Next-Generation Approaches in Sports Medicine: The Role of Genetics, Omics, and Digital Health in Optimizing Athlete Performance and Longevity—A Narrative Review
Abstract
1. Introduction
2. Methods
2.1. Literature Search and Review Process
2.2. Selection Criteria and Data Extraction
2.3. Data Synthesis and Framework Development
2.4. Limitations
3. Results
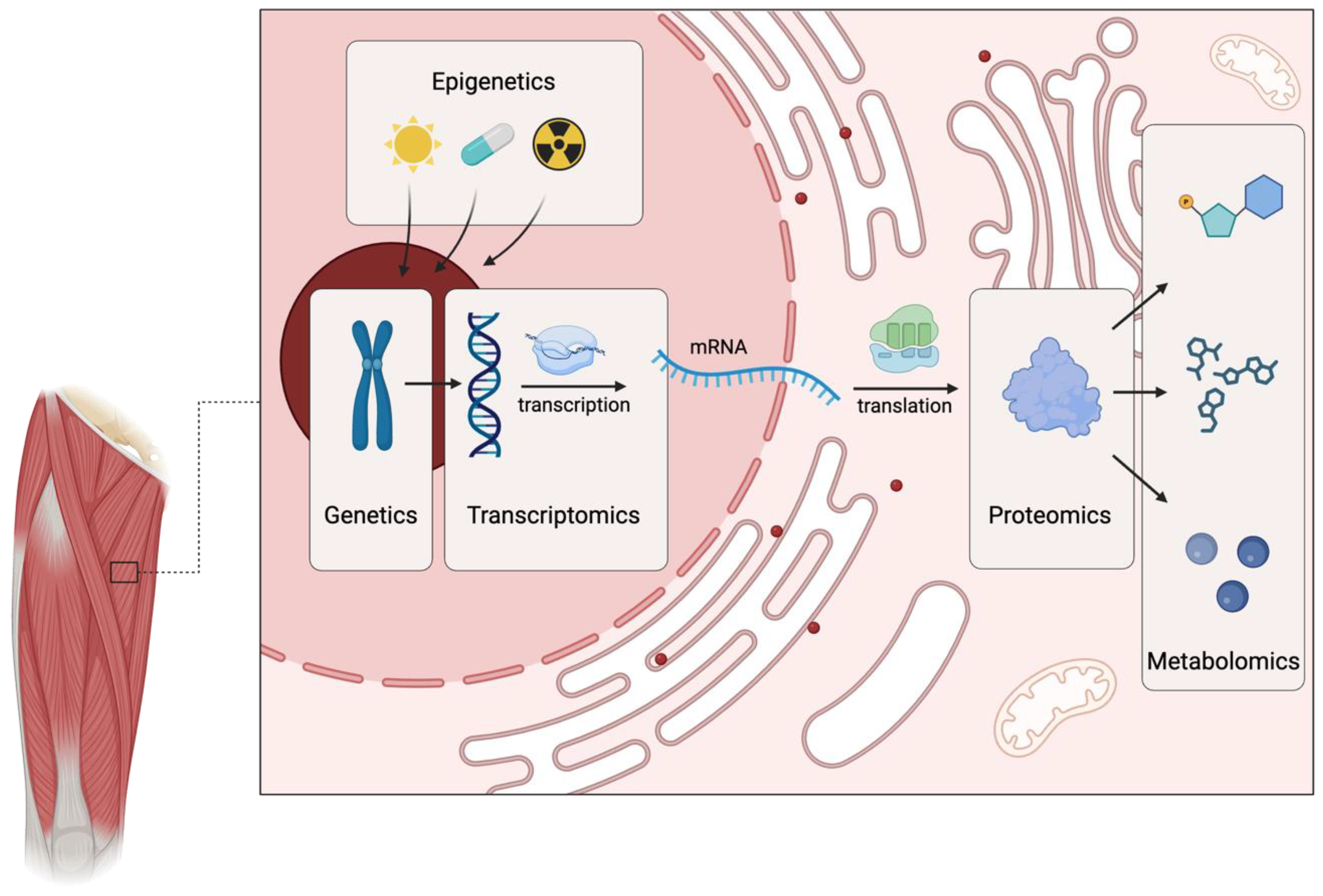
3.1. Genetics
3.2. Pharmacogenomics
3.3. Pain Management
3.4. Cardiovascular Conditions
3.5. Infectious Disease
- -
- Abacavir: Avoid in individuals with HLA-B*57:01 variant due to hypersensitivity risk. The FDA and EMA recommend testing before use in HIV patients [66].
- -
- Atazanavir: CPIC warns UGT1A1 PMs of increased jaundice risk, potentially leading to non-adherence. UGT1A1 PMs often have the Gilbert syndrome phenotype [67].
- -
- Efavirenz: Reduce dose to 200–400 mg/day for CYP2B6 PMs due to side effect risk; start at 400 mg/day for IMs [68].
3.6. Psychotropics: Antidepressants, Anti-Seizure Medications
3.7. Multi-Omics
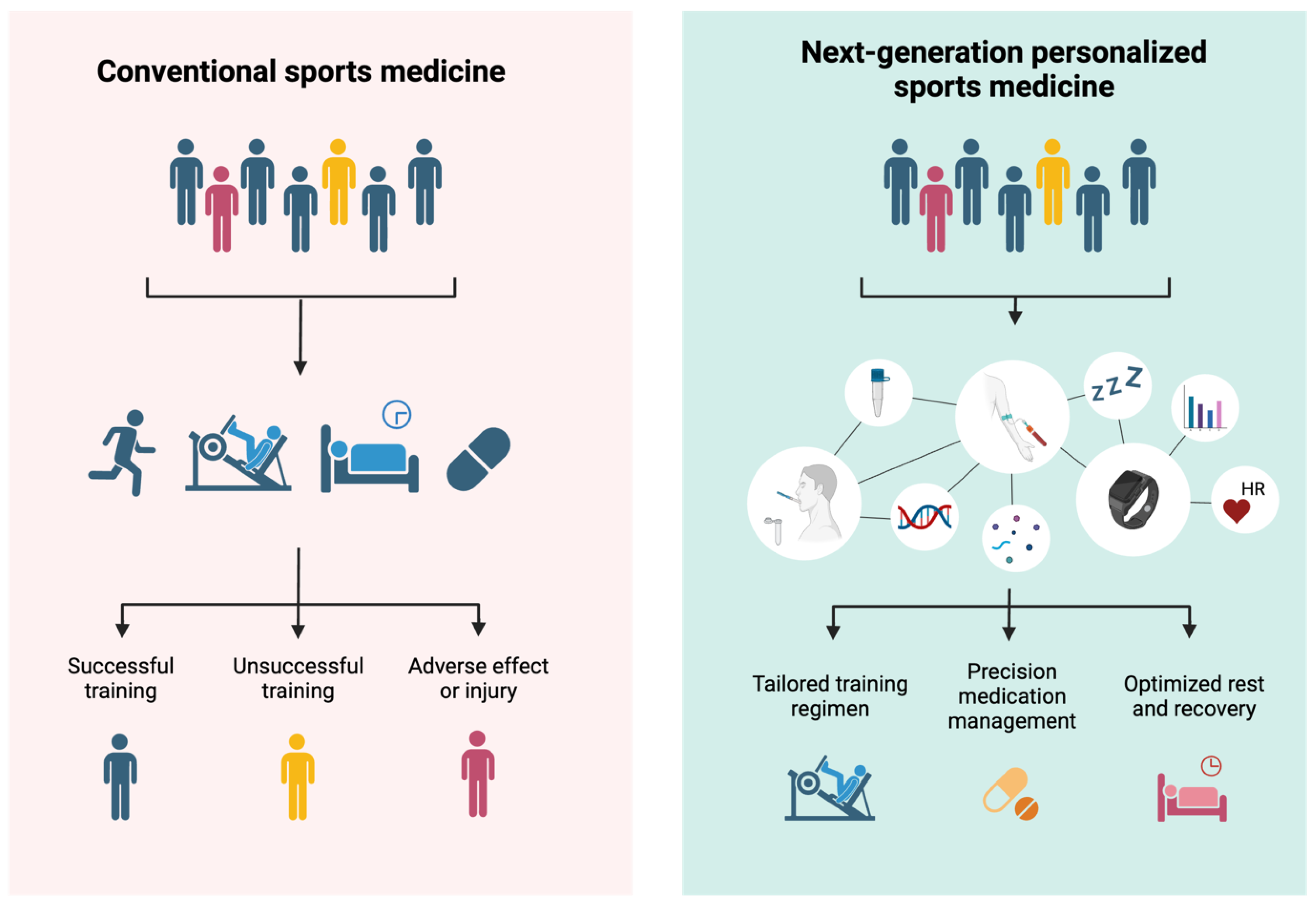
3.8. Digital Health
3.9. Wearable Sensors and Devices
3.10. Telemedicine
3.11. Future Perspectives on Enhancing Athlete Well-Being and Performance Through Digital Health
3.12. Proposed Framework and Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PGx | Pharmacogenomics |
| DH | Digital Health |
| SNP | Single Nucleotide Polymorphism |
| CYP | Cytochrome P450 |
| PM | Poor Metabolizer |
| IM | Intermediate Metabolizer |
| EM | Extensive (Normal) Metabolizer |
| RM | Rapid Metabolizer |
| UM | Ultra-Rapid Metabolizer |
| CPIC | Clinical Pharmacogenetics Implementation Consortium |
| DPWG | Dutch Pharmacogenetics Working Group |
| NSAIDs | Non-Steroidal Anti-Inflammatory Drugs |
| SSRIs | Selective Serotonin Reuptake Inhibitors |
| SNRIs | Serotonin-Norepinephrine Reuptake Inhibitors |
| TCA | Tricyclic Antidepressant |
| ECG | Electrocardiogram |
| EMG | Electromyography |
| AR | Augmented Reality |
| VO2max | Maximal Oxygen Uptake |
References
- Varillas-Delgado, D.; Del Coso, J.; Gutiérrez-Hellín, J.; Aguilar-Navarro, M.; Muñoz, A.; Maestro, A.; Morencos, E. Genetics and Sports Performance: The Present and Future in the Identification of Talent for Sports Based on DNA Testing. Eur. J. Appl. Physiol. 2022, 122, 1811–1830. [Google Scholar] [CrossRef] [PubMed]
- Semenova, E.A.; Hall, E.C.R.; Ahmetov, I.I. Genes and Athletic Performance: The 2023 Update. Genes 2023, 14, 1235. [Google Scholar] [CrossRef] [PubMed]
- Exel, J.; Dabnichki, P. Precision Sports Science: What Is Next for Data Analytics for Athlete Performance and Well-Being Optimization? Appl. Sci. 2024, 14, 3361. [Google Scholar] [CrossRef]
- Griswold, A.J.; Correa, D.; Kaplan, L.D.; Best, T.M. Using Genomic Techniques in Sports and Exercise Science: Current Status and Future Opportunities. Curr. Sports Med. Rep. 2021, 20, 617–623. [Google Scholar] [CrossRef]
- Hasin, Y.; Seldin, M.; Lusis, A. Multi-Omics Approaches to Disease. Genome Biol. 2017, 18, 83. [Google Scholar] [CrossRef]
- Bullock, G.; Collins, G.; Adams, R.; Thigpen, C.; Shanley, E. Personalized Injury Reduction Strategies in Sports Medicine: Lessons Learned from Advances in Breast Cancer Treatment: A Clinical Commentary. Int. J. Sports Phys. Ther. 2023, 18, 253–261. [Google Scholar] [CrossRef]
- Kim, H.; Song, K.H.; Kim, C.H. The ACTN3 R577X Variant in Sprint and Strength Performance. J. Exerc. Nutr. Biochem. 2014, 18, 347–353. [Google Scholar] [CrossRef]
- Lv, Z.T.; Gao, S.T.; Cheng, P.; Liang, S.; Yu, S.Y.; Yang, Q.; Chen, A.M. Association between polymorphism rs12722 in COL5A1 and musculoskeletal soft tissue injuries: A systematic review and meta-analysis. Oncotarget 2017, 9, 15365–15374. [Google Scholar] [CrossRef]
- Micaglio, E.; Locati, E.T.; Monasky, M.M.; Romani, F.; Heilbron, F.; Pappone, C.; Pappone, A. Role of Pharmacogenetics in Adverse Drug Reactions: An Update towards Personalized Medicine. Front. Pharmacol. 2021, 12, 651720. [Google Scholar] [CrossRef]
- Sadee, W.; Wang, D.; Hartmann, K.; Toland, A.E. Pharmacogenomics: Driving Personalized Medicine. Pharmacol. Rev. 2023, 75, 789–814. [Google Scholar] [CrossRef]
- Li, R.T.; Kling, S.R.; Salata, M.J.; Cupp, S.A.; Sheehan, J.; Voos, J.E. Wearable Performance Devices in Sports Medicine. Sports Health 2016, 8, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Seshadri, D.R.; Drummond, C.; Craker, J.; Rowbottom, J.R.; Voos, J.E. Wearable Devices for Sports: New Integrated Technologies Allow Coaches, Physicians, and Trainers to Better Understand the Physical Demands of Athletes in Real Time. IEEE Pulse 2017, 8, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Bongiovanni, T.; Lacome, M.; Fanos, V.; Martera, G.; Cione, E.; Cannataro, R. Metabolomics in Team-Sport Athletes: Current Knowledge, Challenges, and Future Perspectives. Proteomes 2022, 10, 27. [Google Scholar] [CrossRef] [PubMed]
- Muniz-Santos, R.; Magno-França, A.; Jurisica, I.; Cameron, L.C. From Microcosm to Macrocosm: The -Omics, Multiomics, and Sportomics Approaches in Exercise and Sports. OMICS 2023, 27, 499–518. [Google Scholar] [CrossRef]
- Hunter, S.K.; Senefeld, J.W. Sex Differences in Human Performance. J. Physiol. 2024, 602, 4129–4156. [Google Scholar] [CrossRef]
- Hunter, S.K.; Angadi, S.S.; Bhargava, A.; Harper, J.; Hirschberg, A.L.; Levine, B.D. The Biological Basis of Sex Differences in Athletic Performance: Consensus Statement for the American College of Sports Medicine. Med. Sci. Sports Exerc. 2023, 55, 2328–2360. [Google Scholar] [CrossRef]
- Pataky, M.W.; Dasari, S.; Michie, K.L.; Sevits, K.J.; Kumar, A.A.; Klaus, K.A.; Heppelmann, C.J.; Robinson, M.M.; Carter, R.E.; Lanza, I.R.; et al. Impact of Biological Sex and Sex Hormones on Molecular Signatures of Skeletal Muscle at Rest and in Response to Distinct Exercise Training Modes. Cell Metab. 2023, 35, 1996–2010.e6. [Google Scholar] [CrossRef]
- Soldin, O.P.; Mattison, D.R. Sex Differences in Pharmacokinetics and Pharmacodynamics. Clin. Pharmacokinet. 2009, 48, 143–157. [Google Scholar] [CrossRef]
- Ahmetov, I.I.; Fedotovskaya, O.N. Current progress in sports genomics. Adv. Clin. Chem. 2015, 70, 247–314. [Google Scholar] [CrossRef]
- Ahmetov, I.I.; Rogozkin, V.A. Genes, athlete status and training—An overview. Med. Sport Sci. 2009, 54, 43–71. [Google Scholar] [CrossRef]
- Guth, L.M.; Roth, S.M. Genetic influence on athletic performance. Curr. Opin. Pediatr. 2013, 25, 653–658. [Google Scholar] [CrossRef] [PubMed]
- Semenova, E.A.; Zempo, H.; Miyamoto-Mikami, E.; Kumagai, H.; Larin, A.K.; Sultanov, R.I.; Babalyan, K.A.; Zhelankin, A.V.; Tobina, T.; Shiose, K.; et al. Genome-wide association study identifies CDKN1A as a novel locus associated with muscle fiber composition. Cells 2022, 11, 3910. [Google Scholar] [CrossRef] [PubMed]
- Al-Khelaifi, F.; Yousri, N.A.; Diboun, I.; Semenova, E.A.; Kostryukova, E.S.; Kulemin, N.A.; Borisov, O.V.; Andryushchenko, L.B.; Larin, A.K.; Generozov, E.V.; et al. Genome-wide association study reveals a novel association between MYBPC3 gene polymorphism, endurance athlete status, aerobic capacity and steroid metabolism. Front. Genet. 2020, 11, 595. [Google Scholar] [CrossRef] [PubMed]
- Pickering, C.; Kiely, J.; Suraci, B.; Collins, D. A genome-wide association study of sprint performance in elite youth football players. J. Strength Cond. Res. 2019, 33, 2344–2351. [Google Scholar] [CrossRef]
- Díaz Ramírez, J.; Álvarez-Herms, J.; Castañeda-Babarro, A.; Larruskain, J.; Ramírez de la Piscina, X.; Borisov, O.V.; Semenova, E.A.; Kostryukova, E.S.; Kulemin, N.A.; Andryushchenko, O.N. The GALNTL6 gene rs558129 polymorphism is associated with power performance. J. Strength Cond. Res. 2020, 34, 3031–3036. [Google Scholar] [CrossRef]
- Walsh, S.; Zmuda, J.M.; Cauley, J.A.; Shea, P.R.; Metter, E.J.; Hurley, B.F.; Ferrell, R.E.; Roth, S.M. Androgen receptor CAG repeat polymorphism is associated with fat-free mass in men. J. Appl. Physiol. 2005, 98, 132–137. [Google Scholar] [CrossRef]
- Guilherme, J.P.L.F.; Shikhova, Y.V.; Dondukovskaya, R.R.; Topanova, A.A.; Semenova, E.A.; Astratenkova, I.V.; Ahmetov, I.I. Androgen receptor gene microsatellite polymorphism is associated with muscle mass and strength in bodybuilders and power athlete status. Ann. Hum. Biol. 2021, 48, 142–149. [Google Scholar] [CrossRef]
- Ginevičienė, V.; Jakaitienė, A.; Pranculis, A.; Milašius, K.; Tubelis, L.; Utkus, A. AMPD1 rs17602729 is associated with physical performance of sprint and power in elite Lithuanian athletes. BMC Genet. 2014, 15, 58. [Google Scholar] [CrossRef]
- Maestro, A.; Del Coso, J.; Aguilar-Navarro, M.; Gutiérrez-Hellín, J.; Morencos, E.; Revuelta, G.; Ruiz Casares, E.; Perucho, T.; Varillas-Delgado, D. Genetic profile in genes associated with muscle injuries and injury etiology in professional soccer players. Front. Genet. 2022, 13, 1035899. [Google Scholar] [CrossRef]
- Ahmetov, I.; Kulemin, N.; Popov, D.; Naumov, V.; Akimov, E.; Bravy, Y.; Egorova, E.; Galeeva, A.; Generozov, E.; Kostryukova, E.; et al. Genome-wide association study identifies three novel genetic markers associated with elite endurance performance. Biol. Sport 2015, 32, 3–9. [Google Scholar] [CrossRef]
- Maciejewska-Skrendo, A.; Mieszkowski, J.; Kochanowicz, A.; Niespodziński, B.; Cieszczyk, P.; Leźnicka, K.; Leońska-Duniec, A.; Kolbowicz, M.; Kaczmarczyk, M.; Piskorska, E.; et al. Does the PPARA intron 7 gene variant (rs4253778) influence performance in power/strength-oriented athletes? A case-control replication study in three cohorts of European gymnasts. J. Hum. Kinet. 2021, 79, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Varillas-Delgado, D. Role of the PPARGC1A gene and its rs8192678 polymorphism on sport performance, aerobic capacity, muscle adaptation and metabolic diseases: A narrative review. Genes 2024, 15, 1631. [Google Scholar] [CrossRef] [PubMed]
- Vincent, B.; De Bock, K.; Ramaekers, M.; Van den Eede, E.; Van Leemputte, M.; Hespel, P.; Thomis, M.A. ACTN3 (R577X) genotype is associated with fiber type distribution. Physiol. Genom. 2007, 32, 58–63. [Google Scholar] [CrossRef]
- Gómez-Gallego, F.; Ruiz, J.R.; Buxens, A.; Artieda, M.; Arteta, D.; Santiago, C.; Rodríguez-Romo, G.; Lao, J.I.; Lucia, A. The– 786 T/C polymorphism of the NOS3 gene is associated with elite performance in power sports. Eur. J. Appl. Physiol. 2009, 107, 565–569. [Google Scholar] [CrossRef]
- Collins, M.; Mokone, G.G.; September, A.V.; van der Merwe, L.; Schwellnus, M.P. The COL5A1 genotype is associated with range of motion measurements. Scand. J. Med. Sci. Sports 2009, 19, 803–810. [Google Scholar] [CrossRef]
- Appel, M.; Zentgraf, K.; Krüger, K.; Meesmann, M. Effects of genetic variation on endurance performance, muscle strength, and injury susceptibility in sports: A systematic review. Front. Physiol. 2021, 12, 694411. [Google Scholar] [CrossRef]
- Maffulli, N.; Margiotti, K.; Longo, U.G.; Loppini, M.; Fazio, V.M.; Denaro, V. The genetics of sports injuries and athletic performance. Muscles Ligaments Tendons J. 2013, 3, 173–189. [Google Scholar] [CrossRef]
- McAuley, A.B.T.; Hughes, D.C.; Tsaprouni, L.G.; Varley, I.; Suraci, B.; Roos, T.R.; Herbert, A.J.; Jackson, D.T.; Kelly, A.L. A systematic review of the genetic predisposition to injury in football. J. Sci. Sport Exerc. 2023, 5, 97–115. [Google Scholar] [CrossRef]
- McCabe, K.; Collins, C. Can genetics predict sports injury? The association of the genes GDF5, AMPD1, COL5A1 and IGF2 on soccer player injury occurrence. Sports 2018, 6, 21. [Google Scholar] [CrossRef]
- Posthumus, M.; September, A.V.; O’Cuinneagain, D.; van der Merwe, W.; Schwellnus, M.P.; Collins, M. The COL5A1 gene is associated with increased risk of anterior cruciate ligament ruptures in female participants. Am. J. Sports Med. 2009, 37, 2234–2240. [Google Scholar] [CrossRef]
- Ginevičienė, V.; Utkus, A.; Pranckevičienė, E.; Semenova, E.A.; Hall, E.C.R.; Ahmetov, I.I. Perspectives in sports genomics. Biomedicines 2022, 10, 298. [Google Scholar] [CrossRef] [PubMed]
- Bielinski, S.J.; Olson, J.E.; Pathak, J.; Weinshilboum, R.M.; Wang, L.; Lyke, K.J.; Ryu, E.; Targonski, P.V.; Van Norstrand, M.D.; Hathcock, M.A.; et al. Preemptive genotyping for personalized medicine: Design of the right drug, right dose, right time—Using genomic data to individualize treatment protocol. Mayo Clin. Proc. 2014, 89, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Yin, P.; Xu, Z. The genetic basis of sudden death in young people—Cardiac and non-cardiac. Gene 2022, 810, 146067. [Google Scholar] [CrossRef]
- Van Driest, S.L.; Shi, Y.; Bowton, E.A.; Schildcrout, J.S.; Peterson, J.F.; Pulley, J.; Denny, J.C.; Roden, D.M. Clinically actionable genotypes among 10,000 patients with preemptive pharmacogenomic testing. Clin. Pharmacol. Ther. 2014, 95, 423–431. [Google Scholar] [CrossRef]
- Lazarou, J.; Pomeranz, B.H.; Corey, P.N. Incidence of adverse drug reactions in hospitalized patients: A meta-analysis of prospective studies. JAMA 1998, 279, 1200–1205. [Google Scholar] [CrossRef]
- Weinshilboum, R.M.; Wang, L. Pharmacogenomics: Precision medicine and drug response. Mayo Clin. Proc. 2017, 92, 1711–1722. [Google Scholar] [CrossRef]
- Watson, S.; Caster, O.; Rochon, P.A.; Den Ruijter, H. Reported adverse drug reactions in women and men: Aggregated evidence from globally collected individual case reports during half a century. EClinicalMedicine 2019, 17, 100188. [Google Scholar] [CrossRef]
- Alaranta, A.; Alaranta, H.; Holmila, J.; Palmu, P.; Pietilä, K.; Helenius, I. Self-reported attitudes of elite athletes towards medication use: Differences between type of sport. Int. J. Sports Med. 2006, 27, 842–846. [Google Scholar] [CrossRef]
- Fayock, M.; Fayock, K.; Fayock, D. Common prescription medications used in athletes. Curr. Sports Med. Rep. 2014, 13, 365–370. [Google Scholar] [CrossRef]
- Stocco, G.; Lucafò, M.; Decorti, G. Pharmacogenomics of antibiotics. Int. J. Mol. Sci. 2020, 21, 5975. [Google Scholar] [CrossRef]
- Leyk, D.; Rüther, T.; Hartmann, N.; Vits, E.; Staudt, M.; Hoffmann, M.A. Analgesic Use in Sports. Dtsch. Arztebl. Int. 2023, 120, 155–161. [Google Scholar] [PubMed]
- Agel, J.; Arendt, E.A.; Bershadsky, B. Anterior cruciate ligament injury in National Collegiate Athletic Association basketball and soccer: A 13-year review. Am. J. Sports Med. 2005, 33, 524–530. [Google Scholar] [CrossRef] [PubMed]
- Suzic Lazic, J.; Dikic, N.; Radivojevic, N.; Mazic, S.; Radovanovic, D.; Mitrovic, N.; Lazic, M.; Zivanic, S.; Suzic, S. Dietary supplements and medications in elite sport—Polypharmacy or real need? Scand. J. Med. Sci. Sports 2011, 21, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Sheu, Y.H.; Chen, L.H.; Hedegaard, H. Sports- and recreation-related injury episodes in the United States, 2011–2014. Natl. Health Stat. Rep. 2016, 1–12. [Google Scholar]
- Ekhtiari, S.; Haghpanah, S.; Gholami, A.; MacDonald, A.; Leroux, T.; Khan, M. Opioid Use in Athletes: A Systematic Review. Sports Health 2020, 12, 534–539. [Google Scholar] [CrossRef]
- Theken, K.N.; Lee, C.R.; Gong, L.; Caudle, K.E.; Formea, C.M.; Gaedigk, A.; Klein, T.E.; Agúndez, J.A.G.; Grosser, T. Clinical Pharmacogenetics Implementation Consortium guideline (CPIC) for CYP2C9 and nonsteroidal anti-inflammatory drugs. Clin. Pharmacol. Ther. 2020, 108, 191–200. [Google Scholar] [CrossRef]
- Crews, K.R.; Gaedigk, A.; Dunnenberger, H.M.; Leeder, J.S.; Klein, T.E.; Caudle, K.E.; Haidar, C.E.; Shen, D.D.; Callaghan, J.T.; Sadhasivam, S.; et al. Clinical Pharmacogenetics Implementation Consortium guideline for cytochrome P450 2D6 genotype and codeine therapy: 2014 update. Clin. Pharmacol. Ther. 2014, 95, 376–382. [Google Scholar] [CrossRef]
- Rosenberg, H.; Pollock, N.; Schiemann, A.; Bulger, T.; Stowell, K. Malignant hyperthermia: A review. Orphanet J. Rare Dis. 2015, 10, 93. [Google Scholar] [CrossRef]
- Newman, W.; Parry-Williams, G.; Wiles, J.; Edwards, J.; Hulbert, S.; Kipourou, K.; Papadakis, M.; Sharma, R.; O’ Driscoll, J. Risk of atrial fibrillation in athletes: A systematic review and meta-analysis. Br. J. Sports Med. 2021, 55, 1233–1238. [Google Scholar] [CrossRef]
- Meyer, T.; Meister, S. Routine blood parameters in elite soccer players. Int. J. Sports Med. 2011, 32, 875–881. [Google Scholar] [CrossRef]
- Pasternak, R.C.; Smith, S.C., Jr.; Bairey-Merz, C.N.; Grundy, S.M.; Cleeman, J.I.; Lenfant, C. ACC/AHA/NHLBI clinical advisory on the use and safety of statins. Circulation 2002, 106, 1024–1028. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.R.; Luzum, J.A.; Sangkuhl, K.; Gammal, R.S.; Sabatine, M.S.; Stein, C.M.; Kisor, D.F.; Limdi, N.A.; Lee, Y.M.; Scott, S.A.; et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) guideline for CYP2C19 and clopidogrel therapy: 2022 update. Clin. Pharmacol. Ther. 2022, 112, 959–967. [Google Scholar] [CrossRef] [PubMed]
- Castaño-Amores, C.; Antúnez-Rodríguez, A.; Pozo-Agundo, A.; García-Rodríguez, S.; Martínez-González, L.J.; Dávila-Fajardo, C.L. Genetic polymorphisms in ADRB1, ADRB2 and CYP2D6 genes and response to beta-blockers in patients with acute coronary syndrome. Biomed. Pharmacother. 2023, 169, 115869. [Google Scholar] [CrossRef] [PubMed]
- ClinGen Aminoglycoside Induced Hearing Loss Clinical Domain Working Group. Clinical Pharmacogenetics Implementation Consortium (CPIC®) guideline for the use of aminoglycosides based on MT-RNR1 genotype. Clin. Pharmacol. Ther. 2022, 112, 727–735. [Google Scholar] [CrossRef]
- Moriyama, B.; Obeng, A.O.; Barbarino, J.; Penzak, S.R.; Henning, S.A.; Scott, S.A.; Agúndez, J.; Wingard, J.R.; McLeod, H.L.; Klein, T.E.; et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) Guidelines for CYP2C19 and Voriconazole Therapy. Clin. Pharmacol. Ther. 2017, 102, 45–51. [Google Scholar] [CrossRef]
- Mallal, S.; Phillips, E.; Carosi, G.; Molina, J.-M.; Workman, C.; Tomažič, J.; Jägel-Guedes, E.; Rugina, S.; Kozyrev, O.; Cid, J.F.; et al. HLA-B5701* screening for hypersensitivity to abacavir. N. Engl. J. Med. 2008, 358, 568–579. [Google Scholar] [CrossRef]
- Gammal, R.S.; Court, M.H.; Haidar, C.E.; Iwuchukwu, O.F.; Gaur, A.H.; Alvarellos, M.; Guillemette, C.; Lennox, J.L.; Whirl-Carrillo, M.; Brummel, S.S.; et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) guideline for UGT1A1 and atazanavir prescribing. Clin. Pharmacol. Ther. 2016, 99, 363–369. [Google Scholar] [CrossRef]
- Desta, Z.; Gammal, R.S.; Gong, L.; Whirl-Carrillo, M.; Gaur, A.H.; Sukasem, C.; Hockings, J.; Myers, A.; Swart, M.; Tyndale, R.F.; et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) Guideline for CYP2B6 and Efavirenz-Containing Antiretroviral Therapy. Clin. Pharmacol. Ther. 2019, 106, 726–733. [Google Scholar] [CrossRef]
- Hicks, J.K.; Sangkuhl, K.; Swen, J.J.; Müller, D.J.; Ji, Y.; Leckband, S.G.; Leeder, J.S.; Graham, R.L.; Chiulli, D.L.; Llerena, A.; et al. Clinical Pharmacogenetics Implementation Consortium guideline (CPIC) for CYP2D6 and CYP2C19 genotypes and dosing of selective serotonin reuptake inhibitors. Clin. Pharmacol. Ther. 2015, 98, 127–134. [Google Scholar] [CrossRef]
- Hicks, J.K.; Bishop, J.R.; Sangkuhl, K.; Ellingrod, V.L.; Müller, D.J.; Shimoda, K.; Bishop, J.R.; Kharasch, E.D.; Skaar, T.C.; Gaedigk, A.; et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) guideline for CYP2D6 and CYP2C19 genotypes and dosing of tricyclic antidepressants: 2016 update. Clin. Pharmacol. Ther. 2017, 102, 37–44. [Google Scholar] [CrossRef]
- Phillips, E.J.; Sukasem, C.; Whirl-Carrillo, M.; Müller, D.J.; Dunnenberger, H.M.; Chantratita, W.; Goldspiel, B.; Chen, Y.T.; Carleton, B.C.; George, A.L.; et al. Clinical Pharmacogenetics Implementation Consortium Guideline for HLA Genotype and Use of Carbamazepine and Oxcarbazepine: 2017 Update. Clin. Pharmacol. Ther. 2018, 103, 574–581. [Google Scholar] [CrossRef] [PubMed]
- Karnes, J.H.; Rettie, A.E.; Somogyi, A.A.; Huddart, R.; Fohner, A.E.; Formea, C.M.; Ta Michael Lee, M.; Llerena, A.; Whirl-Carrillo, M.; Klein, T.E.; et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) Guideline for CYP2C9 and HLA-B Genotypes and Phenytoin Dosing: 2020 Update. Clin. Pharmacol. Ther. 2021, 109, 302–309. [Google Scholar] [CrossRef]
- Lindholm, M.E.; Marabita, F.; Gomez-Cabrero, D.; Rundqvist, H.; Ekström, T.J.; Tegnér, J.; Sundberg, C.J. An integrative analysis reveals coordinated reprogramming of the epigenome and the transcriptome in human skeletal muscle after training. Epigenetics 2014, 9, 1557–1569. [Google Scholar] [CrossRef]
- Dickinson, J.M.; D’Lugos, A.C.; Naymik, M.A.; Siniard, A.L.; Wolfe, A.J.; Curtis, D.R.; Huentelman, M.J.; Carroll, C.C. Transcriptome response of human skeletal muscle to divergent exercise stimuli. J. Appl. Physiol. 2018, 124, 1529–1540. [Google Scholar] [CrossRef]
- Polakovičová, M.; Musil, P.; Laczo, E.; Hamar, D.; Kyselovič, J. Circulating MicroRNAs as Potential Biomarkers of Exercise Response. Int. J. Mol. Sci. 2016, 17, 1553. [Google Scholar] [CrossRef]
- Hoffman, N.J.; Parker, B.L.; Chaudhuri, R.; Fisher-Wellman, K.H.; Kleinert, M.; Humphrey, S.J.; Yang, P.; Holliday, M.; Trefely, S.; Fazakerley, D.J.; et al. Global phosphoproteomic analysis of human skeletal muscle reveals a network of exercise-regulated kinases and AMPK substrates. Cell Metab. 2015, 22, 922–935. [Google Scholar] [CrossRef]
- Holloway, T.M.; Bloemberg, D.; Quadrilatero, J. High-intensity interval and endurance training have opposing effects on markers of muscle fiber remodeling in human skeletal muscle. PLoS ONE 2018, 13, e0190320. [Google Scholar] [CrossRef]
- Khoramipour, K.; Sandbakk, Ø.; Keshteli, A.H.; Gaeini, A.A.; Wishart, D.S.; Chamari, K. Metabolomics in Exercise and Sports: A Systematic Review. Sports Med. 2022, 52, 547–583. [Google Scholar] [CrossRef]
- Robbins, J.M.; Peterson, B.; Schranner, D.; Tahir, U.A.; Rienmüller, T.; Deng, S.; Keyes, M.J.; Katz, D.H.; Beltran, P.M.J.; Barber, J.L.; et al. Human plasma proteomic profiles indicative of cardiorespiratory fitness. Nat. Metab. 2021, 3, 786–797. [Google Scholar] [CrossRef]
- Zierath, J.R.; Barrès, R. Epigenetic regulation in skeletal muscle in response to exercise and environmental changes. J. Exp. Biol. 2016, 219, 206–216. [Google Scholar] [CrossRef]
- Peffers, M.J.; Liu, X.; Clegg, P.D. Transcriptomic and metabolomic analysis of human Achilles tendinopathy. J. Orthop. Res. 2020, 38, 1300–1311. [Google Scholar] [CrossRef]
- Reitzner, S.M.; Emanuelsson, E.B.; Arif, M.; Kaczkowski, B.; Kwon, A.T.; Mardinoglu, A.; Arner, E.; Chapman, M.A.; Sundberg, C.J. Molecular profiling of high-level athlete skeletal muscle after acute endurance or resistance exercise—A systems biology approach. Mol. Metab. 2024, 79, 101857. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, N.J.; Parker, B.L.; Chaudhuri, R.; Fisher-Wellman, K.H.; Kleinert, M.; Humphrey, S.J.; Yang, P.; Trefely, S.; Fazakerley, D.J.; Stöckli, J.; et al. Global temporal analysis of skeletal muscle proteome after exercise reveals critical regulators of tissue repair and adaptation. Cell Metab. 2015, 22, 922–935. [Google Scholar] [CrossRef] [PubMed]
- Akazawa, N.; Kobayashi, N.; Nakamura, Y.; Kumagai, H.; Choi, Y.; Maeda, S. Effect of sleep efficiency on salivary metabolite profile and cognitive function during exercise in volleyball athletes. Eur. J. Appl. Physiol. 2019, 119, 2215–2223. [Google Scholar] [CrossRef]
- Depner, C.M.; Melanson, E.L.; McHill, A.W.; Wright, K.P., Jr. Mistimed food intake and sleep alters 24-hour time-of-day patterns of the human plasma proteome. Proc. Natl. Acad. Sci. USA 2018, 115, E5390–E5399. [Google Scholar] [CrossRef]
- Peake, J.M.; Kerr, G.; Sullivan, J.P. A critical review of consumer wearables, mobile applications, and equipment for providing biofeedback, monitoring stress, and sleep in physically active populations. Front. Physiol. 2018, 9, 743. [Google Scholar] [CrossRef]
- Pegoraro, N.; Rossini, B.; Giganti, M.; Brymer, E.; Monasterio, E.; Bouchat, P.; Feletti, F. Telemedicine in Sports under Extreme Conditions: Data Transmission, Remote Medical Consultations, and Diagnostic Imaging. Int. J. Environ. Res. Public Health 2023, 20, 6371. [Google Scholar] [CrossRef]
- Sperlich, B.; Aminian, K.; Düking, P.; Holmberg, H.C. Editorial: Wearable Sensor Technology for Monitoring Training Load and Health in the Athletic Population. Front. Physiol. 2020, 10, 1520. [Google Scholar] [CrossRef]
- Channa, A.; Popescu, N.; Skibinska, J.; Burget, R. The rise of wearable devices during the COVID-19 pandemic: A systematic review. Sensors 2021, 21, 5787. [Google Scholar] [CrossRef]
- Gao, W.; Emaminejad, S.; Nyein, H.Y.Y.; Challa, S.; Chen, K.; Peck, A.; Fahad, H.M.; Ota, H.; Shiraki, H.; Kiriya, D.; et al. Fully integrated wearable sensor arrays for multiplexed in situ perspiration analysis. Nature 2016, 529, 509–514. [Google Scholar] [CrossRef]
- Ferrari, M.; Mottola, L.; Quaresima, V. Principles, techniques, and limitations of near-infrared spectroscopy. Can. J. Appl. Physiol. 2004, 29, 463–487. [Google Scholar] [CrossRef] [PubMed]
- Heikenfeld, J.; Jajack, A.; Rogers, J.; Gutruf, P.; Tian, L.; Pan, T.; Li, R.; Khine, M.; Kim, J.; Wang, J.; et al. Wearable sensors: Modalities, challenges, and prospects. Lab Chip 2018, 18, 217–248. [Google Scholar] [CrossRef]
- Seshadri, D.R.; Li, R.T.; Voos, J.E.; Rowbottom, J.R.; Alfes, C.M.; Zorman, C.A.; Drummond, C.K. Wearable sensors for monitoring the internal and external workload of the athlete. NPJ Digit. Med. 2019, 2, 71. [Google Scholar] [CrossRef] [PubMed]
- Giggins, O.M.; Sweeney, K.T.; Caulfield, B. Rehabilitation exercise assessment using inertial sensors: A cross-sectional analytical study. J. Neuroeng. Rehabil. 2014, 11, 158. [Google Scholar] [CrossRef]
- Miller, D.J.; Lastella, M.; Scanlan, A.T.; Bellenger, C.; Halson, S.L.; Roach, G.D.; Sargent, C. A validation study of the WHOOP strap against polysomnography to assess sleep. J. Sports Sci. 2020, 38, 2631–2636. [Google Scholar] [CrossRef]
- Svensson, T.; Madhawa, K.; Nt, H.; Chung, U.I.; Svensson, A.K. Validity and reliability of the Oura Ring Generation 3 (Gen3) with Oura sleep staging algorithm 2.0 (OSSA 2.0) when compared to multi-night ambulatory polysomnography: A validation study of 96 participants and 421,045 epochs. Sleep Med. 2024, 115, 251–263. [Google Scholar] [CrossRef]
- Cook, J.D.; Charest, J. Sleep and Performance in Professional Athletes. Curr. Sleep Med. Rep. 2023, 9, 56–81. [Google Scholar] [CrossRef]
- Walsh, N.P.; Halson, S.L.; Sargent, C.; Roach, G.D.; Nédélec, M.; Gupta, L.; Leeder, J.; Fullagar, H.H.; Coutts, A.J.; Edwards, B.J.; et al. Sleep and the athlete: Narrative review and 2021 expert consensus recommendations. Br. J. Sports Med. 2021, 55, 356–368. [Google Scholar] [CrossRef]
- Gabbett, T.J. The training—Injury prevention paradox: Should athletes be training smarter and harder? Br. J. Sports Med. 2016, 50, 273–280. [Google Scholar] [CrossRef]
- Hulin, B.T.; Gabbett, T.J.; Lawson, D.W.; Caputi, P.; Sampson, J.A. The acute:chronic workload ratio predicts injury: High chronic workload may decrease injury risk in elite rugby league players. Br. J. Sports Med. 2016, 50, 231–236. [Google Scholar] [CrossRef]
- Ekstrand, J.; Hägglund, M.; Waldén, M. Injury incidence and injury patterns in professional football: The UEFA injury study. Br. J. Sports Med. 2011, 45, 553–558. [Google Scholar] [CrossRef]
- WHOOP, Inc. WHOOP Performance Optimization System Goes on Sale to Consumers July 18. Available online: https://www.whoop.com/us/en/press-center/whoop-performance-optimization-system-goes-on-sale-to-consumers-july-18/ (accessed on 21 April 2025).
- O’Driscoll, R.; Turicchi, J.; Hopkins, M.; Horgan, G.W.; Finlayson, G.; Stubbs, J.R. Improving energy expenditure estimates from wearable devices: A machine learning approach. J. Sports Sci. 2020, 38, 1496–1505. [Google Scholar] [CrossRef] [PubMed]
- Ezeamii, V.C.; Okobi, O.E.; Wambai-Sani, H.; Perera, G.S.; Zaynieva, S.; Okonkwo, C.C.; Ohaiba, M.M.; William-Enemali, P.C.; Obodo, O.R.; Obiefuna, N.G. Revolutionizing Healthcare: How Telemedicine Is Improving Patient Outcomes and Expanding Access to Care. Cureus 2024, 16, e63881. [Google Scholar] [CrossRef] [PubMed]
- Dijkstra, H.P.; Pollock, N.; Chakraverty, R.; Alonso, J.M. Managing the health of the elite athlete: A new integrated performance health management and coaching model. Br. J. Sports Med. 2014, 48, 523–531. [Google Scholar] [CrossRef] [PubMed]
- Orlando, J.F.; Beard, M.; Kumar, S. Systematic review of patient and caregivers’ satisfaction with telehealth videoconferencing as a mode of service delivery in managing patients’ health. PLoS ONE 2019, 14, e0221848. [Google Scholar] [CrossRef]
- Dellaserra, C.L.; Gao, Y.; Ransdell, L. Use of Integrated Technology in Team Sports: A Review of Opportunities, Challenges, and Future Directions for Athletes. J. Strength Cond. Res. 2014, 28, 556–573. [Google Scholar] [CrossRef]
- Thakkar, A.; Gupta, A.; De Sousa, A. Artificial intelligence in positive mental health: A narrative review. Front. Digit. Health 2024, 6, 1280235. [Google Scholar] [CrossRef]
| Genetic Marker | Associated Role |
|---|---|
| AMPD1 rs17602729 C |
|
| CDKN1A rs236448 A | |
| CKM rs8111989 | |
| MYBPC3 rs1052373 G |
|
| ACE rs4646994 D |
|
| NFIA-AS2 rs1572312 C | |
| PPARA rs4253778 G | |
| PPARGC1A rs8192678 G |
|
| ACTN3 rs1815739 C |
|
| CPNE5 rs3213537 G |
|
| GALNTL6 rs558129 T |
|
| NOS3 rs2070744 T |
|
| AR ≥ 21 CAG repeats | |
| COL5A1 rs12722 T |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Juginović, A.; Kekić, A.; Aranza, I.; Biloš, V.; Armanda, M. Next-Generation Approaches in Sports Medicine: The Role of Genetics, Omics, and Digital Health in Optimizing Athlete Performance and Longevity—A Narrative Review. Life 2025, 15, 1023. https://doi.org/10.3390/life15071023
Juginović A, Kekić A, Aranza I, Biloš V, Armanda M. Next-Generation Approaches in Sports Medicine: The Role of Genetics, Omics, and Digital Health in Optimizing Athlete Performance and Longevity—A Narrative Review. Life. 2025; 15(7):1023. https://doi.org/10.3390/life15071023
Chicago/Turabian StyleJuginović, Alen, Adrijana Kekić, Ivan Aranza, Valentina Biloš, and Mirko Armanda. 2025. "Next-Generation Approaches in Sports Medicine: The Role of Genetics, Omics, and Digital Health in Optimizing Athlete Performance and Longevity—A Narrative Review" Life 15, no. 7: 1023. https://doi.org/10.3390/life15071023
APA StyleJuginović, A., Kekić, A., Aranza, I., Biloš, V., & Armanda, M. (2025). Next-Generation Approaches in Sports Medicine: The Role of Genetics, Omics, and Digital Health in Optimizing Athlete Performance and Longevity—A Narrative Review. Life, 15(7), 1023. https://doi.org/10.3390/life15071023






