Optimal Low-Frequency Parameter of Percutaneous Electrical Nerve Stimulation in Patients with Lower Back Pain: A Pilot Study
Abstract
1. Introduction
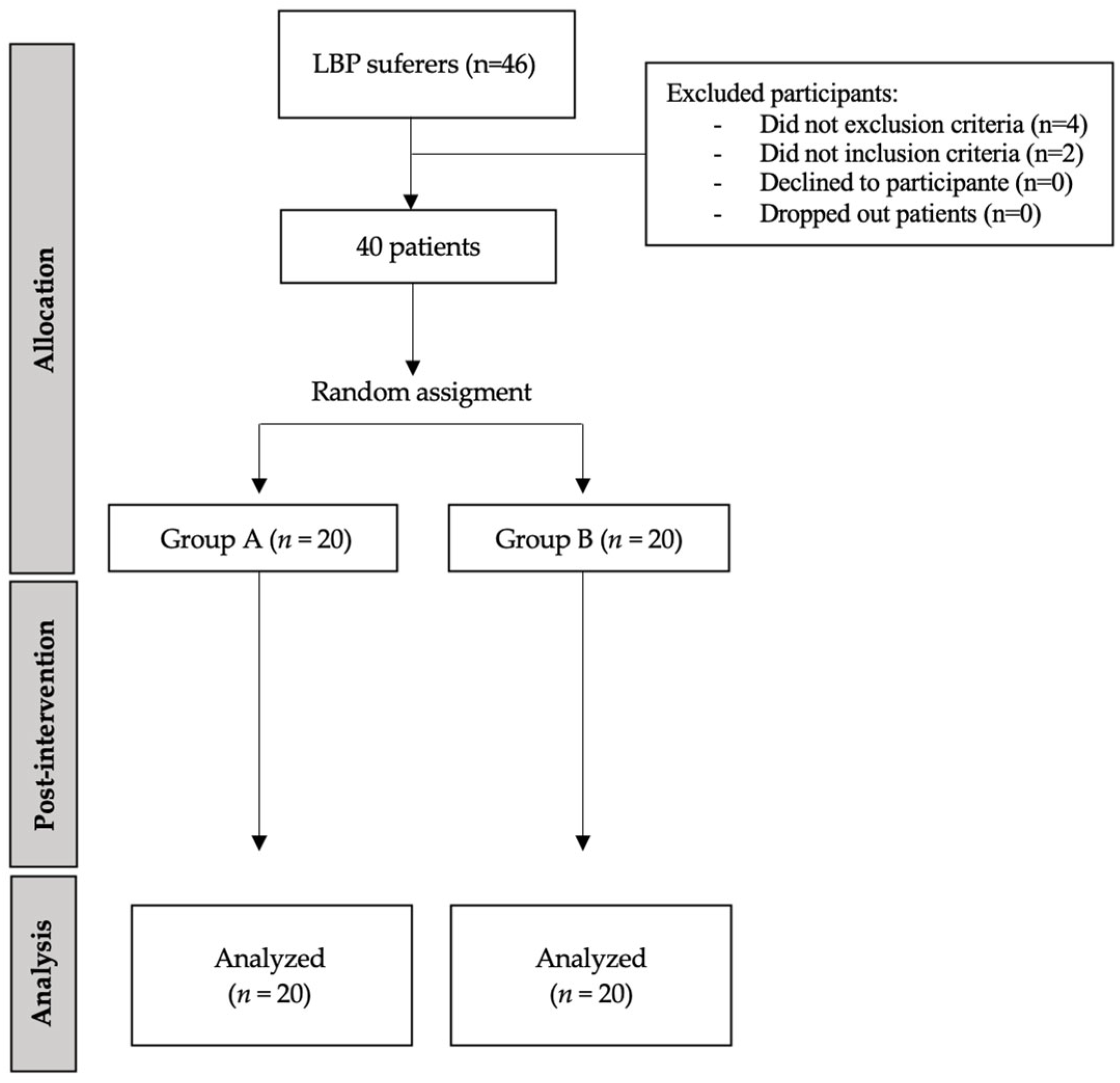
2. Materials and Methods
2.1. Design and Institutional Review Board
2.2. Sample Size Calculation
2.3. Participants
2.4. Outcome Measures
- -
- Pain intensity in the lumbar region was measured using the numerical rating scale (NRS).
- -
- Disability was assessed using the Oswestry Disability Index (ODI) [36], a validated 10-item questionnaire evaluating the impact of LBP on daily life. ODI scores are categorized as minimal (0–20), moderate (21–40), severe (41–60), crippled (61–80), or bed-bound/exaggerated symptoms (81–100).
- -
- Dynamic balance was assessed using the Y Balance Test (YBT), evaluating the anterior (ANT), posteromedial (PM), and posterolateral (PL) reach distances [37,38]. The values were recorded bilaterally in centimeters. Patients with chronic LBP typically present with reductions in the PM and PL reach directions [38].
- -
- Passive hip internal rotation range of motion (IR-ROM) was assessed bilaterally using a universal goniometer [39]. The participants were placed prone with their knees flexed to 90°, hips in a neutral position, and pelvis stabilized with a belt. Two experienced raters performed the measurements following standardized procedures. Only internal rotation was assessed due to its documented relevance to LBP [40].
- -
- Hip isometric muscle strength was evaluated for abduction, internal rotation, external rotation, flexion, and extension using a hand-held dynamometer (HHD) (Power Track II Commander; JTECH Medical, USA) [41]. Positions were selected based on clinical standards [42]: prone for extension, sitting for rotation, and supine for flexion and abduction. The participants were familiarized with submaximal isometric contractions prior to testing.
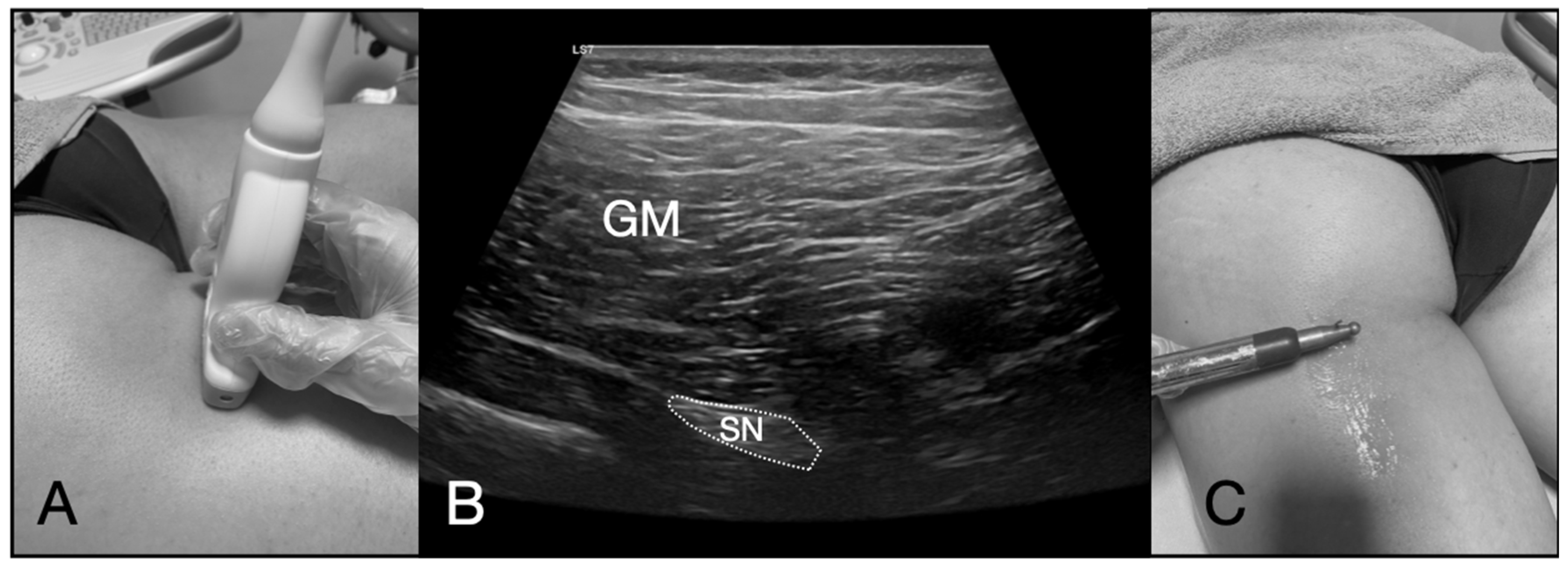
2.5. Ultrasound-Guided PNM Intervention
2.6. Data Analysis
3. Results
4. Discussion
4.1. Clinical Applications
4.2. Limitations and Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Scott, N.; Moga, C.; Harstall, C. Managing low back pain in the primary care setting: The know-do gap. Pain Res. Manag. 2010, 15, 392–400. [Google Scholar] [CrossRef]
- Hoy, D.; Bain, C.; Williams, G.; March, L.; Brooks, P.; Blyth, F.; Woolf, A.; Vos, T.; Buchbinder, R. A systematic review of the global prevalence of low back pain. Arthritis Rheum. 2012, 64, 2028–2037. [Google Scholar] [CrossRef]
- Popescu, A.; Lee, H. Neck pain and lower back pain. Med. Clin. N. Am. 2020, 104, 279–292. [Google Scholar] [CrossRef]
- Baron, R.; Binder, A.; Attal, N.; Casale, R.; Dickenson, A.H.; Treede, R.D. Neuropathic low back pain in clinical practice. Eur. J. Pain 2016, 20, 861–873. [Google Scholar] [CrossRef] [PubMed]
- Brinjikji, W.; Luetmer, P.H.; Comstock, B.; Bresnahan, B.; Chen, L.; Deyo, R.; Halabi, S.; Turner, J.; Avins, A.; James, K.; et al. Systematic literature review of imaging features of spinal degeneration in asymptomatic populations. AJNR Am. J. Neuroradiol. 2015, 36, 811–816. [Google Scholar] [CrossRef]
- Quintner, J.L.; Bove, G.M.; Cohen, M.L. A critical evaluation of the trigger point phenomeno. Rheumatology 2015, 54, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Wall, P.; Sweet, W. Temporary abolition of pain in man. Science 1967, 155, 108–109. [Google Scholar] [CrossRef] [PubMed]
- Gilmore, C.A.; Kapural, L.; McGee, M.J.; Boggs, J.W. Percutaneous peripheral nerve stimulation for the treatment of chronic low back pain provides sustained relief. Neuromodulation 2019, 22, 615–620. [Google Scholar] [CrossRef]
- Cohen, S.; Gilmore, C.; Kapural, L.; Hanling, S.; Plunkett, A.; McGee, M.; Boggs, J. Percutaneous peripheral nerve stimulation for pain reduction and improvements in functional outcomes in chronic low back pain. Mil. Med. 2019, 184 (Suppl. 1), 537–541. [Google Scholar] [CrossRef]
- Kumar, K.; Rizvi, S. Historical and present state of neuromodulation in chronic pain. Curr. Pain Headache Rep. 2014, 18, 387. [Google Scholar] [CrossRef]
- De Groot, J.; Zhou, S.; Carlton, S. Peripheral glutamate release in the hindpaw following low and high intensity sciatic stimulation. Neuroreport 2000, 2, 497–502. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.I.; Walsh, D.M. Transcutaneous electrical nerve stimulation: Mechanisms and clinical application. Phys. Ther. Rev. 2010, 15, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Han, J.S. Acupuncture and endorphins. Neurosci. Lett. 2004, 361, 258–261. [Google Scholar] [CrossRef]
- Valera-Garrido, F.; Minaya-Muñoz, F. Fisioterapia Invasiva; Elsevier: Barcelona, Spain, 2016. [Google Scholar]
- San-Emeterio-Iglesias, R.; Minaya-Muñoz, F.; Romero-Morales, C.; De-la-Cruz-Torres, B. Correct sciatic nerve management to apply ultrasound-guided percutaneous neuromodulation in patients with chronic low back pain: A pilot study. Neuromodulation 2021, 24, 1067–1074. [Google Scholar] [CrossRef]
- San-Emeterio-Iglesias, R.; De-la-Cruz-Torres, B.; Romero-Morales, C.; Minaya-Muñoz, F. Effect of ultrasound-guided percutaneous neuromodulation of sciatic nerve on hip muscle strength in chronic low back pain sufferers: A pilot study. J. Clin. Med. 2022, 11, 6672. [Google Scholar] [CrossRef]
- De-la-Cruz-Torres, B.; Abuín-Porras, V.; Navarro-Flores, E.; Calvo-Lobo, C.; Romero-Morales, C. Ultrasound-guided percutaneous neuromodulation in patients with chronic lateral epicondylalgia: A pilot randomized clinical trial. Int. J. Environ. Res. Public Health 2021, 18, 4877. [Google Scholar] [CrossRef] [PubMed]
- García-Bermejo, P.; De-la-Cruz-Torres, B.; Romero-Morales, C. Ultrasound-guided percutaneous neuromodulation in patients with unilateral anterior knee pain: A randomised clinical trial. Appl. Sci. 2020, 10, 4647. [Google Scholar] [CrossRef]
- De-la-Cruz-Torres, B.; Carrasco-Iglesias, C.; Minaya-Muñoz, F.; Romero-Morales, C. Crossover effects of ultrasound-guided percutaneous neuromodulation on contralateral hamstring flexibility. Acupunct. Med. 2021, 39, 512–521. [Google Scholar] [CrossRef]
- De-la-Cruz-Torres, B.; Barrera-García-Martín, I.; Albornoz-Cabello, M. Immediate effects of ultrasound-guided percutaneous neuromodulation versus physical exercise on performance of the flexor hallucis longus muscle in professional dancers: A randomised clinical trial. Acupunct. Med. 2019, 37, 91–97. [Google Scholar] [CrossRef]
- De-la-Cruz-Torres, B.; Barrera-García-Martín, I.; Romero-Morales, C. Comparative effects of one-shot electrical stimulation on performance of the flexor hallucis longus muscle in professional dancers: Percutaneous versus transcutaneous? Neuromodulation 2020, 23, 865–870. [Google Scholar] [CrossRef]
- Gallego-Sendarrubias, G.M.; Arias-Buría, J.L.; Úbeda-D’Ocasar, E.; Hervás-Pérez, J.P.; Rubio-Palomino, M.A.; Fernández-de-las-Peñas, C.; Valera-Calero, J.A. Effects of percutaneous electrical nerve stimulation on countermovement jump and squat performance speed in male soccer players: A pilot randomized clinical trial. J. Clin. Med. 2021, 10, 690. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Lao, L.; Ren, K.; Berman, B.M. Mechanisms of acupuncture-electroacupuncture on persistent pain. Anesthesiology 2014, 120, 482–503. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, J.V.L.; Calvo-Lobo, C.; Zugasti, A.M.; Fernandez-Carnero, J.; Beltran Alacreu, H. Effectiveness of dry needling with percutaneous electrical nerve stimulation of high frequency versus low frequency in patients with myofascial neck pain. Pain Physician 2021, 24, 135–143. [Google Scholar] [PubMed]
- Fernández-de-Las-Peñas, C.; Arias-Buría, J.L.; El Bachiri, Y.R.; Plaza-Manzano, G.; Cleland, J.A. Ultrasound-guided percutaneous electrical stimulation for a patient with cubital tunnel syndrome: A case report with a one-year follow-up. Physiother. Theory Pract. 2022, 38, 1564–1569. [Google Scholar] [CrossRef]
- D’Souza, R.S.; Jin, M.Y.; Abd-Elsayed, A. Peripheral nerve stimulation for low back pain: A systematic review. Curr. Pain Headache Rep. 2023, 27, 117–128. [Google Scholar] [CrossRef]
- Han, J.S.; Terenius, L. Neurochemical basis of acupuncture analgesia. Annu. Rev. Pharmacol. Toxicol. 1982, 22, 193–220. [Google Scholar] [CrossRef]
- Han, J.S. Acupuncture: Neuropeptide release produced by electrical stimulation of different frequencies. Trends Neurosci. 2003, 26, 17–22. [Google Scholar] [CrossRef]
- Carmona, L.; Ballina, J.; Gabriel, R.; Laffon, A. The burden of musculoskeletal diseases in the general population of Spain: Results from a national survey. Ann. Rheum. Dis. 2001, 60, 1040–1045. [Google Scholar] [CrossRef]
- Deyo, R.A.; Dworkin, S.F.; Amtmann, D.; Andersson, G.; Borenstein, D.; Carragee, E.; Carrino, J.; Chou, R.; Cook, K.; Delitto, A.; et al. Report of the NIH task force on research standards for chronic low back pain. Spine J. 2014, 14, 1375–1391. [Google Scholar] [CrossRef]
- Grande-Alonso, M.; Suso-Martí, L.; Cuenca-Martínez, F.; Pardo-Montero, J.; Gil-Martínez, A.; La Touche, R. Physiotherapy based on a biobehavioral approach with or without orthopedic manual physical therapy in the treatment of nonspecific chronic low back pain: A randomized controlled trial. Pain Med. 2019, 20, 2571–2587. [Google Scholar] [CrossRef]
- Avman, M.A.; Osmotherly, P.G.; Snodgrass, S.; Rivett, D.A. Is there an association between hip range of motion and nonspecific low back pain? A systematic review. Musculoskelet. Sci. Pract. 2019, 42, 38–51. [Google Scholar] [CrossRef] [PubMed]
- Saltychev, M.; Mattie, R.; Laimi, K. Hip biomechanics in patients with low back pain: What do we know? BMC Musculoskelet. Disord. 2024, 24, 74. [Google Scholar]
- Scholtes, S.A.; Sahrmann, S.A. Hip rotation range of motion in people with and without low back pain who participate in rotation-related sports. Phys. Ther. Sport. 2008, 9, 72–81. [Google Scholar]
- Chen, T.; Romero, J.; Guzmán, J. The association of hip range of movement, and its side-to-side asymmetry, with non-specific low back pain: A systematic review. NASS Open Access 2025, 1, 1–10. [Google Scholar]
- Fairbank, J.C.; Pynsent, P.B. The Oswestry disability index. Spine 2000, 25, 2940–2952. [Google Scholar] [CrossRef]
- Teyhen, D.S.; Shaffer, S.W.; Lorenson, C.L.; Greenberg, M.D.; Rogers, S.M.; Koreerat, C.M.; Villena, S.L.; Zosel, K.L.; Walker, M.J.; Childs, J.C. Clinical measures associated with dynamic balance and functional movement. J. Strength Cond. Res. 2014, 28, 1272–1283. [Google Scholar] [CrossRef]
- Hooper, T.L.; James, C.R.; Brismée, J.M.; Rogers, T.J.; Gilbert, K.K.; Browne, K.L.; Sizer, P.S. Dynamic balance as measured by the Y-Balance Test is reduced in individuals with low back pain: A cross-sectional comparative study. Phys. Ther. Sport 2016, 22, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Lea, R.D.; Gerhardt, J.J. Range-of-motion measurements. J. Bone Joint Surg. Am. 1995, 77, 784–798. [Google Scholar] [CrossRef] [PubMed]
- Sadeghisani, M.; Manshadi, F.D.; Kalantari, K.K.; Rahimi, A.; Namnik, N.; Karimi, M.T.; Oskouei, A.E. Correlation between hip rotation range-of-motion impairment and low back pain: A literature review. Ortop. Traumatol. Rehabil. 2015, 17, 455–462. [Google Scholar] [CrossRef]
- Thorborg, K.; Petersen, J.; Magnusson, S.P.; Hölmich, P. Clinical assessment of hip strength using a hand-held dynamometer is reliable. Scand. J. Med. Sci. Sports 2010, 20, 493–501. [Google Scholar] [CrossRef]
- Pua, Y.H.; Wrigley, T.V.; Cowan, S.M.; Bennell, K.L. Intrarater test-retest reliability of hip range of motion and hip muscle strength measurements in persons with hip osteoarthritis. Arch. Phys. Med. Rehabil. 2008, 89, 1146–1154. [Google Scholar] [CrossRef] [PubMed]
- Maughan, E.F.; Lewis, J.S. Outcome measures in chronic low back pain. Eur. Spine J. 2010, 19, 1484–1494. [Google Scholar] [CrossRef] [PubMed]
| Variable | Group A (3 Hz) | Group B (10 Hz) | p-Value |
|---|---|---|---|
| Age (years) | 42.4 ± 12.1 | 41.3 ± 10.9 | >0.05 |
| Weight (kg) | 73.3 ± 13.2 | 68.9 ± 12.6 | >0.05 |
| Height (m) | 1.72 ± 0.09 | 1.69 ± 0.08 | >0.05 |
| Body mass index (kg/m2) | 24.6 ± 3.24 | 23.9 ± 3.08 | >0.05 |
| Sex (female/male) | 6/14 | 12/8 | — |
| Dominant limb (right/left) | 17/3 | 14/6 | — |
| Intervention side (right/left) | 9/11 | 11/9 | — |
| Intrasubject Effects | ||||
|---|---|---|---|---|
| Measure | Group A (3 Hz) | Group B (10 Hz) | Time Value F; p (Eta2) | Treatment × Time F; p (Eta2) |
| Pain (NRS) | F = 94.6; P = 0.001 (0.713) | F = 18.2; P = 0.001 (0.324) | ||
| Baseline | 4.85 ± 1.3 | 5.40 ± 1.4 | ||
| 1 week | 1.90 ± 1.2 | 4.25 ± 1.6 | ||
| ODI (%) | F = 120.49; P = 0.001 (0.760) | F = 3.21; P = 0.081 (0.078) | ||
| Baseline | 16.3 ± 5.28 | 19.8 ± 5.69 | ||
| 1 week | 8.10 ± 4.88 | 13.9 ± 6.73 | ||
| IR Sitting Non-Intervention limb (º) | F = 18.8968; P = 0.001 (0.332) | F = 0.0992; P = 0.754 (0.003) | ||
| Baseline | 37.4 ± 5.13 | 37.1 ± 5.72 | ||
| 1 week | 39.2 ± 3.78 | 38.8 ± 4.91 | ||
| IR Sitting Intervention limb (º) | F = 54.542; P = 0.001 (0.589) | F = 0.334; P = 0.567 (0.009) | ||
| Baseline | 36.0 ± 5.13 | 35.8 ± 6.00 | ||
| 1 week | 39.1 ± 4.85 | 38.5 ± 5.08 | ||
| Intrasubject Effects | ||||
|---|---|---|---|---|
| Measure (Centimeters, cm) | Group A (3 Hz) | Group B (10 Hz) | Time Value F; p (Eta2) | Treatment × Time F; p (Eta2) |
| Anterior Non-Intervention limb | F = 160.732; P = 0.001 (0.809) | F = 0.373; P = 0.545 (0.010) | ||
| Baseline | 54.6 ± 4.43 | 54.8 ± 4.76 | ||
| 1 week | 57.4 ± 4.87 | 56.3 ± 5.63 | ||
| Anterior Intervention limb | F = 28.94; P = 0.001 (0.432) | F = 2.78; P = 0.104 (0.068) | ||
| Baseline | 54.4 ± 4.44 | 54.7 ± 6.54 | ||
| 1 week | 57.8 ± 4.23 | 56.4 ± 6.16 | ||
| Posteromedial Non-Intervention limb | F = 40.11; P = 0.001 (0.513) | F = 4.91; P = 0.053 (0.114) | ||
| Baseline | 99.3 ± 11.9 | 96.2 ± 8.71 | ||
| 1 week | 108 ± 10.6 | 103 ± 9.40 | ||
| Posteromedial Intervention limb | F = 51.725; P = 0.001 (0.576) | F = 0.926; P = 0.342 (0.024) | ||
| Baseline | 101 ± 12.5 | 97.0 ± 8.85 | ||
| 1 week | 110 ± 11.5 | 104 ± 8.88 | ||
| Posterolateral Non-Intervention limb | F = 63.852; P = 0.001 (0.627) | F = 0.570; P = 0.455 (0.015) | ||
| Baseline | 95.7 ± 12.5 | 95.3 ± 9.53 | ||
| 1 week | 104 ± 12.5 | 99.8 ± 9.34 | ||
| Posterolateral Intervention limb | F = 36.49; P = 0.001 (0.490) | F = 3.22; P = 0.081 (0.078) | ||
| Baseline | 95.1 ± 10.2 | 94.5 ± 7.91 | ||
| 1 week | 104 ± 11.5 | 101 ± 7.95 | ||
| Intrasubject Effects | ||||
|---|---|---|---|---|
| Measure (Kgf) | Group A (3 Hz) | Group B (10 Hz) | Time Value F (Df); p (Eta2) | Treatment × Time F (Df); p (Eta2) |
| Flexion Non-Intervention limb | F = 67.88; P = 0.001 (0.641) | F = 1.08; P = 0.305 (0.028) | ||
| Baseline | 15.3 ± 3.03 | 13.9 ± 2.89 | ||
| 1 week | 17.8 ± 3.73 | 17.1 ± 4.05 | ||
| Flexion Intervention limb | F = 171.16; P = 0.001 (0.818) | F = 7.02; P = 0.012 (0.156) | ||
| Baseline | 14.4 ± 3.36 | 13.0 ± 2.57 | ||
| 1 week | 17.8 ± 5.10 | 18.3 ± 3.43 | ||
| ER-prone Non-Intervention limb | F = 80.611; P = 0.001 (0.680) | F = 0.199; P = 0.658 (0.005) | ||
| Baseline | 12.3 ± 2.93 | 10.5 ± 1.99 | ||
| 1 week | 13.5 ± 3.60 | 12.3 ± 2.11 | ||
| ER-prone Intervention limb | F = 65.62; P = 0.001 (0.633) | F = 2.35; P = 0.134 (0.058) | ||
| Baseline | 11.6 ± 2.22 | 10.6 ± 2.17 | ||
| 1 week | 13.6 ± 3.37 | 13.6 ± 2.51 | ||
| IR-prone Non-Intervention limb | F = 82.74; P = 0.001 (0.685) | F = 2.50; P = 0.122 (0.062) | ||
| Baseline | 10.6 ± 2.49 | 9.97 ± 1.52 | ||
| 1 week | 12.8 ± 3.28 | 12.1 ± 2.49 | ||
| IR-prone Intervention limb | F = 54.1368; P = 0.001 (0.588) | F = 0.0141; P = 0.906 (0.000) | ||
| Baseline | 9.14 ± 2.36 | 9.10 ± 1.59 | ||
| 1 week | 12.8 ± 3.74 | 13.2 ± 2.74 | ||
| Abduction Non-Intervention limb | F = 65.958; P = 0.001 (0.634) | F = 0.171; P = 0.682 (0.004) | ||
| Baseline | 12.6 ± 2.18 | 11.4 ± 1.62 | ||
| 1 week | 14.8 ± 3.51 | 13.4 ± 2.07 | ||
| Abduction Intervention limb | F = 65.958; P = 0.001 (0.634) | F = 0.171; P = 0.682 (0.004) | ||
| Baseline | 11.3 ± 1.92 | 10.5 ± 1.52 | ||
| 1 week | 15.3 ± 3.68 | 15.8 ± 2.45 | ||
| Extension Non-Intervention limb | F = 177.78; P = 0.001 (0.824) | F = 3.63; P = 0.065 (0.087) | ||
| Baseline | 19.4 ± 4.40 | 16.6 ± 3.11 | ||
| 1 week | 24.9 ± 9.50 | 22.5 ± 4.90 | ||
| Extension Intervention limb | F = 53.3473; P = 0.001 (0.584) | F = 0.0797; P = 0.779 (0.002) | ||
| Baseline | 17.5 ± 4.68 | 16.2 ± 2.95 | ||
| 1 week | 24.7 ± 9.57 | 24.1 ± 5.30 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
San-Emeterio-Iglesias, R.; Romero-Morales, C.; Minaya-Muñoz, F.; De-la-Cruz-Torres, B. Optimal Low-Frequency Parameter of Percutaneous Electrical Nerve Stimulation in Patients with Lower Back Pain: A Pilot Study. Life 2025, 15, 1005. https://doi.org/10.3390/life15071005
San-Emeterio-Iglesias R, Romero-Morales C, Minaya-Muñoz F, De-la-Cruz-Torres B. Optimal Low-Frequency Parameter of Percutaneous Electrical Nerve Stimulation in Patients with Lower Back Pain: A Pilot Study. Life. 2025; 15(7):1005. https://doi.org/10.3390/life15071005
Chicago/Turabian StyleSan-Emeterio-Iglesias, Roberto, Carlos Romero-Morales, Francisco Minaya-Muñoz, and Blanca De-la-Cruz-Torres. 2025. "Optimal Low-Frequency Parameter of Percutaneous Electrical Nerve Stimulation in Patients with Lower Back Pain: A Pilot Study" Life 15, no. 7: 1005. https://doi.org/10.3390/life15071005
APA StyleSan-Emeterio-Iglesias, R., Romero-Morales, C., Minaya-Muñoz, F., & De-la-Cruz-Torres, B. (2025). Optimal Low-Frequency Parameter of Percutaneous Electrical Nerve Stimulation in Patients with Lower Back Pain: A Pilot Study. Life, 15(7), 1005. https://doi.org/10.3390/life15071005







