Millet in Bioregenerative Life Support Systems: Hypergravity Resilience and Predictive Yield Models
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Plants for the Experiment
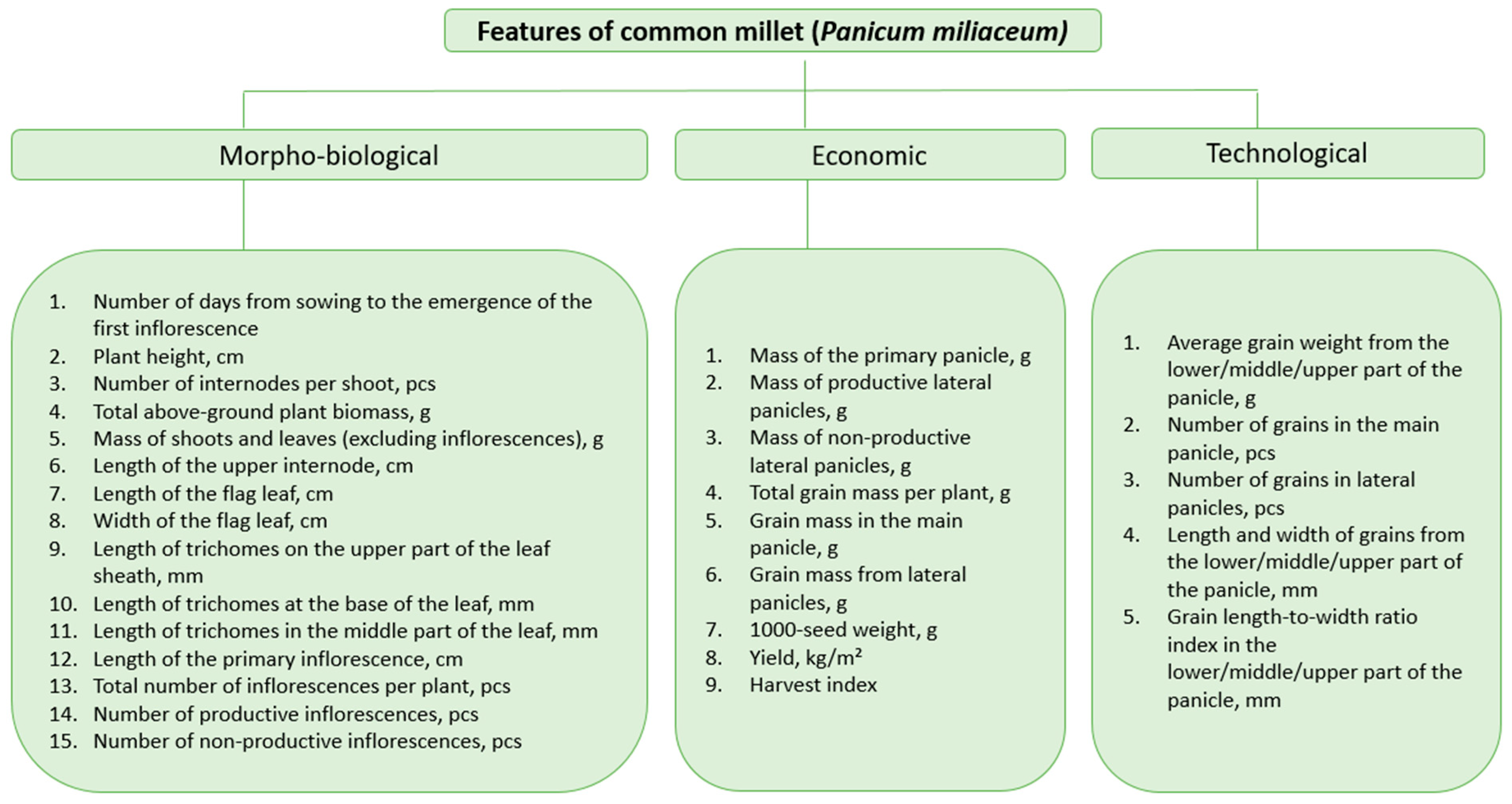
2.2. Data Collection
2.3. Statistical Analysis
3. Results
3.1. The Effect of Hypergravity on Plants
3.2. Predictive Models of Millet Biomass Accumulation on the 10th and 20th Days After Sowing
3.3. Calculation of Prediction Models for Millet Traits Related to Yield When Grown in Closed Systems
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Features | Mean | Standard Deviation | Minimum | Maximum | Variation Coefficient, % |
|---|---|---|---|---|---|
| Shoot weight on day 10, g | 0.06 | 0.02 | 0.02 | 0.12 | 34 |
| Shoot weight on day 20, g | 0.25 | 0.10 | 0.01 | 0.65 | 38 |
| Shoot length on day 10, cm | 6.3 | 1.5 | 3.5 | 11.3 | 23 |
| Shoot length on day 20, cm | 17.0 | 5.4 | 3 | 27.9 | 31 |
| Number of days from sowing to the emergence of the first inflorescence | 105.6 | 34.6 | 78 | 197 | 33 |
| Plant height, cm | 121.6 | 21 | 50 | 145 | 41 |
| Number of internodes per shoot, pcs | 8 | 1.1 | 6 | 10 | 14 |
| Total above-ground plant biomass, g | 6.2 | 2.6 | 0.5 | 12.8 | 42 |
| Mass of shoots and leaves (excluding inflorescences), g | 4.4 | 1.7 | 0.4 | 7.8 | 39 |
| Length of the upper internode, cm | 12.8 | 4.5 | 3.1 | 21.9 | 35 |
| Length of the flag leaf, cm | 34.4 | 9.9 | 17 | 43 | 29 |
| Width of the flag leaf, cm | 1.2 | 0.3 | 0.3 | 1.7 | 25 |
| Length of trichomes on the upper part of the leaf sheath, mm | 1.9 | 0.5 | 1.2 | 3.2 | 24 |
| Length of trichomes at the base of the leaf, mm | 2.2 | 0.4 | 1.6 | 3.1 | 18 |
| Length of trichomes in the middle part of the leaf, mm | 2.3 | 0.5 | 1.5 | 3.4 | 22 |
| Length of the primary inflorescence, cm | 22.9 | 4.8 | 13 | 29 | 18 |
| Total number of inflorescences per plant, pcs | 2.8 | 1.5 | 1 | 5 | 54 |
| Number of productive inflorescences, pcs | 2.6 | 1.5 | 1 | 5 | 58 |
| Number of non-productive inflorescences, pcs | 0.20 | 0.17 | 0 | 1 | 85 |
| Features | Mean | Standard Deviation | Minimum | Maximum | Variation Coefficient, % |
|---|---|---|---|---|---|
| Mass of the primary panicle, g | 0.85 | 0.47 | 0.10 | 1.73 | 55 |
| Mass of productive lateral panicles, g | 0.93 | 0.72 | 0 | 3.40 | 78 |
| Mass of non-productive lateral panicles, g | 0.06 | 0.06 | 0 | 0.39 | 94 |
| Total grain mass per plant, g | 0.34 | 0.29 | 0 | 1.37 | 86 |
| Grain mass in the main panicle, g | 0.22 | 0.18 | 0 | 0.74 | 83 |
| Grain mass from lateral panicles, g | 0.23 | 0.18 | 0 | 0.76 | 78 |
| 1000-seed weight, g | 8.61 | 0.65 | 7.49 | 9.56 | 8 |
| Harvest index | 0.06 | 0.01 | 0.01 | 0.22 | 23 |
| Yield, kg/m2 | 0.31 | 0.19 | 0.08 | 0.78 | 63 |
| Features | Mean | Standard Deviation | Minimum | Maximum | Variation Coefficient, % |
|---|---|---|---|---|---|
| Average grain weight from the lower part of the panicle, g | 0.009 | 0.002 | 0.005 | 0.011 | 18 |
| Average grain weight from the middle part of the panicle, g | 0.009 | 0.001 | 0.005 | 0.011 | 16 |
| Average grain weight from the upper part of the panicle, g | 0.009 | 0.002 | 0.003 | 0.010 | 18 |
| Number of grains in the main panicle, pcs | 24.4 | 17.4 | 0 | 80 | 71 |
| Number of grains in lateral panicles, pcs | 23.7 | 19.4 | 0 | 77 | 82 |
| Length of grains from the lower part of the panicle, mm | 3.2 | 0.2 | 3.0 | 3.6 | 5 |
| Length of grains from the middle part of the panicle, mm | 3.2 | 0.2 | 2.8 | 3.5 | 6 |
| Length of grains from the upper part of the panicle, mm | 3.2 | 0.2 | 2.8 | 3.5 | 6 |
| Width of grains from the lower part of the panicle, mm | 2.7 | 0.2 | 2.4 | 3.0 | 6 |
| Width of grains from the middle part of the panicle, mm | 2.6 | 0.2 | 2.3 | 2.9 | 6 |
| Width of grains from the upper part of the panicle, mm | 2.6 | 0.2 | 2.2 | 2.9 | 7 |
| Grain length-to-width ratio index in the lower part of the panicle, mm | 0.83 | 0.06 | 0.75 | 0.95 | 7 |
| Grain length-to-width ratio index in the middle part of the panicle, mm | 0.83 | 0.06 | 0.73 | 0.95 | 7 |
| Grain length-to-width ratio index in the upper part of the panicle, mm | 0.83 | 0.05 | 0.71 | 0.9 | 6 |
References
- Manturov, D. State Program ‘Space Activities of Russia’. Available online: http://government.ru/news/54169/ (accessed on 14 April 2025).
- Creech, S.; Guidi, J.; Elburn, D. Artemis: An overview of NASA’s activities to return humans to the Moon. In Proceedings of the 2022 IEEE Aerospace Conference (Aero), Big Sky, MT, USA, 7–14 March 2020; IEEE: Piscataway, NJ, USA, 2020; pp. 1–7. [Google Scholar]
- Shaw, R.; Soma, T. To the farm, Mars, and beyond: Technologies for growing food in space, the future of long-duration space missions, and earth implications in English news media coverage. Front. Commun. 2022, 7, 1007567. [Google Scholar] [CrossRef]
- Fuller, S.; Lehnhardt, E.; Zaid, C.; Halloran, K. Gateway program status and overview. J. Space Saf. Eng. 2022, 9, 625–628. [Google Scholar] [CrossRef]
- SpaseX. Smallsat Rideshare Program. Available online: https://www.spacex.com/rideshare (accessed on 27 July 2025).
- Smith, S.M.; Zwart, S.R.; Heer, M. Human adaptation to space flight: The role of nutrition. In National Aeronautics and Space Administration; Lyndon, B., Ed.; Johnson Space Center: Houston, TX, USA, 2014. [Google Scholar]
- Benas, A.D.; Bilash, D.; Chiavetta, P.; Cottee, J.; Aloy, S.F.; Farrow, J.; Zanus, E. Feasibility study for a commercial space station in low earth orbit. Acta Astronaut. 2025, 229, 523–533. [Google Scholar] [CrossRef]
- Lisovsky, G.M. Closed System: Man-Higher Plants; Nauka Press: Novosibirsk, Russia, 1979. (In Russia). [Available in English as a NASA Technical Translation, 1981] [Google Scholar]
- Chunxiao, X.; Hong, L. Crop candidates for the bioregenerative life support systems in China. Acta Astronaut. 2008, 63, 1076–1080. [Google Scholar] [CrossRef]
- Baranova, E.N.; Levinskikh, M.A.; Gulevich, A.A. Wheat Space Odyssey: “From Seed to Seed”. Kernel Morphology. Life 2019, 9, 81. [Google Scholar] [CrossRef] [PubMed]
- Carillo, P.; Morrone, B.; Fusco, G.M.; De Pascale, S.; Rouphael, Y. Challenges for a sustainable food production system on board of the international space station: A technical review. Agronomy 2020, 10, 687. [Google Scholar] [CrossRef]
- Aniskina, T.S.; Sudarikov, K.A.; Levinskikh, M.A.; Gulevich, A.A.; Baranova, E.N. Bread wheat in space flight: Is there a difference in kernel quality? Plants 2023, 13, 73. [Google Scholar] [CrossRef] [PubMed]
- Habiyaremye, C.; Matanguihan, J.B.; D’Alpoim Guedes, J.; Ganjyal, G.M.; Whiteman, M.R.; Kidwell, K.K.; Murphy, K.M. Proso millet (Panicum miliaceum L.) and its potential for cultivation in the Pacific Northwest, US: A review. Front. Plant Sci. 2017, 7, 1961. [Google Scholar] [CrossRef] [PubMed]
- Rajasekaran, R.; Francis, N. Genetic and genomic resources for improving proso millet (Panicum miliaceum L.): A potential crop for food and nutritional security. Nucleus 2021, 64, 21–32. [Google Scholar] [CrossRef]
- Pathak, H.C. Role of Millets in Nutritional Security of India; National Academy of Agricultural Sciences: New Delhi, India, 2013; pp. 1–16. [Google Scholar]
- Leder, I. Sorghum and millets. In Cultivated Plants, Primarily as Food Sources; University of Agricultural Sciences: Gödöllö, Hungary, 2004; Volume 1, pp. 66–84. [Google Scholar]
- Amadou, I.; Gounga, M.E.; Le, G.W. Millets: Nutritional composition, some health benefits and processing-A review. Emirates J. Food Agric. 2013, 25, 501–508. [Google Scholar] [CrossRef]
- Changmei, S.; Dorothy, J. Millet-the frugal grain. Int. J. Sci. Res. Rev. 2014, 3, 75–90. [Google Scholar]
- Zadoks, J.C.; Chang, T.T.; Konzak, C.F. A decimal code for the growth stages of cereals. Weed Res. 1974, 14, 415–421. [Google Scholar] [CrossRef]
- Schindelin, J.; Rueden, C.T.; Hiner, M.C.; Eliceiri, K.W. The ImageJ ecosystem: An open platform for biomedical image analysis. Mol. Reprod. Dev. 2015, 82, 518–529. [Google Scholar] [CrossRef]
- Tretyakov, N.N.; Koshkin, E.I.; Novikov, N.N.; Makrushin, N.M.; Pilshchikova, N.V.; Karnaukhova, T.V.; Loseva, A.S. Physiology and Biochemistry of Agricultural Plants; Kolos: Moscow, Russia, 2000; 640p. (In Russian) [Google Scholar]
- Mshelmbula, B.; Akomolafe, G. Preliminary Effect of Centrifugal Force on Germination and Early Growth of Maize (Zea mays L.). Trans. Sci. Technol. 2019, 6, 328–333. [Google Scholar]
- Jagtap, S.S.; Vidyasagar, P.B. Effects of High g Values on Growth and Chlorophyll Content in Hydrated and Dehydrated Wheat Seeds. Bul. Fis. 2020, 21, 82. [Google Scholar] [CrossRef]
- Brown, A.H.; Dahl, A.O.; Chapman, D.K. Morphology of Arabidopsis Grown under Chronic Centrifugation and on the Clinostat. Plant Physiol. 1976, 57, 358–364. [Google Scholar] [CrossRef]
- Waldron, K.W.; Brett, C.T. Effects of extreme acceleration on the germination, growth and cell wall composition of pea epicotyls. J. Exp. Bot. 1990, 41, 71–77. [Google Scholar] [CrossRef]
- Dommes, J.; Van de Walle, C. Newly Synthesized mRNA is translated during the Initial Imbibition Phase in Germinating Maize Embryo. Plant Physiol. 1983, 73, 484–487. [Google Scholar] [CrossRef]
- Asahi, T.; Maeshima, M. Biogenesis of Mitochondria in Higher Plant Cells. In The New Frontiers in Plant Biochemistry; Akazawa, T., Ed.; Nijhoff: Hague, The Netherlands; Junk Publ.: Hague, The Netherlands, 1983; pp. 119–132. [Google Scholar]
- Palchetti, E.; Moretta, M.; Calamai, A.; Mancini, M.; Dell’Acqua, M.; Brilli, L.; Masoni, A. Effects of nitrogen fertilization and plant density on proso millet (Panicum miliaceum L.) growth and yield under mediterranean pedoclimatic conditions. Agriculture 2023, 13, 1657. [Google Scholar] [CrossRef]
- Saruhan, V.; Kusvuran, A.; Babat, S. The effect of different humic acid fertilization on yield and yield components performances of common millet (Panicum miliaceum L.). Sci. Res. Essays 2011, 6, 663–669. [Google Scholar]
- Basilio-Apolinar, A.; Vara, L.E.G.D.L.; Ramírez-Pimentel, J.G.; Aguirre-Mancilla, C.L.; Iturriaga, G.; Covarrubias-Prieto, J.; Raya-Pérez, J.C. Silicon induces changes in the antioxidant system of millet cultivated in drought and salinity. Chil. J. Agric. Res. 2021, 81, 655–663. [Google Scholar] [CrossRef]
- Seghatoleslami, M.; Kafi, M.; Majidi, E. Effect of drought stress at different growth stages on yield and water use efficiency of five proso millet (Panicum miliaceum L.) genotypes. Pak. J. Bot. 2008, 40, 1427–1432. [Google Scholar]
- Mastrorilli, M.; Katerji, N.; Rana, G. Water efficiency and stress on grain sorghum at different reproductive stages. Agric. Water Manag. 1995, 28, 23–34. [Google Scholar] [CrossRef]
- Calamai, A.; Masoni, A.; Marini, L.; Dell’Acqua, M.; Ganugi, P.; Boukail, S.; Palchetti, E. Evaluation of the agronomic traits of 80 accessions of proso millet (Panicum miliaceum L.) under Mediterranean pedoclimatic conditions. Agriculture 2020, 10, 578. [Google Scholar] [CrossRef]
- Yadav, A.; Srivastava, D.P. Path analysis in Panicum miliare. Indian J. Genet. Plant Breed. 1976, 36, 64–68. [Google Scholar]
- Singh, A.; Kashyap, S.C.; Kumar, B.; Kumawat, R. Deciphering Trait Association and their Effect on Yield, Yield Attributing and Quality Traits in Barnyard Millet [Echinochloa frumentacea (Roxb.) Link]. Int. J. Plant Soil. Sci. 2023, 35, 718–724. [Google Scholar] [CrossRef]
- Zhi, H.; He, Q.; Tang, S.; Yang, J.; Zhang, W.; Liu, H.; Diao, X. Genetic control and phenotypic characterization of panicle architecture and grain yield-related traits in foxtail millet (Setaria italica). Theor. Appl. Genet. 2021, 134, 3023–3036. [Google Scholar] [CrossRef]
- Qu, Y.; Wang, K.; Luo, Y.; Vus, N.; Feng, B. Plant-type characteristics and seed yield of proso millet (Panicum miliaceum L.) response to plant density. Russ. J. Agric. Socio-Econ. Sci. 2020, 98, 152–160. [Google Scholar] [CrossRef]
- Swamy, B.K.; Hosamani, R.; Sathasivam, M. Novel hypergravity treatment enhances root phenotype and positively influences physio-biochemical parameters in bread wheat (Triticum aestivum L.). Sci. Rep. 2021, 11, 15303. [Google Scholar] [CrossRef]
| Graph | Model | Regression Model | Coefficient of Determination (R2) | Standard Error of Estimation |
|---|---|---|---|---|
| 10 | Linear | y = 0.012x − 0.015 | 0.749 | 0.011 |
| 10 | Quadratic | y = (−0.021)x2 + 0.001x − 0.043 | 0.758 | 0.010 |
| 20 | Linear | y = 0.019x − 0.077 | 0.806 | 0.051 |
| 20 | Quadratic | y = (−0.004)x2 + 0.0005x + 0.034 | 0.825 | 0.048 |
| Dependent Variable (y) | Model Predictors | Regression Model | Coefficient of Determination (R2) | Standard Error of Estimation |
|---|---|---|---|---|
| Weight of 1000 seeds, g | a—length of the main inflorescence, cm; b—number of all inflorescences on the plant, pcs; constant | y = 0.96a − 0.243b + 7.053 | 0.647 | 0.411 |
| Weight of 1000 seeds, g | a—mass of grains in the main inflorescence, g; b—number of grains in the main inflorescence, pcs; c—length of the main inflorescence, cm; constant | y = 25.68a − 0.227b + 0.052c + 7.3 | 0.799 | 0.320 |
| Number of productive inflorescences, pcs | a—number of all inflorescences on the plant, pcs; constant | y = 1.022a − 0.222 | 0.942 | 0.384 |
| Total above-ground plant weight, g | a—number of all inflorescences on the plant, pcs; mass of the main inflorescence with grain, g; constant | y = 1.145a + 2.334b + 0.914 | 0.674 | 1.193 |
| Total number of grains per plant, pcs | a—mass of grains in the main inflorescence, g; constant | y = 105.071a + 1.709 | 0.996 | 1.333 |
| Weight of grains per plant, pcs | a—number of grains in the main inflorescence, pcs | y = 0.018a − 0.118 | 0.992 | 0.035 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aniskina, T.S.; Kudritsky, A.N.; Shchuklina, O.A.; Andreev, N.E.; Baranova, E.N. Millet in Bioregenerative Life Support Systems: Hypergravity Resilience and Predictive Yield Models. Life 2025, 15, 1261. https://doi.org/10.3390/life15081261
Aniskina TS, Kudritsky AN, Shchuklina OA, Andreev NE, Baranova EN. Millet in Bioregenerative Life Support Systems: Hypergravity Resilience and Predictive Yield Models. Life. 2025; 15(8):1261. https://doi.org/10.3390/life15081261
Chicago/Turabian StyleAniskina, Tatiana S., Arkady N. Kudritsky, Olga A. Shchuklina, Nikita E. Andreev, and Ekaterina N. Baranova. 2025. "Millet in Bioregenerative Life Support Systems: Hypergravity Resilience and Predictive Yield Models" Life 15, no. 8: 1261. https://doi.org/10.3390/life15081261
APA StyleAniskina, T. S., Kudritsky, A. N., Shchuklina, O. A., Andreev, N. E., & Baranova, E. N. (2025). Millet in Bioregenerative Life Support Systems: Hypergravity Resilience and Predictive Yield Models. Life, 15(8), 1261. https://doi.org/10.3390/life15081261







