The Role of Predictive Biomarkers in Modern Prostate Cancer Radiotherapy: A Literature Review on Personalised Treatment Strategies and the Prediction of Adverse Effects
Abstract
1. Introduction
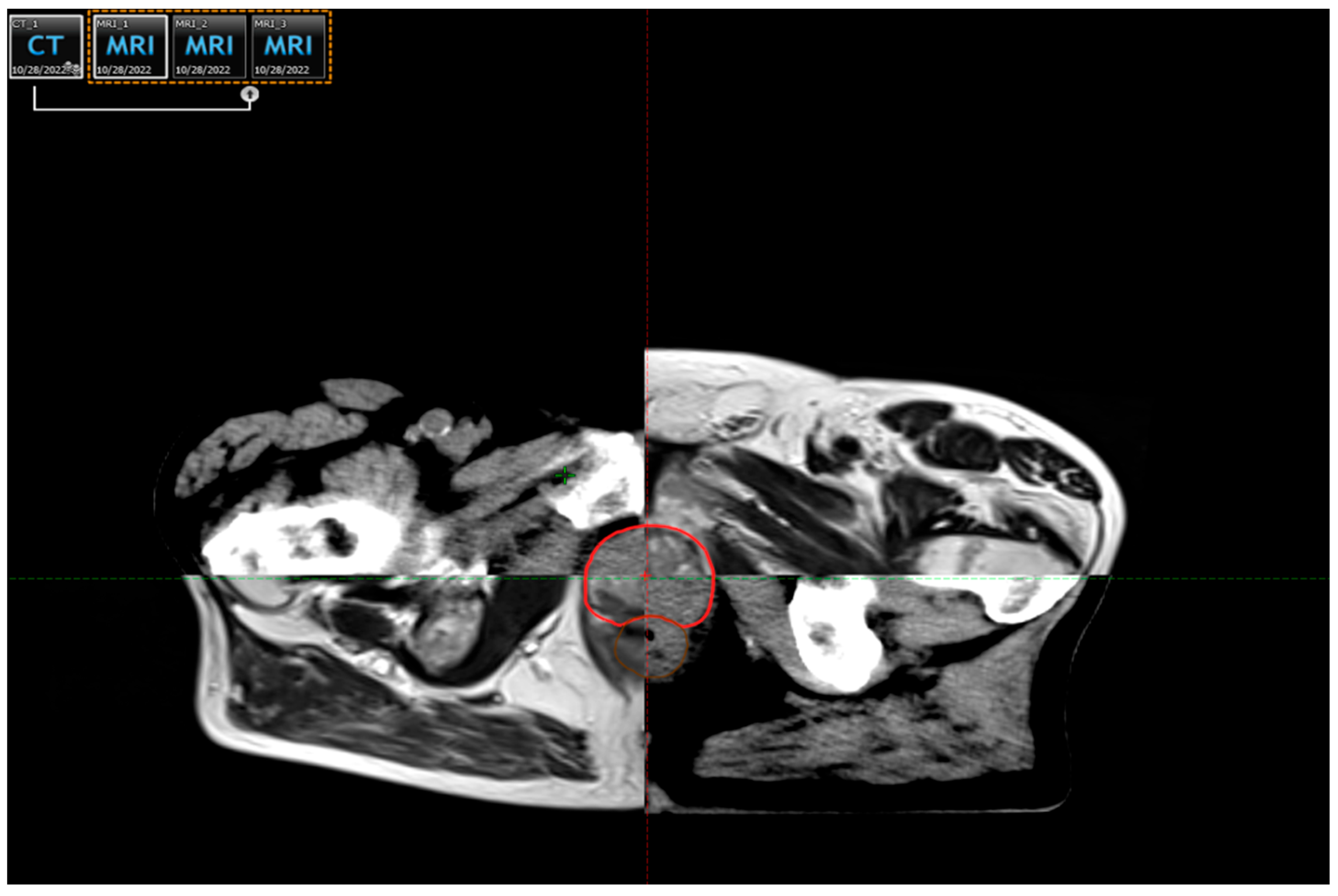
2. The Modern Radiotherapy of Prostate Cancer
3. Individual Radiosensitivity: The Role of Biomarkers and Predictive Assays in Personalising Radiotherapy for Prostate Cancer Patients
3.1. The Role of Cytokines as Potential Biomarkers for Radiation Toxicity
3.2. The RILA Assay: A Tool for Stratifying Patients by Radiosensitivity to Anticipate Radiation-Induced Toxicity
3.3. Genetic Insights into Radiotherapy-Induced Toxicity
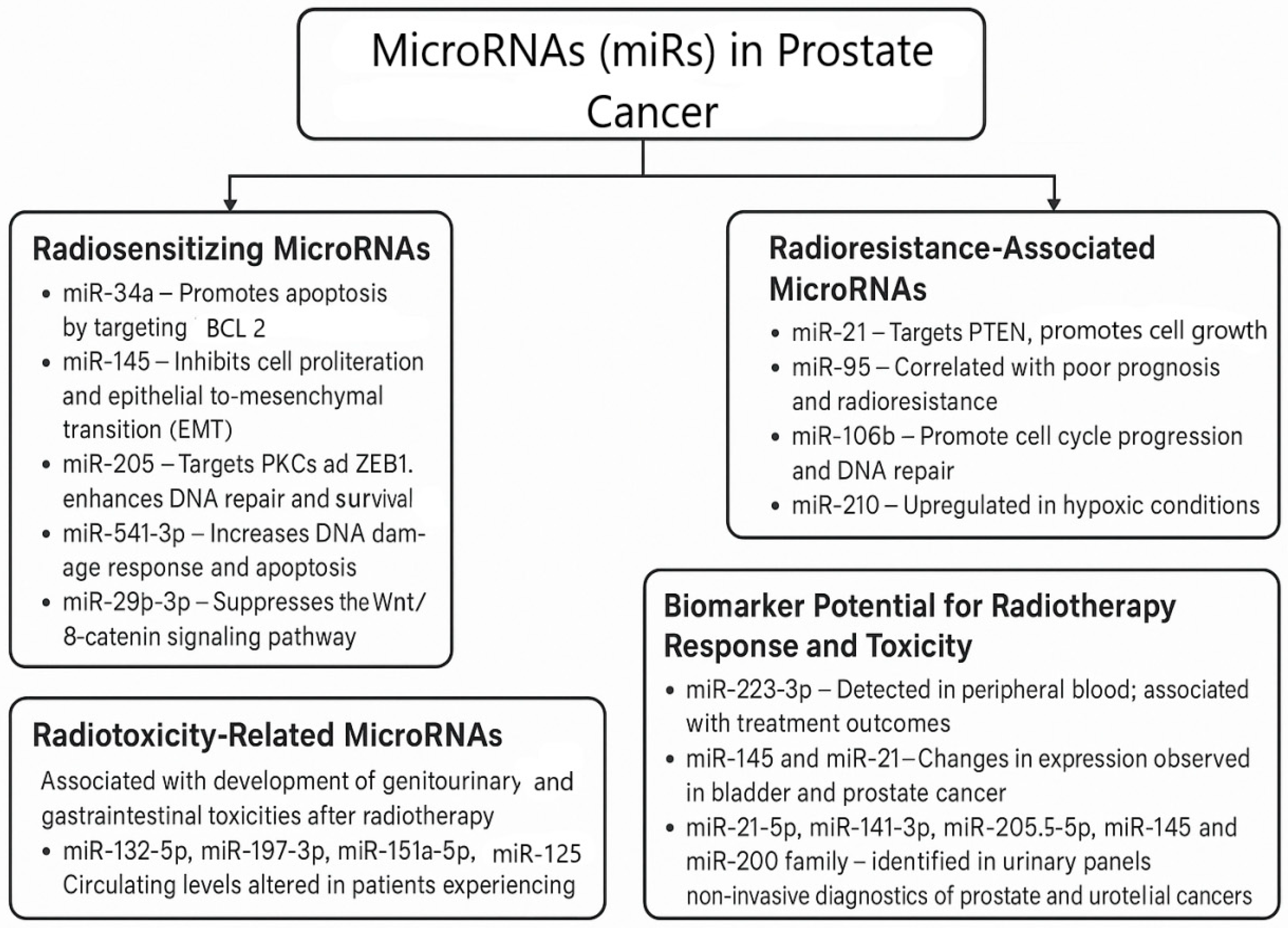
3.4. MicroRNAs and Their Role in Radiation-Induced Toxicity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer Statistics, 2024. CA A Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef] [PubMed]
- National Cancer Institute. Cancer Stat Facts: Prostate Cancer. Available online: https://seer.cancer.gov/statfacts/html/prost.html (accessed on 27 June 2024).
- GLOBOCAN. Population Fact Sheet. Chrome Extension. 2020. Available online: https://backlinko.com/chrome-users (accessed on 29 June 2025).
- Batut, Institute of Public Health of Serbia. Malignant Tumors in Serbia 2022. 2022. Available online: https://www.batut.org.rs/index.php?content=2096 (accessed on 4 May 2025).
- International Agency for Research on Cancer. Cancer Tomorrow; World Health Organization, Beijing, China. 2025. Available online: https://gco.iarc.fr/tomorrow/en/dataviz/isotype?cancers=27&single_unit=50000 (accessed on 27 June 2024).
- Mottet, N.; van den Bergh, R.C.N.; Briers, E.; Van den Broeck, T.; Cumberbatch, M.G.; De Santis, M.; Fanti, S.; Fossati, N.; Gandaglia, G.; Gillessen, S.; et al. Prostate Cancer; European Association of Urology: Arnhem, The Netherlands, 2020. [Google Scholar]
- Stanić, J.; Stanković, V.; Nikitović, M. Radiation Toxicity in Prostate Cancer Patients. Medic. Podml. 2021, 72, 26–33. [Google Scholar] [CrossRef]
- Stanić, J.; Stanković, V.; Nikitović, M. Modern Radiotherapy in the Treatment of Localized Prostate Cancer. Srp. Arh. Za Celok. Lek. 2021, 149, 117–121. [Google Scholar] [CrossRef]
- NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®): Prostate Cancer. Version 1.2024. National Comprehensive Cancer Network. Available online: https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf (accessed on 4 May 2025).
- Gillessen, S.; Attard, G.; Beer, T.M.; Beltran, H.; Bjartell, A.; Bossi, A.; Briganti, A.; Bristow, R.G.; Chi, K.N.; Clarke, N.; et al. Management of patients with advanced prostate cancer: Report of the Advanced Prostate Cancer Consensus Conference 2019. Eur Urol. 2020, 77, 508–547. [Google Scholar] [CrossRef] [PubMed]
- Cheng, B.; He, H.; Chen, B.; Zhou, Q.; Luo, T.; Li, K.; Du, T.; Huang, H. Assessment of treatment outcomes: Cytoreductive surgery compared to radiotherapy in oligometastatic prostate cancer—An in-depth quantitative evaluation and retrospective cohort analysis. Int. J. Surg. 2024, 110, 3190–3202. [Google Scholar] [CrossRef]
- American Cancer Society. Cancer Facts & Figures 2024. Available online: https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/global-cancer-facts-and-figures/global-cancer-facts-and-figures-4th-edition.pdf (accessed on 29 June 2025).
- Schack, L.M.H.; Petersen, S.E.; Nielsen, S.; Lundby, L.; Høyer, M.; Bentzen, L.; Overgaard, J.; Andreassen, C.N.; Alsner, J. Validation of Genetic Predictors of Late Radiation-Induced Morbidity in Prostate Cancer Patients. Acta Oncol. 2017, 56, 1514–1521. [Google Scholar] [CrossRef] [PubMed]
- Davda, R.; Al-Abdullah, A.; Ricketts, K. Advances in External Beam Radiotherapy for Prostate Cancer. Trends Urol. Men’s Health 2016, 7, 13–16. [Google Scholar] [CrossRef]
- Pereira, G.C.; Traughber, M.; Muzic, R.F., Jr. The Role of Imaging in Radiation Therapy Planning: Past, Present, and Future. Biomed. Res. Int. 2014, 2014, 231090. [Google Scholar] [CrossRef]
- Hoffman, K.E.; Voong, K.R.; Levy, L.B.; Allen, P.K.; Choi, S.; Schlembach, P.J.; Lee, A.K.; McGuire, S.E.; Nguyen, Q.; Pugh, T.J.; et al. Randomized Trial of Hypofractionated, Dose-Escalated, Intensity-Modulated Radiation Therapy (IMRT) versus Conventionally Fractionated IMRT for Localized Prostate Cancer. J. Clin. Oncol. 2018, 36, 2943–2949. [Google Scholar] [CrossRef]
- Jovanović, L.; Filipović, P.; Dedović, S.J.; Milovanović, Z.; Labudović, B.M.; Tanasijević, J.; Petrašinović, P.; Marinković, T.; Plešinac, K.V. The Role of c-MYC Expression in the Diagnostic and Clinical Confirmation of Radiation-Induced Angiosarcoma. Vojnosanit. Pregl. 2022, 79, 825–829. [Google Scholar] [CrossRef]
- Michalski, J.M.; Yan, Y.; Watkins-Bruner, D.; Bosch, W.R.; Winter, K.; Galvin, J.M.; Bahary, J.P.; Morton, G.P.; Parliament, M.B.; Sandler, H.M. Preliminary Toxicity Analysis of 3-Dimensional Conformal Radiation Therapy versus Intensity Modulated Radiation Therapy on the High-Dose Arm of the Radiation Therapy Oncology Group 0126 Prostate Cancer Trial. Int. J. Radiat. Oncol. Biol. Phys. 2013, 87, 932–938. [Google Scholar] [CrossRef] [PubMed]
- Hall, W.H.; Jani, A.B.; Ryu, J.K.; Narayan, S.; Vijayakumar, S. The Impact of Age and Comorbidity on Survival Outcomes and Treatment Patterns in Prostate Cancer. Prostate Cancer Prostatic Dis. 2005, 8, 22–30. [Google Scholar] [CrossRef]
- Valicenti, R.K.; Winter, K.; Cox, J.D.; Roach, M.; Markoe, A.; Perez, C.A.; Parliament, M.; Sandler, H.M. RTOG 94-06: Is the Addition of Neoadjuvant Hormonal Therapy to Dose-Escalated 3D Conformal Radiation Therapy for Prostate Cancer Associated with Treatment Toxicity? Int. J. Radiat. Oncol. Biol. Phys. 2003, 57, 614–620. [Google Scholar] [CrossRef]
- Solanki, A.A.; Liauw, S.L. Tobacco Use and EBRT for Prostate Cancer: Influence on Biochemical Control and Late Toxicity. Cancer 2013, 119, 2807–2814. [Google Scholar] [CrossRef]
- Kalakota, K.; Liauw, S.L. Toxicity After EBRT for Prostate Cancer: An Analysis of Late Morbidity in Men with Diabetes Mellitus. Urology 2013, 81, 1196–1201. [Google Scholar] [CrossRef]
- De Langhe, S.; De Ruyck, K.; Ost, P.; Thierens, H.; Vral, A. Acute Radiation-Induced Nocturia in Prostate Cancer Patients Is Associated with Pretreatment Symptoms, Radical Prostatectomy, and Genetic Markers in the TGFβ1 Gene. Int. J. Radiat. Oncol. Biol. Phys. 2013, 85, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Mathieu, R.; Arango, J.D.; Beckendorf, V.; Delobel, J.B.; Messai, T.; Chira, C.; Bossi, A.; Le Prisé, E.; Guerif, S.; Simon, J.M.; et al. Nomograms to Predict Late Urinary Toxicity after Prostate Cancer Radiotherapy. World J. Urol. 2014, 32, 743–751. [Google Scholar] [PubMed][Green Version]
- Yahya, N.; Ebert, M.A.; Bulsara, M.; Haworth, A.; Kennedy, A. Dosimetry, Clinical Factors and Medication Intake Influencing Urinary Symptoms after Prostate Radiotherapy: An Analysis of Data from the RADAR Prostate Radiotherapy Trial. Radiother. Oncol. 2015, 116, 112–118. [Google Scholar] [CrossRef]
- Brengues, M.; Lapierre, A.; Bourgier, C.; Pèlegrin, A.; Özsahin, M.; Azria, D. T Lymphocytes to Predict Radiation-Induced Late Effects in Normal Tissues. Expert Rev. Mol. Diagn. 2017, 17, 119–127. [Google Scholar] [CrossRef]
- Herskind, C.; Talbot, C.J.; Kerns, S.L.; Veldwijk, M.R.; Rosenstein, B.S.; West, C.M. Radiogenomics: A Systems Biology Approach to Understanding Genetic Risk Factors for Radiotherapy Toxicity? Cancer Lett. 2016, 382, 95–109. [Google Scholar] [CrossRef]
- Wang, K.; Tepper, J.E. Radiation Therapy-Associated Toxicity: Etiology, Management, and Prevention. CA A Cancer J. Clin. 2021, 71, 437–454. [Google Scholar] [CrossRef] [PubMed]
- Ainsbury, E.A.; Abrantes, A.M.; Baatout, S.; Baeyens, A.; Botelho, M.F.; Frey, B.; Foray, N.; Lyng, F.M.; Milic, M.; Pires, A.S.; et al. Individual Radiation Sensitivity and Biomarkers: Molecular Radiation Biology. In Radiobiology Textbook; Baatout, S., Ed.; Springer: Cham, Switzerland, 2023; pp. 7–23. [Google Scholar] [CrossRef]
- Christensen, E.; Pintilie, M.; Evans, K.R.; Lenarduzzi, M.; Ménard, C.; Catton, C.N.; Diamandis, E.P.; Bristow, R.G. Longitudinal Cytokine Expression during IMRT for Prostate Cancer and Acute Treatment Toxicity. Clin. Cancer Res. 2009, 15, 5576–5583. [Google Scholar] [CrossRef]
- Di Maggio, F.M.; Minafra, L.; Forte, G.I.; Cammarata, F.P.; Lio, D.; Messa, C.; Gilardi, M.C.; Bravatà, V. Portrait of Inflammatory Response to Ionizing Radiation Treatment. J. Inflamm. 2015, 12, 1. [Google Scholar] [CrossRef]
- Rubin, P.; Johnston, C.J.; Williams, J.P.; McDonald, S.; Finkelstein, J.N. A Perpetual Cascade of Cytokines Postirradiation Leads to Pulmonary Fibrosis. Int. J. Radiat. Oncol. Biol. Phys. 1995, 33, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Fessé, P.; Svensson, P.; Zackrisson, B.; Valdman, A.; Fransson, P.; Grankvist, K.; Kristensen, I.; Langegård, U.; Ohlsson-Nevo, E.; Sjövall, K.; et al. Association of Circulating Inflammatory Biomarker Levels and Toxicity in Patients Undergoing Pelvic Radiation for Cancer: A Critical Review. Adv. Radiat. Oncol. 2025, 2025, 101766. [Google Scholar] [CrossRef]
- Bonkhof, H. Factors Implicated in Radiation Therapy Failure and Radiosensitization of Prostate Cancer. Prostate Cancer 2012, 2012, 593241. [Google Scholar] [CrossRef]
- Miao, L.; Holley, A.K.; Zhao, Y.; St Clair, W.H.; St Clair, D.K. Redox-Mediated and Ionizing Radiation-Induced Inflammatory Mediators in Prostate Cancer Development and Treatment. Antioxid. Redox Signal. 2014, 20, 1481–1500. [Google Scholar] [CrossRef]
- Wu, C.T.; Chen, M.F.; Chen, W.C.; Hsieh, C.C. The Role of IL-6 in the Radiation Response of Prostate Cancer. Radiat. Oncol. 2013, 8, 1. [Google Scholar] [CrossRef] [PubMed]
- Muroyama, Y.; Nirschl, T.R.; Kochel, C.M.; Lopez-Bujanda, Z.; Theodros, D.; Mao, W.; Carrera-Haro, M.A.; Ghasemzadeh, A.E.; Marciscano, A.E.; Velarde, E.; et al. Stereotactic Radiotherapy Increases Functionally Suppressive Regulatory T Cells in the Tumor Microenvironment. Cancer Immunol. Res. 2017, 5, 992–1004. [Google Scholar] [CrossRef]
- Stanojković, T.P.; Matić, I.Z.; Petrović, N.; Stanković, V.; Kopčalić, K.; Besu, I.; Đorđić Crnogorac, M.; Mališić, E.; Mirjačić-Martinović, K.; Vuletić, A.; et al. Evaluation of Cytokine Expression and Circulating Immune Cell Subsets as Potential Parameters of Acute Radiation Toxicity in Prostate Cancer Patients. Sci. Rep. 2020, 10, 19002. [Google Scholar] [CrossRef]
- Kopčalić, K.; Matić, I.Z.; Besu, I.; Stanković, V.; Bukumirić, Z.; Stanojković, T.P.; Stepanović, A.; Nikitović, M. Circulating Levels of IL-6 and TGF-β1 in Patients with Prostate Cancer Undergoing Radiotherapy: Associations with Acute Radiotoxicity and Fatigue Symptoms. BMC Cancer 2022, 22, 1167. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Sohal, S.S.; Ahuja, K.; Lim, A.; Duncan, H.; Thachil, T.; De Ieso, P. Investigation of circulatory cytokines in patients undergoing intensity-modulated radiotherapy (IMRT) for adenocarcinoma of the prostate and association with acute RT-induced toxicity: A prospective clinical study. Cytokine 2020, 131, 155108. [Google Scholar] [CrossRef] [PubMed]
- Stankovic, V.; Džamic, Z.; Pekmezovic, T.; Tepavcevic, D.K.; Dozic, M.; Saric, M.; Vuckovic, S.; Nikitovic, M. Acute and Late Genitourinary Toxicity after 72 Gy of Conventionally Fractionated Conformal Radiotherapy for Localised Prostate Cancer: Impact of Individual and Clinical Parameters. Clin. Oncol. R Coll Radiol. 2016, 28, 577–586. [Google Scholar] [CrossRef]
- Paganetti, H. A Review on Lymphocyte Radiosensitivity and Its Impact on Radiotherapy. Front. Oncol. 2023, 13, 1201500. [Google Scholar] [CrossRef] [PubMed]
- Özsahin, M.; Özsahin, H.; Shi, Y.; Larsson, B.; Wurgler, F.E.; Crompton, N.E. Rapid Assay of Intrinsic Radiosensitivity Based on Apoptosis in Human CD4 and CD8 T-Lymphocytes. Int. J. Radiat. Oncol. Biol. Phys. 1997, 38, 429–440. [Google Scholar] [CrossRef]
- Özsahin, M.; Crompton, N.E.; Gourgou, S.; Kramar, A.; Li, L.; Shi, Y. CD4 and CD8 T-Lymphocyte Apoptosis Can Predict Radiation-Induced Late Toxicity: A Prospective Study in 399 Patients. Clin. Cancer Res. 2005, 11, 7426–7433. [Google Scholar] [CrossRef] [PubMed]
- Azria, D.; Belkacemi, Y.; Romieu, G.; Gourgou, S.; Gutowski, M.; Zaman, K.; Moscardo, C.L.; Lemanski, C.; Coelho, M.; Rosenstein, B.; et al. Concurrent or Sequential Adjuvant Letrozole and Radiotherapy after Conservative Surgery for Early-Stage Breast Cancer (CO-HO-RT): A Phase 2 Randomised Trial. Lancet Oncol. 2010, 11, 258–265. [Google Scholar] [CrossRef]
- Azria, D.; Riou, O.; Castan, F.; Nguyen, T.D.; Peignaux, K.; Lemanski, C. Radiation-Induced CD8 T-Lymphocyte Apoptosis as a Predictor of Breast Fibrosis after Radiotherapy: Results of the Prospective Multicenter French Trial. eBioMedicine 2015, 2, 1965–1973. [Google Scholar] [CrossRef]
- Foro, P.; Algara, M.; Lozano, J.; Rodriguez, N.; Sanz, X.; Torres, E. Relationship between Radiation-Induced Apoptosis of T Lymphocytes and Chronic Toxicity in Patients with Prostate Cancer Treated by Radiation Therapy: A Prospective Study. Int. J. Radiat. Oncol. Biol. Phys. 2014, 88, 1057–1063. [Google Scholar] [CrossRef]
- Pinkawa, M.; Brzozowska, K.; Kriehuber, R.; Eble, M.J.; Schmitz, S. Prediction of Radiation-Induced Toxicity by In Vitro Radiosensitivity of Lymphocytes in Prostate Cancer Patients. Future Oncol. 2016, 12, 617–624. [Google Scholar] [CrossRef]
- Schnarr, K.; Boreham, D.; Sathya, J.; Julian, J.; Dayes, I.S. Radiation-Induced Lymphocyte Apoptosis to Predict Radiation Therapy Late Toxicity in Prostate Cancer Patients. Int. J. Radiat. Oncol. Biol. Phys. 2009, 74, 1424–1430. [Google Scholar] [CrossRef]
- Talbot, C.J.; Veldwijk, M.R.; Azria, D.; Goujon, M.; Dufresne, A.; Rancati, T.; Giraud, P.; Martin, E.; Gosselin, M.; de Vathaire, F.; et al. Multi-Centre Technical Evaluation of the Radiation-Induced Lymphocyte Apoptosis Assay as a Predictive Test for Radiotherapy Toxicity. Clin. Transl. Radiat. Oncol. 2019, 18, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Azria, D.; Créhange, G.; Castan, F.; Belkacemi, Y.; Lagrange, J.; Nguyen, T.; Chapet, O.; Mornex, F.; Noel, G.; Lartigau, E.; et al. Le taux d’apoptose lymphocytaire radio-induit CD8 prédicteur de la toxicité pelvienne après radiothérapie prostatique: Résultats de l’étude prospective multicentrique française. Progrès En Urol. 2019, 29, 745. [Google Scholar] [CrossRef]
- Mališić, E.; Petrović, N.; Brengues, M.; Azria, D.; Matić, I.Z.; Srbljak Ćuk, I.; Kopčalić, K.; Stanojković, T.; Nikitović, M. Association of polymorphisms in TGFB1, XRCC1, XRCC3 genes and CD8 T-lymphocyte apoptosis with adverse effect of radiotherapy for prostate cancer. Sci. Rep. 2022, 12, 21306. [Google Scholar] [CrossRef] [PubMed]
- West, C.; Azria, D.; Chang-Claude, J.; Davidson, S.; Lambin, P.; Rosenstein, B.; De Ruysscher, D.; Talbot, C.; Thierens, H.; Valdagni, R.; et al. The REQUITE Project: Validating Predictive Models and Biomarkers of Radiotherapy Toxicity to Reduce Side-Effects and Improve Quality of Life in Cancer Survivors. Clin. Oncol. 2014, 26, 739–742. [Google Scholar] [CrossRef]
- Azria, D.; Michalet, M.; Riou, O.; Bourgier, C.; Brengues, M.; Sroussi, Y.; Gourgou, S.; Farcy-Jacquet, M.P.; Kotzki, L.; Ozsahin, M. Radiation-induced lymphocyte apoptosis assay: Primetime for routine clinical use? Cancer Radiother. 2024, 28, 442–448. [Google Scholar] [CrossRef]
- Lapierre, A.; Bourillon, L.; Larroque, M.; Gouveia, T.; Bourgier, C.; Ozsahin, M.; Pèlegrin, A.; Azria, D.; Brengues, M. Improving patients’ life quality after radiotherapy treatment by predicting late toxicities. Cancers 2022, 14, 2097. [Google Scholar] [CrossRef]
- West, C.M.; Barnett, G.C. Genetics and genomics of radiotherapy toxicity: Towards prediction. Genome Med. 2011, 3, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Kleinerman, R.A. Radiation-sensitive genetically susceptible pediatric sub-populations. Pediatr. Radiol. 2009, 39 (Suppl. S1), S27–S31. [Google Scholar] [CrossRef]
- Tamulevicius, P.; Wang, M.; Iliakis, G. Homology-directed repair is required for the development of radioresistance during S phase: Interplay between double-strand break repair and checkpoint response. Radiat. Res. 2007, 167, 1–11. [Google Scholar] [CrossRef]
- Kerns, S.L.; Stock, R.G.; Stone, N.N.; Rath, L.; Vega, A.; Fachal, L.; Gómez-Caamaño, A.; De Ruysscher, D.; Lammering, G.; Parliament, M.; et al. Genome-Wide Association Study Identifies a Region on Chromosome 11q14.3 Associated with Late Rectal Bleeding Following Radiation Therapy for Prostate Cancer. Radiother. Oncol. 2013, 107, 372–376. [Google Scholar] [CrossRef] [PubMed]
- Damaraju, S.; Murray, D.; Dufour, J.; Carandang, D.; Myrehaug, S.; Fallone, G.; Field, C.; Greiner, R.; Hanson, J.; Cass, C.E.; et al. Association of DNA repair and steroid metabolism gene polymorphisms with clinical late toxicity in patients treated with conformal radiotherapy for prostate cancer. Clin. Cancer Res. 2006, 12, 2545–2554. [Google Scholar] [CrossRef] [PubMed]
- Hümmerich, J.; Werle-Schneider, G.; Popanda, O.; Celebi, O.; Chang-Claude, J.; Kropp, S.; Mayer, C.; Debus, J.; Bartsch, H.; Schmezer, P. Constitutive mRNA expression of DNA repair-related genes as a biomarker for clinical radio-resistance: A pilot study in prostate cancer patients receiving radiotherapy. Int. J. Radiat. Biol. 2006, 82, 593–604. [Google Scholar] [CrossRef]
- Fachal, L.; Gómez-Caamaño, A.; Barnett, G.C.; Peleteiro, P.; Carballo, A.M.; Calvo-Crespo, P.; Kerns, S.L.; Sánchez-García, M.; Lobato-Busto, R.; Dorling, L.; et al. A Three-Stage Genome-Wide Association Study Identifies a Susceptibility Locus for Late Radiotherapy Toxicity at 2q24.1. Nat. Genet. 2014, 46, 891–894. [Google Scholar] [CrossRef]
- Kerns, S.L.; Dorling, L.; Fachal, L.; Bentzen, S.; Pharoah, P.D.; Barnes, D.R.; Gómez-Caamaño, A.; Carballo, A.M.; Dearnaley, D.P.; Peleteiro, P.; et al. Meta-Analysis of Genome-Wide Association Studies Identifies Genetic Markers of Late Toxicity Following Radiotherapy for Prostate Cancer. eBioMedicine 2016, 10, 150–163. [Google Scholar] [CrossRef]
- West, C.; Rosenstein, B.S. Establishment of a Radiogenomics Consortium. Int. J. Radiat. Oncol. Biol. Phys. 2010, 76, 1295–1296. [Google Scholar] [CrossRef] [PubMed]
- Andreassen, C.N.; Rosenstein, B.S.; Kerns, S.L.; Ostrer, H.; De Ruysscher, D.; Cesaretti, J.A.; Barnett, G.C.; Dunning, A.M.; Dorling, L.; West, C.M.L.; et al. Individual Patient Data Meta-Analysis Shows a Significant Association Between the ATM rs1801516 SNP and Toxicity After Radiotherapy in 5456 Breast and Prostate Cancer Patients. Radiother. Oncol. 2016, 121, 431–439. [Google Scholar] [CrossRef]
- Cesaretti, J.A.; Stock, R.G.; Atencio, D.P.; Peters, S.A.; Peters, C.A.; Burri, R.J.; Stone, N.N.; Rosenstein, B.S. A Genetically Determined Dose-Volume Histogram Predicts Rectal Bleeding Among Patients Treated with Prostate Brachytherapy. Int. J. Radiat. Oncol. Biol. Phys. 2007, 68, 1410–1416. [Google Scholar] [CrossRef]
- Valdagni, R.; Rancati, T.; Ghilotti, M.; Cozzarini, C.; Vavassori, V.; Fellin, G.; Fiorino, C.; Girelli, G.; Barra, S.; Zaffaroni, N.; et al. To Bleed or Not to Bleed: A Prediction Based on Individual Gene Profiling Combined with Dose-Volume Histogram Shapes in Prostate Cancer Patients Undergoing Three-Dimensional Conformal Radiation Therapy. Int. J. Radiat. Oncol. Biol. Phys. 2009, 74, 1431–1440. [Google Scholar] [CrossRef]
- Cintra, H.S.; Pinezi, J.C.; Machado, G.D.; de Carvalho, G.M.; Carvalho, A.T.; dos Santos, T.E.; Marciano, R.D.; Soares, R.d.B. Investigation of Genetic Polymorphisms Related to the Outcome of Radiotherapy for Prostate Cancer Patients. Dis. Markers 2013, 35, 701–710. [Google Scholar] [CrossRef]
- Petrović, N.; Stanojković, T.P.; Nikitović, M. MicroRNAs in Prostate Cancer Following Radiotherapy: Towards Predicting Response to Radiation Treatment. Curr. Med. Chem. 2022, 29, 1543–1560. [Google Scholar] [CrossRef] [PubMed]
- Keller, A.; Rounge, T.; Backes, C.; Ludwig, N.; Gislefoss, R.; Leidinger, P.; Langseth, H.; Meese, E. Sources to variability in circulating human miRNA signatures. RNA Biol. 2017, 14, 1791–1798. [Google Scholar] [CrossRef] [PubMed]
- Stang, A.; Weilert, H.; Lipp, M.J.; Oldhafer, K.J.; Hoheisel, J.D.; Zhang, C.; Bauer, A.S. MicroRNAs in blood act as biomarkers of colorectal cancer and indicate potential therapeutic targets. Mol. Oncol. 2021, 15, 2480–2490. [Google Scholar] [CrossRef]
- Aveta, A.; Cilio, S.; Contieri, R.; Spena, G.; Napolitano, L.; Manfredi, C.; Franco, A.; Crocerossa, F.; Cerrato, C.; Ferro, M.; et al. Urinary MicroRNAs as Biomarkers of Urological Cancers: A Systematic Review. Int. J. Mol. Sci. 2023, 24, 10846. [Google Scholar] [CrossRef]
- Ho, P.T.B.; Clark, I.M.; Le, L.T.T. MicroRNA-Based Diagnosis and Therapy. Int. J. Mol. Sci. 2022, 23, 7167. [Google Scholar] [CrossRef]
- Soares, S.; Guerreiro, S.G.; Cruz-Martins, N.; Faria, I.; Baylina, P.; Sales, M.G.; Correa-Duarte, M.A.; Fernandes, R. The Influence of miRNAs on Radiotherapy Treatment in Prostate Cancer—A Systematic Review. Front. Oncol. 2021, 11, 704664. [Google Scholar] [CrossRef] [PubMed]
- Gounaris-Shannon, S.; Chevassut, T. The Role of MicroRNAs in Radiotherapy Response in Cancer. Bone Marrow Res. 2013, 2013, 269107. [Google Scholar]
- Bartel, D.P. MicroRNAs: Target Recognition and Regulatory Functions. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef]
- Abdelaal, A.M.; Sohal, I.S.; Iyer, S.; Sudarshan, K.; Kothandaraman, H.; Lanman, N.A.; Low, P.S.; Kasinski, A.L. A first-in-class fully modified version of miR-34a with outstanding stability, activity, and anti-tumor efficacy. Oncogene 2023, 42, 2985–2999. [Google Scholar] [CrossRef]
- Slabáková, E.; Culig, Z.; Remšík, J.; Souček, K. Alternative mechanisms of miR-34a regulation in cancer. Cell Death Dis. 2017, 8, e3100. [Google Scholar] [CrossRef]
- Chen, C.Z. MicroRNAs and Cancer: From Bench to Bedside. N. Engl. J. Med. 2005, 353, 1768–1771. [Google Scholar] [CrossRef]
- Rahman, M.S.; Ghorai, S.; Panda, K.; Santiago, M.J.; Aggarwal, S.; Wang, T.; Rahman, I.; Chinnapaiyan, S.; Unwalla, H.J., Dr. Jekyll or Mr. Hyde: The multifaceted roles of miR-145-5p in human health and disease. Noncoding RNA Res. 2024, 11, 22–37. [Google Scholar] [CrossRef]
- El Bezawy, R.; Tinelli, S.; Tortoreto, M.; Doldi, V.; Zuco, V.; Folini, M.; Stucchi, C.; Rancati, T.; Valdagni, R.; Gandellini, P.; et al. miR-205 enhances radiation sensitivity of prostate cancer cells by impairing DNA damage repair through PKCε and ZEB1 inhibition. J. Exp. Clin. Cancer Res. 2019, 38, 51. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Shen, F.; Qi, P.; Zhai, Z.; Wang, Z. miR-541-3p enhances the radiosensitivity of prostate cancer cells by inhibiting HSP27 expression and downregulating β-catenin. Cell Death Discov. 2021, 7, 18. [Google Scholar] [CrossRef] [PubMed]
- Mao, A.; Tang, J.; Tang, D.; Wang, F.; Liao, S.; Yuan, H.; Tian, C.; Sun, C.; Si, J.; Zhang, H.; et al. MicroRNA-29b-3p enhances radiosensitivity through modulating WISP1-mediated mitochondrial apoptosis in prostate cancer cells. J. Cancer 2020, 11, 6356–6364. [Google Scholar] [CrossRef]
- Jenike, A.E.; Halushka, M.K. miR-21: A non-specific biomarker of all maladies. Biomark. Res. 2021, 9, 18. [Google Scholar] [CrossRef]
- Chawra, H.S.; Agarwal, M.; Mishra, A.; Chandel, S.S.; Singh, R.P.; Dubey, G.; Kukreti, N.; Singh, M. MicroRNA-21’s role in PTEN suppression and PI3K/AKT activation: Implications for cancer biology. Pathol. Res. Pract. 2024, 254, 155091. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.K.; Rajak, N.; Singh, Y.; Singh, A.K.; Giri, R.; Garg, N. Role of MicroRNA-21 in prostate cancer progression and metastasis: Molecular mechanisms to therapeutic targets. Ann. Surg. Oncol. 2024, 31, 4795–4808. [Google Scholar] [CrossRef]
- Huang, X.; Taeb, S.; Jahangiri, S.; Emmenegger, U.; Tran, E.; Bruce, J.; Mesci, A.; Korpela, E.; Vesprini, D.; Wong, C.S.; et al. miRNA-95 mediates radioresistance in tumors by targeting the sphingolipid phosphatase SGPP1. Cancer Res. 2013, 73, 6972–6986. [Google Scholar] [CrossRef]
- Pedroza-Torres, A.; Romero-Córdoba, S.L.; Montaño, S.; Peralta-Zaragoza, O.; Vélez-Uriza, D.E.; Arriaga-Canon, C.; Guajardo-Barreto, X.; Bautista-Sánchez, D.; Sosa-León, R.; Hernández-González, O.; et al. Radio-miRs: A comprehensive view of radioresistance-related microRNAs. Genetics 2024, 227, iyae097. [Google Scholar] [CrossRef]
- Jia, M.; Wang, Z. MicroRNAs as Biomarkers for Ionizing Radiation Injury. Front. Cell Dev. Biol. 2022, 10, 861451. [Google Scholar] [CrossRef] [PubMed]
- Ni, J.; Bucci, J.; Chang, L.; Malouf, D.; Graham, P.; Li, Y. Targeting MicroRNAs in Prostate Cancer Radiotherapy. Theranostics 2017, 7, 3243–3259. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhang, Y.; Liu, F.; Ye, Y.; Liu, X.; Chen, R.; Luo, G.; Shi, C.; Xu, J.; Liu, C.; et al. circNEIL3 Regulatory Loop Promotes Pancreatic Ductal Adenocarcinoma Progression via miR-432-5p/ADAR1/GLI1 Axis. Mol. Cancer 2021, 20, 80. [Google Scholar] [CrossRef]
- Yuan, Z.; Chen, S.; Duan, S.; Dong, Y.; Li, Q.; Zhang, J.; Ma, X.; Wu, J.; Xu, L.; Yang, H. Loss of NEIL3 Activates Radiotherapy Resistance in Prostate Cancer Progression. Cancer Biol. Med. 2022, 19, 1160–1176. [Google Scholar] [CrossRef]
| Risk Group | TNM Stage | PSA (ng/mL) | Gleason Score/Grade Group | Treatment Options |
|---|---|---|---|---|
| Very low | T1c | <10 | GS ≤ 6 (Grade Group 1), <3 positive biopsy cores, PSA density < 0.15 | - Active surveillance (preferred) - Radical prostatectomy (selected cases) - Brachytherapy (rare) |
| Low | T1-T2a | <10 | GS ≤ 6 (Grade Group 1) | - Active surveillance (preferred) - Radical prostatectomy - EBRT or brachytherapy |
| Favourable intermediate | T1-T2b | 10–20 | GS 3 + 4 (Grade Group 2) OR <50% biopsy cores positive | - Radical prostatectomy ± lymph node dissection - EBRT ± short-term ADT (4–6 mo) - Brachytherapy ± EBRT |
| Unfavourable intermediate | T2c or ≥50% cores + or GS 4 + 3 (Grade Group 3) or PSA 10–20 | 10–20 | GS 4 + 3 (Grade Group 3) or multiple intermediate factors | - Radical prostatectomy ± pelvic LN dissection - EBRT + short-term ADT - EBRT + brachytherapy boost |
| High | T3a or PSA > 20 or GS 8 (Grade Group 4) | >20 | GS 8 (Grade Group 4) | - EBRT + long-term ADT (2–3 years) - EBRT + brachytherapy + ADT - Radical prostatectomy (select cases, as part of multimodal treatment) |
| Very high | T3b-T4 or GS 9–10 (Grade Group 5) or >4 biopsy cores with Grade Group 4 or 5 | Any | GS 9–10 (Grade Group 5) | - EBRT + long-term ADT ± abiraterone - EBRT + brachytherapy + ADT - Radical prostatectomy (select cases) |
| Regional (N1) | Any T, N1, M0 | Any | Any | - EBRT + long-term ADT - ADT alone (non-curative setting) - Consider abiraterone + ADT |
| Metastatic (M1) | Any T, any N, M1 | Any | Any | - ADT + novel hormonal therapy (abiraterone, enzalutamide, and apalutamide) - ±Docetaxel (in high-volume disease) - Bone-protecting agents (zoledronic acid or denosumab) - Palliative radiotherapy-Lu-177 PSMA, PARP inhibitors (selected mCRPC) |
| Cytokine | Role | Association with Adverse Radiotherapy Effects |
|---|---|---|
| TGF-β1 (Transforming Growth Factor Beta 1) | Modulates immune responses and fibrosis | Elevated TGF-β1 levels after radiotherapy can contribute to fibrosis and toxicity in prostate cancer patients [33,39] |
| TNF-α (Tumour Necrosis Factor Alpha) | Proinflammatory cytokine, regulates apoptosis | High TNF-α levels after radiotherapy may be linked to increased inflammation and risk of late toxicities [33] |
| IL-6 (Interleukin-6) | Mediates inflammatory responses and tissue repair | Elevated IL-6 can be a marker of poor prognoses, contributing to inflammation and fibrosis post-RT [30,36] |
| IL-1β (Interleukin-1 Beta) | Promotes inflammation and tissue damage | Associated with radiation-induced fibrosis and increased late toxicities in prostate cancer radiotherapy [39] |
| IFN-γ (Interferon Gamma) | Enhances immune response and regulates apoptosis | Higher IFN-γ levels may correlate with increased lymphocyte apoptosis and lower late toxicity risk [30,38] |
| IL-2 (Interleukin-2) | Stimulates T-cell activation and proliferation | Plays a key role in immune recovery after radiotherapy; altered levels may influence long-term toxicities [30] |
| IL-8 (Interleukin-8) | Chemotactic cytokine that attracts neutrophils | Elevated IL-8 levels after radiotherapy may contribute to inflammation and exacerbate toxicities [33] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stanić, J.; Šović, I.; Jovanovic, L.; Matić, I.Z.; Nikić, P.; Nikitović, M. The Role of Predictive Biomarkers in Modern Prostate Cancer Radiotherapy: A Literature Review on Personalised Treatment Strategies and the Prediction of Adverse Effects. Life 2025, 15, 1062. https://doi.org/10.3390/life15071062
Stanić J, Šović I, Jovanovic L, Matić IZ, Nikić P, Nikitović M. The Role of Predictive Biomarkers in Modern Prostate Cancer Radiotherapy: A Literature Review on Personalised Treatment Strategies and the Prediction of Adverse Effects. Life. 2025; 15(7):1062. https://doi.org/10.3390/life15071062
Chicago/Turabian StyleStanić, Jelena, Ivana Šović, Luka Jovanovic, Ivana Z. Matić, Predrag Nikić, and Marina Nikitović. 2025. "The Role of Predictive Biomarkers in Modern Prostate Cancer Radiotherapy: A Literature Review on Personalised Treatment Strategies and the Prediction of Adverse Effects" Life 15, no. 7: 1062. https://doi.org/10.3390/life15071062
APA StyleStanić, J., Šović, I., Jovanovic, L., Matić, I. Z., Nikić, P., & Nikitović, M. (2025). The Role of Predictive Biomarkers in Modern Prostate Cancer Radiotherapy: A Literature Review on Personalised Treatment Strategies and the Prediction of Adverse Effects. Life, 15(7), 1062. https://doi.org/10.3390/life15071062






