The Effects of Low-Load Resistance Training Combined with Blood Flow Restriction or Hypoxia on Cardiovascular Response: A Randomized Controlled Trial
Abstract
1. Introduction
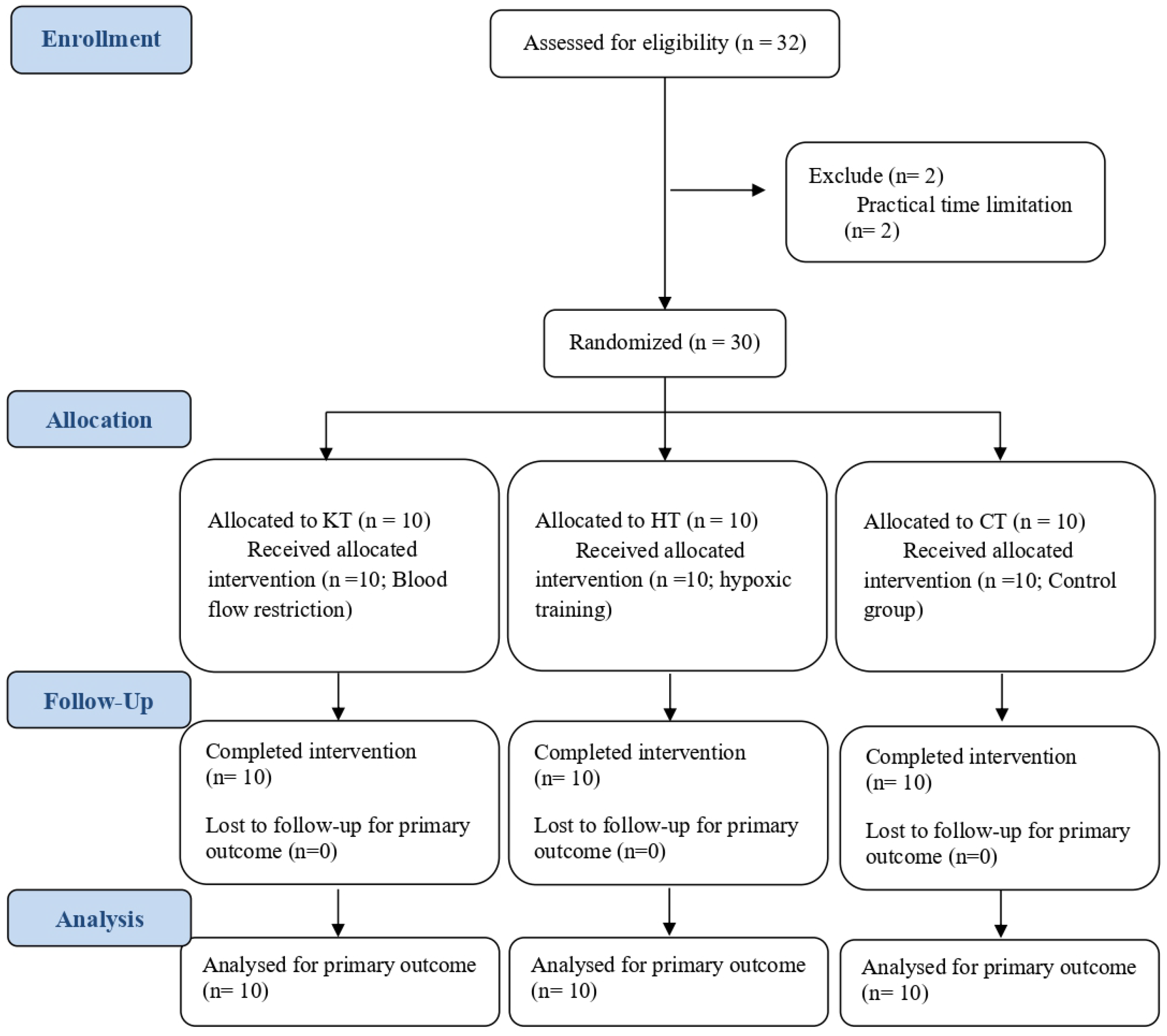
2. Materials and Methods
2.1. Participants
2.2. Inclusion and Exclusion Criteria
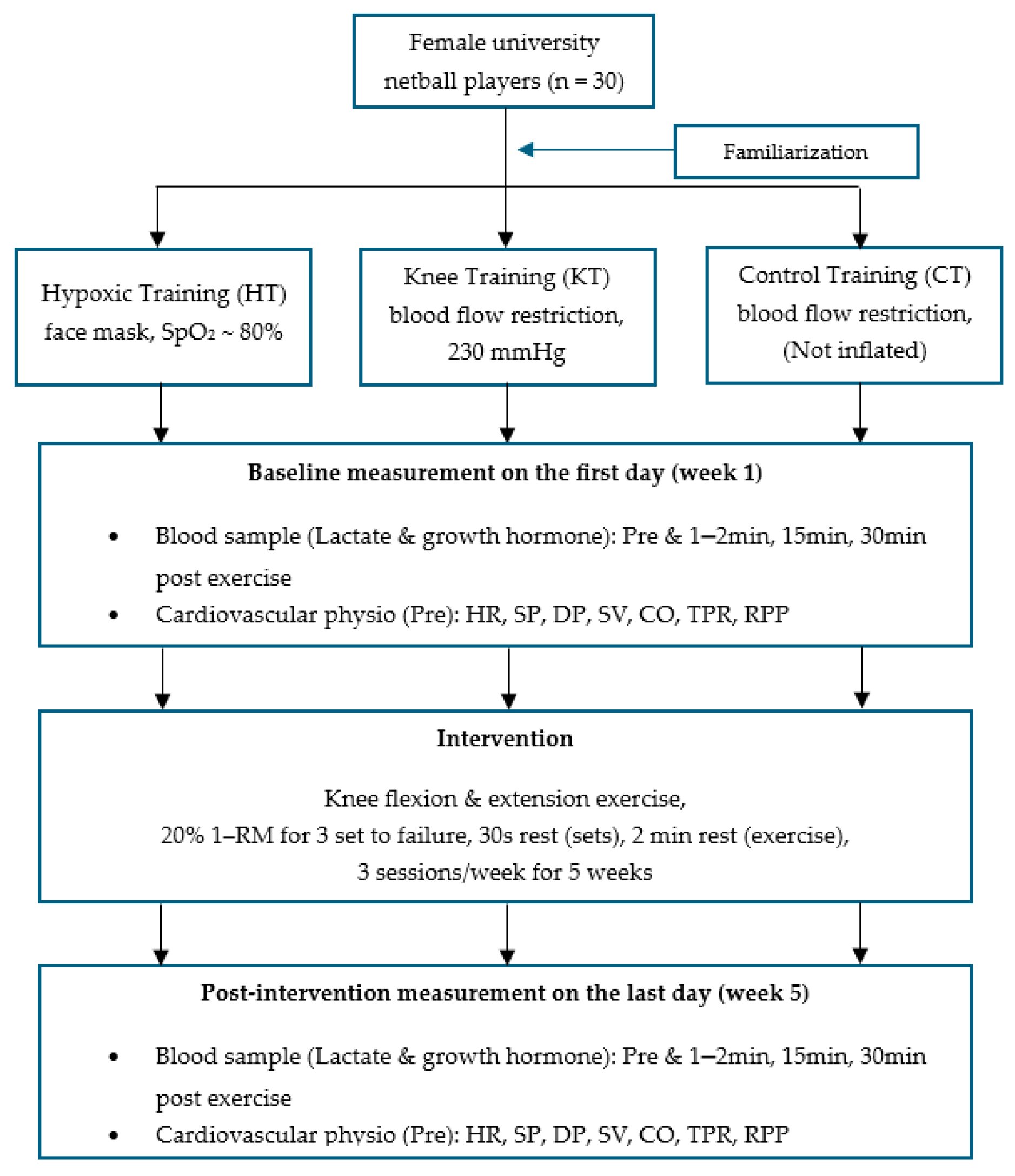
2.3. Protocol
2.4. Measurement
2.5. Statistical Analysis
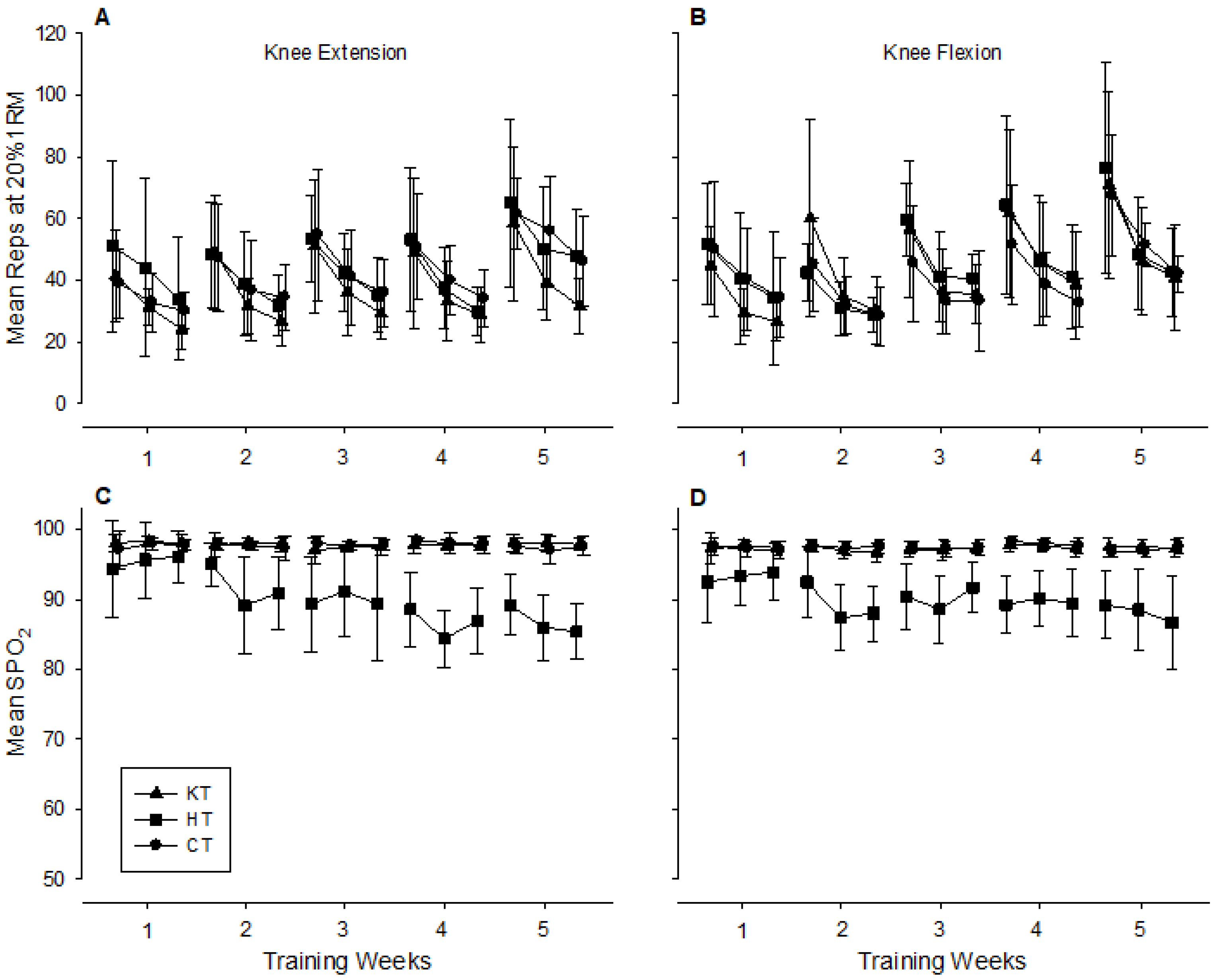
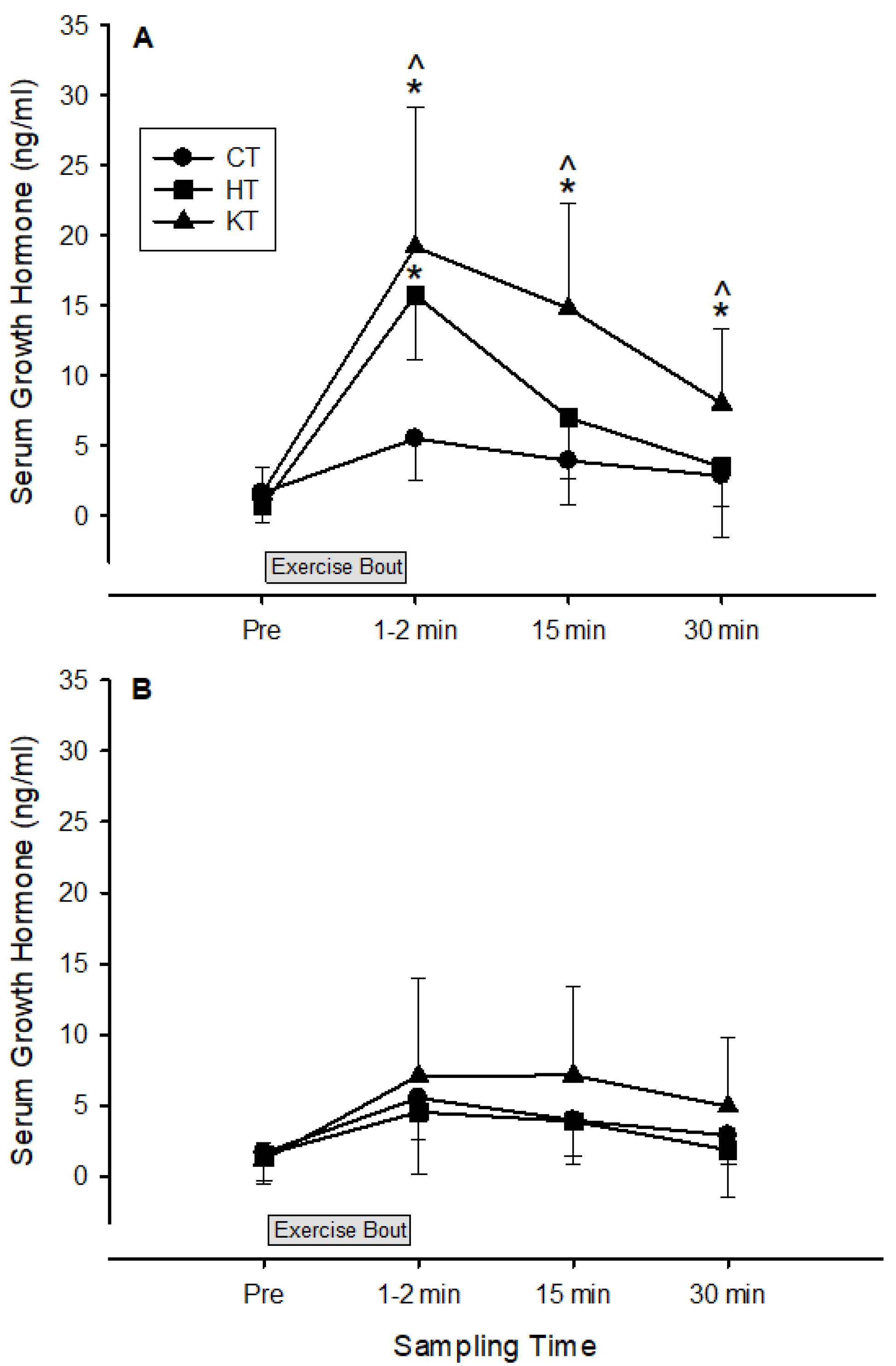
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CSA | Cross-Sectional Area |
| 1-RM | 1 Repetition Maximum |
| MVC | Maximal Voluntary Contraction |
| SBP | Systolic Blood Pressure |
| DBP | Diastolic Blood Pressure |
| MAP | Mean Arterial Pressure |
| SV | Stroke Volume |
| LVET | Left Ventricular Ejection Time |
| NO | Nitric Oxide |
References
- Ratamess, N.A.; Alvar, B.A.; Evetoch, T.K.; Housh, T.J.; Kibler, W.B.; Kraemer, W.J.; Tripplet, N.T. Progression Models in Resistance Training for Healthy Adults. Med. Sci. Sports Exerc. 2009, 41, 687–708. [Google Scholar] [CrossRef]
- Abe, T.; Kearns, C.F.; Sato, Y. Muscle Size and Strength Are Increased Following Walk Training with Restricted Venous Blood Flow from the Leg Muscle, Kaatsu-Walk Training. J. Appl. Physiol. 2006, 100, 1460–1466. [Google Scholar] [CrossRef]
- Wernbom, M.; Augustsson, J.; Raastad, T. Ischemic Strength Training: A Low-Load Alternative to Heavy Resistance Exercise? Scand. J. Med. Sci. Sports 2008, 18, 401–416. [Google Scholar] [CrossRef]
- Takarada, Y.; Nakamura, Y.; Aruga, S.; Onda, T.; Miyazaki, S.; Ishii, N. Rapid Increase in Plasma Growth Hormone after Low-Intensity Resistance Exercise with Vascular Occlusion. J. Appl. Physiol. 2000, 88, 61–65. [Google Scholar] [CrossRef]
- Sjøgaard, G. Physiology in Bicycling; Mouvement Pubns: Ithaca, NY, USA, 1985. [Google Scholar]
- Millar, I.D.; Barber, M.C.; Lomax, M.A.; Travers, M.T.; Shennan, D.B. Mammary Protein Synthesis Is Acutely Regulated by the Cellular Hydration State. Biochem. Biophys. Res. Commun. 1997, 230, 351–355. [Google Scholar] [CrossRef]
- Kawada, S.; Ishii, N. Skeletal Muscle Hypertrophy After Chronic Restriction of Venous Blood Flow in Rats. Med. Sci. Sports Exerc. 2005, 37, 1144–1150. [Google Scholar] [CrossRef]
- Hollander, D.B.; Reeves, G.V.; Clavier, J.D.; Francois, M.R.; Thomas, C.; Kraemer, R.R. Partial Occlusion During Resistance Exercise Alters Effort Sense and Pain. J. Strength Cond. Res. 2010, 24, 235–243. [Google Scholar] [CrossRef]
- Wernbom, M.; Augustsson, J.; Thomeé, R. Effects of Vascular Occlusion on Muscular Endurance in Dynamic Knee Extension Exercise at Different Submaximal Loads. J. Strength Cond. Res. 2006, 20, 372. [Google Scholar] [CrossRef]
- Wernbom, M.; Järrebring, R.; Andreasson, M.A.; Augustsson, J. Acute Effects of Blood Flow Restriction on Muscle Activity and Endurance During Fatiguing Dynamic Knee Extensions at Low Load. J. Strength Cond. Res. 2009, 23, 2389–2395. [Google Scholar] [CrossRef]
- Thijssen, D.H.J.; Dawson, E.A.; Tinken, T.M.; Cable, N.T.; Green, D.J. Retrograde Flow and Shear Rate Acutely Impair Endothelial Function in Humans. Hypertension 2009, 53, 986–992. [Google Scholar] [CrossRef]
- Patterson, S.D.; Hughes, L.; Warmington, S.; Burr, J.; Scott, B.R.; Owens, J.; Abe, T.; Nielsen, J.L.; Libardi, C.A.; Laurentino, G.; et al. Blood Flow Restriction Exercise: Considerations of Methodology, Application, and Safety. Front. Physiol. 2019, 10, 533. [Google Scholar] [CrossRef]
- Gamonales, J.M.; Rojas-Valverde, D.; Vásquez, J.; Martínez-Guardado, I.; Azofeifa-Mora, C.; Sánchez-Ureña, B.; Ibáñez, S.J. An Update to a Comprehensive Assessment of the Methods and Effectiveness of Resistance Training in Normobaric Hypoxia for the Development of Strength and Muscular Hypertrophy. Appl. Sci. 2023, 13, 1078. [Google Scholar] [CrossRef]
- Manimmanakorn, A.; Manimmanakorn, N.; Taylor, R.; Draper, N.; Billaut, F.; Shearman, J.P.; Hamlin, M.J. Effects of Resistance Training Combined with Vascular Occlusion or Hypoxia on Neuromuscular Function in Athletes. Eur. J. Appl. Physiol. 2013, 113, 1767–1774. [Google Scholar] [CrossRef] [PubMed]
- Downs, M.E.; Hackney, K.J.; Martin, D.; Caine, T.L.; Cunningham, D.; O’connor, D.P.; Ploutz-Snyder, L.L. Acute Vascular and Cardiovascular Responses to Blood Flow–Restricted Exercise. Med. Sci. Sports Exerc. 2014, 46, 1489–1497. [Google Scholar] [CrossRef] [PubMed]
- Kon, M.; Ikeda, T.; Homma, T.; Akimoto, T.; Suzuki, Y.; Kawahara, T. Effects of Acute Hypoxia on Metabolic and Hormonal Responses to Resistance Exercise. Med. Sci. Sports Exerc. 2010, 42, 1279–1285. [Google Scholar] [CrossRef] [PubMed]
- Bonetti, D.L.; Hopkins, W.G.; Kilding, A.E. High-Intensity Kayak Performance After Adaptation to Intermittent Hypoxia. Int. J. Sports Physiol. Perform. 2006, 1, 246–260. [Google Scholar] [CrossRef]
- Batterham, A.M.; Hopkins, W.G. Making Meaningful Inferences About Magnitudes. Int. J. Sports Physiol. Perform. 2006, 1, 50–57. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Routledge: Oxfordshire, UK, 2013; ISBN 9781134742707. [Google Scholar]
- Nishimura, A.; Sugita, M.; Kato, K.; Fukuda, A.; Sudo, A.; Uchida, A. Hypoxia Increases Muscle Hypertrophy Induced by Resistance Training. Int. J. Sports Physiol. Perform. 2010, 5, 497–508. [Google Scholar] [CrossRef]
- Laurentino, G.C.; Ugrinowitsch, C.; Roschel, H.; Aoki, M.S.; Soares, A.G.; Neves, M.; Aihara, A.Y.; Da Rocha Correa Fernandes, A.; Tricoli, V. Strength Training with Blood Flow Restriction Diminishes Myostatin Gene Expression. Med. Sci. Sports Exerc. 2012, 44, 406–412. [Google Scholar] [CrossRef]
- Chilibeck, P.D.; Calder, A.W.; Sale, D.G.; Webber, C.E. A Comparison of Strength and Muscle Mass Increases During Resistance Training in Young Women. Eur. J. Appl. Physiol. 1997, 77, 170–175. [Google Scholar] [CrossRef]
- Friedmann, B.; Kinscherf, R.; Borisch, S.; Richter, G.; Bärtsch, P.; Billeter, R. Effects of Low-Resistance/High-Repetition Strength Training in Hypoxia on Muscle Structure and Gene Expression. Pflugers Arch. 2003, 446, 742–751. [Google Scholar] [CrossRef] [PubMed]
- Cardozo, G.G.; Lopes, K.G.; Bottino, D.A.; de Souza, M.D.G.C.; Bouskela, E.; Farinatti, P.; de Oliveira, R.B. Acute Effects of Physical Exercise with Different Levels of Blood Flow Restriction on Vascular Reactivity and Biomarkers of Muscle Hypertrophy, Endothelial Function and Oxidative Stress in Young and Elderly Subjects—A Randomized Controlled Protocol. Contemp. Clin. Trials Commun. 2021, 22, 100740. [Google Scholar] [CrossRef] [PubMed]
- Kraemer, W.J.; Marchitelli, L.; Gordon, S.E.; Harman, E.; Dziados, J.E.; Mello, R.; Frykman, P.; McCurry, D.; Fleck, S.J. Hormonal and Growth Factor Responses to Heavy Resistance Exercise Protocols. J. Appl. Physiol. 1990, 69, 1442–1450. [Google Scholar] [CrossRef]
- Kraemer, W.J.; Fleck, S.J.; Dziados, J.E.; Harman, E.A.; Marchitelli, L.J.; Gordon, S.E.; Mello, R.; Frykman, P.N.; Koziris, L.P.; Triplett, N.T. Changes in Hormonal Concentrations After Different Heavy-Resistance Exercise Protocols in Women. J. Appl. Physiol. 1993, 75, 594–604. [Google Scholar] [CrossRef]
- Vanhelder, W.P.; Radomski, M.W.; Goode, R.C. Growth Hormone Responses During Intermittent Weight Lifting Exercise in Men. Eur. J. Appl. Physiol. Occup. Physiol. 1984, 53, 31–34. [Google Scholar] [CrossRef]
- Gordon, S.E.; Kraemer, W.J.; Vos, N.H.; Lynch, J.M.; Knuttgen, H.G. Effect of Acid-Base Balance on the Growth Hormone Response to Acute High-Intensity Cycle Exercise. J. Appl. Physiol. 1994, 76, 821–829. [Google Scholar] [CrossRef]
- Fernandes, R.V.; Tricoli, V.; Soares, A.G.; Miyabara, E.H.; Aoki, M.S.; Laurentino, G. Low-Load Resistance Exercise with Blood Flow Restriction Increases Hypoxia-Induced Angiogenic Genes Expression. J. Hum. Kinet. 2022, 84, 82–91. [Google Scholar] [CrossRef]
- Laurentino, G.; Loenneke, J.; Ugrinowitsch, C.; Aoki, M.; Soares, A.; Roschel, H.; Tricoli, V. Blood-Flow-Restriction-Training-Induced Hormonal Response Is Not Associated with Gains in Muscle Size and Strength. J. Hum. Kinet. 2022, 83, 235–243. [Google Scholar] [CrossRef]
- Horiuchi, M.; Ni-I-Nou, A.; Miyazaki, M.; Ando, D.; Koyama, K. Impact of Resistance Exercise Under Hypoxia on Postexercise Hemodynamics in Healthy Young Males. Int. J. Hypertens. 2018, 2018, 1456972. [Google Scholar] [CrossRef]
- Auer, J.; Berent, R.; Prenninger, M.; Weber, T.; Kritzinger, K.; Veits, M.; Lamm, G.; Lassnig, E.; Eber, B. Short-Term Effects of a Single Exercise Bout at Moderate Altitude on Blood Pressure. J. Sport. Rehabil. 2004, 13, 19–30. [Google Scholar] [CrossRef]
- Greie, S.; Humpeler, E.; Gunga, H.C.; Koralewski, E.; Klingler, A.; Mittermayr, M.; Fries, D.; Lechleitner, M.; Hoertnagl, H.; Hoffmann, G.; et al. Improvement of Metabolic Syndrome Markers Through Altitude Specific Hiking Vacations. J. Endocrinol. Investig. 2006, 29, 497–504. [Google Scholar] [CrossRef]
- Bailey, D.M.; Davies, B.; Baker, J. Training in Hypoxia: Modulation of Metabolic and Cardiovascular Risk Factors in Men. Med. Sci. Sports Exerc. 2000, 32, 1058–1066. [Google Scholar] [CrossRef]
- Lyamina, N.P.; Lyamina, S.V.; Senchiknin, V.N.; Mallet, R.T.; Downey, H.F.; Manukhina, E.B. Normobaric Hypoxia Conditioning Reduces Blood Pressure and Normalizes Nitric Oxide Synthesis in Patients with Arterial Hypertension. J. Hypertens. 2011, 29, 2265–2272. [Google Scholar] [CrossRef] [PubMed]
- Jung, W.-S.; Kim, S.-W.; Kim, J.-W.; Park, H.-Y. Resistance Training in Hypoxia as a New Therapeutic Modality for Sarcopenia—A Narrative Review. Life 2021, 11, 106. [Google Scholar] [CrossRef] [PubMed]
- Hurley, R.S.; Bossetti, B.M.; O’Dorisio, T.M.; Welch, M.A.; Rice, R.R.; Tenison, E.B.; Wasson, C.J.; Malarkey, W.B. The Response of Serum Growth Hormone and Prolactin to Training in Weight-Maintaining Healthy Males. J. Sports Med. Phys. Fit. 1990, 30, 45–48. [Google Scholar]
- Mccall, G.E.; Byrnes, W.C.; Fleck, S.J.; Dickinson, A.; Kraemer, W.J. Acute and Chronic Hormonal Responses to Resistance Training Designed to Promote Muscle Hypertrophy. Can. J. Appl. Physiol. 1999, 24, 96–107. [Google Scholar] [CrossRef]
- Craig, B.W.; Brown, R.; Everhart, J. Effects of Progressive Resistance Training on Growth Hormone and Testosterone Levels in Young and Elderly Subjects. Mech. Ageing Dev. 1989, 49, 159–169. [Google Scholar] [CrossRef]
- Wideman, R. Cardio-Pulmonary Hemodynamics and Ascites in Broiler Chickens. Avian Poult. Biol. Rev. 2000, 11, 21–43. [Google Scholar]
- Hartley, L.H.; Mason, J.W.; Hogan, R.P.; Jones, L.G.; Kotchen, T.A.; Mougey, E.H.; Wherry, F.E.; Pennington, L.L.; Ricketts, P.T. Multiple Hormonal Responses to Graded Exercise in Relation to Physical Training. J. Appl. Physiol. 1972, 33, 602–606. [Google Scholar] [CrossRef]
- Weltman, A.; Weltman, J.Y.; Womack, C.J.; Davis, S.E.; Blumer, J.L.; Gaesser, G.A.; Hartman, M.L. Exercise Training Decreases the Growth Hormone (GH) Response to Acute Constant-Load Exercise. Med. Sci. Sports Exerc. 1997, 29, 669–676. [Google Scholar] [CrossRef]

| Muscles | Effect of KT (KT vs. CT) (cm2, ±90% CL) | Practical Outcome | Effect of HT (HT vs. CT) (cm2, ±90% CL) | Practical Outcome |
|---|---|---|---|---|
| Extensors | ||||
| Rectus femoris | 2.1 ± 1.6 | Very likely | 0.7 ± 1.1 | Unclear |
| Vastus lateralis | 1.7 ± 1.6 | Likely | 0.4 ± 0.9 | Unclear |
| Vastus medialis | 1.7 ± 1.5 | Likely | 0.4 ± 1.2 | Unclear |
| Flexors | ||||
| Biceps femoris | 0.7 ± 0.8 | Likely | 1.3 ± 0.9 | Very likely |
| Semitendinosus | 1.1 ± 0.8 | Very likely | 1.1 ± 0.8 | Very likely |
| Semimembranosus | 0.7 ± 0.7 | Likely | 1.4 ± 0.8 | Very likely |
| Parameters | Effect of KT (KT vs. CT) (±90% CL) | Practical Outcome | Effect of HT (HT vs. CT) (±90% CL) | Practical Outcome |
|---|---|---|---|---|
| Systolic (mmHg) | −1.8 ± 8.9 | Unclear | −11.7 ± 11.3 | Almost certainly |
| Diastolic (mmHg) | 9.8 ± 10.8 | Likely | 1.6 ± 12.5 | Unclear |
| Mean (mmHg) | 5.7 ± 6.5 | Likely | 2.4 ± 11.7 | Unclear |
| Heart Rate (bpm) | −2.4 ± 15.1 | Unclear | 1.3 ± 12.1 | Unclear |
| Stroke Volume (mL) | −17.2 ± 19.5 | Likely | −1.2 ± 19.7 | Unclear |
| LVET (ms) | −3.8 ± 6.9 | Unclear | 4.6 ± 5.9 | Very likely |
| Pulse Interval (ms) | 0.5 ± 17.1 | Unclear | −2.8 ± 13.8 | Unclear |
| Maximum Slope (mmHg/s) | 1.2 ± 18.3 | Unclear | 8.3 ± 27.4 | Unclear |
| Cardiac Output (L/min) | −18.0 ± 21.9 | Likely | 6.4 ± 23.9 | Unclear |
| TPR (dyn·s/cm5) | 68.8 ± 40.4 | Very likely | −1.3 ± 37.0 | Unclear |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manimmanakorn, A.; Manimmanakorn, P.; Srisaphonphusitti, L.; Sumethanurakkhakun, W.; Nithisup, P.; Muangritdech, N.; Thuwakum, W. The Effects of Low-Load Resistance Training Combined with Blood Flow Restriction or Hypoxia on Cardiovascular Response: A Randomized Controlled Trial. Life 2025, 15, 1162. https://doi.org/10.3390/life15081162
Manimmanakorn A, Manimmanakorn P, Srisaphonphusitti L, Sumethanurakkhakun W, Nithisup P, Muangritdech N, Thuwakum W. The Effects of Low-Load Resistance Training Combined with Blood Flow Restriction or Hypoxia on Cardiovascular Response: A Randomized Controlled Trial. Life. 2025; 15(8):1162. https://doi.org/10.3390/life15081162
Chicago/Turabian StyleManimmanakorn, Apiwan, Pudis Manimmanakorn, Lertwanlop Srisaphonphusitti, Wirakan Sumethanurakkhakun, Peeraporn Nithisup, Nattha Muangritdech, and Worrawut Thuwakum. 2025. "The Effects of Low-Load Resistance Training Combined with Blood Flow Restriction or Hypoxia on Cardiovascular Response: A Randomized Controlled Trial" Life 15, no. 8: 1162. https://doi.org/10.3390/life15081162
APA StyleManimmanakorn, A., Manimmanakorn, P., Srisaphonphusitti, L., Sumethanurakkhakun, W., Nithisup, P., Muangritdech, N., & Thuwakum, W. (2025). The Effects of Low-Load Resistance Training Combined with Blood Flow Restriction or Hypoxia on Cardiovascular Response: A Randomized Controlled Trial. Life, 15(8), 1162. https://doi.org/10.3390/life15081162







