Preclinical Parkinson’s Disease Models for Non-Motor Symptoms: Research Recommendations from a Systematic Review
Abstract
1. Introduction
2. Methods
2.1. Eligibility Criteria
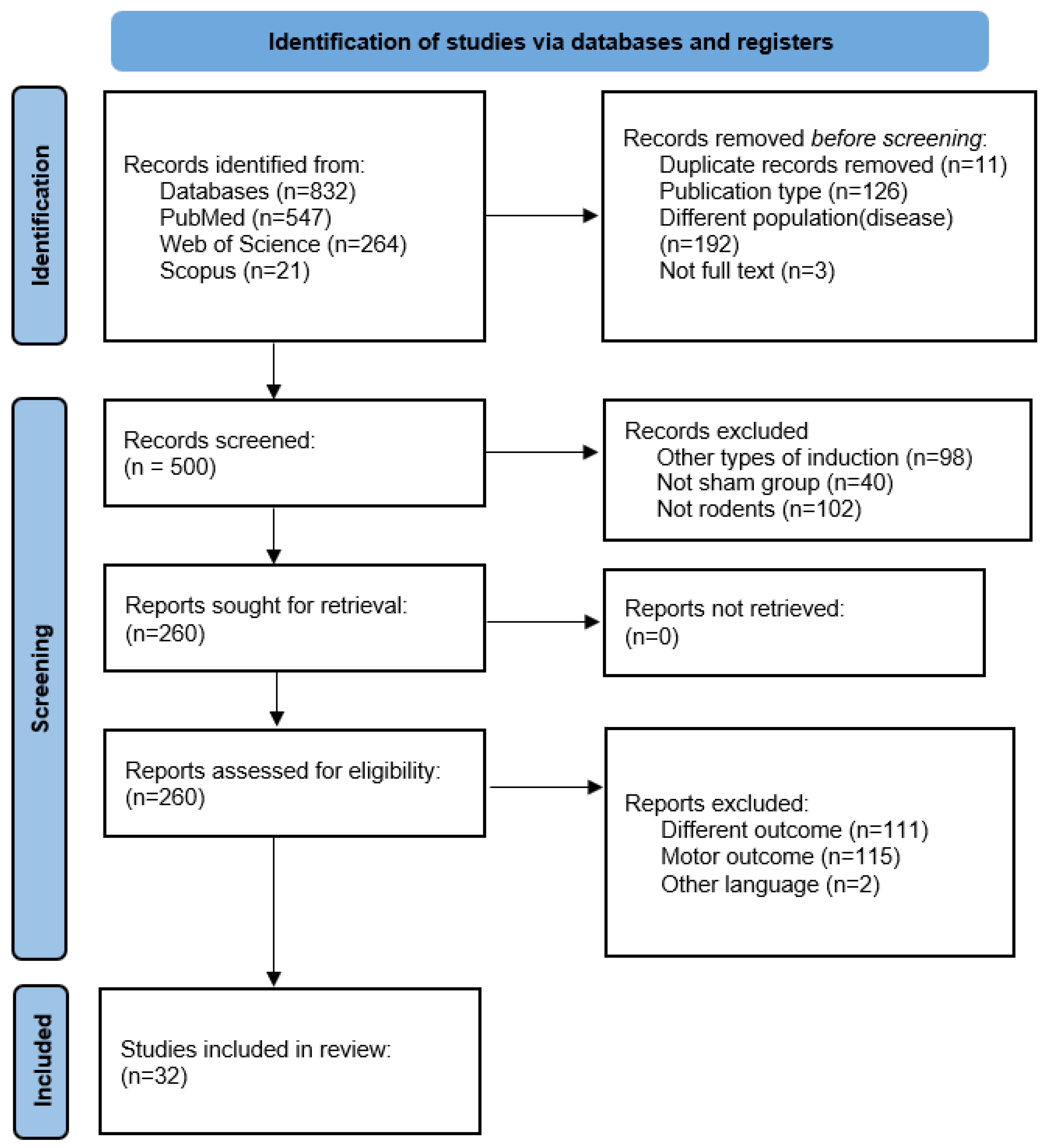
2.2. Information Sources and Selection Procedure
2.3. Outcomes
2.4. Search Strategy
2.5. Data Synthesis
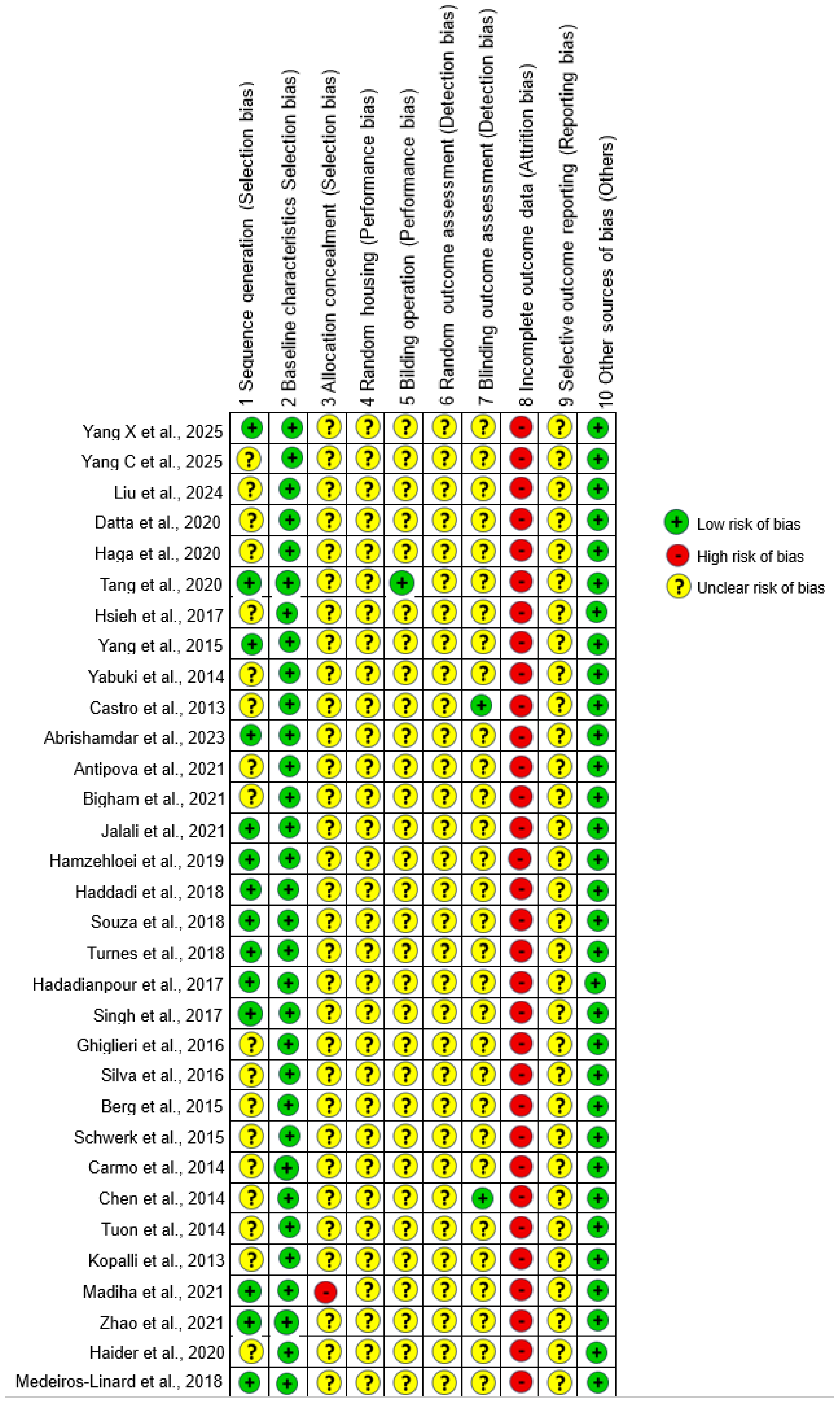
2.6. Methodological Quality and Confidence in the Evidence
3. Results
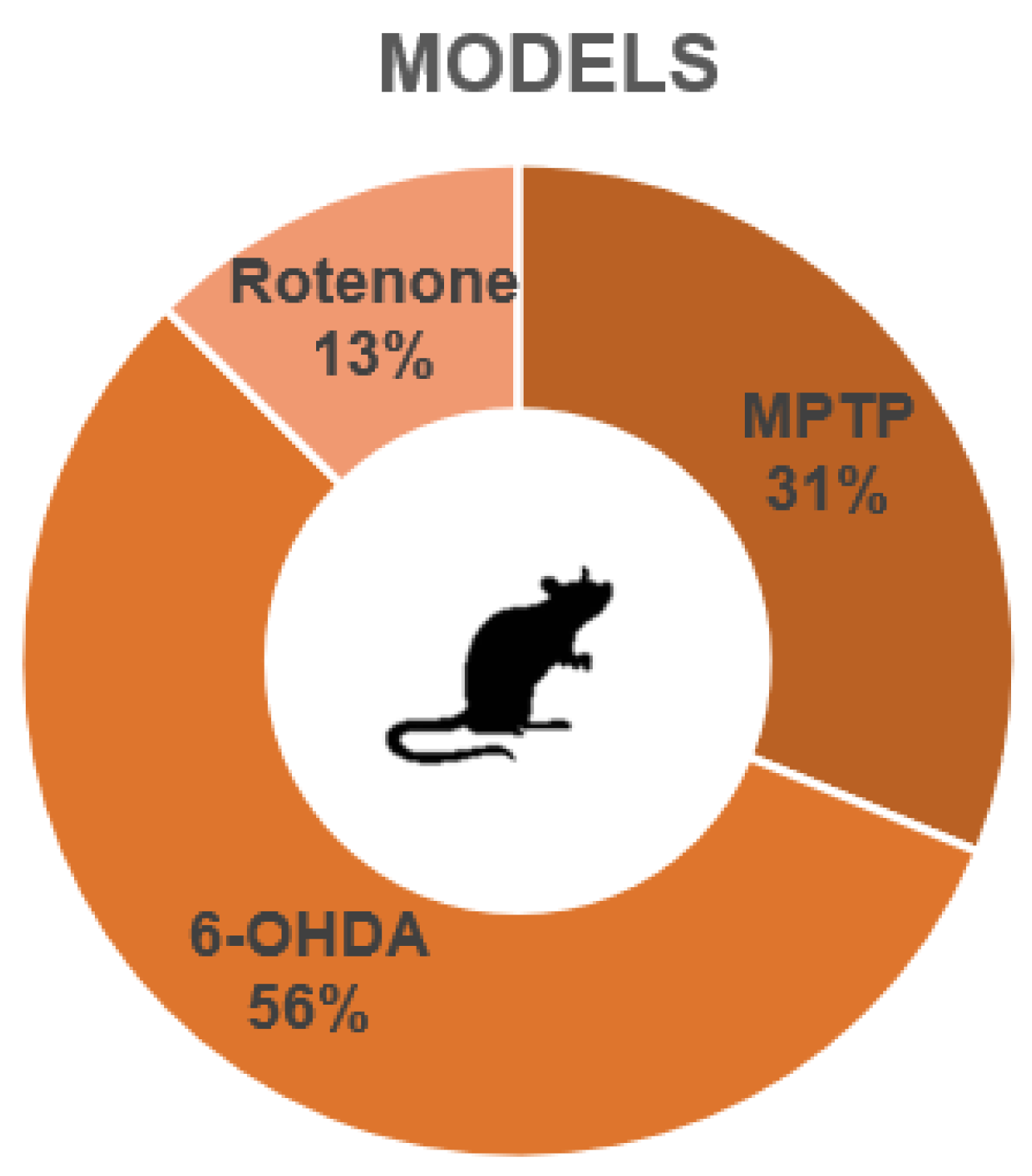
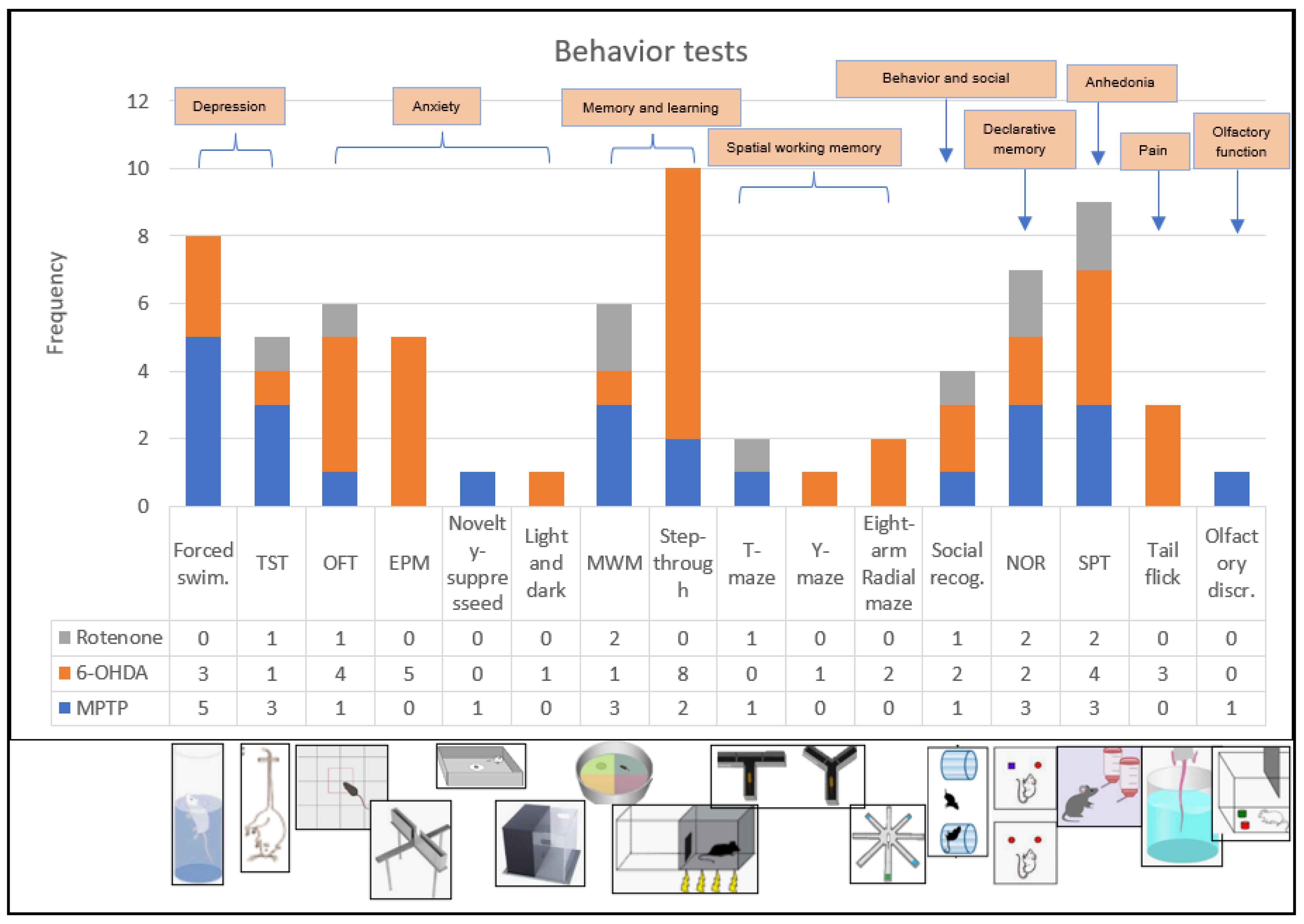
3.1. Overall Characteristics of the Studies
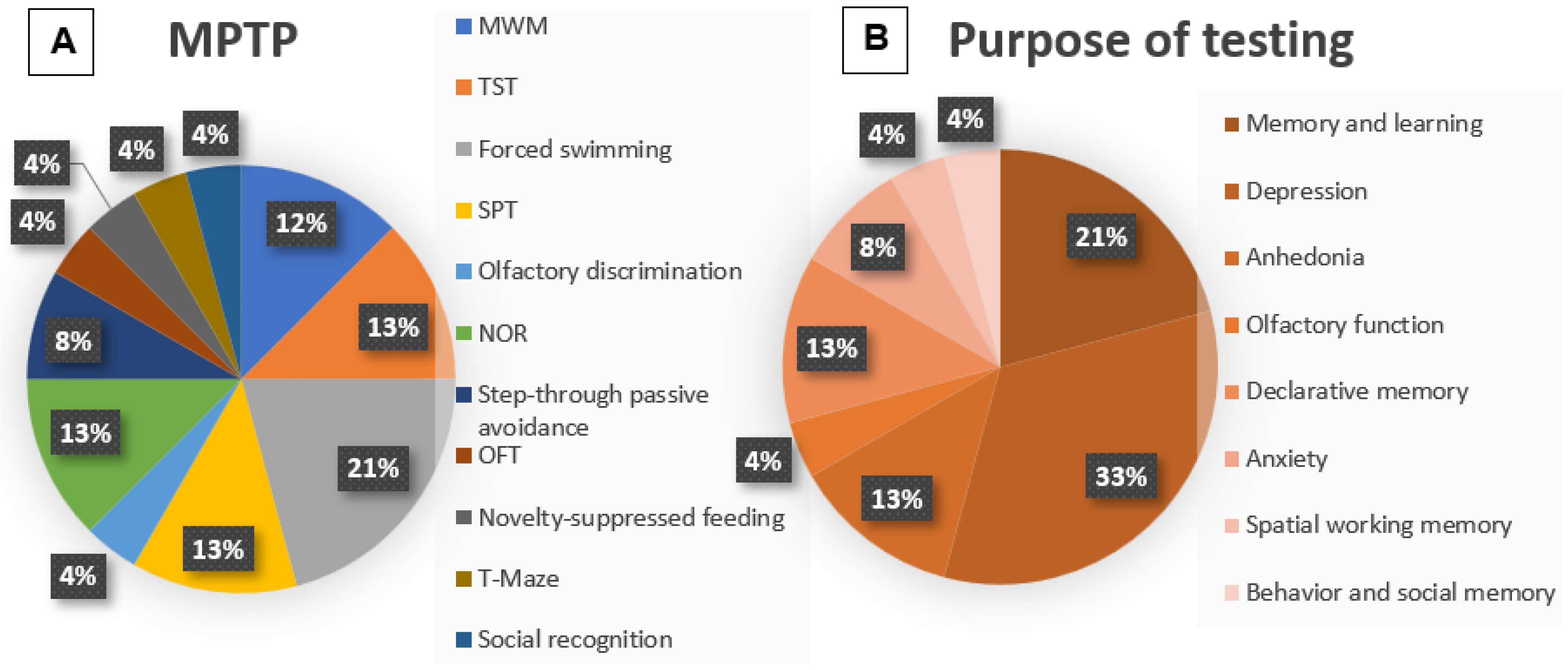
3.2. Methodologies: MPTP
3.3. Outcome: MPTP
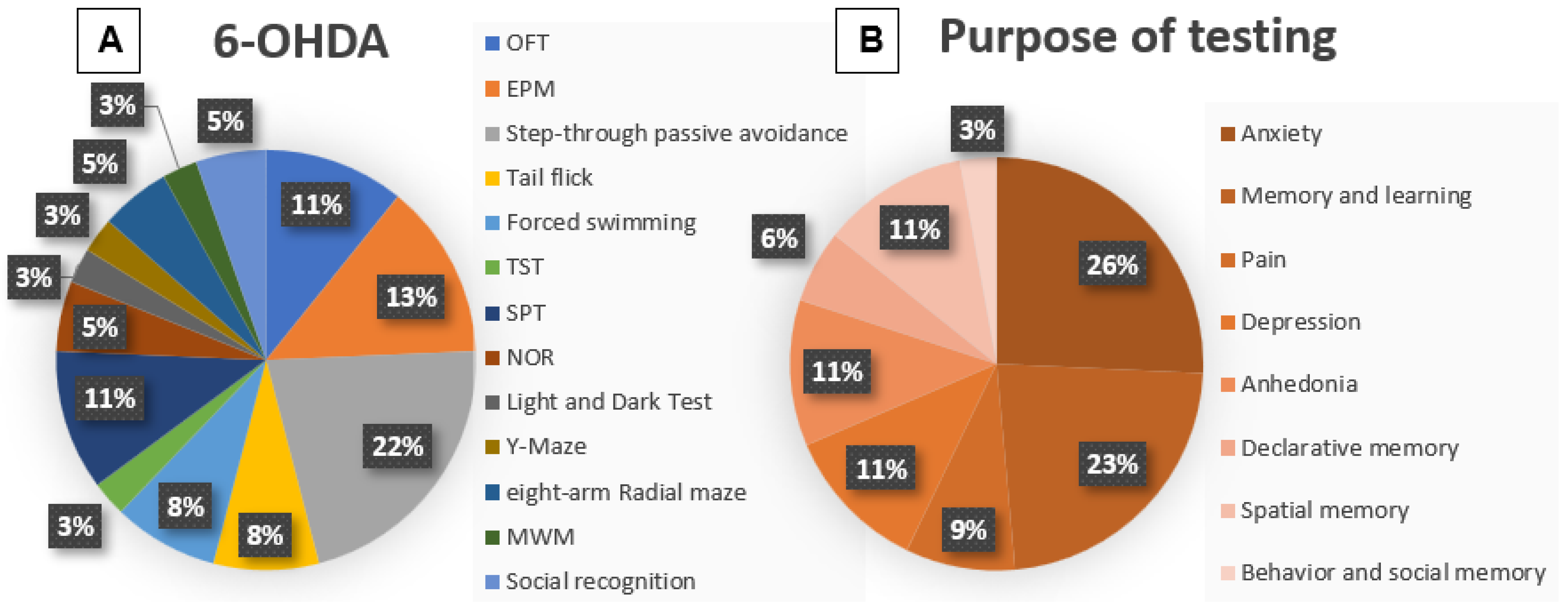
3.4. Methodologies: 6-OHDA
3.5. Outcome 6-OHDA
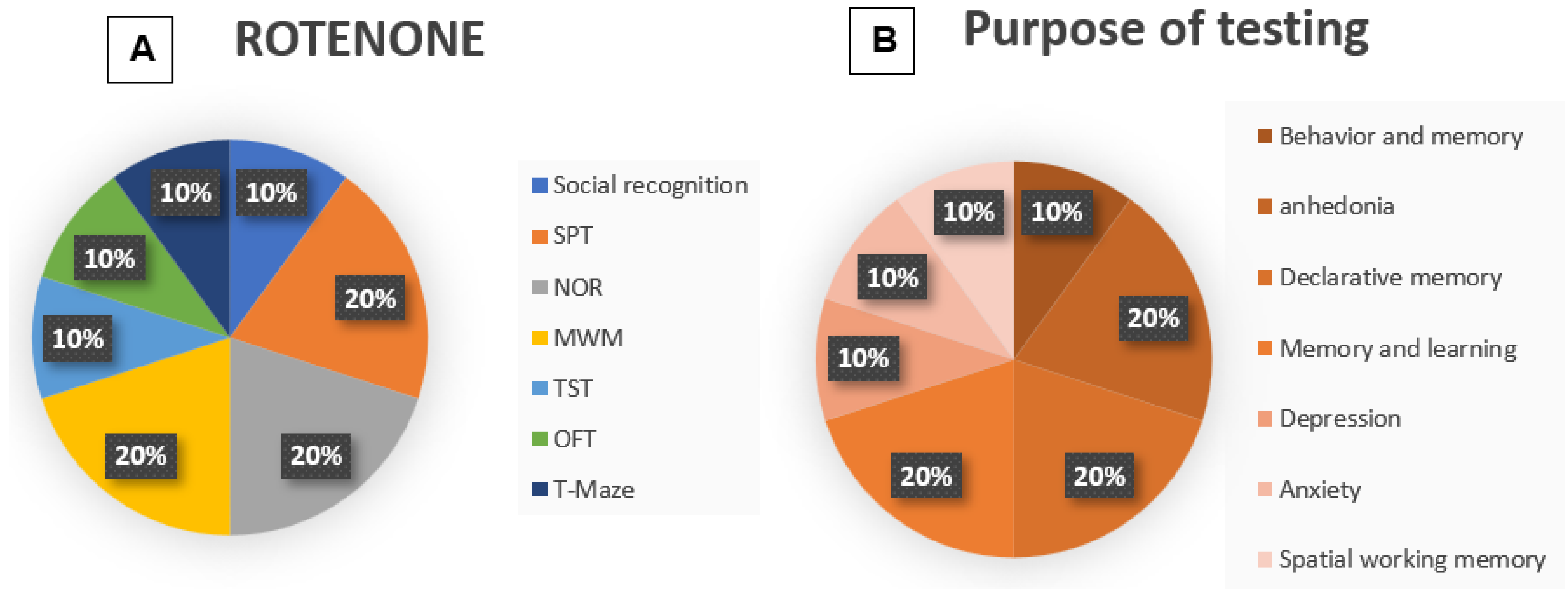
3.6. Methodologies: Rotenone
3.7. Outcome Rotenone
3.8. Quality Assessment
4. Discussion
4.1. Model-Specific Observations
4.1.1. MPTP
4.1.2. 6-OHDA
4.1.3. Rotenone
4.1.4. Comparison of Models and Concerns About Reproducibility
4.2. Risk of Bias and Reporting Transparency
4.3. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Simon, D.K.; Tanner, C.M.; Brundin, P. Parkinson Disease Epidemiology, Pathology, Genetics, and Pathophysiology. Clin. Geriatr. Med. 2020, 36, 1–12. [Google Scholar] [CrossRef]
- Raza, C.; Anjum, R.; Shakeel, N.U.A. Parkinson’s disease: Mechanisms, translational models and management strategies. Life Sci. 2019, 226, 77–90. [Google Scholar] [CrossRef]
- Galet, B.; Ingallinesi, M.; Pegon, J.; Thi, A.D.; Ravassard, P.; Biguet, N.F.; Meloni, R. G-protein coupled receptor 88 knockdown in the associative striatum reduces psychiatric symptoms in a translational male rat model of Parkinson disease. J. Psychiatry Neurosci. 2021, 46, E44–E55. [Google Scholar] [CrossRef]
- Murueta-Goyena, A.; Andikoetxea, A.; Gómez-Esteban, J.C.; Gabilondo, I. Contribution of the GABAergic System to Non-Motor Manifestations in Premotor and Early Stages of Parkinson’s Disease. Front. Pharmacol. 2019, 10, 1294. [Google Scholar] [CrossRef]
- Tansey, M.G.; Wallings, R.L.; Houser, M.C.; Herrick, M.K.; Keating, C.E.; Joers, V. Inflammation and immune dysfunction in Parkinson disease. Nat. Rev. Immunol. 2022, 22, 657–673. [Google Scholar] [CrossRef]
- Ray Chaudhuri, K.; Poewe, W.; Brooks, D. Motor and Nonmotor Complications of Levodopa: Phenomenology, Risk Factors, and Imaging Features. Mov. Disord. 2018, 33, 909–919. [Google Scholar] [CrossRef]
- Prasad, E.M.; Hung, S.Y. Behavioral Tests in Neurotoxin-Induced Animal Models of Parkinson’s Disease. Antioxidants 2020, 9, 1007. [Google Scholar] [CrossRef]
- Zhang, T.D.; Kolbe, S.C.; Beauchamp, L.C.; Woodbridge, E.K.; Finkelstein, D.I.; Burrows, E.L. How Well Do Rodent Models of Parkinson’s Disease Recapitulate Early Non-Motor Phenotypes? A Systematic Review. Biomedicines 2022, 10, 3026. [Google Scholar] [CrossRef]
- Quiroga-Varela, A.; Aguilar, E.; Iglesias, E.; Obeso, J.A.; Marin, C. Short- and long-term effects induced by repeated 6-OHDA intraventricular administration: A new progressive and bilateral rodent model of Parkinson’s disease. Neuroscience 2017, 361, 144–156. [Google Scholar] [CrossRef]
- Vieira, J.C.F.; Bassani, T.B.; Santiago, R.M.; Guaita, G.d.O.; Zanoveli, J.M.; da Cunha, C.; Vital, M.A. Anxiety-like behavior induced by 6-OHDA animal model of Parkinson’s disease may be related to a dysregulation of neurotransmitter systems in brain areas related to anxiety. Behav. Brain Res. 2019, 371, 111981. [Google Scholar] [CrossRef]
- Branchi, I.; D’ANdrea, I.; Armida, M.; Cassano, T.; Pèzzola, A.; Potenza, R.L.; Morgese, M.G.; Popoli, P.; Alleva, E. Nonmotor symptoms in Parkinson’s disease: Investigating early-phase onset of behavioral dysfunction in the 6-hydroxydopamine-lesioned rat model. J. Neurosci. Res. 2008, 86, 2050–2061. [Google Scholar] [CrossRef] [PubMed]
- Carnicella, S.; Drui, G.; Boulet, S.; Carcenac, C.; Favier, M.; Duran, T.; Savasta, M. Implication of dopamine D3 receptor activation in the reversion of Parkinson’s disease-related motivational deficits. Transl. Psychiatry 2014, 4, e401. [Google Scholar] [CrossRef]
- Hooijmans, C.R.; Rovers, M.M.; de Vries, R.B.M.; Leenaars, M.; Ritskes-Hoitinga, M.; Langendam, M.W. SYRCLE’s risk of bias tool for animal studies. BMC Med. Res. Methodol. 2014, 14, 43. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhao, Y.; Liang, L.; Qu, Y.; Yu, C.; Zhang, J.; Lian, W.; Zhao, Y. Protective effect of ginsenoside CK against MPTP-induced Parkinson’ s disease mouse model by suppressing oxidative stress and inflammation, and modulating the gut microbiota. Microb. Pathog. 2025, 202, 107409. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Song, Y.; Luo, M.; Wang, Q.; Zhang, Y.; Cen, J.; Du, G.; Shi, J. Exosomes-encapsulated biomimetic polydopamine carbon dots with dual-targeting effect alleviate motor and non-motor symptoms of Parkinson’s disease via anti-neuroinflammation. Int. J. Biol. Macromol. 2025, 296, 139724. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, X.; Xue, K.; Sun, R.; Tang, Y.; Tang, C. Reviving: Restoring depression-like behaviour through glial cell-derived neurotrophic factor treatment in the medial prefrontal cortex. J. Psychiatry Neurosci. 2024, 49, E23–E34. [Google Scholar] [CrossRef]
- Datta, I.; Mekha, S.R.; Kaushal, A.; Ganapathy, K.; Razdan, R. Influence of intranasal exposure of MPTP in multiple doses on liver functions and transition from non-motor to motor symptoms in a rat PD model. Naunyn Schmiedebergs Arch. Pharmacol. 2020, 393, 147–165. [Google Scholar] [CrossRef]
- Haga, H.; Yamada, R.; Izumi, H.; Shinoda, Y.; Kawahata, I.; Miyachi, H.; Fukunaga, K. Novel fatty acid-binding protein 3 ligand inhibits dopaminergic neuronal death and improves motor and cognitive impairments in Parkinson’s disease model mice. Pharmacol. Biochem. Behav. 2020, 191, 172891. [Google Scholar] [CrossRef]
- Tang, J.; Lu, L.; Wang, Q.; Liu, H.; Xue, W.; Zhou, T.; Xu, L.; Wang, K.; Wu, D.; Wei, F.; et al. Crocin Reverses Depression-Like Behavior in Parkinson Disease Mice via VTA-mPFC Pathway. Mol. Neurobiol. 2020, 57, 3158–3170. [Google Scholar] [CrossRef]
- Hsieh, M.-H.; Meng, W.-Y.; Liao, W.-C.; Weng, J.-C.; Li, H.-H.; Su, H.-L.; Lin, C.-L.; Hung, C.-S.; Ho, Y.-J. Ceftriaxone reverses deficits of behavior and neurogenesis in an MPTP-induced rat model of Parkinson’s disease dementia. Brain Res. Bull. 2017, 132, 129–138. [Google Scholar] [CrossRef]
- Yang, W.; Chen, Y.H.; Liu, H.; Qu, H.D. Neuroprotective effects of piperine on the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced Parkinson’s disease mouse model. Int. J. Mol. Med. 2015, 36, 1369–1376. [Google Scholar] [CrossRef]
- Yabuki, Y.; Ohizumi, Y.; Yokosuka, A.; Mimaki, Y.; Fukunaga, K. Nobiletin treatment improves motor and cognitive deficits seen in MPTP-induced Parkinson model mice. Neuroscience 2014, 259, 126–141. [Google Scholar] [CrossRef] [PubMed]
- Castro, A.A.; Wiemes, B.P.; Matheus, F.C.; Lapa, F.R.; Viola, G.G.; Santos, A.R.; Tasca, C.I.; Prediger, R.D. Atorvastatin improves cognitive, emotional and motor impairments induced by intranasal 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) administration in rats, an experimental model of Parkinson’s disease. Brain Res. 2013, 1513, 103–116. [Google Scholar] [CrossRef] [PubMed]
- Abrishamdar, M.; Farbood, Y.; Sarkaki, A.; Rashno, M.; Badavi, M. Evaluation of betulinic acid effects on pain, memory, anxiety, catalepsy, and oxidative stress in animal model of Parkinson’s disease. Metab. Brain Dis. 2023, 38, 467–482. [Google Scholar] [CrossRef]
- Antipova, V.; Holzmann, C.; Hawlitschka, A.; Witt, M.; Wree, A. Antidepressant-Like Properties of Intrastriatal Botulinum Neurotoxin-A Injection in a Unilateral 6-OHDA Rat Model of Parkinson’s Disease. Toxins 2021, 13, 505. [Google Scholar] [CrossRef]
- Bigham, M.; Mohammadipour, A.; Hosseini, M.; Malvandi, A.M.; Ebrahimzadeh-Bideskan, A. Neuroprotective effects of garlic extract on dopaminergic neurons of substantia nigra in a rat model of Parkinson’s disease: Motor and non-motor outcomes. Metab. Brain Dis. 2021, 36, 927–937. [Google Scholar] [CrossRef]
- Jalali, M.S.; Saki, G.; Farbood, Y.; Azandeh, S.S.; Mansouri, E.; Dehcheshmeh, M.G.; Sarkaki, A. Therapeutic effects of Wharton’s jelly-derived Mesenchymal Stromal Cells on behaviors, EEG changes and NGF-1 in rat model of the Parkinson’s disease. J. Chem. Neuroanat. 2021, 113, 101921. [Google Scholar] [CrossRef]
- Hamzehloei, L.; Rezvani, M.E.; Rajaei, Z. Effects of carvacrol and physical exercise on motor and memory impairments associated with Parkinson’s disease. Arq. Neuropsiquiatr. 2019, 77, 493–500. [Google Scholar] [CrossRef]
- Haddadi, H.; Rajaei, Z.; Alaei, H.; Shahidani, S. Chronic treatment with carvacrol improves passive avoidance memory in a rat model of Parkinson’s disease. Arq. Neuropsiquiatr. 2018, 76, 71–77. [Google Scholar] [CrossRef]
- Souza, L.C.; Martynhak, B.J.; Bassani, T.B.; Turnes, J.d.M.; Machado, M.M.; Moura, E.; Andreatini, R.; Vital, M.A. Agomelatine’s effect on circadian locomotor rhythm alteration and depressive-like behavior in 6-OHDA lesioned rats. Physiol. Behav. 2018, 188, 298–310. [Google Scholar] [CrossRef]
- Turnes, J.M.; Bassani, T.B.; Souza, L.C.; Vital, M.A.B.F. Ineffectiveness of saxagliptin as a neuroprotective drug in 6-OHDA-lesioned rats. J. Pharm. Pharmacol. 2018, 70, 1059–1068. [Google Scholar] [CrossRef]
- Hadadianpour, Z.; Fatehi, F.; Ayoobi, F.; Kaeidi, A.; Shamsizadeh, A.; Fatemi, I. The effect of orexin-A on motor and cognitive functions in a rat model of Parkinson’s disease. Neurol. Res. 2017, 39, 845–851. [Google Scholar] [CrossRef]
- Singh, S.; Mishra, A.; Srivastava, N.; Shukla, S. MK-801 (Dizocilpine) Regulates Multiple Steps of Adult Hippocampal Neurogenesis and Alters Psychological Symptoms via Wnt/β-Catenin Signaling in Parkinsonian Rats. ACS Chem. Neurosci. 2017, 8, 592–605. [Google Scholar] [CrossRef]
- Ghiglieri, V.; Mineo, D.; Vannelli, A.; Cacace, F.; Mancini, M.; Pendolino, V.; Napolitano, F.; di Maio, A.; Mellone, M.; Stanic, J.; et al. Modulation of serotonergic transmission by eltoprazine in L-DOPA-induced dyskinesia: Behavioral, molecular, and synaptic mechanisms. Neurobiol. Dis. 2016, 86, 140–153. [Google Scholar] [CrossRef]
- Silva, T.P.; Poli, A.; Hara, D.B.; Takahashi, R.N. Time course study of microglial and behavioral alterations induced by 6-hydroxydopamine in rats. Neurosci. Lett. 2016, 622, 83–87. [Google Scholar] [CrossRef]
- Berg, J.; Roch, M.; Altschüler, J.; Winter, C.; Schwerk, A.; Kurtz, A.; Steiner, B. Human adipose-derived mesenchymal stem cells improve motor functions and are neuroprotective in the 6-hydroxydopamine-rat model for Parkinson’s disease when cultured in monolayer cultures but suppress hippocampal neurogenesis and hippocampal memory function when cultured in spheroids. Stem Cell Rev. Rep. 2015, 11, 133–149. [Google Scholar] [CrossRef]
- Schwerk, A.; Altschüler, J.; Roch, M.; Gossen, M.; Winter, C.; Berg, J.; Kurtz, A.; Akyüz, L.; Steiner, B. Adipose-derived human mesenchymal stem cells induce long-term neurogenic and anti-inflammatory effects and improve cognitive but not motor performance in a rat model of Parkinson’s disease. Regen. Med. 2015, 10, 431–446. [Google Scholar] [CrossRef]
- Carmo, M.R.; Menezes, A.P.F.; Nunes, A.C.L.; Pliássova, A.; Rolo, A.P.; Palmeira, C.M.; Cunha, R.A.; Canas, P.M.; Andrade, G.M. The P2X7 receptor antagonist Brilliant Blue G attenuates contralateral rotations in a rat model of Parkinsonism through a combined control of synaptotoxicity, neurotoxicity and gliosis. Neuropharmacology 2014, 81, 142–152. [Google Scholar] [CrossRef]
- Chen, L.; Deltheil, T.; Turle-Lorenzo, N.; Liberge, M.; Rosier, C.; Watabe, I.; Sreng, L.; Amalric, M.; Mourre, C. SK channel blockade reverses cognitive and motor deficits induced by nigrostriatal dopamine lesions in rats. Int. J. Neuropsychopharmacol. 2014, 17, 1295–1306. [Google Scholar] [CrossRef]
- Tuon, T.; Valvassori, S.; Pont, G.D.; Paganini, C.; Pozzi, B.; Luciano, T.; Souza, P.; Quevedo, J.; Souza, C.; Pinho, R. Physical training prevents depressive symptoms and a decrease in brain-derived neurotrophic factor in Parkinson’s disease. Brain Res. Bull. 2014, 108, 106–112. [Google Scholar] [CrossRef]
- Kopalli, S.R.; Noh, S.J.; Koppula, S.; Suh, Y.H. Methylparaben protects 6-hydroxydopamine-induced neurotoxicity in SH-SY5Y cells and improved behavioral impairments in mouse model of Parkinson’s disease. Neurotoxicology 2013, 34, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Madiha, S.; Batool, Z.; Tabassum, S.; Liaquat, L.; Sadir, S.; Shahzad, S.; Naqvi, F.; Saleem, S.; Yousuf, S.; Nawaz, A.; et al. Quercetin exhibits potent antioxidant activity, restores motor and non-motor deficits induced by rotenone toxicity. PLoS ONE 2021, 16, e0258928. [Google Scholar] [CrossRef]
- Zhao, X.; Kong, D.; Zhou, Q.; Wei, G.; Song, J.; Liang, Y.; Du, G. Baicalein alleviates depression-like behavior in rotenone- induced Parkinson’s disease model in mice through activating the BDNF/TrkB/CREB pathway. Biomed. Pharmacother. 2021, 140, 111556. [Google Scholar] [CrossRef] [PubMed]
- Haider, S.; Madiha, S.; Batool, Z. Amelioration of motor and non-motor deficits and increased striatal APoE levels highlight the beneficial role of pistachio supplementation in rotenone-induced rat model of PD. Metab. Brain Dis. 2020, 35, 1189–1200. [Google Scholar] [CrossRef] [PubMed]
- Medeiros-Linard, C.F.B.; Andrade-Da-Costa, B.L.d.S.; Augusto, R.L.; Sereniki, A.; Trevisan, M.T.S.; Perreira, R.d.C.R.; de Souza, F.T.C.; Braz, G.R.F.; Lagranha, C.J.; de Souza, I.A.; et al. Anacardic Acids from Cashew Nuts Prevent Behavioral Changes and Oxidative Stress Induced by Rotenone in a Rat Model of Parkinson’s Disease. Neurotox. Res. 2018, 34, 250–262. [Google Scholar] [CrossRef]
- Vingill, S.; Connor-Robson, N.; Wade-Martins, R. Are rodent models of Parkinson’s disease behaving as they should? Behav. Brain Res. 2018, 352, 133–141. [Google Scholar] [CrossRef]
- Yin, R.; Zhang, K.; Li, Y.; Tang, Z.; Zheng, R.; Ma, Y.; Chen, Z.; Lei, N.; Xiong, L.; Guo, P.; et al. Lipopolysaccharide-induced depression-like model in mice: Meta-analysis and systematic evaluation. Front. Immunol. 2023, 14, 1181973. [Google Scholar] [CrossRef]
- Kaluve, A.M.; Le, J.T.; Graham, B.M. Female rodents are not more variable than male rodents: A meta-analysis of preclinical studies of fear and anxiety. Neurosci. Biobehav. Rev. 2022, 143, 104962. [Google Scholar] [CrossRef]
| Database | String or Combined Search Terms |
|---|---|
| PubMed | ((((parkinson’s disease) AND (animal model)) AND (behavior)) AND (motor activity OR no motor activity)) AND (therapeutics) |
| Scopus | (TITLE-ABS-KEY “parkinson’s disease”) AND TITLE-ABS-KEY (“animal model”) AND TITLE-ABS-KEY (behavior) AND TITLE-ABS-KEY (“motor activity”) OR TITLE-ABS-KEY (“not motor activity”) AND TITLE-ABS-KEY therapeutics)) |
| Web of Science | (((((ALL = (parkinson’s disease)) AND ALL = (animal model)) AND ALL = (behavior)) AND ALL = (motor activity)) OR ALL = (no motor activity)) AND ALL = (therapeutics) |
| Author/ Year | Sample | Intervention Elements | Recovery Time | Measurement Non Motor Test | Significant Results |
|---|---|---|---|---|---|
| Yang X et al., 2025 [14] | n = ? male C57BL/6 mice | 35 mg/kg MPTP i.p. 28 short | 56 days | MWM | ↑ time and movement distance |
| Yang C et al., 2025 [15] | n = 5/group male C57BL/6 mice | 30 mg/kg MPTP i.p. 7 short | 7 days | TST, FST and Coat score assay | ↑ immobility TST and FST |
| Liu et al., 2024 [16] | n = 8/group male C57BL/6J mice | 7 mg/mL MPTP i.p. 5 short | 5 to 9 days | SPT, FST and TST | ↑ immobility FST and TST. ↓ sucrose SPT |
| Datta et al., 2020 [17] | n = 8/group male Wistar, albino rats | 0.1 mg/nostril MPTP. 1, 2 and 3 short | 7–14–21 days | Olfactory discrimination and FST | ↓ time spent in familiar compartments, ↑ immobility FST |
| Haga et al., 2020 [18] | n = 6/group male C57BL/6N mice | 25 mg/kg MPTP i.p 5 short | 28 days | NOR and step-through passive avoidance test | No ≠ NOR. ↓ step-through |
| Tang et al., 2020 [19] | n = 9–11/group male C57BL/6 mice | 30 mg/kg MPTP i.p 7 short | 1 day to include and 7 days other tests | OFT, TST, FST, SPT and novelty-suppressed feeding | ↓ SPT. ↑ immobility TST and FST. No ≠ OFT and novelty- suppressed feeding |
| Hsieh et al., 2017 [20] | n = 13/group male Wistar rats | 1 μmol in 2 μL of saline MPTP substantia nigra pars compacta. 1 short | 8–9 T-maze test 14 = Object recognition test | T-maze test and NOR | ↓ T-maze and NOR |
| Yang et al., 2015 [21] | n = 9/group male C57BL/6 mice | 30 mg/kg MPTP i.p. 7 short | 7 days | MWM | ↓ latency |
| Yabuki et al., 2014 [22] | n = 10–11/group male C57BL/6N mice | 25 mg/kg MPTP i.p. 5 short | 28 days | Step-through passive avoidance task and NOR | ↓ discrimination NOR |
| Castro et al., 2013 [23] | n = 6–7/group male Wistar rats | 1 mg/nostril MPTP intranasally bilateral and | 7 days = Social recognition 14 days = forced swimming, SPT 21 days = water maze | Social recognition, FST, SPT, and MWM | No ≠ social recognition, SPT and MWM. ↓ social recognition. ↑ FST |
| Author/ Year | Sample | Intervention Elements | Recovery Time | Measurement | Significant Results |
|---|---|---|---|---|---|
| Abrishamdar et al., 2023 [24] | n = 8/group male Wistar rats | 20 µg/4 µL 6-OHDA into right MFB. 25 mg/kg of desipramine | 2 weeks = rotation test 3 weeks = others | Apomorphine-induced rotation test, OFT, EPM, passive avoidance test and tail-flick test | ↑ immobility OFT. ↓ EPM and passive avoidance. ↓ tail-flick test. |
| Antipova et al., 2021 [25] | n = 10–11/group male Wistar rats | 24 μg 6-OHDA into right MFB | 4 weeks | Apomorphine-Induced Rotation Test, amphetamine-Induced Rotation Test, OFT, EPM, FST and TST | = OFT, EPM and TST. ↑ frequency of immobility FST. |
| Bigham et al., 2021 [26] | n = 10/group male Wistar rats | 2 µL/min 6-OHDA in the left MFB | 2 weeks | Apomorphine-induced rotational test, OFT and passive avoidance test | ↑ central zone OFT. ↓ latency passive avoidance. |
| Jalali et al., 2021 [27] | n = 10/group male Wistar rats | 16 μg/2 μL 6-OHDA into right MFB. 15 mg/kg of desipramine | 2 weeks = rotation test 4 months = others | Apomorphine-Induced Rotation Test, tail flick test, EPM and OFT | ↓ tail flick latencies, EPM and OFT |
| Hamzehloei et al., 2019 [28] | n = 8/group male Wistar rats | 16 μg/4 μL 6-OHDA into left MFB | 6 weeks | Apomorphine-induced rotation test and passive avoidance memory | ↓ latency passive avoidance |
| Haddadi et al., 2018 [29] | n = 7/group male Wistar rats | 16 μg/4 μL 6-OHDA into the left MFB | 2–6 weeks = rotation test 6 weeks = others | Apomorphine-induced rotations, passive avoidance memory and Tail-flick test | ↓ latency passive avoidance and tail-flick |
| Souza et al., 2018 [30] | n = 10–12/group male Wistar rats | 1 μL 6-OHDA into the SNpc | 1–2–3 weeks | SPT | ↓ sucrose |
| Turnes et al., 2018 [31] | n = 10–13/ group male Wistar rats | 6 μg/μL 6-OHDA into SN | 20–21 = NOR | NOR | ↓ discrimination index |
| Hadadianpour et al., 2017 [32] | n = 7/group male Wistar rats | 200 μg/5 μL 6-OHDA intracerebroventricul. 25 mg/kg desipramine | 2 weeks | Passive avoidance learning | ↓ latency |
| Singh et al., 2017 [33] | n = 6/group male Sprague– Dawley rats | 2 μL 6-OHDA into right MFB | 3 weeks | Light and Dark Test, social interaction test and FST | ↓ time spent in the light. ↓ social interaction. ↑ immobility FST. |
| Ghiglieri et al., 2016 [34] | n = 6–8/group male Wistar rats | 3 μg/μL 6-OHDA into the left MFB | 2 weeks = rotation test 7 weeks = others | Apomorphine-induced rotation test, SPT and Active avoidance | ↓ shock avoidance behavior. |
| Silva et al., 2016 [35] | n = 12/group male Wistar rats | 12 μg 6-OHDA bilaterally into dorsal striatum. Desipramine | 1–2–3 weeks | EPM and SPT | ↓ fluid consumption SPT |
| Berg et al., 2015 [36] | n = 11–16/group male Wistar rats | 6.5 μg 6-OHDA in the left MFB | 1–3 weeks = Rotation test 3 weeks = other | D-Amphetamine Induced Rotation and eight-arm Radial maze | ↓ time spent EPM and sucrose in SPT |
| Schwerk et al., 2015 [37] | n = 7/group male Wistar rats | 2 μL 6-OHDA into the MFB | 1–4 weeks = Rotation test 3 weeks = others | D-amphetamine-induced rotations and eight-arm radial maze | ↓ short-term memory |
| Carmo et al., 2014 [38] | n = 8/group male Wistar rats | 18 μg/3 μL 6-OHDA unilateral intrastriatal | 15 to 18 days | Apomorphine-induced rotation test, passive avoidance test and MWM | ↓ latency passive avoidance. ↓ performance MWM |
| Chen et al., 2014 [39] | n = 8–10/group male Wistar rats | 12 µg/3 µL/side 6-OHDA bilaterally into the striatum | 14 to 25 days | Cylinder test, Apomorphine-induced rotation test, EPM, SPT, social interaction and NOR | ↓ EPM and sucrose SPT. = NOR. ↓ social interaction |
| Tuon et al., 2014 [40] | n = 12/group male C57BL mice | 8 μg 6-OHDA unilateral in the terminal region of the striatum | 1 week | Apomorphine-induced rotations and FST | ↑ immobility FST |
| Kopalli et al., 2013 [41] | n = 6/group male C57BL/6N mice | 10 μg 6-OHDA unilaterally into the SN. 25 mg/Kg desipramine | 2 weeks | Apomorphine-induced rotational behavior test, passive avoidance test and Y-maze task | ↓ latency passive avoidance. ↓ performance Y-maze |
| Author/ Year | Sample | Intervention Elements | Recovery Time | Measurement | Significant Results |
|---|---|---|---|---|---|
| Madiha et al., 2021 [42] | n = 8/group male Wistar rats. | 1.5 mg/kg rotenone subcutaneously 8 short | 2 weeks | Social interaction test, SPT, NOR and MWM | ↓ interaction social. ↓ sucrose SPT. ↓ NOR. ↑ escape latency MWM |
| Zhao et al., 2021 [43] | n = 8–12/group male C57BL/6J mice. | 2.5 mg/kg rotenone i.p. 14 short | 1 day = include 4 weeks = others | TST and SPT | ↑ immobility TST. ↓ sucrose SPT |
| Haider et al., 2020 [44] | n = 6/group Wistar rats. | 1.5 mg/kg rotenone s.c. 8 short | 3 weeks | MWM and NOR | ↑ escape latency MWM. ↓ preference index NOR |
| Medeiros-Linard et al., 2018 [45] | n = 8/group male Wistar rats. | 3 mg/kg/day rotenone s.c. 5 shorts | 1 day | OFT and T-maze test | ↑ immobility OFT. ↓ memory retention T-maze |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zambetta, M.L.; Fernandes, E.B.; Kim, A.; Russo, T.L.; Gianlorenço, A.C. Preclinical Parkinson’s Disease Models for Non-Motor Symptoms: Research Recommendations from a Systematic Review. Life 2025, 15, 1034. https://doi.org/10.3390/life15071034
Zambetta ML, Fernandes EB, Kim A, Russo TL, Gianlorenço AC. Preclinical Parkinson’s Disease Models for Non-Motor Symptoms: Research Recommendations from a Systematic Review. Life. 2025; 15(7):1034. https://doi.org/10.3390/life15071034
Chicago/Turabian StyleZambetta, Mariana Lara, Elayne Borges Fernandes, Allison Kim, Thiago Luiz Russo, and Anna Carolyna Gianlorenço. 2025. "Preclinical Parkinson’s Disease Models for Non-Motor Symptoms: Research Recommendations from a Systematic Review" Life 15, no. 7: 1034. https://doi.org/10.3390/life15071034
APA StyleZambetta, M. L., Fernandes, E. B., Kim, A., Russo, T. L., & Gianlorenço, A. C. (2025). Preclinical Parkinson’s Disease Models for Non-Motor Symptoms: Research Recommendations from a Systematic Review. Life, 15(7), 1034. https://doi.org/10.3390/life15071034







