Staphylococcus Strains in Atopic Dermatitis in Children: Toxins Production and Resistance Properties
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Microbial Isolation Patterns
- ○
- S. aureus: 160 strains (53.3%).
- ○
- CoNS: 140 strains (46.6%):
- ▪
- S. epidermidis: 87 (29.0%);
- ▪
- S. haemolyticus: 22 (7.3%);
- ▪
- S. hominis: 15 (5.0%);
- ▪
- S. capitis: 7 (2.3%);
- ▪
- S. warneri: 5 (1.7%);
- ▪
- S. cohnii: 2 (0.7%);
- ▪
- Single isolates of S. simulans and S. saprophyticus.
- ○
- Most frequent: S. aureus + S. epidermidis (n = 29);
- ○
- Moderate frequency: S. epidermidis + S. haemolyticus (n = 5), S. aureus + S. haemolyticus (n = 4), S. epidermidis + S. hominis (n = 4);
- ○
- Rare associations (n = 1 each): S. aureus + S. saprophyticus/capitis, S. epidermidis + S. warneri/cohnii/capitis, S. haemolyticus + S. capitis;
- ○
- Triple colonization: S. aureus + S. epidermidis + S. haemolyticus/hominis.
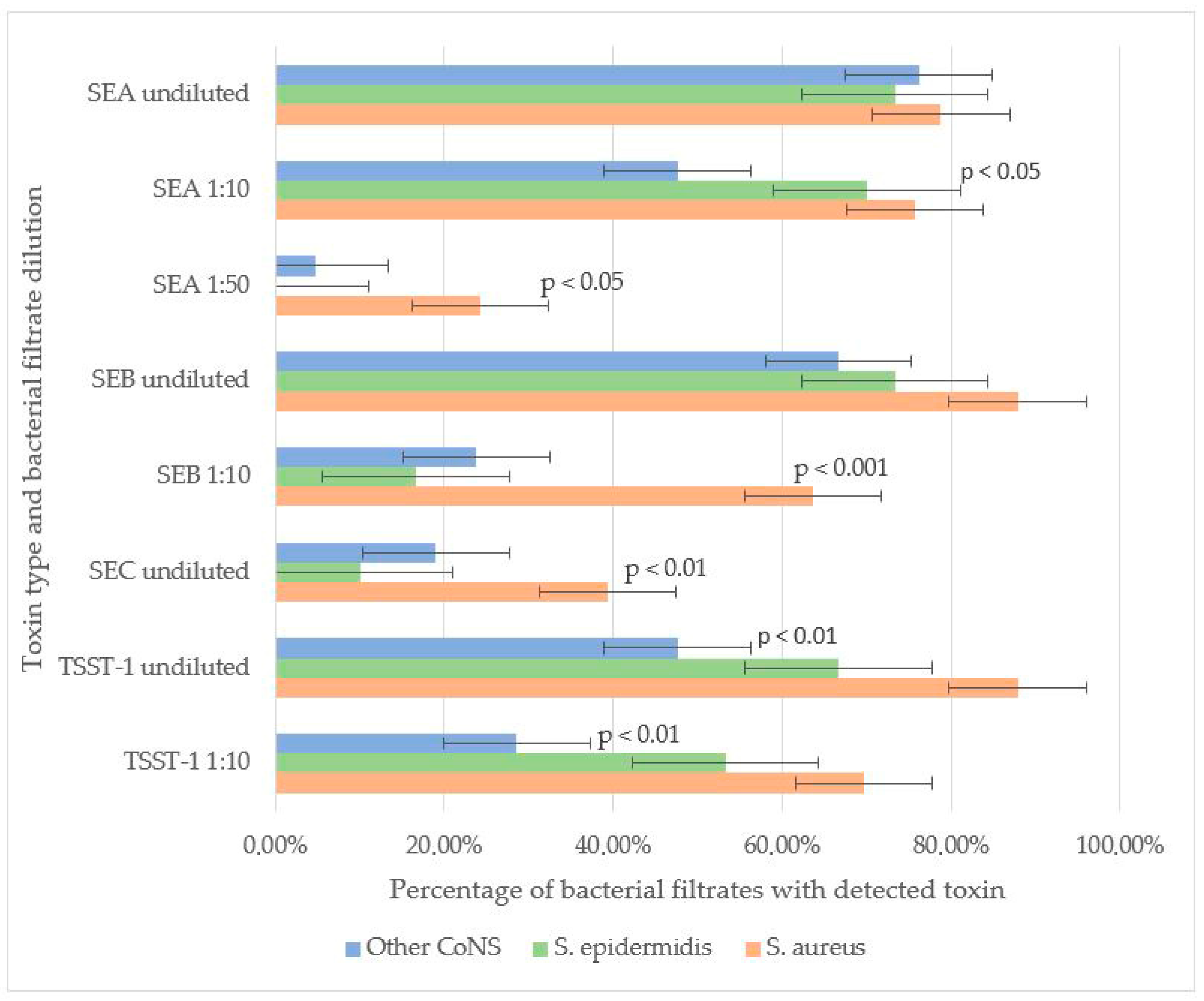
3.2. Toxin-Producing Properties of CoNS vs. CoPS
3.3. Antibiotic Susceptibility of CoNS vs. CoPS
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AD | Atopic dermatitis |
| CoNS | Coagulase-negative staphylococci |
| MRSA | Methicillin-resistant Staphylococcus aureus |
| MRSE | Methicillin-resistant Staphylococcus epidermidis |
| CoPS | Coagulase-positive staphylococci |
| SEA | Staphylococcal enterotoxin A |
| SEB | Staphylococcal enterotoxin B |
| SEC | Staphylococcal enterotoxin C |
| TSST-1 | Toxic shock syndrome toxin 1 |
References
- Langan, S.M.; Irvine, A.D.; Weidinger, S. Atopic dermatitis. Lancet 2020, 396, 345–360. [Google Scholar] [CrossRef]
- Bay, L.; Barnes, C.J.; Fritz, B.G.; Ravnborg, N.; Ruge, I.F.; Halling-Sønderby, A.-S.; Søeborg, S.R.; Langhoff, K.H.; Lex, C.; Hansen, A.J.; et al. Unique dermal bacterial signature differentiates atopic dermatitis skin from healthy. Msphere 2025, 10, e0015625. [Google Scholar] [CrossRef]
- Wang, Z.; Hülpüsch, C.; Traidl-Hoffmann, C.; Reiger, M.; Schloter, M. Understanding the role of Staphylococcus aureus in atopic dermatitis: Strain diversity, microevolution, and prophage influences. Front. Med. 2024, 11, 1480257. [Google Scholar] [CrossRef]
- Özdemir, E.; Öksüz, L. Effect of Staphylococcus aureus colonization and immune defects on the pathogenesis of atopic dermatitis. Arch. Microbiol. 2024, 206, 410. [Google Scholar] [CrossRef]
- Schachner, L.A.; Andriessen, A.; Gonzalez, M.E.; Lal, K.; Hebert, A.A.; Eichenfield, L.F.; Lio, P. A Consensus on Staphylococcus aureus Exacerbated Atopic Dermatitis and the Need for a Novel Treatment. J. Drugs Dermatol. JDD 2024, 23, 825–832. [Google Scholar] [CrossRef]
- Svitich, O.A.; Soboleva, V.A.; Abramova, N.D.; Gelezhe, K.A.; Kudryavtseva, A.V. Expression of HNP1 gene in children with atopic dermatitis. Vopr. Prakt. Pediatr. 2022, 17, 31–36. [Google Scholar] [CrossRef]
- Ogonowska, P.; Gilaberte, Y.; Barańska-Rybak, W.; Nakonieczna, J. Colon. with Staphylococcus aureus in Atopic Dermatitis Patients: Attempts to Reveal the Unknown. Front. Microbiol. 2021, 11, 567090. [Google Scholar] [CrossRef]
- Edslev, S.M.; Olesen, C.M.; Nørreslet, L.B.; Ingham, A.C.; Iversen, S.; Lilje, B.; Clausen, M.L.; Jensen, J.S.; Stegger, M.; Agner, T.; et al. Staphylococcal Communities on Skin Are Associated with Atopic Dermatitis and Disease Severity. Microorganisms 2021, 9, 432. [Google Scholar] [CrossRef]
- Kong, H.H.; Oh, J.; Deming, C.; Conlan, S.; Grice, E.A.; Beatson, M.A.; Nomicos, E.; Polley, E.C.; Komarow, H.D.; NISC Comparative Sequence Program; et al. Temporal shifts in the skin microbiome associated with disease flares and treatment in children with atopic dermatitis. Genome Res. 2012, 22, 850–859. [Google Scholar] [CrossRef] [PubMed]
- Gelezhe, K.A.; Kudryavtseva, A.V.; Svitich, O.A. Dynamics of skin colonization by staphylococcus spp. in children and adolescents with atopic dermatitis. Pediatr. J. Named After G.N. Speransky 2019, 98, 88–93. [Google Scholar] [CrossRef]
- Guimarães, L.C.; Garcia, G.D.; Cavalcante, F.S.; Dias, G.M.; de Farias, F.M.; Saintive, S.; Abad, E.D.; Ferreira, D.C.; Dos Santos, K.R.N. Methicillin-resistant Staphylococcus aureus and coagulase-negative Staphylococcus produce antimicrobial substances against members of the skin microbiota in children with atopic dermatitis. FEMS Microbiol. Ecol. 2024, 100, fiae070. [Google Scholar] [CrossRef] [PubMed]
- Lei, X.; Ring, S.; Jin, S.; Singh, S.; Mahnke, K. Extracellular Vesicles and Their Role in Skin Inflammatory Diseases: From Pathogenesis to Therapy. Int. J. Mol. Sci. 2025, 26, 3827. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Seiti Yamada Yoshikawa, F.; Feitosa de Lima, J.; Notomi Sato, M.; Álefe Leuzzi Ramos, Y.; Aoki, V.; Leao Orfali, R. Exploring the Role of Staphylococcus aureus Toxins in Atopic Dermatitis. Toxins 2019, 11, 321. [Google Scholar] [CrossRef] [PubMed]
- Bier, K.; Schittek, B. Beneficial effects of coagulase-negative Staphylococci on Staphylococcus aureus skin colonization. Exp. Dermatol. 2021, 30, 1442–1452. [Google Scholar] [CrossRef]
- Chajęcka-Wierzchowska, W.; Gajewska, J.; Wiśniewski, P.; Zadernowska, A. Enterotoxigenic Potential of Coagulase-Negative Staphylococci from Ready-to-Eat Food. Pathogens 2020, 9, 734. [Google Scholar] [CrossRef]
- Saheb Kashaf, S.; Harkins, C.P.; Deming, C.; Joglekar, P.; Conlan, S.; Holmes, C.J.; NISC Comparative Sequencing Program; Almeida, A.; Finn, R.D.; Segre, J.A.; et al. Staphylococcal diversity in atopic dermatitis from an individual to a global scale. Cell Host Microbe 2023, 31, 578–592.e6. [Google Scholar] [CrossRef]
- Nakashima, C.; Otsuka, A.; Kabashima, K. Interleukin-31 and interleukin-31 receptor: New therapeutic targets for atopic dermatitis. Exp. Dermatol. 2018, 27, 327–331. [Google Scholar] [CrossRef]
- Di Domenico, E.G.; Cavallo, I.; Bordignon, V.; Prignano, G.; Sperduti, I.; Gurtner, A.; Trento, E.; Toma, L.; Pimpinelli, F.; Capitanio, B.; et al. Inflammatory cytokines and biofilm production sustain Staphylococcus aureus outgrowth and persistence: A pivotal interplay in the pathogenesis of Atopic Dermatitis. Sci. Rep. 2018, 8, 9573. [Google Scholar] [CrossRef]
- Abdurrahman, G.; Schmiedeke, F.; Bachert, C.; Bröker, B.M.; Holtfreter, S. Allergy-A New Role for T Cell Superantigens of Staphylococcus aureus? Toxins 2020, 12, 176. [Google Scholar] [CrossRef]
- Hon, K.L.; Tsang, K.Y.; Kung, J.S.; Leung, T.F.; Lam, C.W.; Wong, C.K. Clinical Signs, Staphylococcus and Atopic Eczema-Related Seromarkers. Molecules 2017, 22, 291. [Google Scholar] [CrossRef]
- Fišarová, L.; Botka, T.; Du, X.; Mašlaňová, I.; Bárdy, P.; Pantůček, R.; Benešík, M.; Roudnický, P.; Winstel, V.; Larsen, J.; et al. Staphylococcus epidermidis Phages Transduce Antimicrobial Resistance Plasmids and Mobilize Chromosomal Islands. Msphere 2021, 6, e00223-21. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, K.; Moriwaki, M.; Miyake, R.; Hide, M. Staphylococcus aureus in atopic dermatitis: Strain-specific cell wall proteins and skin immunity. Allergol. Int. Off. J. Jpn. Soc. Allergol. 2019, 68, 309–315. [Google Scholar] [CrossRef]
- Stadler, J.F. Severity scoring of atopic dermatitis: The SCORAD index. Consensus Report of the European Task Force on Atopic Dermatitis. Dermatology 1993, 186, 23–31. [Google Scholar] [CrossRef]
- Fluer, F.S.; Prokhorov, V.I.; Vesnina, A.F. Enzyme immunoassay system for detection of staphylococcal enterotoxin, type C. Zhurnal Mikrobiol. Epidemiol. Immunobiol. 2002, 6, 65–68. [Google Scholar]
- Akatov, A.K.; Fluer, F.S.; Mikheeva, G.V.; Pavlova, I.P.; Chachanina, K.L.; Bobkova, E.V.; Melnikov, N.V. Enzyme immunoassay system for detection of staphylococcal enterotoxin, type A. Temporarily Pharm. Norms Regul. 1989, 89, 42–235. [Google Scholar]
- Akatov, A.K.; Fluer, F.S.; Mikheeva, G.V.; Chachanina, K.L. Enzyme immunoassay system for detection of staphylococcal enterotoxin, type B. Temporarily Pharm. Norms Regul. 1989, 89, 42–236. [Google Scholar]
- Fluer, F.S.; Pozhar, P.F.; Ratgauz, G.L.; Akatov, A.K. A rapid method of detecting the staphylococcal exotoxin of toxic shock. Zhurnal Mikrobiol. Epidemiol. Immunobiol. 1990, 12, 70–73. [Google Scholar]
- Zhou, Y.; Xu, X.; Liu, Y.; Wang, A.; Luo, Y.; Liu, X.; Wang, X.; Li, W.; Yao, X. Heterogeneous Regulation of Staphylococcus aureus by Different Staphylococcus epidermidis agr Types in Atopic Dermatitis. J. Investig. Dermatol. 2023, 143, 2484–2493.e11. [Google Scholar] [CrossRef]
- Nakatsuji, T.; Chen, T.H.; Narala, S.; Chun, K.A.; Two, A.M.; Yun, T.; Shafiq, F.; Kotol, P.F.; Bouslimani, A.; Melnik, A.V.; et al. Antimicrobials from human skin commensal bacteria protect against Staphylococcus aureus and are deficient in atopic der-matitis. Sci. Transl. Med. 2017, 9, eaah4680. [Google Scholar] [CrossRef]
- Byrd, A.L.; Deming, C.; Cassidy, S.K.B.; Harrison, O.J.; Ng, W.I.; Conlan, S.; NISC Comparative Sequencing Program; Belkaid, Y.; Segre, J.A.; Kong, H.H. Staphylococcus aureus and Staphylococcus epidermidis strain diversity underlying pediatric atopic dermatitis. Sci. Transl. Med. 2017, 9, eaal4651. [Google Scholar] [CrossRef]
- Landemaine, L.; Da Costa, G.; Fissier, E.; Francis, C.; Morand, S.; Verbeke, J.; Michel, M.L.; Briandet, R.; Sokol, H.; Gueniche, A.; et al. Staphylococcus epidermidis isolates from atopic or healthy skin have opposite effect on skin cells: Potential implication of the AHR pathway modulation. Front. Immunol. 2023, 14, 1098160. [Google Scholar] [CrossRef] [PubMed]
- Williams, M.R.; Bagood, M.D.; Enroth, T.J.; Bunch, Z.L.; Jiang, N.; Liu, E.; Almoughrabie, S.; Khalil, S.; Li, F.; Brinton, S.; et al. Staphylococcus epidermidis activates keratinocyte cytokine expression and promotes skin inflammation through the production of phenol-soluble modulins. Cell Rep. 2023, 42, 113024. [Google Scholar] [CrossRef]
- Cau, L.; Williams, M.R.; Butcher, A.M.; Nakatsuji, T.; Kavanaugh, J.S.; Cheng, J.Y.; Shafiq, F.; Higbee, K.; Hata, T.R.; Horswill, A.R.; et al. Staphylococcus epidermidis protease EcpA can be a deleterious component of the skin microbiome in atopic dermatitis. J. Allergy Clin. Immunol. 2021, 147, 955–966.e16. [Google Scholar] [CrossRef] [PubMed]
- Abdurrahman, G.; Pospich, R.; Steil, L.; Gesell Salazar, M.; Izquierdo González, J.J.; Normann, N.; Mrochen, D.; Scharf, C.; Völker, U.; Werfel, T.; et al. The extracellular serine protease from Staphylococcus epidermidis elicits a type 2-biased immune response in atopic dermatitis patients. Front. Immunol. 2024, 15, 1352704. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, T.; Stevens, M.L.; Baatyrbek Kyzy, A.; Alarcon, R.; He, H.; Kroner, J.W.; Spagna, D.; Grashel, B.; Sidler, E.; Martin, L.J.; et al. Biofilm propensity of Staphylococcus aureus skin isolates is associated with increased atopic dermatitis severity and barrier dysfunction in the MPAACH pediatric cohort. Allergy 2021, 76, 302–313. [Google Scholar] [CrossRef]
- Burke, Ó.; Zeden, M.S.; O’Gara, J.P. The pathogenicity and virulence of the opportunistic pathogen Staphylococcus epidermidis. Virulence 2024, 15, 2359483. [Google Scholar] [CrossRef]


| Atopic Dermatitis Patients | ||||
|---|---|---|---|---|
| Mild | Moderate | Severe | Total | |
| Patients n (%) | 68 (20.6%) | 124 (37.7%) | 137 (41.6%) | 329 |
| Male n (%) | 43 (25.8%) | 53 (31.9%) | 70 (42.1%) | 166 |
| Female n (%) | 25 (15.3%) | 71 (43.5%) | 67 (41.1%) | 163 |
| Age (yr) | 2.9 ± 0.6 | 5.3 ± 0.9 | 5.5 ± 3.1 | 4.89 ± 0.9 |
| SCORAD | 15.1 ± 4.2 | 38.8 ± 4.7 | 71.1 ± 6.5 | 47.8 ± 5.1 |
| Staphylococci strais | 61 (20.4%) | 109 (36.3%) | 130 (43.3%) | 300 |
| S. aureus | 22 (13.7%) | 55 (34.3%) | 83 (51.8%) | 160 (53.3%) |
| CoNS total | 39 (27.8%) | 54 (38.5%) | 47 (33.5%) | 140 (46.6%) |
| S. epidermidis | 24 (27.5%) | 39 (44.8%) | 24 (27.5%) | 87 |
| S. haemolyticus | 4 (18.18%) | 8 (36.36%) | 10 (45.45%) | 22 |
| S. hominis | 9 (60.0%) | 4 (26.6%) | 2 (13.3%) | 15 |
| S. warneri | 1 | 1 | 3 | 5 |
| S. saprophyticus | 1 | 1 | ||
| S. capitis | 7 | 7 | ||
| S. simulans | 1 | 1 | ||
| S. cohnii | 1 | 1 | 2 | |
| No staphylococcal growth | 20 (23.5%) | 33 (38.8%) | 32 (37.6%) | 85 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kudryavtseva, A.; Fluer, F.; Khachatryan, L.; Makarova, S.; Osipenko, O.; Ryzhii, E.; Titarev, S.; Zaslavsky, D.; Gelezhe, K. Staphylococcus Strains in Atopic Dermatitis in Children: Toxins Production and Resistance Properties. Life 2025, 15, 1120. https://doi.org/10.3390/life15071120
Kudryavtseva A, Fluer F, Khachatryan L, Makarova S, Osipenko O, Ryzhii E, Titarev S, Zaslavsky D, Gelezhe K. Staphylococcus Strains in Atopic Dermatitis in Children: Toxins Production and Resistance Properties. Life. 2025; 15(7):1120. https://doi.org/10.3390/life15071120
Chicago/Turabian StyleKudryavtseva, Asya, Fyodor Fluer, Lusine Khachatryan, Svetlana Makarova, Oksana Osipenko, Elena Ryzhii, Sergei Titarev, Denis Zaslavsky, and Katerina Gelezhe. 2025. "Staphylococcus Strains in Atopic Dermatitis in Children: Toxins Production and Resistance Properties" Life 15, no. 7: 1120. https://doi.org/10.3390/life15071120
APA StyleKudryavtseva, A., Fluer, F., Khachatryan, L., Makarova, S., Osipenko, O., Ryzhii, E., Titarev, S., Zaslavsky, D., & Gelezhe, K. (2025). Staphylococcus Strains in Atopic Dermatitis in Children: Toxins Production and Resistance Properties. Life, 15(7), 1120. https://doi.org/10.3390/life15071120






