Growth Performance, Carcass Quality and Gut Microbiome of Finishing Stage Pigs Fed Formulated Protein-Energy Nutrients Balanced Diet with Banana Agro-Waste Silage
Abstract
1. Introduction
2. Materials and Methods
2.1. Banana Agro-Waste Silage Preparation
2.2. Animal Experimentation
2.3. Feed Intake and Growth Performance
2.4. Carcass and Meat Quality
2.5. Metagenomics Analysis of the Gut Microbiome
2.5.1. Sample Collection and DNA Extraction
2.5.2. Metagenome Library Preparation and Sequencing
2.5.3. Bioinformatics Analysis
2.6. Statistics
3. Results
3.1. Feeding Value of BAWS
3.2. Dietary Intervention with BAWS Formulated Feed in Finishing Stage Pigs
3.2.1. Growth Performances
3.2.2. Improvement of Carcass Characteristics and Lean Composition, and Sensory of Taste
3.3. Metagenomics Analysis of Gut Microbiomes via Next Generation Sequencer
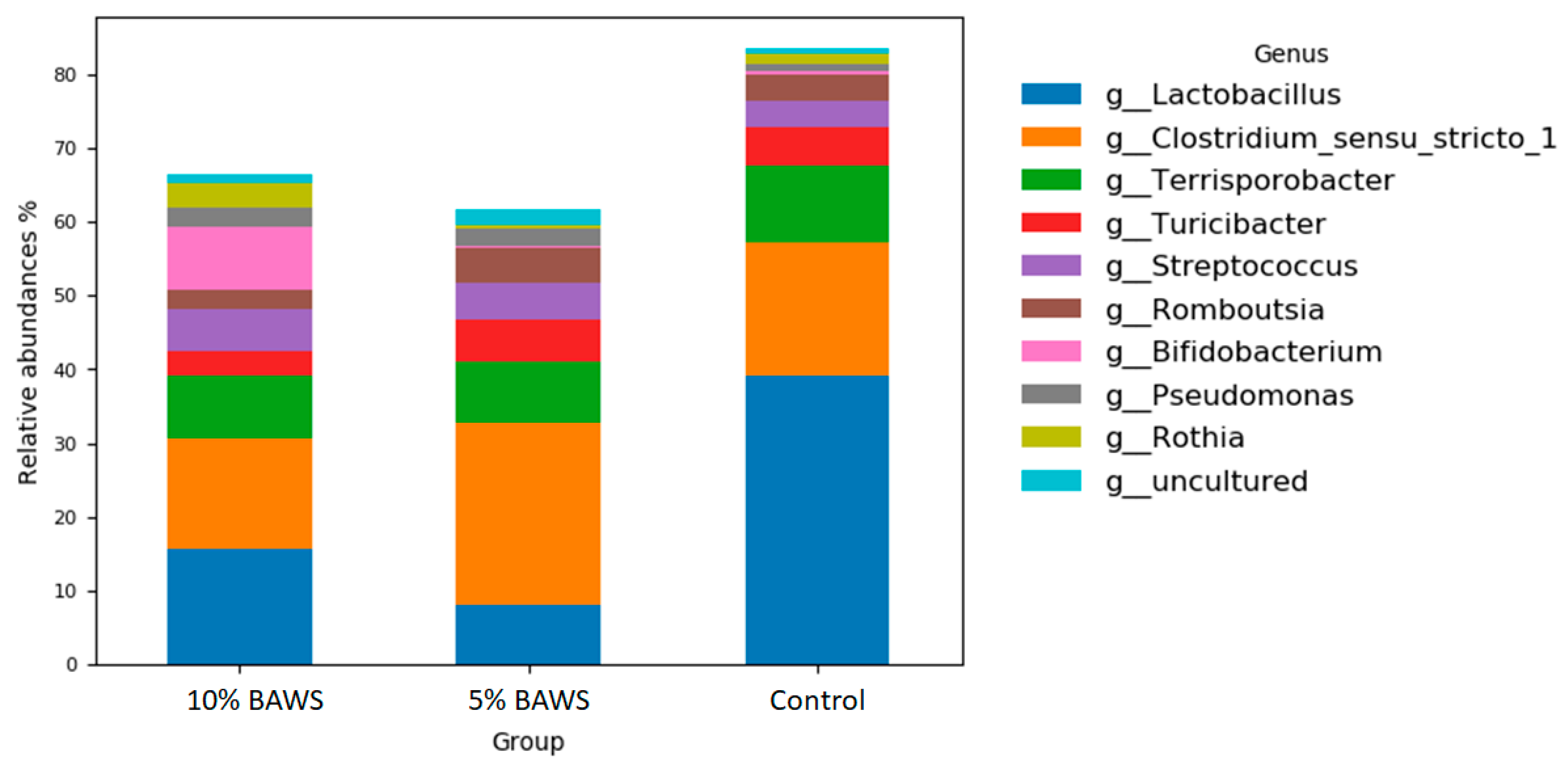
3.3.1. Fore Gut—Reduction in the Abundance of Genus Lactobacillus
3.3.2. Hind Gut—Increase in the Abundance of Streptococcaceae and Prevotellaceae Families
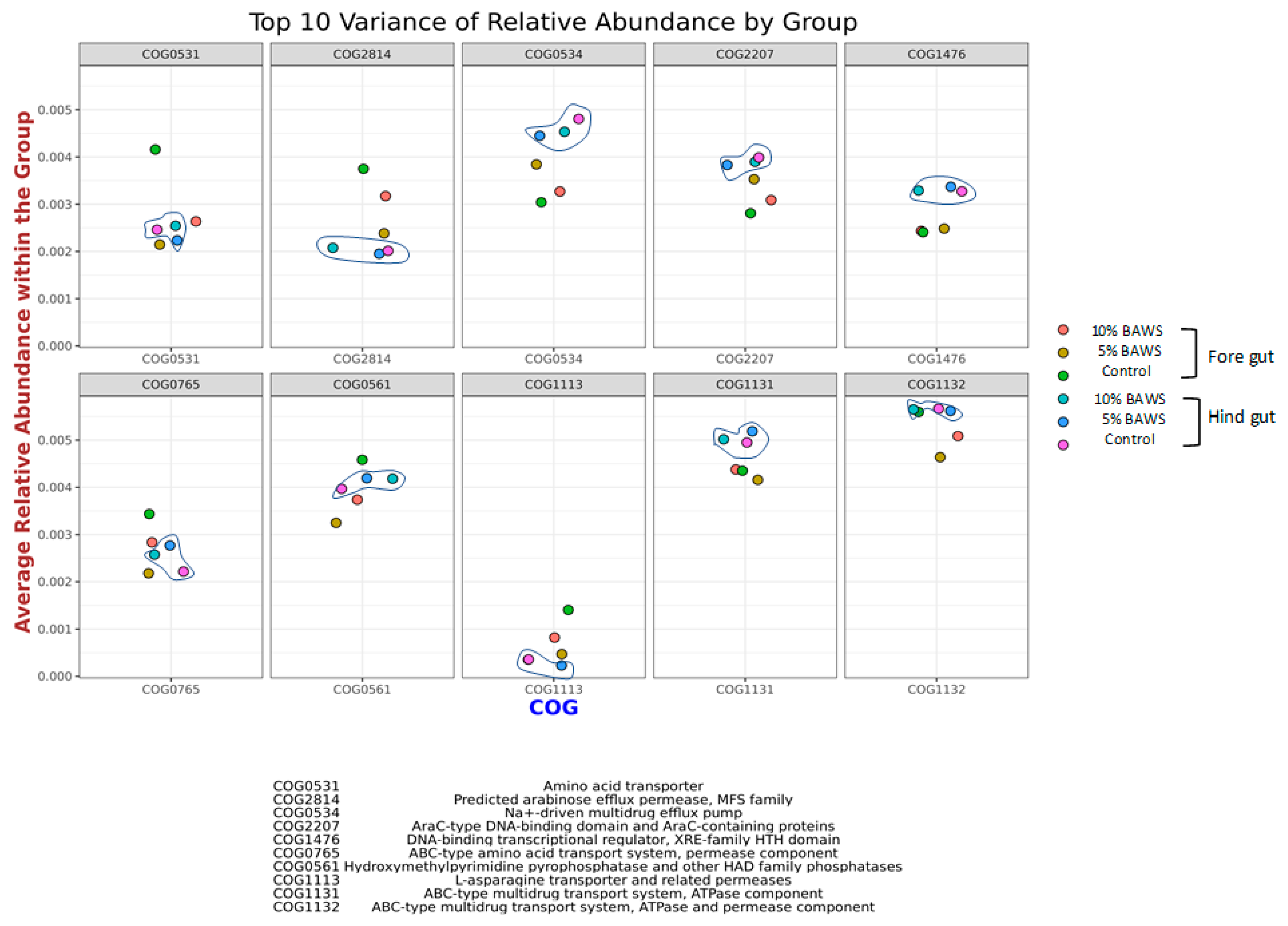
3.3.3. Predicting the Functional Distribution of Homologous Proteins with Similar Functions and Interpreting Their Significance
4. Discussion
4.1. Feed Banana Agro-Waste Silage Had No Impact on Growth, Carcass, and Meat Quality
4.2. Gut Microbiome of KHAPS Pigs and Populations Shift with Dietary BAWS
4.3. Clusters of Orthologous Genes (COG) Database for Microbial Genome Annotation on Predicted Functional Proteins
4.4. Study’s Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Taiwan Banana Research Institute Publicized Information. Available online: https://www.banana.org.tw/Default.aspx (accessed on 25 June 2023).
- Roibás, L.; Elbehri, A.; Hospido, A. Carbon footprint along the EcuadorianBanana Supply Chain: Methodological Improvements and Calculation Tool. J. Clean. Prod. 2016, 112, 2441–2451. [Google Scholar] [CrossRef]
- Shalaby, A.S.; Hamza, A.S.; Ali, M.A.; El-Shinnawy, A.M. Use of Banana Wastes as Hay or Silage for Feeding Rumi-nants: 1-Influence of Different Additives on The Quality and Feeding Value of Silage Made from Banana Waste, Com-pared with Banana Hay. J. Anim. Poult. Prod. 2009, 34, 10481–10496. [Google Scholar]
- 4. Preparation Method of Banana stem Leaf Biological Feed. 2009. CN101579041B. Available online: https://patents.google.com/patent/CN101579041B/en (accessed on 25 June 2023).
- Hashim, M.; Akbar, A.; Gul, Z.; Sadiq, M.B.; Achakzai, J.K.; Khan, N.A. Fermentation Impact: A Comparative Study on the Functional and Biological Properties of Banana Peel Waste. Heliyon 2024, 10, e36095. [Google Scholar] [CrossRef] [PubMed]
- Fatmawati, A.; Lidiawati, T.; Hadinata, S.; Adiarto, M. Solid-State Fermentation of Banana Peels Potential Study for Feed Additive. MATEC Web Conf. 2018, 215, 01027. [Google Scholar] [CrossRef]
- Jiang, S.; Quan, W.; Luo, J.; Lou, A.; Zhou, X.; Li, F.; Shen, Q.W. Low-Protein Diets Supplemented with Glycine Improves Pig Growth Performance and Meat Quality: An Untargeted Metabolomic Analysis. Front. Vet. Sci. 2023, 10, 1170573. [Google Scholar] [CrossRef]
- Zhou, L.; Fang, L.; Sun, Y.; Su, Y.; Zhu, W. Effects of the dietary protein level on the microbial composition and metabolomic profile in the hindgut of the pig. Anaerobe 2016, 38, 61–69. [Google Scholar] [CrossRef]
- Guevarra, R.B.; Lee, J.H.; Lee, S.H.; Seok, M.J.; Kim, D.W.; Kang, B.N.; Johnson, T.J.; Richard EIsaacson, R.J.; Kim, H.B. Piglet gut microbial shifts early in life: Causes and effects. J. Anim. Sci. Biotechnol. 2019, 10, 1. [Google Scholar] [CrossRef]
- Gao, J.; Liu, Z.; Wang, C.; Ma, L.; Chen, Y.; Li, T. Effects of Dietary Protein Level on the Microbial Composition and Metabolomic Profile in Postweaning Piglets. Oxid. Med. Cell Longev. 2022, 2022, e3355687. [Google Scholar] [CrossRef]
- Lee, H.L.; Lin, M.Y.; Wang, H.S.; Hsu, C.B.; Lin, C.Y.; Chang, S.C.; Shen, P.C.; Chang, H.L. Direct–Maternal Genetic Parameters for Litter Size and Body Weight of Piglets of a New Black Breed for the Taiwan Black Hog Market. Animals 2022, 12, 3295. [Google Scholar] [CrossRef]
- Flieg, O. A key for the evaluation of silage samples. Futterb. Und Giirfutterbereitung 1938, 1, 112–128. [Google Scholar]
- Huang, H.J.; Weng, B.C.; Hsuuw, Y.D.; Lee, Y.S.; Chen, K.L. Dietary Supplementation of Two-Stage Fermented Feather-Soybean Meal Product on Growth Performance and Immunity in Finishing Pigs. Animals 2021, 6, 1527. [Google Scholar] [CrossRef] [PubMed]
- NRC. Nutrient Requirements of Swine, 10th ed.; National Academy Press: Washington, DC, USA, 1998. [Google Scholar]
- Chemists AOAC. Official Methods of Analysis of the Association of Official Analytical Chemists: The Association; Chemists AOAC: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J. 2011, 17, 3. [Google Scholar] [CrossRef]
- Lozupone, C.A.; Hamady, M.; Kelley, S.T.; Knight, R. Quantitative and Qualitative β Diversity Measures Lead to Different Insights into Factors That Structure Microbial Communities. Appl. Environ. Microbiol. 2007, 73, 1576–1585. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Julia KGoodrich, J.K.; Jeffrey IGordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods. 2010, 7, 335–336. [Google Scholar] [CrossRef] [PubMed]
- Glöckner, F.O.; Yilmaz, P.; Quast, C.; Gerken, J.; Beccati, A.; Ciuprina, A.; Gerrit Bruns, G.; Pablo Yarza, P.; Jörg Peplies, P.; Ralf Westram, R.; et al. 25 years of serving the community with ribosomal RNA gene reference databases and tools. J. Biotechnol. 2017, 261, 169–176. [Google Scholar] [CrossRef]
- Ondov, B.D.; Bergman, N.H.; Phillippy, A.M. Interactive metagenomic visualization in a Web browser. BMC Bioinform. 2011, 12, 385. [Google Scholar] [CrossRef]
- Douglas, G.M.; Maffei, V.J.; Zaneveld, J.R.; Yurgel, S.N.; Brown, J.R.; Taylor, C.M.; Curtis Huttenhower, C.; Langille, M.G.I. PICRUSt2 for prediction of metagenome functions. Nat. Biotechnol. 2020, 38, 685–688. [Google Scholar] [CrossRef]
- Wemheuer, F.; Taylor, J.A.; Daniel, R.; Johnston, E.; Meinicke, P.; Thomas, T.; Wemheuer, B. Tax4Fun2: Prediction of habitat-specific functional profiles and functional redundancy based on 16S rRNA gene sequences. Environ. Microbiomes 2020, 15, 11. [Google Scholar] [CrossRef]
- Fu, J.; Wang, Y.; Tan, S.; Wang, J. Effects of Banana Resistant Starch on the Biochemical Indexes and Intestinal Flora of Obese Rats Induced by a High-Fat Diet and Their Correlation Analysis. Front. Bioeng. Biotechnol. 2021, 9, 575724. [Google Scholar] [CrossRef]
- Munir, H.; Hamza Alam, H.; Nadeem, M.T.; Almalki, R.S.; Muhammad Sajid Arshad, M.S.; Suleria, H.A.R. Green Banana Resistant Starch: A Promising Potential as Functional Ingredient against Certain Maladies. Food Sci. Nutr. 2024, 12, 3787–3805. [Google Scholar] [CrossRef] [PubMed]
- Falcomer, A.L.; Riquette, R.F.R.; de Lima, B.R.; Ginani, V.C.; Zandonadi, R.P. Health Benefits of Green Banana Consumption: A Systematic Review. Nutrients 2019, 11, 1222. [Google Scholar] [CrossRef] [PubMed]
- Rochana, A.; Dhalika, T.; Budiman, A.; Kamil, K.A. Nutritional Value of a Banana Stem (Musa paradisiaca Val) of Anaerobic Fermentation Product Supplemented with Nitrogen, Sulphur and Phosphorus Sources. Pak. J. Nutri. 2017, 16, 5. [Google Scholar] [CrossRef]
- Arjin, C.; Souphannavong, C.; Sartsook, A.; Seel-audom, M.; Mekchay, S.; Sringarm, K. Efficiency of Fresh and Fermented Banana Stem in Low Protein Diet on Nutrient Digestibility, Productive Performance and Intestinal Morphology of Crossbred Pig ((Thai Native x Meishan) x Duroc)). Vet. Integr. Sci. 2021, 19, 51–64. [Google Scholar] [CrossRef]
- Dhema, M.; Nalley, W.M.; Aryanta, I.M.S.; Suryani, N.N.; Hine, T.M. Effects of Fermented Banana Stem as Corn Substitutes on Performance and Protein Utilization in Growing-Finishing Pigs. Int. J. Sci. Adv. 2022, 3, 645–650. [Google Scholar] [CrossRef]
- Lee, A.-Y.; Chen, C.-H.; Liou, J.-S.; Lin, Y.-C.; Hamada, M.; Wang, Y.-T.; Peng, L.L.; Chang, S.C.; Chen, C.C.; Lin, C.F.; et al. Micrococcus porci Sp. Nov., Isolated from Feces of Black Pig (Sus scrofa). Life 2022, 12, 1749. [Google Scholar] [CrossRef]
- Dong, B.; Lin, X.; Jing, X.; Hu, T.; Zhou, J.; Chen, J.; Xiao, L.; Wang, B.; Chen, Z.; Liu, J.; et al. A Bacterial Genome and Culture Collection of Gut Microbial in Weanling Piglet. Microbiol. Spectr. 2022, 10, e02417-21. [Google Scholar] [CrossRef]
- Liao, S.F.; Feng, J.; Fan, P.; Kristin Denryter, K. Swine Gastrointestinal Microbiota and the Effects of Dietary Amino Acids on Its Composition and Metabolism. Int. J. Mol. Sci. 2024, 25, 1237. [Google Scholar] [CrossRef]
- Yang, J.; Fan, Y.; Jin, R.; Peng, Y.; Chai, J.; Wei, X.; Zhao, Y.; Deng, F.; Zhao, J.; Ying Li, Y. Exploring the Intestinal Microbial Community of Lantang Pigs through Metagenome-Assembled Genomes and Carbohydrate Degradation Genes. Fermentation 2024, 10, 207. [Google Scholar] [CrossRef]
- Yang, J.; Chen, R.; Peng, Y.; Chai, J.; Li, Y.; Deng, F. The Role of Gut Archaea in the Pig Gut Microbiome: A Mini-Review. Front. Microbiol. 2023, 14, 1284603. [Google Scholar] [CrossRef]
- Rahman, R.; Fouhse, J.M.; Ju, T.; Fan, Y.; Marcolla, C.S.; Pieper, R.; Brook, R.K.; Willing, B.P. A Comparison of Wild Boar and Domestic Pig Microbiota Does Not Reveal a Loss of Microbial Species but an Increase in Alpha Diversity and Opportunistic Genera in Domestic Pigs. Microbiol. Spectr. 2024, 12, e0084324. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Leero, D.D.; Yin, C.; Yang, L.; Zhu, L.; Zhu, Z.; Jiang, L. Clostridium as Microbial Cell Factory to Enable the Sustainable Utilization of Three Generations of Feedstocks. Bioresour. Technol. 2022, 361, 127656. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Dai, Z.; Liu, N.; Ji, Y.; Chen, J.; Zhang, Y.; Yang, Y.; Li, J.; Wu, Z.; Wu, G. Dietary L-Tryptophan Modulates the Structural and Functional Composition of the Intestinal Microbiome in Weaned Piglets. Front. Microbiol. 2018, 9, 1736. [Google Scholar] [CrossRef] [PubMed]
- Bradley, P.H.; Pollard, K.S. Proteobacteria Explain Significant Functional Variability in the Human Gut Microbiome. Microbiome 2017, 5, 36. [Google Scholar] [CrossRef]
- Stamboulian, M.; Canderan, J.; Ye, Y. Metaproteomics as a Tool for Studying the Protein Landscape of Human-Gut Bacterial Species. PLoS Comput. Biol. 2022, 18, e1009397. [Google Scholar] [CrossRef]
- Blakeley-Ruiz, J.A.; Bartlett, A.; McMillan, A.S.; Awan, A.; Vanhoy, W.M.; Meyerhoffer, A.K.; Vintila, S.; Maier, J.L.; Richie, T.; Theriot, C.M.; et al. Dietary Protein Source Alters Gut Microbiota Composition and Function. ISME J. 2025, 19, wraf048. [Google Scholar] [CrossRef]


| Ingredient (%) | Control | 5% BAWS | 10% BAWS |
|---|---|---|---|
| Corn meal | 68.09 | 63.09 | 58.09 |
| Soybean meal, 44% | 21.94 | 21.89 | 21.91 |
| Banana agro-waste Silage BAWS | 0.00 | 5.00 | 10.00 |
| Wheat bran | 3.00 | 2.31 | 1.53 |
| Dicalcium phosphate | 0.31 | 0.31 | 0.33 |
| Ground limestone | 0.81 | 0.82 | 0.83 |
| Molasses | 3.00 | 3.00 | 2.70 |
| Salt | 0.30 | 0.30 | 0.30 |
| Soybean oil | 2.27 | 3.00 | 4.04 |
| Vitamin premix 1 | 0.10 | 0.10 | 0.10 |
| Mineral premix 2 | 0.15 | 0.15 | 0.15 |
| L-Lysine-HCl (78%) | 0.03 | 0.03 | 0.03 |
| Feed Analysis composition (dry matter basis) | |||
| ME, Kcal/kg | 3250.58 | 3250.19 | 3250.43 |
| Crude protein, % | 15.56 | 15.56 | 15.57 |
| Crude fiber, % | 1.95 | 1.88 | 1.80 |
| Ca, % | 0.68 | 0.69 | 0.68 |
| P, % | 0.52 | 0.53 | 0.51 |
| Lysine, % | 0.98 | 0.98 | 0.99 |
| Methionine, % | 0.31 | 0.31 | 0.31 |
| Threonine, % | 0.60 | 0.60 | 0.60 |
| Sulfur-containing AA % | 0.56 | 0.57 | 0.56 |
| Item/Analyse(%) | DM | CP | NDF | ADF | ADL | WSC | P | K | Ca | Mg | TOC | NH4-N (ppm) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Disqualified banana | 15.81 | 6.68 | 9.09 | 5.31 | 1.95 | 3.83 | 0.11 | 2.27 | 0.09 | 0.24 | 55.09 | 139.7 |
| Pseudostem | 23.70 | 6.10 | 37.43 | 25.00 | 4.65 | 17.23 | 0.11 | 2.20 | 0.83 | 0.93 | 51.85 | 158.1 |
| BAWS (whole mixture) | 23.69 | 12.43 | 28.08 | 15.24 | 2.33 | 1.40 | 0.53 | 1.40 | 0.16 | 0.38 | 54.79 | 1911.6 |
| Quality | pH | Acetic acid | Butyric acid | Lactic acid | Flieg’s score | |||||||
| BAWS | 4.33 ± 0.27 | 4.41 ± 0.26 | ND | 2.46 ± 0.69 | 54.0 ± 3.0 | |||||||
| Finishing Period (Day) | Control Group | 5% BAWS | 10% BAWS | p-Value |
|---|---|---|---|---|
| Body weight (kg) | ||||
| 0 | 78 | 78 | 78 | 0.999 |
| 21 | 90 | 90 | 89 | 0.9586 |
| 42 | 102 | 101 | 99 | 0.8188 |
| 70 | 117 | 116 | 113 | 0.6961 |
| Average daily weight gain (ADG) (kg) | ||||
| 0–21 | 0.57 | 0.56 | 0.52 | 0.599 |
| 21–42 | 0.54 a | 0.51 ab | 0.47 b | 0.0059 |
| 42–70 | 0.73 a | 0.72 a | 0.69 b | 0.0008 |
| 0–70 | 0.62 | 0.60 | 0.56 | 0.0613 |
| Average daily feed intake (ADFI) (kg) | ||||
| 0–21 | 2.40 a | 2.37 a | 2.31 b | 0.0002 |
| 21–42 | 2.84 b | 2.89 a | 2.90 a | 0.0017 |
| 42–70 | 2.91 | 2.90 | 2.92 | 0.4875 |
| 0–70 | 2.71 | 2.72 | 2.71 | 0.7852 |
| Feed conversion ratio (FCR) (ADFI/ADG) | ||||
| 0–21 | 3.97 | 5.01 | 4.48 | 0.662 |
| 21–42 | 5.29 b | 5.64 b | 6.19 a | 0.0007 |
| 42–70 | 3.99 b | 4.04 b | 4.29 a | 0.0006 |
| 0–70 | 4.43 | 4.59 | 4.88 | 0.1286 |
| Items | Control Group | 5% BAWS | 10% BAWS | p-Value |
|---|---|---|---|---|
| Carcass | ||||
| Live weight, kg | 115 | 114 | 112 | 0.7507 |
| Slaughter weight, kg | 99 | 98 | 95 | 0.7406 |
| Dressing % | 86 | 86 | 85 | 0.9222 |
| Carcass length, cm | 101.1 | 100.0 | 100.8 | 0.9027 |
| Back fat thickness, cm | 3.29 | 2.84 | 2.63 | 0.1950 |
| First rib fat | 3.99 | 3.61 | 3.82 | 0.6475 |
| Last rib fat | 3.29 | 2.70 | 2.30 | 0.1139 |
| Lumbar fat | 2.59 | 2.21 | 1.77 | 0.0638 |
| Meat quality | ||||
| Color score 1 | 3.7 | 3.5 | 3.7 | 0.5156 |
| Marbling score 2 | 2.7 | 2.6 | 2.7 | 0.9142 |
| Firmness score 3 | 3.1 | 3.4 | 3.1 | 0.4930 |
| Lumbar meat area 4 | 4.98 | 6.06 | 6.02 | 0.4832 |
| Senory score 5 | ||||
| Flavor | 3.23 | 3.20 | 3.22 | 0.9404 |
| Juiciness | 3.05 | 3.12 | 3.10 | 0.5089 |
| Tenderness | 3.44 | 3.37 | 3.15 | 0.2770 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chou, L.-S.; Lo, C.-Y.; Huang, C.-J.; Huang, H.-J.; Chang, S.-C.; Weng, B.B.-C.; Hsieh, C.-W. Growth Performance, Carcass Quality and Gut Microbiome of Finishing Stage Pigs Fed Formulated Protein-Energy Nutrients Balanced Diet with Banana Agro-Waste Silage. Life 2025, 15, 1033. https://doi.org/10.3390/life15071033
Chou L-S, Lo C-Y, Huang C-J, Huang H-J, Chang S-C, Weng BB-C, Hsieh C-W. Growth Performance, Carcass Quality and Gut Microbiome of Finishing Stage Pigs Fed Formulated Protein-Energy Nutrients Balanced Diet with Banana Agro-Waste Silage. Life. 2025; 15(7):1033. https://doi.org/10.3390/life15071033
Chicago/Turabian StyleChou, Lan-Szu, Chih-Yu Lo, Chien-Jui Huang, Hsien-Juang Huang, Shen-Chang Chang, Brian Bor-Chun Weng, and Chia-Wen Hsieh. 2025. "Growth Performance, Carcass Quality and Gut Microbiome of Finishing Stage Pigs Fed Formulated Protein-Energy Nutrients Balanced Diet with Banana Agro-Waste Silage" Life 15, no. 7: 1033. https://doi.org/10.3390/life15071033
APA StyleChou, L.-S., Lo, C.-Y., Huang, C.-J., Huang, H.-J., Chang, S.-C., Weng, B. B.-C., & Hsieh, C.-W. (2025). Growth Performance, Carcass Quality and Gut Microbiome of Finishing Stage Pigs Fed Formulated Protein-Energy Nutrients Balanced Diet with Banana Agro-Waste Silage. Life, 15(7), 1033. https://doi.org/10.3390/life15071033








