Microbiome Against the Background of the Complex Aetiology in Sarcoidosis—What Do We Already Know?
Abstract
1. Introduction
2. Epidemiology
3. Etiopathogenesis of Sarcoidosis
3.1. Genetic Factors
3.2. Environmental Abiotic Risk Factors
3.3. Environmental Infectious Microbial Agents
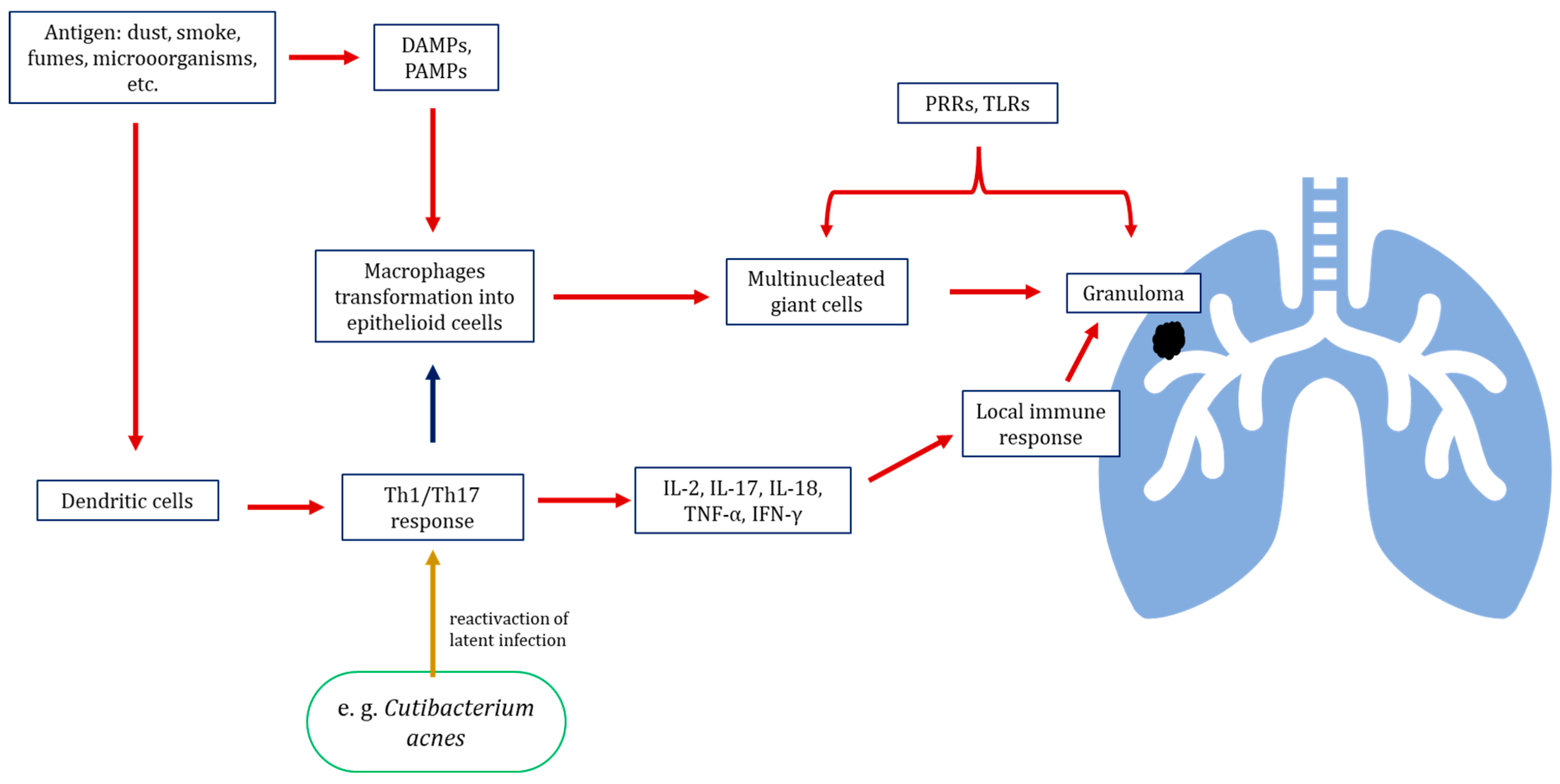
3.3.1. Infection as Potential Factors Contributing to the Development of Sarcoidosis—Microorganisms as a Triggering Factor
3.3.2. Microbiome of the Lower Respiratory Tract and Lung
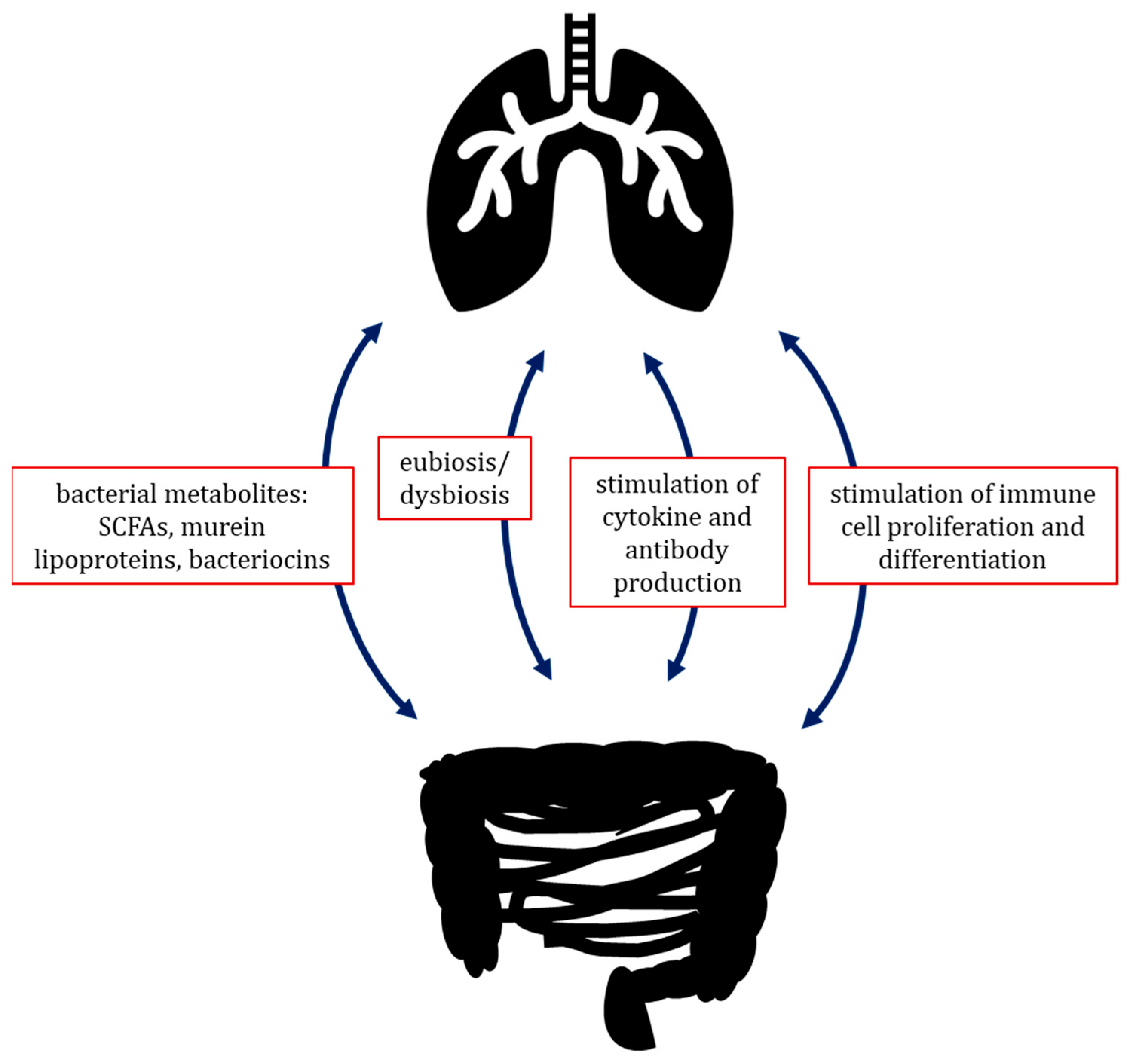
3.3.3. Gut–Lung Axis
3.3.4. Blood Microbiome Dysbiosis
3.3.5. The Mycobiome in Sarcoidosis
4. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Weeratunga, P.; Moller, D.R.; Ho, L.P. Immune mechanisms of granuloma formation in sarcoidosis and tuberculosis. J. Clin. Investig. 2024, 134, e175264. [Google Scholar] [CrossRef] [PubMed]
- Melani, A.S.; Simona, A.; Armati, M.; d’Alessandro, M.; Bargagli, E. A Comprehensive Review of Sarcoidosis Diagnosis and Monitoring for the Pulmonologist. Pulm. Ther. 2021, 7, 309–324. [Google Scholar] [CrossRef] [PubMed]
- Brown, F.; Modi, P.; Tanner, L.S. Lofgren Syndrome. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Ungprasert, P.; Crowson, C.S.; Matteson, E.L. Epidemiology and Clinical Characteristics of Sarcoidosis: An Update from a Population-Based Cohort Study from Olmsted County, Minnesota. Reumatismo 2017, 69, 16–22. [Google Scholar] [CrossRef]
- Waly, Y.M.; Sharafeldin, A.-B.K.; Akhtar, M.U.; Chilmeran, Z.; Fredericks, S. A review of sarcoidosis etiology, diagnosis and treatment. Front. Med. 2025, 12, 1558049. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Y.; Yang, Y.; Wu, Y.; Yang, Q. Exploring the Dynamic Changes between Pulmonary and Cutaneous Sarcoidosis Based on Gene Expression. Médecine/Sciences 2018, 34, 121–133. [Google Scholar] [CrossRef]
- Takeuchi, S.; Kawada, J.I.; Suzuki, A.; Sakamoto, K.; Fukuda, Y.; Horiba, K.; Suzuki, T.; Torii, Y.; Shindo, Y.; Ito, Y. Metagenomic Analysis of Lung Microbiome in Patients with Interstitial Lung Diseases and Sarcoidosis: An Experimental Study. Health Sci. Rep. 2025, 8, e70328. [Google Scholar] [CrossRef]
- Becker, A.; Vella, G.; Galata, V.; Rentz, K.; Beisswenger, C.; Herr, C.; Walter, J.; Tierling, S.; Slevogt, H.; Keller, A.; et al. The composition of the pulmonary microbiota in sarcoidosis–an observational study. Respir. Res. 2019, 20, 46. [Google Scholar] [CrossRef]
- Hodzhev, Y. Analysis of blood microbiome dysbiosis in pulmonary sarcoidosis by decision tree model. Biotechnol. Biotechnol. Equip. 2023, 37, 2283133. [Google Scholar] [CrossRef]
- Hodzhev, Y.; Tsafarova, B.; Tolchkov, V.; Youroukova, V.; Ivanova, S.; Kostadinov, D.; Yanev, N.; Zhelyazkova, M.; Tsonev, S.; Kalfin, R.; et al. Visualization of the Individual Blood Microbiome to Study the Etiology of Sarcoidosis. Comput. Struct. Biotechnol. J. 2023, 22, 50–57. [Google Scholar] [CrossRef]
- Chen, S.; Li, Y. Global health inequalities in the burden of interstitial lung disease and pulmonary sarcoidosis from 1990 to 2021. BMC Public Health 2024, 24, 2892. [Google Scholar] [CrossRef]
- Rossides, M.; Darlington, P.; Kullberg, S.; Arkema, E.V. Sarcoidosis: Epidemiology and clinical insights. J. Intern. Med. 2023, 293, 668680. [Google Scholar] [CrossRef] [PubMed]
- Neves, F.S.; Pereira, I.A.; Sztajnbok, F.; Neto, N.S.R. Sarcoidosis: A general overview. Adv. Rheumatol. 2024, 64, 57. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Gerke, A.K.; Lower, E.E.; Vizel, A.; Talwar, D.; Strambu, I.; Francesqui, J.; Sellares, J.; Sawahata, M.; Obi, O.N.; et al. The impact of demographic disparities in the presentation of sarcoidosis: A multicenter prospective study. Respir. Med. 2021, 187, 106564. [Google Scholar] [CrossRef] [PubMed]
- Arkema, E.V.; Cozier, Y.C. Epidemiology of Sarcoidosis: Current Findings and Future Directions. Ther. Adv. Chronic Dis. 2018, 9, 227–240. [Google Scholar] [CrossRef]
- Inaoka, P.T.; Shono, M.; Kamada, M.; Espinoza, J.L. Host-Microbe Interactions in the Pathogenesis and Clinical Course of Sarcoidosis. J. Biomed. Sci. 2019, 26, 45. [Google Scholar] [CrossRef]
- Ungprasert, P.; Crowson, C.S.; Matteson, E.L. Seasonal Variation in Incidence of Sarcoidosis: A Population-Based Study, 1976–2013. Thorax 2016, 71, 1164–1166. [Google Scholar] [CrossRef]
- Chen, C.; Luo, N.; Dai, F.; Zhou, W.; Wu, X.; Zhang, J. Advance in pathogenesis of sarcoidosis: Triggers and progression. Heliyon 2024, 10, e27612. [Google Scholar] [CrossRef]
- Sivalokanathan, S. Exploring the Role of Genetics in Sarcoidosis and Its Impact on the Development of Cardiac Sarcoidosis. Cardiogenetics 2024, 14, 106–121. [Google Scholar] [CrossRef]
- Kishore, A.; Petrek, M. Next-Generation Sequencing Based HLA Typing: Deciphering Immunogenetic Aspects of Sarcoidosis. Front. Genet. 2018, 9, 503. [Google Scholar] [CrossRef]
- Watad, A.; Rosenberg, V.; Tiosano, S.; Cohen Tervaert, J.W.; Yavne, Y.; Shoenfeld, Y.; Shalev, V.; Chodick, G.; Amital, H. Silicone Breast Implants and the Risk of Autoimmune/Rheumatic Disorders: A Real-World Analysis. Int. J. Epidemiol. 2018, 47, 1846–1854. [Google Scholar] [CrossRef]
- Jordan, H.T.; Stellman, S.D.; Prezant, D.; Teirstein, A.; Osahan, S.S.; Cone, J.E. Sarcoidosis Diagnosed after September 11, 2001, among Adults Exposed to the World Trade Center Disaster. J. Occup. Environ. Med. 2011, 53, 966–974. [Google Scholar] [CrossRef] [PubMed]
- Culver, D.A. Beryllium Disease and Sarcoidosis: Still Besties after All These Years? Eur. Respir. J. 2016, 47, 1625–1628. [Google Scholar] [CrossRef]
- Cozier, Y.C.; Govender, P.; Berman, J.S. Obesity and Sarcoidosis Risk: An Index Under Suspicion. Chest 2022, 162, 954–956. [Google Scholar] [CrossRef] [PubMed]
- Della Zoppa, M.; Bertuccio, F.R.; Campo, I.; Tousa, F.; Crescenzi, M.; Lettieri, S.; Mariani, F.; Corsico, A.G.; Piloni, D.; Stella, G.M. Phenotypes and Serum Biomarkers in Sarcoidosis. Diagnostics 2024, 14, 709. [Google Scholar] [CrossRef]
- Judson, M.A.; Tiwari, A.; Gemoets, D.E. The relationship of obesity and OSA to the development of sarcoidosis: A large retrospective case-control US Veterans administration analysis. Chest 2022, 162, 1086–1092. [Google Scholar] [CrossRef]
- Ungprasert, P.; Crowson, C.S.; Matteson, E.L. Smoking, Obesity and Risk of Sarcoidosis: A Population-Based Nested Case-Control Study. Respir. Med. 2016, 120, 87–90. [Google Scholar] [CrossRef]
- Dehara, M.; Nguyen, N.V.; Arkema, E.V. Smoking and the risk of sarcoidosis: A systematic review and meta-analysis. Respir. Med. 2025, 241, 108089. [Google Scholar] [CrossRef] [PubMed]
- Miedema, J.; Cinetto, F.; Smed-Sörensen, A.; Spagnolo, P. The Immunopathogenesis of Sarcoidosis. J. Autoimmun. 2024, 149, 103247. [Google Scholar] [CrossRef]
- Dubaniewicz, A. Microbial and Human Heat Shock Proteins as ‘Danger Signals’ in Sarcoidosis. Hum. Immunol. 2013, 74, 1550–1558. [Google Scholar] [CrossRef]
- Gabrilovich, M.I.; Walrath, J.; van Lunteren, J.; Nethery, D.; Seifu, M.; Kern, J.A.; Harding, C.V.; Tuscano, L.; Lee, H.; Williams, S.D.; et al. Disordered Toll-like Receptor 2 Responses in the Pathogenesis of Pulmonary Sarcoidosis. Clin. Exp. Immunol. 2013, 173, 512–522. [Google Scholar] [CrossRef]
- Jain, R.; Kumari, R.; Chakraborty, S.; Mitra, D.K.; Mohan, A.; Hadda, V.; Madan, K.; Guleria, R. T-Cell Signature Cytokines Distinguish Pulmonary Sarcoidosis from Pulmonary Tuberculosis. Eur. J. Immunol. 2023, 53, e2250255. [Google Scholar] [CrossRef] [PubMed]
- Ostadkarampour, M.; Eklund, A.; Moller, D.; Glader, P.; Olgart Höglund, C.; Lindén, A.; Grunewald, J.; Wahlström, J. Higher Levels of Interleukin IL-17 and Antigen-Specific IL-17 Responses in Pulmonary Sarcoidosis Patients with Löfgren’s Syndrome. Clin. Exp. Immunol. 2014, 178, 342–352. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Lu, Z.; Jiang, C.; Liu, J.; Wang, Y.; Xu, Z. Imbalance between Th17 and Regulatory T-Cells in Sarcoidosis. Int. J. Mol. Sci. 2013, 14, 21463–21473. [Google Scholar] [CrossRef]
- Baughman, R.P.; Lower, E.E. Who Dies from Sarcoidosis and Why? Am. J. Respir. Crit. Care Med. 2011, 183, 1446–1447. [Google Scholar] [CrossRef] [PubMed]
- Eishi, Y. Potential Association of Cutibacterium acnes with Sarcoidosis as an Endogenous Hypersensitivity Infection. Microorganisms 2023, 11, 289. [Google Scholar] [CrossRef]
- Starshinova, A.A.; Malkova, A.M.; Basantsova, N.Y.; Zinchenko, Y.S.; Kudryavtsev, I.V.; Ershov, G.A.; Soprun, L.A.; Mayevskaya, V.A.; Churilov, L.P.; Yablonskiy, P.K. Sarcoidosis as an Autoimmune Disease. Front. Immunol. 2019, 10, 2933. [Google Scholar] [CrossRef]
- Akata, K.; Yamasaki, K.; Nemoto, K.; Ikegami, H.; Kawaguchi, T.; Noguchi, S.; Kawanami, T.; Fukuda, K.; Mukae, H.; Yatera, K. Sarcoidosis Associated with Enlarged Mediastinal Lymph Nodes with the Detection of Streptococcus gordonii and Cutibacterium acnes Using a Clone Library Method. Intern. Med. 2024, 63, 299–304. [Google Scholar] [CrossRef]
- Esteves, T.; Aparicio, G.; Garcia-Patos, V. Is There Any Association between Sarcoidosis and Infectious Agents?: A Systematic Review and Meta-Analysis. BMC Pulm. Med. 2016, 16, 165. [Google Scholar] [CrossRef]
- Kraaijvanger, R.; Veltkamp, M. The Role of Cutibacterium acnes in Sarcoidosis: From Antigen to Treatable Trait? Microorganisms 2022, 10, 1649. [Google Scholar] [CrossRef]
- Correa-González, N.; Díaz, M.C.; Ángel, K.J.T.; Polania, M.D.; Murillo, N.R.; Robles, P.A. Clinical convergence: An exceptional case of sarcoidosis and tuberculosis with multiple organic manifestations. Case report. Rev. Colomb. Reumatol. 2025, 32, 95–102. [Google Scholar] [CrossRef]
- Yang, W.; Jiang, J.; Zhao, Q.; Ren, H.Q.; Yao, X.X.; Sun, S.Y.; Zhang, L.; Fu, A.S.; Ge, Y.L. A Case of Tuberculosis Misdiagnosed as Sarcoidosis and then Confirmed by NGS Testing. Clin. Lab. 2024, 70, p579. [Google Scholar] [CrossRef]
- Dai, G.; Yin, C.; Chen, S.; Gao, W.; Tang, X.; Wang, T.; Zeng, Y. Coexistence of tuberculosis and sarcoidosis: A description of two cases. Quant. Imaging Med. Surg. 2024, 14, 3755–3761. [Google Scholar] [CrossRef]
- Dow, C.T.; Lin, N.W.; Chan, E.D. Sarcoidosis, Mycobacterium paratuberculosis and Noncaseating Granulomas: Who Moved My Cheese. Microorganisms 2023, 11, 829. [Google Scholar] [CrossRef]
- Dickson, R.P.; Erb-Downward, J.R.; Martinez, F.J.; Huffnagle, G.B. The Microbiome and the Respiratory Tract. Annu. Rev. Physiol. 2016, 78, 481–504. [Google Scholar] [CrossRef] [PubMed]
- Moffatt, M.F.; Cookson, W.O. The Lung Microbiome in Health and Disease. Clin. Med. Lond. Engl. 2017, 17, 525–529. [Google Scholar] [CrossRef] [PubMed]
- Mathieu, E.; Escribano-Vazquez, U.; Descamps, D.; Cherbuy, C.; Langella, P.; Riffault, S.; Remot, A.; Thomas, M. Paradigms of Lung Microbiota Functions in Health and Disease, Particularly, in Asthma. Front. Physiol. 2018, 9, 1168. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Li, J.; Zhou, X. Lung Microbiome: New Insights into the Pathogenesis of Respiratory Diseases. Signal Transduct. Target. Ther. 2024, 9, 19. [Google Scholar] [CrossRef]
- Nguyen, L.D.N.; Viscogliosi, E.; Delhaes, L. The Lung Mycobiome: An Emerging Field of the Human Respiratory Microbiome. Front. Microbiol. 2015, 6, 89. [Google Scholar] [CrossRef]
- Hartmann, J.E.; Albrich, W.C.; Dmitrijeva, M.; Kahlert, C.R. The Effects of Corticosteroids on the Respiratory Microbiome: A Systematic Review. Front. Med. 2021, 8, 588584. [Google Scholar] [CrossRef]
- Wang, J.; Li, F.; Tian, Z. Role of Microbiota on Lung Homeostasis and Diseases. Sci. China Life Sci. 2017, 60, 1407–1415. [Google Scholar] [CrossRef]
- D’Argenio, V.; Casaburi, G.; Precone, V.; Moccia, L.G.; Postiglione, I.; Bocchino, M.; Sanduzzi, A. A Common Microbial Signature Is Present in the Lower Airways of Interstitial Lung Diseases Including Sarcoidosis. Sarcoidosis Vasc. Diffus. Lung Dis. 2018, 35, 354–362. [Google Scholar] [CrossRef]
- Gupta, S.; Shariff, M.; Chaturvedi, G.; Sharma, A.; Goel, N.; Yadav, M.; Mortensen, M.S.; Sørensen, S.J.; Mukerji, M.; Chauhan, N.S. Comparative Analysis of the Alveolar Microbiome in COPD, ECOPD, Sarcoidosis, and ILD Patients to Identify Respiratory Illnesses Specific Microbial Signatures. Sci. Rep. 2021, 11, 3963. [Google Scholar] [CrossRef]
- Estaki, M.; Jiang, L.; Bokulich, N.A.; McDonald, D.; González, A.; Kosciolek, T.; Martino, C.; Zhu, Q.; Birmingham, A.; Vázquez-Baeza, Y.; et al. QIIME 2 enables comprehensive end-to-end analysis of diverse microbiome data and comparative studies with publicly available data. Curr. Protoc. Bioinform. 2020, 70, e100. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, K.S.; Lehmann, S.; Nielsen, R.; Tangedal, S.; Paytuvi-Gallart, A.; Sanseverino, W.; Martinsen, E.M.H.; Hiemstra, P.S.; Eagan, T.M. The Lower Airways Microbiota and Antimicrobial Peptides Indicate Dysbiosis in Sarcoidosis. Microbiome 2022, 10, 175. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, A.; Knecht, H.; Häsler, R.; Zissel, G.; Gaede, K.I.; Hofmann, S.; Nebel, A.; Müller-Quernheim, J.; Schreiber, S.; Fischer, A. Atopobium and Fusobacterium as Novel Candidates for Sarcoidosis-Associated Microbiota. Eur. Respir. J. 2017, 50, 1600746. [Google Scholar] [CrossRef]
- Zhu, H.; Li, M.; Bi, D.; Yang, H.; Gao, Y.; Song, F.; Zheng, J.; Xie, R.; Zhang, Y.; Liu, H.; et al. Fusobacterium nucleatum promotes tumor progression in KRAS p.G12D-mutant colorectal cancer by binding to DHX15. Nat. Commun. 2024, 15, 1688. [Google Scholar] [CrossRef]
- Muraoka, A.; Suzuki, M.; Hamaguchi, T.; Watanabe, S.; Iijima, K.; Murofushi, Y.; Shinjo, K.; Osuka, S.; Hariyama, Y.; Ito, M.; et al. Fusobacterium infection facilitates the development of endometriosis through the phenotypic transition of endometrial fibroblasts. Sci. Transl. Med. 2023, 15, eadd1531. [Google Scholar] [CrossRef]
- Carabotti, M.; Scirocco, A.; Maselli, M.A.; Severi, C. The gut-brain axis: Interactions between enteric microbiota, central and enteric nervous systems. Ann. Gastroenterol. 2015, 28, 203–209. [Google Scholar]
- Haldar, S.; Jadhav, S.R.; Gulati, V.; Beale, D.J.; Balkrishna, A.; Varshney, A.; Palombo, E.A.; Karpe, A.V.; Shah, R.M. Unravelling the gut-lung axis: Insights into microbiome interactions and Traditional Indian Medicine’s perspective on optimal health. FEMS Microbiol. Ecol. 2023, 99, fiad103. [Google Scholar] [CrossRef]
- Anand, S.; Mande, S.S. Diet, Microbiota and Gut-Lung Connection. Front. Microbiol. 2018, 9, 2147. [Google Scholar] [CrossRef]
- Shukla, S.D.; Budden, K.F.; Neal, R.; Hansbro, P.M. Microbiome Effects on Immunity, Health and Disease in the Lung. Clin. Transl. Immunol. 2017, 6, e133. [Google Scholar] [CrossRef]
- Farahat, R.A.; Nazir, A.; Ochani, S.; Khan, S.H.; Mahjabin, A.; Mohammed, S.; Jahan, I.; Kubra, K.; Swed, S.; Dhama, K. Hidden Relationship between Sarcoidosis and Gut Microbiota: Recent Evidence and Future Implications. Egypt. J. Intern. Med. 2023, 35, 17. [Google Scholar] [CrossRef]
- Lee, J.; Chen, Y.; Huang, K.; Vagts, C.; Ascoli, C.; Sun, J.; Sweiss, N.; Perkins, D.; Finn, P. Investigating the Interplay Between Gut Microbiome and Immune Landscape in Sarcoidosis. J. Allergy Clin. Immunol. 2023, 151, AB322. [Google Scholar] [CrossRef]
- Cheng, H.S.; Tan, S.P.; Wong, D.M.K.; Koo, W.L.Y.; Wong, S.H.; Tan, N.S. The Blood Microbiome and Health: Current Evidence, Controversies, and Challenges. Int. J. Mol. Sci. 2023, 24, 5633. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.C.S.; Ko, K.K.K.; Chen, H.; Liu, J.; Loh, M.; SG10K_Health Consortium; Chia, M.; Nagarajan, N. No evidence for a common blood microbiome based on a population study of 9,770 healthy humans. Nat. Microbiol. 2023, 8, 973–985. [Google Scholar] [CrossRef]
- Góralska, K.; Dzikowiec, M. Role of microbiota in maintaining the homeostasis in the human body. Post. Mikrobiol.-Adv. Microbiol. 2018, 57, 5–11. [Google Scholar] [CrossRef]
- Tipton, L.; Ghedin, E.; Morris, A. The Lung Mycobiome in the Next-Generation Sequencing Era. Virulence 2017, 8, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Weaver, D.; Gago, S.; Bromley, M.; Bowyer, P. The Human Lung Mycobiome in Chronic Respiratory Disease: Limitations of Methods and Our Current Understanding. Curr. Fungal Infect. Rep. 2019, 13, 109–119. [Google Scholar] [CrossRef]
- Clarke, E.L.; Lauder, A.P.; Hofstaedter, C.E.; Hwang, Y.; Fitzgerald, A.S.; Imai, I.; Biernat, W.; Rękawiecki, B.; Majewska, H.; Dubaniewicz, A.; et al. Microbial Lineages in Sarcoidosis. A Metagenomic Analysis Tailored for Low-Microbial Content Samples. Am. J. Respir. Crit. Care Med. 2018, 197, 225–234. [Google Scholar] [CrossRef]
- Greaves, S.A.; Ravindran, A.; Santos, R.G.; Chen, L.; Falta, M.T.; Wang, Y.; Mitchell, A.M.; Atif, S.M.; Mack, D.G.; Tinega, A.N.; et al. CD4+ T cells in the lungs of acute sarcoidosis patients recognize an Aspergillus nidulans epitope. J. Exp. Med. 2021, 218, e20210785. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szymański, M.; Góralska, K.; Brzeziańska-Lasota, E. Microbiome Against the Background of the Complex Aetiology in Sarcoidosis—What Do We Already Know? Life 2025, 15, 1069. https://doi.org/10.3390/life15071069
Szymański M, Góralska K, Brzeziańska-Lasota E. Microbiome Against the Background of the Complex Aetiology in Sarcoidosis—What Do We Already Know? Life. 2025; 15(7):1069. https://doi.org/10.3390/life15071069
Chicago/Turabian StyleSzymański, Maciej, Katarzyna Góralska, and Ewa Brzeziańska-Lasota. 2025. "Microbiome Against the Background of the Complex Aetiology in Sarcoidosis—What Do We Already Know?" Life 15, no. 7: 1069. https://doi.org/10.3390/life15071069
APA StyleSzymański, M., Góralska, K., & Brzeziańska-Lasota, E. (2025). Microbiome Against the Background of the Complex Aetiology in Sarcoidosis—What Do We Already Know? Life, 15(7), 1069. https://doi.org/10.3390/life15071069





