Impact of Thermal Variation on Egg Hatching and the Life Cycle of Aedes (Protomacleaya) terrens (Diptera: Culicidae) in a Laboratory Environment
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statement
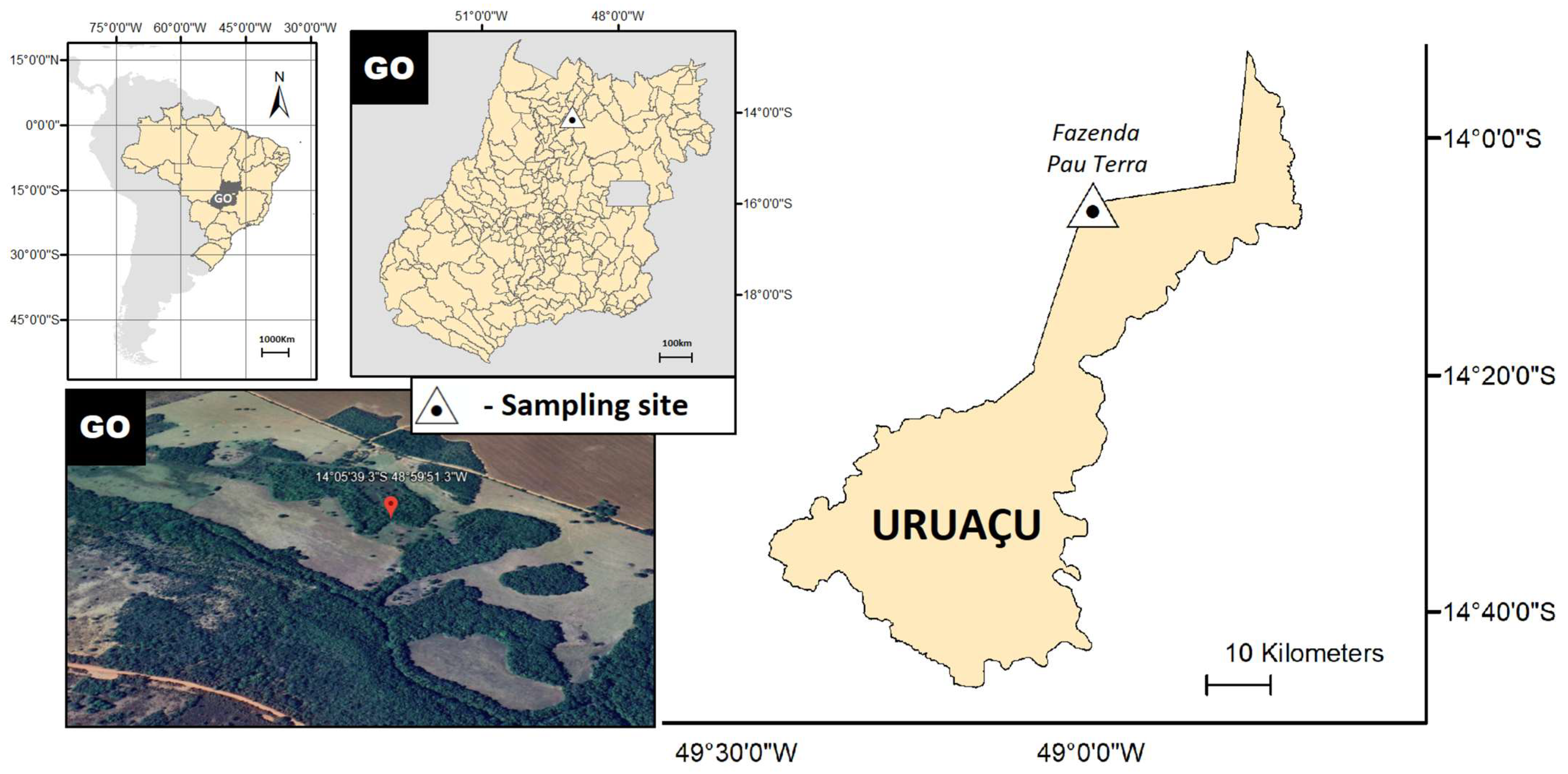
2.2. Study Area
2.3. Laboratory Experimental Design
2.4. Statistical Analyses
3. Results
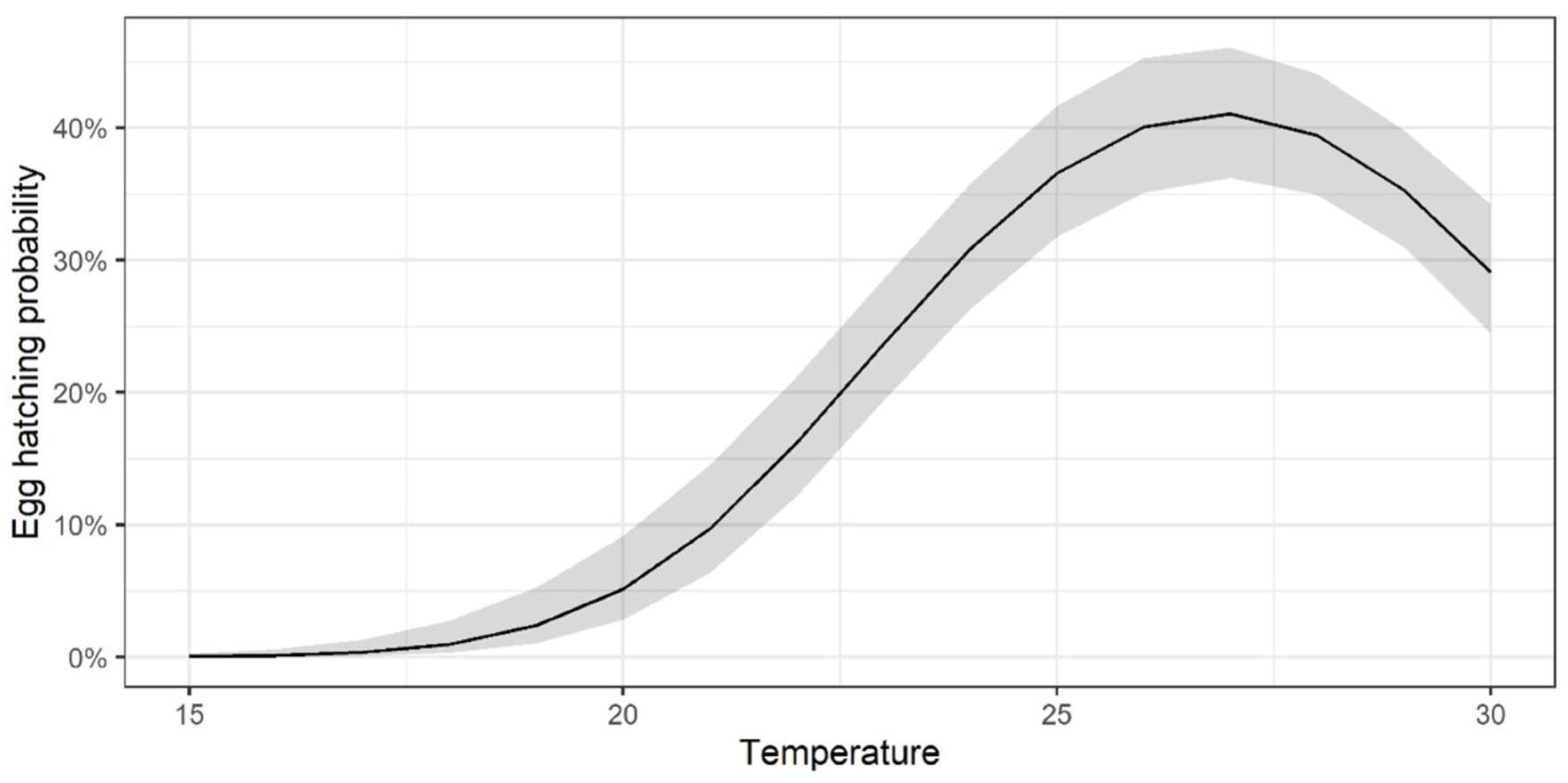
3.1. Egg Hatching × Temperature
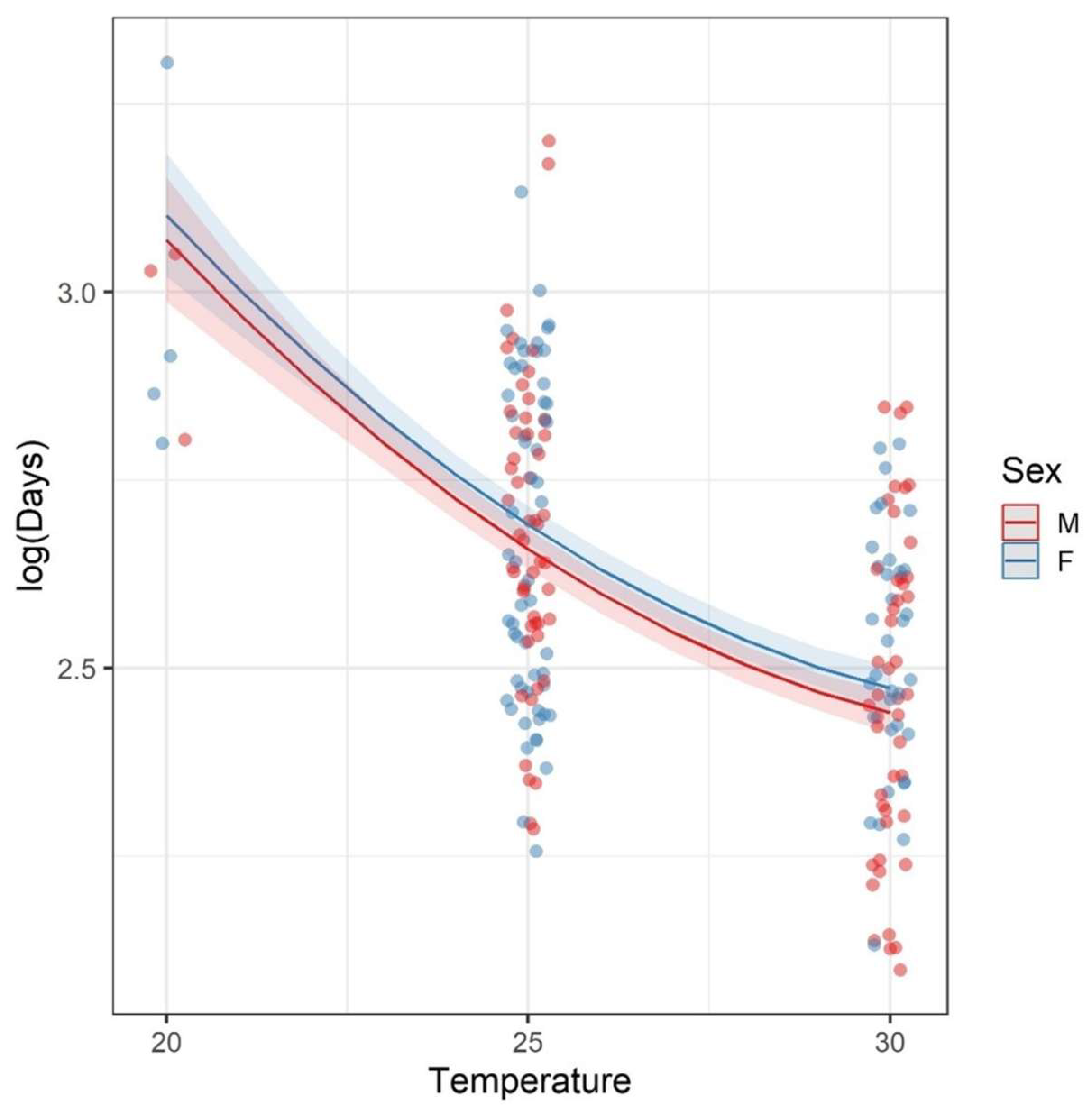
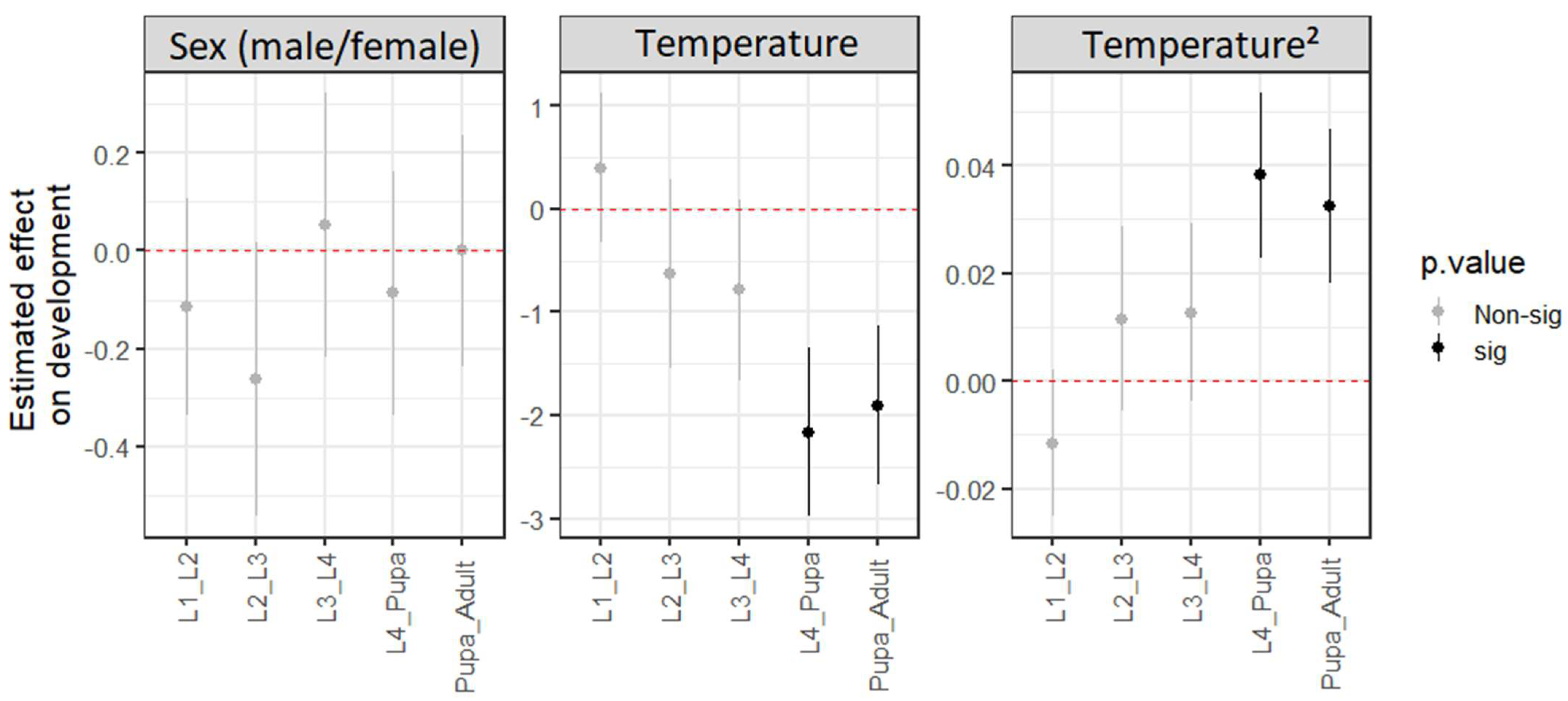
3.2. Development × Temperature
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Calado, D.C.; Silva, M.A. Avaliação da influência da temperatura sobre o desenvolvimento de Aedes albopictus [Evaluation of the temperature influence on the development of Aedes albopictus]. Rev. Saude Publica 2002, 36, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Calado, D.C.; Navarro-Silva, M.A. Influência da temperatura sobre a longevidade, fecundidade e atividade hematofágica de Aedes (Stegomya) albopictus (Skuse), 1894 (Diptera, Culicidae) sob condições de laboratório. Rev. Bras. Entomol. 2002, 46, 93–98. [Google Scholar] [CrossRef]
- Beserra, E.B.; Fernandes, C.R.M.; Silva, S.A.O.; Silva, L.A.; Santos, J.W. Effects of temperature on life cycle, thermal exigency and number of generations per year estimation of Aedes aegypti (Diptera, Culicidae). Iheringia Ser. Zool. 2009, 99, 142–148. [Google Scholar] [CrossRef]
- Ribeiro, P.B.; Costa, P.R.; Loeck, A.E.; Vianna, É.E.; Silveira Júnior, P. Thermal requirements of Culex quinquefasciatus (Diptera, Culicidae) in Pelotas, Rio Grande do Sul, Brazil. Iheringia. Ser. Zool. 2004, 94, 177–180. [Google Scholar] [CrossRef]
- Kumm, H.W.; Novis, B.H. Transmission of yellow fever to laboratory animals by bites of Haemagogus mosquitoes. Public Health Rep. 1938, 53, 1924–1936. [Google Scholar]
- Davis, N.C. The effect of various temperatures in modifying the extrinsic incubation period of the yellow fever virus in Aedes aegypti. Am. J. Hyg. 1944, 39, 45–67. [Google Scholar] [CrossRef]
- Davis, N.C. Modification of the extrinsic incubation period of yellow fever virus in Aedes aegypti. Am. J. Trop. Med. Hyg. 1945, 25, 153–176. [Google Scholar]
- Neves, J.; Faria, J.B. Observações sobre a biologia de Haemagogus janthinomys Dyar, 1921 (Diptera: Culicidae). Rev. Bras. Biol. 1977, 37, 313–318. [Google Scholar]
- Lourenço-de-Oliveira, R.; Deane, L.M.; Da Rosa, A.P.A.T.; De Souza Lopes, O. Studies on wild yellow fever virus vectors at the National Park of Serra dos Órgãos, State of Rio de Janeiro. II—Vertical distribution of potential mosquito vectors. Mem. Inst. Oswaldo Cruz 1986, 81, 287–293. [Google Scholar]
- Guimarães, A.E.; Arlé, M.; Machado, R.N.M. Mosquitos no Parque Nacional da Serra dos Órgãos, Estado do Rio de Janeiro, Brasil. II—Distribuição vertical. Mem. Inst. Oswaldo Cruz 1985, 80, 171–185. [Google Scholar] [CrossRef]
- Dias, R.; de Mello, C.F.; Santos, G.S.; Carbajal-de-la-Fuente, A.L.; Alencar, J. Vertical Distribution of Oviposition and Temporal Segregation of Arbovirus Vector Mosquitoes (Diptera: Culicidae) in a Fragment of the Atlantic Forest, State of Rio de Janeiro, Brazil. Trop. Med. Infect. Dis. 2023, 8, 256. [Google Scholar] [CrossRef] [PubMed]
- Lourenço-de-Oliveira, R.; Failloux, A.B. High risk for chikungunya virus to initiate an enzootic sylvatic cycle in the tropical Americas. PLoS Negl. Trop. Dis. 2017, 11, e0005698. [Google Scholar] [CrossRef] [PubMed]
- Galindo, P.; Trapido, H.; Carpenter, S.J. Ecological Observations on Forest Mosquitoes of an Endemic Yellow Fever Area in Panama. Am. J. Trop. Med. Hyg. 1951, 31, 303–324. [Google Scholar] [CrossRef] [PubMed]
- Galindo, P.; Carpenter, S.J.; Trapido, H. The Effect of Repeated Immersion on Hatching of Eggs of Aedes terrens. Mosq. News 1955, 15, 234–239. [Google Scholar]
- Ribeiro, J.F.; Walter, B.M.T. As matas de galeria no contexto do bioma Cerrado. In Cerrado: Caracterização e Recuperação de Matas de Galeria; Ribeiro, J.F., Fonseca, C.E.L., Sousa-Silva, J.C., Eds.; Embrapa Cerrados: Planaltina, Brazil, 2001; pp. 29–47. [Google Scholar]
- Lane, J. Neotropical Culicidae; Universidade de São Paulo: São Paulo, Brazil, 1953; Volume 1. [Google Scholar]
- Consoli, R.A.G.B.; Lourenço-De-Oliveira, R. Principais Mosquitos de Importância Sanitária do Brasil; Editora FIOCRUZ: Rio de Janeiro, Brazil, 1994. [Google Scholar] [CrossRef]
- Forattini, O.P. Culicidologia Médica: Identificação, Biologia, Epidemiologia; Edusp—Editora da Universidade de São Paulo: São Paulo, Brazil, 2002. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024; Available online: https://www.R-project.org/ (accessed on 13 July 2024).
- Bartoń, K. MuMIn: Inferência Multi-Modelo; R Package Version 1.48.4. 2024. Available online: https://CRAN.R-project.org/package=MuMIn (accessed on 13 July 2024).
- Burnham, K.P.; Anderson, D.R. Inferência multimodelo: Compreendendo AIC e BIC na seleção de modelos. Métodos Sociológicos Pesquisa 2004, 33, 261–304. [Google Scholar] [CrossRef]
- Hartig, F. DHARMa: Diagnóstico Residual para Modelos de Regressão Hierárquicos (Multi-Nível/Mistos); R Package Version 0.4.6. 2022. Available online: https://CRAN.R-project.org/package=DHARMa (accessed on 13 July 2024).
- INMET—Instituto Nacional de Meteorologia. Normais Climatológicas do Brasil (1991–2020); INMET: Brasília, Brazil, 2022. Available online: https://portal.inmet.gov.br/normais (accessed on 28 November 2024).
- de Souza, W.M.; Weaver, S.C. Effects of climate change and human activities on vector-borne diseases. Nat. Rev. Microbiol. 2024, 22, 476–491. [Google Scholar] [CrossRef]
- Rodrigues, W.C. Fatores que influenciam no desenvolvimento dos insetos. Info Insetos 2004, 1, 1–4. [Google Scholar]
- Prasad, P.; Gupta, S.K.; Mahto, K.K.; Kumar, G.; Rani, A.; Velan, I.; Arya, D.K.; Singh, H. Influence of climatic factors on the life stages of Aedes mosquitoes and vectorial transmission: A review. J. Vector Borne Dis. 2024, 61, 158–166. [Google Scholar] [CrossRef]
- Alvares, C.A.; Stape, J.L.; Sentelhas, P.C.; De Moraes Gonçalves, J.L.; Sparovek, G. Köppen’s climate classification map for Brazil. Meteorol. Z. 2013, 22, 711–728. [Google Scholar] [CrossRef]
- WeatherSpark. Clima Característico em Goiás, Brasil Durante o Ano. Available online: https://pt.weatherspark.com/y/29864/Clima-caracter%C3%ADstico-em-Goi%C3%A1s-Brasil-durante-o-ano (accessed on 6 March 2025).
- Ryan, S.J.; Carlson, C.J.; Mordecai, E.A.; Johnson, L.R. Global expansion and redistribution of Aedes-borne virus transmission risk with climate change. PLoS Negl. Trop. Dis. 2019, 13, e0007213. [Google Scholar] [CrossRef]
- Farnesi, L.C.; Martins, A.J.; Valle, D.; Rezende, G.L. Embryonic development of Aedes aegypti (Diptera: Culicidae): Influence of different constant temperatures. Mem. Inst. Oswaldo Cruz 2009, 104, 679–685. [Google Scholar] [CrossRef]
- Beserra, E.B.; Castro, F.P., Jr.; Santos, J.W.; Santos, T.S.; Fernandes, C.R.M. Biologia e exigências térmicas de Aedes aegypti (L.) (Diptera: Culicidae) provenientes de quatro regiões bioclimáticas da Paraíba. Neotrop. Entomol. 2006, 35, 853–860. [Google Scholar] [CrossRef]
| Temperature | Eggs | Hatching Rate | CI 95% |
|---|---|---|---|
| 15 | 214 | 0.00 | 0.00–0.00 |
| 20 | 181 | 0.05 | 0.03–0.09 |
| 25 | 226 | 0.37 | 0.32–0.42 |
| 30 | 236 | 0.29 | 0.24–0.34 |
| Temperature (°C) | L1–L2 (SD) | L2–L3 (SD) | L3–L4 (SD) | L4–Pupa (SD) | Pupa–Adult (SD) | L1–Adult (SD) | Total Development (Days) | |
|---|---|---|---|---|---|---|---|---|
| Min. | Max. | |||||||
| 20 | 4.43 (0.98) | 2.43 (0.53) | 3.43 (1.13) | 6.86 (1.07) | 4.71 (1.95) | 21.9 (1.95) | 20 | 25 |
| 25 | 3.71 (0.99) | 1.97 (0.84) | 2.34 (1.03) | 3.79 (1.21) | 2.79 (0.89) | 14.6 (1.63) | 12 | 22 |
| 30 | 2.30 (0.84) | 1.99 (0.75) | 1.89 (0.89) | 3.21 (1.33) | 2.24 (0.78) | 11.6 (1.81) | 8 | 15 |
| Model | df | AICc | ∆AICc | Weight |
|---|---|---|---|---|
| log(Days)~Sex + Temperature + Temperature2 | 5 | −308.8 | 0.00 | 0.759 |
| log(Days)~Temperature + Temperature2 | 4 | −306.5 | 2.30 | 0.241 |
| log(Days)~Sex + Temperature | 4 | −294.5 | 14.30 | 0.001 |
| log(Days)~Temperature | 3 | −292.7 | 16.02 | 0.000 |
| log(Days)~Sex + Temperature2 | 4 | −286.3 | 22.49 | 0.000 |
| log(Days)~Temperature2 | 3 | −284.7 | 24.10 | 0.000 |
| log(Days)~Sex | 3 | −117.4 | 191.35 | 0.000 |
| log(Days)~1 (null model) | 2 | −114.1 | 194.65 | 0.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dias, R.; Leite, M.P.C.; Corrêa-do-Nascimento, G.S.; Santos, G.S.; de Mello, C.F.; Menezes de Almeida, N.; Alencar, J. Impact of Thermal Variation on Egg Hatching and the Life Cycle of Aedes (Protomacleaya) terrens (Diptera: Culicidae) in a Laboratory Environment. Life 2025, 15, 1038. https://doi.org/10.3390/life15071038
Dias R, Leite MPC, Corrêa-do-Nascimento GS, Santos GS, de Mello CF, Menezes de Almeida N, Alencar J. Impact of Thermal Variation on Egg Hatching and the Life Cycle of Aedes (Protomacleaya) terrens (Diptera: Culicidae) in a Laboratory Environment. Life. 2025; 15(7):1038. https://doi.org/10.3390/life15071038
Chicago/Turabian StyleDias, Rayane, Manuella Pereira Cerqueira Leite, Guilherme Sanches Corrêa-do-Nascimento, Gabriel Silva Santos, Cecilia Ferreira de Mello, Nathália Menezes de Almeida, and Jeronimo Alencar. 2025. "Impact of Thermal Variation on Egg Hatching and the Life Cycle of Aedes (Protomacleaya) terrens (Diptera: Culicidae) in a Laboratory Environment" Life 15, no. 7: 1038. https://doi.org/10.3390/life15071038
APA StyleDias, R., Leite, M. P. C., Corrêa-do-Nascimento, G. S., Santos, G. S., de Mello, C. F., Menezes de Almeida, N., & Alencar, J. (2025). Impact of Thermal Variation on Egg Hatching and the Life Cycle of Aedes (Protomacleaya) terrens (Diptera: Culicidae) in a Laboratory Environment. Life, 15(7), 1038. https://doi.org/10.3390/life15071038







