiol. 2021, 12, 656269. [Google Scholar] [CrossRef]
- Lambais, M.R.; Barrera, S.E.; Santos, E.C.; Crowley, D.E.; Jumpponen, A. Phyllosphere metaproteomes of trees from the Brazilian Atlantic forest show high levels of functional redundancy. Microb. Ecol. 2017, 73, 123–134. [Google Scholar] [CrossRef]
- Batool, F.; Rehman, Y.; Hasnain, S. Phylloplane associated plant bacteria of commercially superior wheat varieties exhibit superior plant growth promoting abilities. Front. Life Sci. 2016, 9, 313–322. [Google Scholar] [CrossRef]
- Scavino, A.F.; Pedraza, R.O. The role of siderophores in plant growth-promoting bacteria. In Bacteria in Agrobiology: Crop Productivity; Maheshwari, D.K., Saraf, M., Aeron, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 265–285. [Google Scholar]
- Kouzuma, A.; Watanabe, K. Exploring the potential of algae/bacteria interactions. Curr. Opin. Biotechnol. 2015, 33, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Lant, P.; Pratt, S. The contribution of bacteria to algal growth by carbon cycling. Biotechnol. Bioeng. 2015, 112, 688–695. [Google Scholar] [CrossRef]
- Cole, J.J. Interactions between bacteria and algae in aquatic ecosystems. Annu. Rev. Ecol. Syst. 1982, 13, 291–314. [Google Scholar] [CrossRef]
- Qu, L.; Wang, R.; Zhao, P.; Chen, R.; Zhou, W.; Tang, L.; Tang, X. Interaction between Chlorella vulgaris and bacteria: Interference and resource competition. Acta Oceanol. Sin. 2014, 33, 135–140. [Google Scholar] [CrossRef]
- Guo, Z.; Tong, Y.W. The interactions between Chlorella vulgaris and algal symbiotic bacteria under photoautotrophic and photoheterotrophic conditions. J. Appl. Phycol. 2014, 26, 1483–1492. [Google Scholar] [CrossRef]
- Kim, H.J.; Choi, Y.K.; Jeon, H.J.; Bhatia, S.K.; Kim, Y.H.; Kim, Y.G.; Choi, K.Y.; Kim, H.J.; Lee, S.H.; Lee, Y.K.; et al. Growth promotion of Chlorella vulgaris by modification of nitrogen source composition with symbiotic bacteria, Microbacterium sp. HJ1. Biomass Bioenergy 2015, 74, 213–219. [Google Scholar] [CrossRef]
- Yilmaz, M.; Soran, H.; Beyatli, Y. Antimicrobial activities of some Bacillus spp. strains isolated from the soil. Microbiol. Res. 2006, 161, 127–131. [Google Scholar] [CrossRef]
- Velho, R.V.; Medina, L.F.C.; Segalin, J.; Brandelli, A. Production of lipopeptides among Bacillus strains showing growth inhibition of phytopathogenic fungi. Folia Microbiol. 2011, 56, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Fulbright, S.P.; Chisholm, S.; Reardon, K.F. Growth inhibition of Nannochloropsis species by Bacillus pumilus. Algal Res. 2016, 20, 70–76. [Google Scholar] [CrossRef]
- Ismail, A.; Ktari, L.; Ahmed, M.; Bolhuis, H.; Boudabbous, A.; Stal, L.J.; Cretoiu, M.S.; El Bour, M. Antimicrobial activities of bacteria associated with the brown alga Padina pavonica. Front. Microbiol. 2016, 7, 1072. [Google Scholar] [CrossRef] [PubMed]
- Rooney, A.P.; Price, N.P.J.; Ehrhardt, C.; Swezey, J.L.; Bannan, J.D. Phylogeny and molecular taxonomy of the Bacillus subtilis species complex and description of Bacillus subtilis subsp. inaquosorum subsp. nov. Int. J. Syst. Evol. Microbiol. 2009, 59 Pt 10, 2429–2436. [Google Scholar] [CrossRef]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef]
- Tamura, K.; Peterson, D.; Peterson, N.; Stecher, G.; Nei, M.; Kumar, S. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 2011, 28, 2731–2739. [Google Scholar] [CrossRef]
- Bold, H.C. The morphology of Chlamydomonas chlamydogama, Sp. Nov. Bull. Torrey Bot. Club 1949, 76, 101. [Google Scholar] [CrossRef]
- Godoy-Hernández, G.; Vázquez-Flota, F.A. Growth measurements: Estimation of cell division and cell expansion. Methods Mol. Biol. 2012, 877, 41–48. [Google Scholar]
- Gojon, C.; Dureault, B. Spectrophotometric study of the reaction between hydrazine and p.dimethylaminobenzaldehyde. J. Nucl. Sci. Technol. 1996, 33, 731–735. [Google Scholar] [CrossRef]
- Peters, M.; Kumpfert, J.; Ward, C.H.; Leyens, C. Titanium alloys for aerospace applications. In Titanium and Titanium Alloys: Fundamentals and Applications; Leyens, C., Peters, M., Eds.; Wiley-VCH Verlag GmbH & Co. KgaA: Weinheim, Germany, 2003; pp. 333–350. [Google Scholar]
- Sato, E.; Sawai, S.; Uesugi, K.; Takami, T.; Furukawa, K.; Kamada, M.; Kondo, M. Superplastic titanium tanks for propulsion system of satellites. In Superplasticity in Advanced Materials; Trans Tech Publications Ltd.: Stafa, Switzerland, 2007; pp. 43–48. [Google Scholar]
- Nguyen, H.N.; Chenoweth, J.A.; Bebarta, V.S.; Albertson, T.E.; Nowadly, C.D. The toxicity, pathophysiology, and treatment of acute hydrazine propellant exposure: A systematic review. Mil Med. 2021, 186, e319–e326. [Google Scholar] [CrossRef] [PubMed]
- Mantel, C.; London, S. Adaptation of a soil bacterium to hydrazine propellants. Bull. Environ. Contam. Toxicol. 1980, 25, 762–770. [Google Scholar] [CrossRef]
- Zekker, I.; Artemchuk, O.; Rikmann, E.; Ohimai, K.; Dhar Bhowmick, G.; Madhao Ghangrekar, M.; Burlakovs, J.; Tenno, T. Start-up of anammox SBR from non-specific inoculum and process acceleration methods by hydrazine. Water 2021, 13, 350. [Google Scholar] [CrossRef]
- Nwankwoala, A.U.; Egiebor, N.O.; Nyavor, K. Enhanced biodegradation of methylhydrazine and hydrazine contaminated NASA wastewater in fixed-film bioreactor. Biodegradation 2001, 12, 1–10. [Google Scholar] [CrossRef]
- Takada, S.T.; Gotou, H.I.; Mawatari, K.; Ishihara, N.; Kai, R. Alternatives to hydrazine in water treatment at thermal power plants. Mitsubishi Heavy Ind. Tech. Rev. 2009, 46, 43. [Google Scholar]
- Gaunt, H.; Wetton, E.A.M. The reaction between hydrazine and oxygen in water. J. Appl. Chem. 1966, 16, 171–176. [Google Scholar] [CrossRef]
- Peschek, G.A. Reduced sulfur and nitrogen compounds and molecular hydrogen as electron donors for anaerobic CO2 photoreduction in Anacystis nidulans. Arch. Microbiol. 1978, 119, 313–322. [Google Scholar] [CrossRef]
- Peschek, A. Electron Transport Chains in Oxygenic Cyanobacteria. In Primary Processes of Photosynthesis, Part 2: Principles and Apparatus; RSC Publishing: Cambridge, UK, 2007. [Google Scholar]
- Šeršeň, F.; Gregáň, F.; Peško, M.; Dvoranová, D.; Kráľová, K.; Matkovičová, Z.; Gregáň, J.; Donovalová, J. Synthesis and herbicidal activity of new hydrazide and hydrazonoyl derivatives. Molecules 2015, 20, 14139–14154. [Google Scholar] [CrossRef]
- Messinger, J.; Renger, G. The reactivity of hydrazine with photosystem II strongly depends on the redox state of the water oxidizing system. FEBS Lett. 1990, 277, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Canaani, O.; Havaux, M.; Malkin, S. Hydroxylamine, hydrazine and methylamine donate electrons to the photooxidizing side of Photosystem II in leaves inhibited in oxygen evolution due to water stress. Biochim. Biophys. Acta 1986, 851, 151–155. [Google Scholar] [CrossRef]
- Eimoori, R.; Zolala, J.; Pourmohiabadi, H.; Noroozian, E.; Mansouri, H. Contribution of Azolla filiculoides to hydrazine elimination from water. Wetl. Ecol. Manag. 2020, 28, 439–447. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kinel-Tahan, Y.; Sorek-Abramovich, R.; Alexander-Shani, R.; Shoval, I.; Hauschner, H.; Corsia, C.; Kedar, A.Z.; Derzy, I.; Sapir, I.; Mastai, Y.; et al. Cometabolic Biodegradation of Hydrazine by Chlorella vulgaris–Bacillus Extremophilic Consortia: Synergistic Potential for Space and Industry. Life 2025, 15, 1197. https://doi.org/10.3390/life15081197
Kinel-Tahan Y, Sorek-Abramovich R, Alexander-Shani R, Shoval I, Hauschner H, Corsia C, Kedar AZ, Derzy I, Sapir I, Mastai Y, et al. Cometabolic Biodegradation of Hydrazine by Chlorella vulgaris–Bacillus Extremophilic Consortia: Synergistic Potential for Space and Industry. Life. 2025; 15(8):1197. https://doi.org/10.3390/life15081197
Chicago/Turabian StyleKinel-Tahan, Yael, Reut Sorek-Abramovich, Rivka Alexander-Shani, Irit Shoval, Hagit Hauschner, Chen Corsia, Ariel Z. Kedar, Igor Derzy, Itsik Sapir, Yitzhak Mastai, and et al. 2025. "Cometabolic Biodegradation of Hydrazine by Chlorella vulgaris–Bacillus Extremophilic Consortia: Synergistic Potential for Space and Industry" Life 15, no. 8: 1197. https://doi.org/10.3390/life15081197
APA StyleKinel-Tahan, Y., Sorek-Abramovich, R., Alexander-Shani, R., Shoval, I., Hauschner, H., Corsia, C., Kedar, A. Z., Derzy, I., Sapir, I., Mastai, Y., Al Ashhab, A., & Yehoshua, Y. (2025). Cometabolic Biodegradation of Hydrazine by Chlorella vulgaris–Bacillus Extremophilic Consortia: Synergistic Potential for Space and Industry. Life, 15(8), 1197. https://doi.org/10.3390/life15081197








