Water Stress Is Differently Tolerated by Fusarium-Resistant and -Susceptible Chickpea Genotypes During Germination
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Treatments and Experimental Materials
2.3. Measurement of the Germination Parameters
2.4. Measurement of the Leaf Proline Content
2.5. Measurement of Leaf Lipid Peroxidation (MDA) Levels
2.6. Statistical Analyses
3. Results
3.1. Results of Germination Parameters
3.2. Selection of Tolerant/Moderately Tolerant and Susceptible Genotypes on the Basis of Their IC50 Values
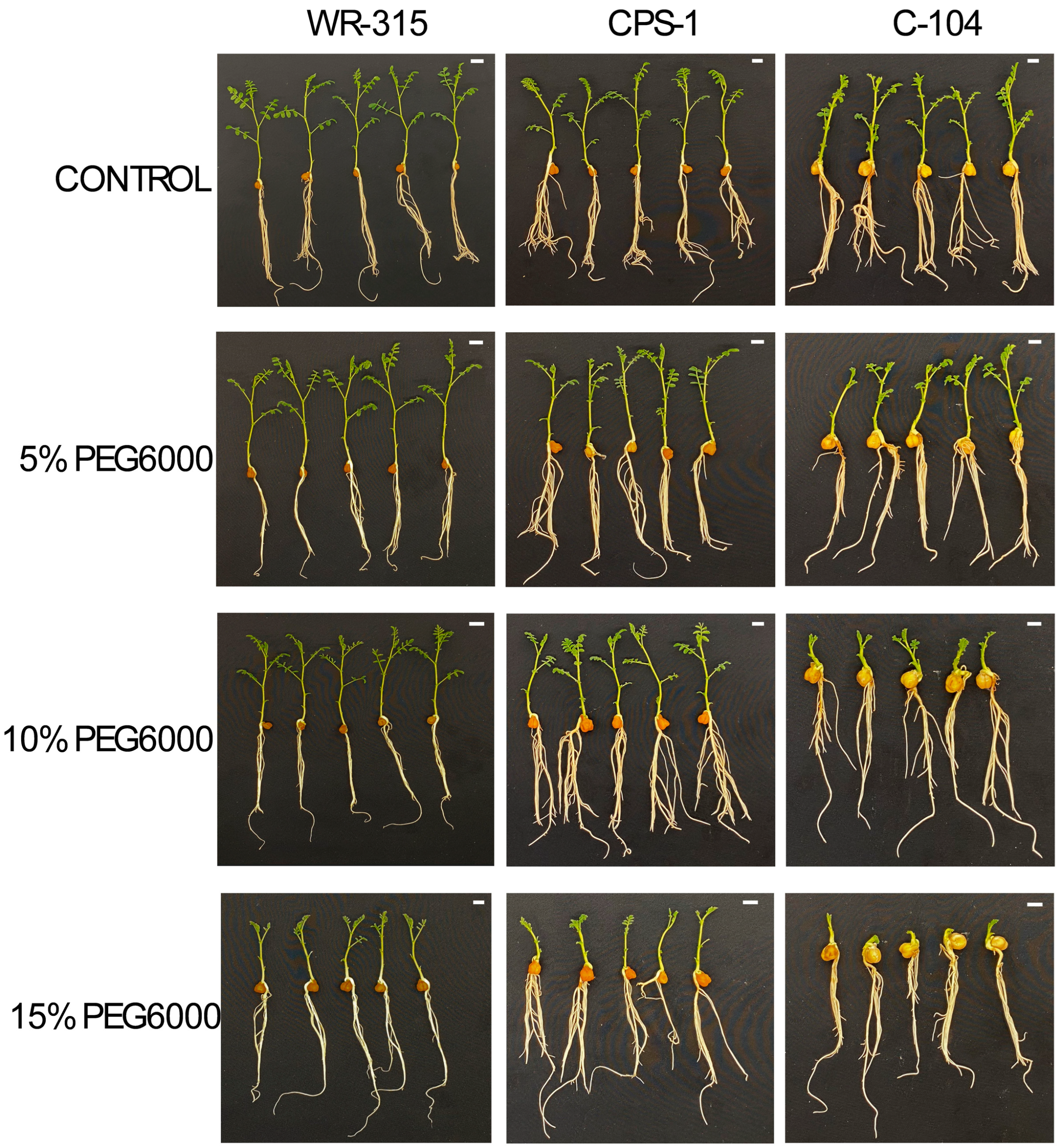
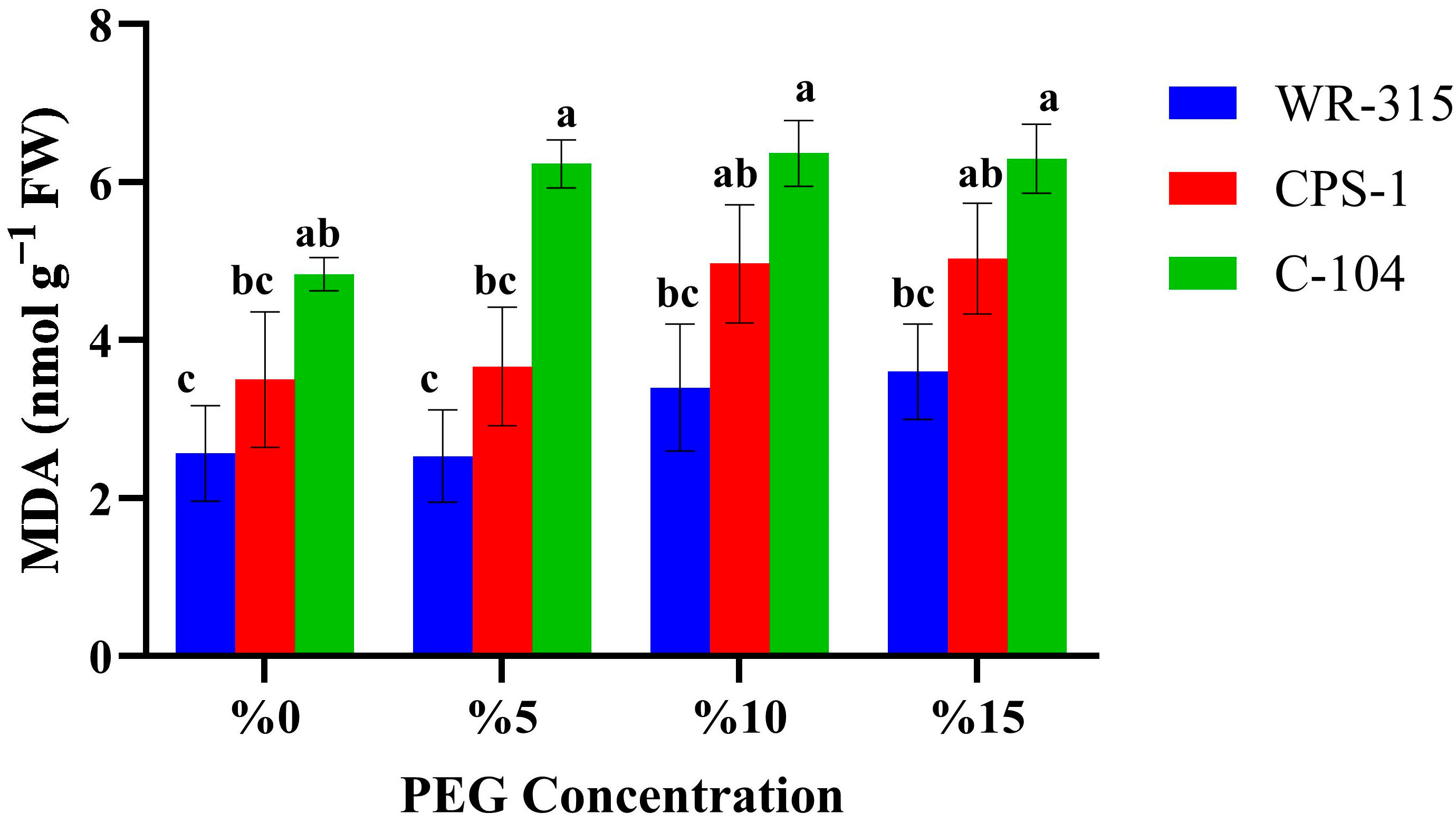
3.3. Biochemical Responses of Selected Genotypes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gaur, P.M.; Samineni, S.; Sajja, S.; Chibbar, R.N. Achievements and Challenges in Improving Nutritional Quality of Chickpea. Legume Perspect. 2015, 9, 31–33. [Google Scholar]
- Alcorta, A.; Porta, A.; Amparo, T.; Dolores Alvarez, M.; Pilar Vaquero, M. Foods for Plant-Based Diets: Challenges and Innovations Alexandra. Foods 2021, 10, 293. [Google Scholar] [CrossRef]
- Gopalakrishnan, S.; Srinivas, V.; Samineni, S. Nitrogen Fixation, Plant Growth and Yield Enhancements by Diazotrophic Growth-Promoting Bacteria in Two Cultivars of Chickpea (Cicer arietinum L.). Biocatal. Agric. Biotechnol. 2017, 11, 116–123. [Google Scholar] [CrossRef]
- Tiwari, S.; Sahu, V.K.; Gupta, N.; Tripathi, M.K.; Yasin, M. Evaluation of Physiological and Biochemical Contents in Desi and Kabuli Chickpea. Legume Res. Int. J. 2020, I, 1–12. [Google Scholar] [CrossRef]
- Arora, N.K. Impact of Climate Change on Agriculture Production and Its Sustainable Solutions. Environ. Sustain. 2019, 2, 95–96. [Google Scholar] [CrossRef]
- Koskosidis, A.; Khah, E.; Mavromatis, A.; Pavli, O.; Vlachostergios, D.N. Effect of PEG-Induced Drought Stress on Germination of Ten Chickpea (Cicer arietinum L.) Genotypes. Not. Bot. Horti Agrobot. Cluj-Napoca 2020, 48, 294–304. [Google Scholar] [CrossRef]
- Devasirvatham, V.; Tan, D.K.Y. Impact of High Temperature and Drought Stresses on Chickpea Production. Agronomy 2018, 8, 145. [Google Scholar] [CrossRef]
- Song, L.; Jin, J.; He, J. Effects of Severe Water Stress on Maize Growth Processes in the Field. Sustainability 2019, 11, 5086. [Google Scholar] [CrossRef]
- Reed, R.C.; Bradford, K.J.; Khanday, I. Seed Germination and Vigor: Ensuring Crop Sustainability in a Changing Climate. Heredity 2022, 128, 450–459. [Google Scholar] [CrossRef]
- Yousefi, A.R.; Rashidi, S.; Moradi, P.; Mastinu, A. Germination and Seedling Growth Responses of Zygophyllum fabago, Salsola kali L. and Atriplex canescens to Peg-Induced Drought Stress. Environments 2020, 7, 107. [Google Scholar] [CrossRef]
- Domozych, D.S.; Kozel, L.; Palacio-Lopez, K. The Effects of Osmotic Stress on the Cell Wall-Plasma Membrane Domains of the Unicellular Streptophyte, Penium margaritaceum. Protoplasma 2021, 258, 1231–1249. [Google Scholar] [CrossRef]
- Kalefetoǧlu Macar, T.; Turan, Ö.; Ekmekçi, Y. Effects of Water Deficit Induced by PEG and NaCl on Chickpea (Cicer arietinum L.) Cultivars and Lines at Early Seedling Stages. Gazi Univ. J. Sci. 2009, 22, 5–14. [Google Scholar]
- Nikitin, D.A.; Ivanova, E.A.; Semenov, M.V.; Zhelezova, A.D.; Ksenofontova, N.A.; Tkhakakhova, A.K.; Kholodov, V.A. Diversity, Ecological Characteristics and Identification of Some Problematic Phytopathogenic Fusarium in Soil: A Review. Diversity 2023, 15, 49. [Google Scholar] [CrossRef]
- Kheiri, A.; Moosawi Jorf, S.A.; Malihipour, A. Infection Process and Wheat Response to Fusarium Head Blight Caused by Fusarium Graminearum. Eur. J. Plant Pathol. 2019, 153, 489–502. [Google Scholar] [CrossRef]
- Bhar, A.; Jain, A.; Das, S. Soil Pathogen, Fusarium oxysporum Induced Wilt Disease in Chickpea: A Review on Its Dynamicity and Possible Control Strategies. Proc. Indian Natl. Sci. Acad. 2021, 87, 260–274. [Google Scholar] [CrossRef]
- Dikilitas, M.; Karakas, S. Crop Plants under Saline-Adapted Fungal Pathogens. In Emerging Technologies and Management of Crop Stress Tolerance; Elsevier: Amsterdam, The Netherlands, 2014; pp. 173–192. ISBN 978-0-12-800875-1. [Google Scholar]
- Baran, B.; Ölmez, F.; Çapa, B.; Dikilitas, M. Defense Pathways of Wheat Plants Inoculated with Zymoseptoria tritici under NaCl Stress Conditions: An Overview. Life 2024, 14, 648. [Google Scholar] [CrossRef]
- Dubey, S.C.; Priyanka, K.; Singh, V.; Singh, B. Race Profiling and Molecular Diversity Analysis of Fusarium oxysporum f. Sp. ciceris Causing Wilt in Chickpea. J. Phytopathol. 2012, 160, 576–587. [Google Scholar] [CrossRef]
- Kocalar, H.; Kafadar, F.N.; Ozkan, A.; Talapov, T.; Demirel, O.; Anay, A.; Mart, D.; Can, C. Current Distribution and Virulence of Fusarium oxysporum f. Sp. Ciceris in Turkey. Legume Res. 2020, 43, 735–741. [Google Scholar] [CrossRef]
- Jiménez-Gasco, M.D.M.; Milgroom, M.G.; Jiménez-Díaz, R.M. Stepwise Evolution of Races in Fusarium oxysporum f. Sp. ciceris Inferred from Fingerprinting with Repetitive DNA Sequences. Phytopathology 2004, 94, 228–235. [Google Scholar] [CrossRef]
- Muche, M.; Yemata, G. Epidemiology and Pathogenicity of Vascular Wilt of Chickpea (Cicer arietinum L.) Caused by Fusarium oxysporum f. Sp. ciceris, and the Host Defense Responses. S. Afr. J. Bot. 2022, 151, 339–348. [Google Scholar] [CrossRef]
- Trapero-Casas, A.; Jimenez-Diaz, R.M. Fungal Wilt and Root Rot Diseases of Chickpea in Southern Spain. Phytopathology 1985, 75, 1146–1151. [Google Scholar] [CrossRef]
- Haware, M.P.; Nene, Y.L. Races of Fusarium oxysporum f. Sp. ciceri. Plant Dis. 1982, 66, 809–810. [Google Scholar] [CrossRef]
- Dikilitas, M.; Karakas, S. Behaviour of Plant Pathogens for Crops Under Stress During the Determination of Physiological, Biochemical, and Molecular Approaches for Salt Stress Tolerance; Ashraf, M., Öztürk, M., Ahmad, M.S.A., Aksoy, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; Volume 9789400741, ISBN 978-94-007-4116-4. [Google Scholar]
- Dikilitas, M.; Karakas, S.; Hashem, A.; Abd Allah, E.F.; Ahmad, P. Oxidative Stress and Plant Responses to Pathogens under Drought Conditions. Water Stress Crop Plants Sustain. Approach 2016, 1–2, 102–123. [Google Scholar] [CrossRef]
- Sinha, R.; Irulappan, V.; Mohan-Raju, B.; Suganthi, A.; Senthil-Kumar, M. Impact of Drought Stress on Simultaneously Occurring Pathogen Infection in Field-Grown Chickpea. Sci. Rep. 2019, 9, 5577. [Google Scholar] [CrossRef]
- Michel, B.E.; Kaufmann, M.R. The Osmotic Potential of Polyethylene Glycol 6000. Plant Physiol. 1973, 51, 914–916. [Google Scholar] [CrossRef]
- Ullah, A.; Sadaf, S.; Ullah, S.; Alshaya, H.; Okla, M.K. Using Halothermal Time Model to Describe Barley (Hordeumvulgare L.) Seed Germination Response to Water Potential and Temperature. Life 2022, 12, 209. [Google Scholar] [CrossRef]
- Itroutwar, P.D.; Kasivelu, G.; Raguraman, V.; Malaichamy, K.; Sevathapandian, S.K. Effects of Biogenic Zinc Oxide Nanoparticles on Seed Germination and Seedling Vigor of Maize (Zea mays). Biocatal. Agric. Biotechnol. 2020, 29, 101778. [Google Scholar] [CrossRef]
- Bakhshandeh, E.; Jamali, M.; Afshoon, E.; Gholamhossieni, M. Using Hydrothermal Time Concept to Describe Sesame (Sesamum indicum L.) Seed Germination Response to Temperature and Water Potential. Acta Physiol. Plant. 2017, 39, 250. [Google Scholar] [CrossRef]
- Carlson, J.R.; Ditterline, R.L.; Martin, J.M.; Sands, D.C.; Lund, R.E. Alfalfa Seed Germination in Antiobiotic Agar Containing NaCl. Crop Sci. 1983, 23, 882–885. [Google Scholar] [CrossRef]
- Dikilitas, M. Effect of Salinity & Its Interactions with Verticillium Albo-Atrum on the Disease Development in Tomato (Lycopersicon esculentum Mill.) and Lucerne (Medicago sativa L. & M. media) Plants. Ph.D. Thesis, Swansea University, Swansea, UK, 2003. [Google Scholar]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid Determination of Free Proline for Water—Stress Studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in Isolated Chloroplasts. II. Role of Electron Transfer. Arch. Biochem. Biophys. 1968, 125, 850–857. [Google Scholar] [CrossRef] [PubMed]
- Dolar, F.S. Determination of the Races of Fusarium oxysporum f. Sp. ciceris in Ankara Province, Turkey. J. Turk. Phytopqthology 1997, 26, 11–15. [Google Scholar]
- Jiménez-Díaz, R.M.; Castillo, P.; del Mar Jiménez-Gasco, M.; Landa, B.B.; Navas-Cortés, J.A. Fusarium Wilt of Chickpeas: Biology, Ecology and Management. Crop Prot. 2015, 73, 16–27. [Google Scholar] [CrossRef]
- Farooq, M.; Ullah, A.; Lee, D.J.; Alghamdi, S.S.; Siddique, K.H.M. Desi Chickpea Genotypes Tolerate Drought Stress Better than Kabuli Types by Modulating Germination Metabolism, Trehalose Accumulation, and Carbon Assimilation. Plant Physiol. Biochem. 2018, 126, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Wahab, A.; Abdi, G.; Saleem, M.H.; Ali, B.; Ullah, S.; Shah, W.; Mumtaz, S.; Yasin, G.; Muresan, C.C.; Marc, R.A. Alleviate the Adverse Effects of Drought Stress: A Comprehensive Review. Plants 2022, 11, 1620. [Google Scholar] [CrossRef]
- Seleiman, M.F.; Al-Suhaibani, N.; Ali, N.; Akmal, M.; Alotaibi, M.; Refay, Y.; Dindaroglu, T.; Abdul-Wajid, H.H.; Battaglia, M.L. Drought Stress Impacts on Plants and Different Approaches to Alleviate Its Adverse Effects. Plants 2021, 10, 259. [Google Scholar] [CrossRef]
- Ahmad, M.A.; Javed, R.; Adeel, M.; Rizwan, M.; Yang, Y. PEG 6000-Stimulated Drought Stress Improves the Attributes of in Vitro Growth, Steviol Glycosides Production, and Antioxidant Activities in Stevia Rebaudiana Bertoni. Plants 2020, 9, 1552. [Google Scholar] [CrossRef] [PubMed]
- Adetunji, A.E.; Sershen; Varghese, B.; Pammenter, N.W. Effects of Inorganic Salt Solutions on Vigour, Viability, Oxidative Metabolism and Germination Enzymes in Aged Cabbage and Lettuce Seeds. Plants 2020, 9, 1164. [Google Scholar] [CrossRef]
- Himaja, R.; Radhika, K.; Reddy, K.B.; Raghavendra, M. Screening of Chickpea (Cicer Arietinum L.) Genotypes for Germination and Early Seedling Growth under PEG 6000 Induced Drought Stress. Legume Res. 2021, 46, 813–821. [Google Scholar] [CrossRef]
- Abderemane, B.A.; Houasli, C.; Mitache, M.; Idrissi, O.; Fakiri, M. Physiological, Agro-Morphological, and Germination Responses of a Worldwide Chickpea (Cicer arietinum) Collection Subjected to Drought Stress by Applying Polyethylene Glycol (PEG) on Germinating Seeds and by Exposure Plants to Water Restriction at the Vegetative Stage. Biocatal. Agric. Biotechnol. 2024, 56, 103011. [Google Scholar] [CrossRef]
- Haghpanah, M.; Hashemipetroudi, S.; Arzani, A.; Araniti, F. Drought Tolerance in Plants: Physiological and Molecular Responses. Plants 2024, 13, 2962. [Google Scholar] [CrossRef]
- Terfa, G.N.; Pan, W.; Hu, L.; Hao, J.; Zhao, Q.; Jia, Y.; Nie, X. Mechanisms of Salt and Drought Stress Responses in Foxtail Millet. Plants 2025, 14, 1215. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Shi, J.; Meng, M.; Xu, K.; Xu, Y.; Ji, D.; Wang, W.; Xie, C. Metabolic Responses of Pyropia haitanensis to Dehydration-Rehydration Cycles Revealed by Metabolomics. Mar. Drugs 2025, 23, 203. [Google Scholar] [CrossRef] [PubMed]
- Adamipour, N.; Nazari, F.; Nalousi, A.M.; Teixeira Da Silva, J.A. Evaluation of the Molecular Mechanism Underlying Proline Metabolic and Catabolic Pathways and Some Morpho-Physiological Traits of Tobacco (Nicotiana tabacum L.) Plants under Arsenic Stress. BMC Plant Biol 2025, 25, 258. [Google Scholar] [CrossRef] [PubMed]
- Gazi, R.; Kumar, S.; Jana, M. Proline Concentration Driven Thermostability and Hydration Properties of Ubiquitin. J. Mol. Liq. 2025, 424, 127108. [Google Scholar] [CrossRef]
- Yan, L.; Lu, M.; Riaz, M.; Tong, K.; Yu, H.; Gao, G.; Niu, Y. Exogenous Proline Enhances Salt Acclimation in Soybean Seedlings: Modifying Physicochemical Properties and Controlling Proline Metabolism through the Ornithine-Glutamate Dual Pathway. Ecotoxicol. Environ. Saf. 2025, 294, 118012. [Google Scholar] [CrossRef]
- Khan, N.; Bano, A.; Rahman, M.A.; Guo, J.; Kang, Z.; Babar, M.A. Comparative Physiological and Metabolic Analysis Reveals a Complex Mechanism Involved in Drought Tolerance in Chickpea (Cicer arietinum L.) Induced by PGPR and PGRs. Sci. Rep. 2019, 9, 2097. [Google Scholar] [CrossRef]
- Furlan, A.L.; Bianucci, E.; Giordano, W.; Castro, S.; Becker, D.F. Proline Metabolic Dynamics and Implications in Drought Tolerance of Peanut Plants. Plant Physiol. Biochem. 2020, 151, 566–578. [Google Scholar] [CrossRef]
- Desoky, E.S.M.; Mansour, E.; El-Sobky, E.S.E.A.; Abdul-Hamid, M.I.; Taha, T.F.; Elakkad, H.A.; Arnaout, S.M.A.I.; Eid, R.S.M.; El-Tarabily, K.A.; Yasin, M.A.T. Physio-Biochemical and Agronomic Responses of Faba Beans to Exogenously Applied Nano-Silicon Under Drought Stress Conditions. Front. Plant Sci. 2021, 12, 637783. [Google Scholar] [CrossRef]
- Machado, J.; Fernandes, A.P.G.; Bokor, B.; Vaculík, M.; Kostoláni, D.; Kokavcová, A.; Heuvelink, E.; Vasconcelos, M.W.; Carvalho, S.M.P. Tomato Responses to Nitrogen, Drought and Combined Stresses: Shared and Specific Effects on Vascular Plant Anatomy, Nutrient Partitioning and Amino Acids Profile. Plant Physiol. Biochem. 2025, 221, 109649. [Google Scholar] [CrossRef]
- Martinez, V.; Mestre, T.C.; Rubio, F.; Girones-Vilaplana, A.; Moreno, D.A.; Mittler, R.; Rivero, R.M. Accumulation of Flavonols over Hydroxycinnamic Acids Favors Oxidative Damage Protection under Abiotic Stress. Front. Plant Sci. 2016, 7, 838. [Google Scholar] [CrossRef] [PubMed]
- Khaleghi, A.; Naderi, R.; Brunetti, C.; Maserti, B.E.; Salami, S.A.; Babalar, M. Morphological, Physiochemical and Antioxidant Responses of Maclura Pomifera to Drought Stress. Sci. Rep. 2019, 9, 19250. [Google Scholar] [CrossRef] [PubMed]
- Sarker, U.; Oba, S. The Response of Salinity Stress-Induced A. Tricolor to Growth, Anatomy, Physiology, Non-Enzymatic and Enzymatic Antioxidants. Front. Plant Sci. 2020, 11, 559876. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Guan, J.; Liang, Q.; Zhang, X.; Hu, H.; Zhang, J. Effects of Cadmium Stress on Growth and Physiological Characteristics of Sassafras Seedlings. Sci. Rep. 2021, 11, 9913. [Google Scholar] [CrossRef]
- Martínez, M.; Arata, A.F.; Lázaro, L.; Stenglein, S.A.; Dinolfo, M.I. Effects of Waterlogging Stress on Plant-Pathogen Interaction between Fusarium poae and Wheat/Barley. Acta Sci. Agron. 2019, 41, e42629. [Google Scholar] [CrossRef]

| Seed Type | Genotype | PEG (%) | WP (MPa) | GP (%) * | GE | VI | GRI | MGT | Hypocotyl Height | Radicle Length |
|---|---|---|---|---|---|---|---|---|---|---|
| Desi | WR-315 | 0 | 0 | 85 ± 0.0 a1 | 82.50 ± 2.5 a | 136.4 ± 20.3 bc | 6.49 ± 0.5 a | 5.25 ± 0.02 a | 0.075 ± 0.075 b | 1.50 ± 0.07 bc |
| 5 | −0.05 | 85 ± 0.0 a | 78.75 ± 6.9 a | 821 ± 265 a | 5.68 ± 0.4 a | 5.43 ± 0.23 a | 2.84 ± 1.11 a | 6.58 ± 1.85 a | ||
| 7.5 | −0.075 | 90 ± 5.77 a | 72.50 ± 6.6 a | 455 ± 42.7 ab | 5.49 ± 0.3 a | 5.36 ± 0.03 a | 1.055 ± 0.35 a | 2.90 ± 0.969 bc | ||
| 10 | −0.15 | 100 ± 0.0 a | 82.50 ± 3.2 a | 455 ± 4.12 ab | 6.62 ± 0.4 a | 5.23 ± 0.03 a | 0.975 ± 0.7 b | 3.57 ± 0.041 ab | ||
| 15 | −0.22 | 85 ± 5.0 a | 65.00 ± 8.4 a | 0 ± 0.0 c | 3.89 ± 0.4 b | 5.75 ± 0.08 a | 0 ± 0.0 b | 0 ± 0.0 c | ||
| 20 | −0.5 | 5 ± 5.0 b | 5.00 ± 0.0 b | 0 ± 0.0 c | 0.43 ± 0.2 c | 4.13 ± 1.66 a | 0 ± 0.0 b | 0 ± 0.0 c | ||
| Desi | BG-212 | 0 | 0 | 40 ± 0.0 c | 28.75 ± 4.27 c | 16.8 ± 16.8 c | 1.29 ± 0.33 cd | 6.3 ± 0.30 a | 0.05 ± 0.05 c | 0.37 ± 0.37 d |
| 5 | −0.05 | 100 ± 0.0 a | 88.75 ± 3.75 a | 1584.5 ± 62.7 a | 5.46 ± 0.57 a | 5.59 ± 0.09 b | 4.36 ± 0.22 a | 11.48 ± 0.48 a | ||
| 7.5 | −0.075 | 85 ± 5.0 b | 68.75 ± 9.44 ab | 1072 ± 124 b | 3.02 ± 0.59 bc | 6.26 ± 0.18 ab | 1.86 ± 0.58 b | 10.64 ± 0.15 a | ||
| 10 | −0.15 | 100 ± 0.0 a | 83.75 ± 3.75 ab | 895 ± 11.8 b | 4.51 ± 0.52 ab | 5.78 ± 0.11 ab | 0.07 ± 0.07 c | 8.88 ± 0.58 b | ||
| 15 | −0.22 | 75 ± 5.0 b | 68.75 ± 3.75 ab | 126.5 ± 30.4 c | 3.49 ± 0.57 ab | 5.90 ± 0.13 ab | 0 ± 0.0 c | 1.75 ± 0.47 c | ||
| 20 | −0.5 | 75 ± 5.0 b | 63.75 ± 5.54 b | 5.60 ± 5.60 c | 3.63 ± 0.58 ab | 5.73 ± 0.14 ab | 0 ± 0.0 c | 0.07 ± 0.07 d | ||
| Desi | JG-74 | 0 | 0 | 100 ± 0.0 a | 87.50 ± 5.2 ab | 1444 ± 164 a | 4.97 ± 0.29 b | 5.67 ± 0.10 ab | 4.46 ± 0.56 a | 9.97 ± 1.08 a |
| 5 | −0.05 | 100 ± 0.0 a | 90 ± 4.08 a | 884 ± 32.3 b | 5.17 ± 0.41 b | 5.69 ± 0.04 ab | 1.77 ± 0.22 b | 7.06 ± 0.20 b | ||
| 7.5 | −0.075 | 75 ± 5.77 b | 72.5 ± 6.61 b | 298 ± 11 cd | 4.41 ± 0.77 b | 5.66 ± 0.10 ab | 0.72 ± 0.41 bc | 3.73 ± 1.25 c | ||
| 10 | −0.15 | 100 ± 0.0 a | 88.75 ± 1.25 a | 546.5 ± 44.5 c | 7.09 ± 0.63 a | 5.22 ± 0.02 b | 0.59 ± 0.19 bc | 4.87 ± 0.28 bc | ||
| 15 | −0.22 | 75 ± 5.0 b | 17.50 ± 2.5 c | 5.6 ± 5.6 d | 0.80 ± 0.30 c | 6.37 ± 0.47 a | 0 ± 0.0 c | 0 ± 0.0 d | ||
| 20 | −0.5 | 75 ± 5.0 b | 0 ± 0.0 d | 0 ± 0.0 d | 0 ± 0.0 c | 0 ± 0.0 c | 0 ± 0.0 c | 0 ± 0.0 d | ||
| Desi | CPS-1 | 0 | 0 | 75 ± 5.0 a | 61.25 ± 6.88 ab | 82.4 ± 36.1 bc | 3.79 ± 0.68 a | 5.79 ± 0.23 a | 0.07 ± 0.07 b | 0.95 ± 0.40 ab |
| 5 | −0.05 | 50 ± 5.77 b | 71.25 ± 6.25 a | 126.5 ± 73.3 bc | 3.61 ± 0.50 a | 5.92 ± 0.14 a | 0.56 ± 0.35 a | 1.54 ± 0.88 ab | ||
| 7.5 | −0.075 | 85 ± 5.00 a | 70 ± 7.91 a | 292.1 ± 28.4 a | 3.78 ± 0.49 a | 5.91 ± 0.14 a | 1.00 ± 0.10 a | 2.42 ± 0.14 a | ||
| 10 | −0.15 | 85 ± 5.00 a | 37.5 ± 5.20 c | 220.9 ± 24.9 ab | 2.53 ± 0.53 a | 5.66 ± 0.25 a | 0.18 ± 0.18 b | 2.42 ± 0.11 a | ||
| 15 | −0.22 | 50 ± 5.77 b | 46.25 ± 2.39 bc | 24.8 ± 18.1 c | 2.21 ± 0.28 a | 5.92 ± 0.08 a | 0 ± 0.0 b | 0.46 ± 0.30 ab | ||
| 20 | −0.5 | 0 ± 0.0 c | 0 ± 0.0 d | 0 ± 0.0 c | 0 ± 0.0 b | 0 ± 0.0 b | 0 ± 0.0 b | 0 ± 0.0 b | ||
| Desi | K-850 | 0 | 0 | 90.0 ± 5.77 a | 81.25 ± 3.15 ab | 529.5 ± 51 a | 5.88 ± 0.30 ab | 5.37 ± 0.03 a | - | 6.39 ± 0.20 a |
| 5 | −0.05 | 100 ± 0.00 a | 93.75 ± 3.75 a | 639.5 ± 8.66 a | 7.19 ± 0.50 a | 5.29 ± 0.06 a | - | 5.61 ± 0.08 a | ||
| 7.5 | −0.075 | 95 ± 5.00 a | 81.25 ± 6.88 ab | 465 ± 118 a | 5.21 ± 0.62 b | 5.61 ± 0.09 a | - | 4.72 ± 1.11 a | ||
| 10 | −0.15 | 85 ± 5.00 a | 71.25 ± 7.74 b | 124.9 ± 51.8 b | 5.18 ± 0.56 b | 5.43 ± 0.11 a | - | 1.40 ± 0.51 b | ||
| 15 | −0.22 | 5 ± 5.00 b | 5.00 ± 0.0 c | 0 ± 0.0 b | 0.15 ± 0.15 c | 1.63 ± 0.11 b | - | 0.375 ± 0.375 b | ||
| 20 | −0.5 | 0 ± 0.0 b | 0 ± 0.0 c | 0 ± 0.0 b | 0 ± 0.0 c | 0 ± 0.0 b | - | 0 ± 0.0 b | ||
| Desi | JG-62 | 0 | 0 | 95 ± 2.89 a | 86.25 ± 5.54 a | 1164 ± 93.9 a | 6.28 ± 0.45 bc | 5.38 ± 0.07 abc | 3.98 ± 0.78 a | 7.66 ± 0.19 a |
| 5 | −0.05 | 75 ± 2.89 b | 77.50 ± 9.68 a | 793 ± 80.8 b | 4.82 ± 0.49 c | 5.71 ± 0.11 ab | 1.90 ± 0.90 b | 8.0 ± 0.45 a | ||
| 7.5 | −0.075 | 80 ± 0.0 b | 91.25 ± 5.91 a | 683 ± 56.4 b | 7.06 ± 0.45 ab | 5.32 ± 0.06 bc | 1.13 ± 0.39 b | 7.40 ± 0.51 a | ||
| 10 | −0.15 | 75 ± 0.0 b | 95 ± 3.54 a | 473 ± 164 b | 8.58 ± 0.22 a | 5.07 ± 0.02 c | 0.74 ± 0.32 b | 3.99 ± 1.38 b | ||
| 15 | −0.22 | 40 ± 0.0 c | 33.75 ± 2.39 b | 51.3 ± 30.6 c | 1.98 ± 0.65 d | 5.78 ± 0.21 a | 0 ± 0.0 b | 1.28 ± 0.76 bc | ||
| 20 | −0.5 | 0 ± 0.0 d | 0 ± 0.0 c | 0 ± 0.0 c | 0 ± 0.0 e | 0 ± 0.0 d | 0 ± 0.0 b | 0 ± 0.0 c | ||
| Desi | CHAFFA | 0 | 0 | 95 ± 5.0 a | 83.75 ± 7.18 a | 1278.1 ± 95.4 a | 5.25 ± 0.46 a | 5.63 ± 0.06 a | 2.92 ± 0.14 a | 10.67 ± 1.23 a |
| 5 | −0.05 | 100 ± 0.0 a | 90 ± 5.40 a | 987 ± 96 b | 6.22 ± 0.62 a | 5.45 ± 0.10 a | 0.90 ± 0.53 b | 8.97 ± 0.78 ab | ||
| 7.5 | −0.075 | 100 ± 0.0 a | 88.75 ± 4.27 a | 852.5 ± 43.3 b | 6.96 ± 0.55 a | 5.27 ± 0.05 a | 0.80 ± 0.29 b | 7.72 ± 0.17 b | ||
| 10 | −0.15 | 95 ± 5.0 a | 88.75 ± 4.73 a | 784.5 ± 84.2 b | 6.35 ± 0.21 a | 5.39 ± 0.06 a | 0 ± 0.0 b | 8.21 ± 0.61 ab | ||
| 15 | −0.22 | 30 ± 5.77 b | 21.25 ± 2.39 b | 14.6 ± 14.6 c | 1.75 ± 0.69 b | 5.79 ± 0.64 a | 0 ± 0.0 b | 0.36 ± 0.36 c | ||
| 20 | −0.5 | 0 ± 0.0 c | 0 ± 0.0 c | 0 ± 0.0 c | 0 ± 0.0 b | 0 ± 0.0 b | 0 ± 0.0 b | 0 ± 0.0 c | ||
| Desi | BG-215 | 0 | 0 | 100 ± 0.0 a | 87.50 ± 5.20 ab | 1633 ± 128 a | 6.72 ± 0.37 a | 5.38 ± 0.05 b | 4.13 ± 0.67 a | 12.19 ± 0.80 a |
| 5 | −0.05 | 95 ± 5.0 a | 90 ± 4.08 a | 1084 ± 193 b | 6.21 ± 0.34 a | 5.40 ± 0.03 b | 1.66 ± 0.36 b | 9.70 ± 1.41 ab | ||
| 7.5 | −0.075 | 95 ± 5.0 a | 72.50 ± 6.61 b | 916 ± 132 b | 4.33 ± 0.45 b | 5.75 ± 0.14 a | 1.60 ± 0.09 b | 7.89 ± 1.05 b | ||
| 10 | −0.15 | 95 ± 5.0 a | 88.75 ± 1.25 a | 243 ± 124 c | 6.53 ± 0.51 a | 5.30 ± 0.04 b | 1.00 ± 0.54 bc | 1.42 ± 0.71 c | ||
| 15 | −0.22 | 65 ± 5.0 b | 17.50 ± 2.50 c | 0 ± 0.0 c | 4.60 ± 0.12 b | 5.33 ± 0.07 b | 0 ± 0.0 c | 0 ± 0.0 c | ||
| 20 | −0.5 | 0 ± 0.0 c | 0 ± 0.0 d | 0 ± 0.0 c | 0 ± 0.0 c | 0 ± 0.0 c | 0 ± 0.0 c | 0 ± 0.0 c | ||
| Desi | ANNIGERI | 0 | 0 | 55 ± 5.0 c | 50 ± 3.54 b | 75.1 ± 28.1 b | 2.78 ± 0.27 b | 5.75 ± 0.22 a | 0.07 ± 0.07 b | 1.31 ± 0.44 b |
| 5 | −0.05 | 70 ± 5.77 bc | 67.50 ± 8.54 ab | 593.5 ± 22.1 a | 3.83 ± 0.88 ab | 5.97 ± 0.23 a | 1.74 ± 0.65 a | 6.89 ± 0.66 a | ||
| 7.5 | −0.075 | 100 ± 0.0 a | 76.25 ± 5.91 a | 600 ± 206 a | 5.36 ± 0.25 a | 5.43 ± 0.03 a | 0.99 ± 0.42 ab | 5.0 ± 1.70 a | ||
| 10 | −0.15 | 80 ± 8.16 b | 62.50 ± 5.95 ab | 153.7 ± 60.9 b | 4.97 ± 0.93 a | 5.36 ± 0.22 a | 0.24 ± 0.13 b | 1.80 ± 0.63 b | ||
| 15 | −0.22 | 10 ± 5.77 d | 7.50 ± 1.44 c | 4.50 ± 4.50 b | 0.37 ± 0.29 c | 3.25 ± 1.92 a | 0 ± 0.0 b | 0.22 ± 0.22 b | ||
| 20 | −0.5 | 0 ± 0.0 d | 0 ± 0.0 c | 0 ± 0.0 b | 0 ± 0.0 c | 0 ± 0.0 b | 0 ± 0.0 b | 0 ± 0.0 b | ||
| Kabuli | C-104 | 0 | 0 | 100 ± 0.0 a | 87.50 ± 5.20 a | 904.5 ± 31.5 a | 5.97 ± 0.39 a | 5.46 ± 0.05 b | 0.66 ± 0.23 a | 8.38 ± 0.23 a |
| 5 | −0.05 | 75 ± 2.04 b | 65 ± 7.91 b | 669 ± 51 b | 3.31 ± 0.41 b | 5.97 ± 0.12 a | 0.44 ± 0.17 a | 7.91 ± 0.78 a | ||
| 7.5 | −0.075 | 70 ± 2.04 c | 67.50 ± 5.20 b | 499.6 ± 91.3 b | 4.17 ± 0.47 b | 5.64 ± 0.10 b | 0 ± 0.0 b | 6.25 ± 1.14 a | ||
| 10 | −0.15 | 0 ± 0.0 d | 0 ± 0.0 c | 0 ± 0.0 c | 0 ± 0.0 c | 0 ± 0.0 c | 0 ± 0.0 b | 0 ± 0.0 b | ||
| 15 | −0.22 | 0 ± 0.0 d | 0 ± 0.0 c | 0 ± 0.0 c | 0 ± 0.0 c | 0 ± 0.0 c | 0 ± 0.0 b | 0 ± 0.0 b | ||
| 20 | −0.5 | 0 ± 0.0 d | 0 ± 0.0 c | 0 ± 0.0 c | 0 ± 0.0 c | 0 ± 0.0 c | 0 ± 0.0 b | 0 ± 0.0 b | ||
| Kabuli | UC-27 | 0 | 0 | 85 ± 5.0 a | 75 ± 4.56 a | 147.7 ± 57.6 a | 5.52 ± 0.33 a | 5.38 ± 0.05 a | - | 1.708 ± 0.67 a |
| 5 | −0.05 | 65 ± 9.57 ab | 60 ± 2.89 b | 69 ± 69 ab | 4.28 ± 0.63 ab | 5.48 ± 0.09 a | - | 0.863 ± 0.86 a | ||
| 7.5 | −0.075 | 45 ± 9.57 bc | 41.25 ± 2.39 c | 0 ± 0.0 b | 3.01 ± 0.22 b | 5.35 ± 0.10 a | - | 0 ± 0.0 a | ||
| 10 | −0.15 | 35 ± 9.57 cd | 30 ± 3.54 d | 0 ± 0.0 b | 0.44 ± 0.27 c | 6.08 ± 0.33 a | - | 0 ± 0.0 a | ||
| 15 | −0.22 | 15 ± 5.0 de | 12.50 ± 1.44 e | 0 ± 0.0 b | 0 ± 0.0 c | 3.23 ± 1.87 a | - | 0 ± 0.0 a | ||
| 20 | −0.5 | 0 ± 0.0 e | 0 ± 0.0 f | 0 ± 0.0 b | 0 ± 0.0 c | 0 ± 0.0 b | - | 0 ± 0.0 a | ||
| Kabuli | ILC-482 | 0 | 0 | 65 ± 5.0 a | 58.75 ± 3.15 ab | 8 ± 8.0 a | 3.69 ± 0.46 ab | 5.60 ± 0.11 a | 0 ± 0.0 b | 0.10 ± 0.10 a |
| 5 | −0.05 | 60 ± 8.16 a | 47.50 ± 6.61 b | 244 ± 150 a | 2.65 ± 0.39 bc | 5.87 ± 0.15 a | 1.87 ± 0.93 a | 1.49 ± 0.92 a | ||
| 7.5 | −0.075 | 60 ± 0.0 a | 51.25 ± 5.91 ab | 189 ± 81.3 a | 2.90 ± 0.40 bc | 5.81 ± 0.18 a | 0.37 ± 0.37 ab | 2.78 ± 1.22 a | ||
| 10 | −0.15 | 70 ± 5.77 a | 65 ± 2.04 a | 172 ± 121 a | 4.64 ± 0.44 a | 5.38 ± 0.05 a | 0.26 ± 0.26 ab | 1.91 ± 1.25 a | ||
| 15 | −0.22 | 30 ± 5.77 b | 25 ± 2.89 c | 0 ± 0.0 a | 1.62 ± 0.54 c | 5.78 ± 0.30 a | 0 ± 0.0 b | 0 ± 0.0 a | ||
| 20 | −0.5 | 0 ± 0.0 c | 0 ± 0.0 d | 0 ± 0.0 a | 0 ± 0.0 d | 0 ± 0.0 b | 0 ± 0.0 b | 0 ± 0.0 a |
| Seed Type | Genotypes | IC50 | Race0 | Race1A | Race2 | Race4 | Race5 |
|---|---|---|---|---|---|---|---|
| Desi | WR-315 | 13.93 a | R | R | R | R | R |
| Desi | BG-212 | 17.76 a | R | R | S | M | R |
| Desi | JG-74 | 16.83 a | R | R | S | R | M |
| Desi | CPS-1 | 10.04 b | R | R | S | M | M |
| Desi | K-850 | 11.49 b | - | - | |||
| Desi | JG-62 | 11.54 b | R | S | S | S | S |
| Desi | CHAFFA | 15.24 a | S | R | S | ||
| Desi | BG-215 | 13.93 a | |||||
| Desi | Annigeri | 10.13 b | R | S | S | ||
| Kabuli | C-104 | 7.38 c | R | M | S | S | S |
| Kabuli | UC-27 | 6.95 c | M | M | |||
| Kabuli | ILC-482 | 8.53 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaşıkcı Şimşek, Ü.; Dikilitas, M.; Talapov, T.; Can, C. Water Stress Is Differently Tolerated by Fusarium-Resistant and -Susceptible Chickpea Genotypes During Germination. Life 2025, 15, 1050. https://doi.org/10.3390/life15071050
Kaşıkcı Şimşek Ü, Dikilitas M, Talapov T, Can C. Water Stress Is Differently Tolerated by Fusarium-Resistant and -Susceptible Chickpea Genotypes During Germination. Life. 2025; 15(7):1050. https://doi.org/10.3390/life15071050
Chicago/Turabian StyleKaşıkcı Şimşek, Ümmühan, Murat Dikilitas, Talap Talapov, and Canan Can. 2025. "Water Stress Is Differently Tolerated by Fusarium-Resistant and -Susceptible Chickpea Genotypes During Germination" Life 15, no. 7: 1050. https://doi.org/10.3390/life15071050
APA StyleKaşıkcı Şimşek, Ü., Dikilitas, M., Talapov, T., & Can, C. (2025). Water Stress Is Differently Tolerated by Fusarium-Resistant and -Susceptible Chickpea Genotypes During Germination. Life, 15(7), 1050. https://doi.org/10.3390/life15071050





