Biochemical Study of Bilberry Extract Potential in Preventing Retinal Damage in Rat Model of Diabetes Induced by Streptozotocin/Nicotinamide
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Bilberry Extract Preparation and Content Determination
2.3. Animals and Housing
2.4. Establishing Animal Model of Hyperglycemia
2.5. Bilberry Extract Treatment
2.6. Sample Collection and Preparation
2.7. Determination of Serum Parameters
2.8. Determination of Thiobarbiturate Reacting Substances
2.9. Determination of Advanced Oxidative Protein Products
2.10. Determination of iNOS, VEGF, and MMP-9 in Retinal Homogenates
2.11. Statistical Analysis
3. Results
3.1. Anthocyanin Content in the Bilberry Extract
3.2. Effect of Bilberry Extract on Body Weight, Food, and Water Intake
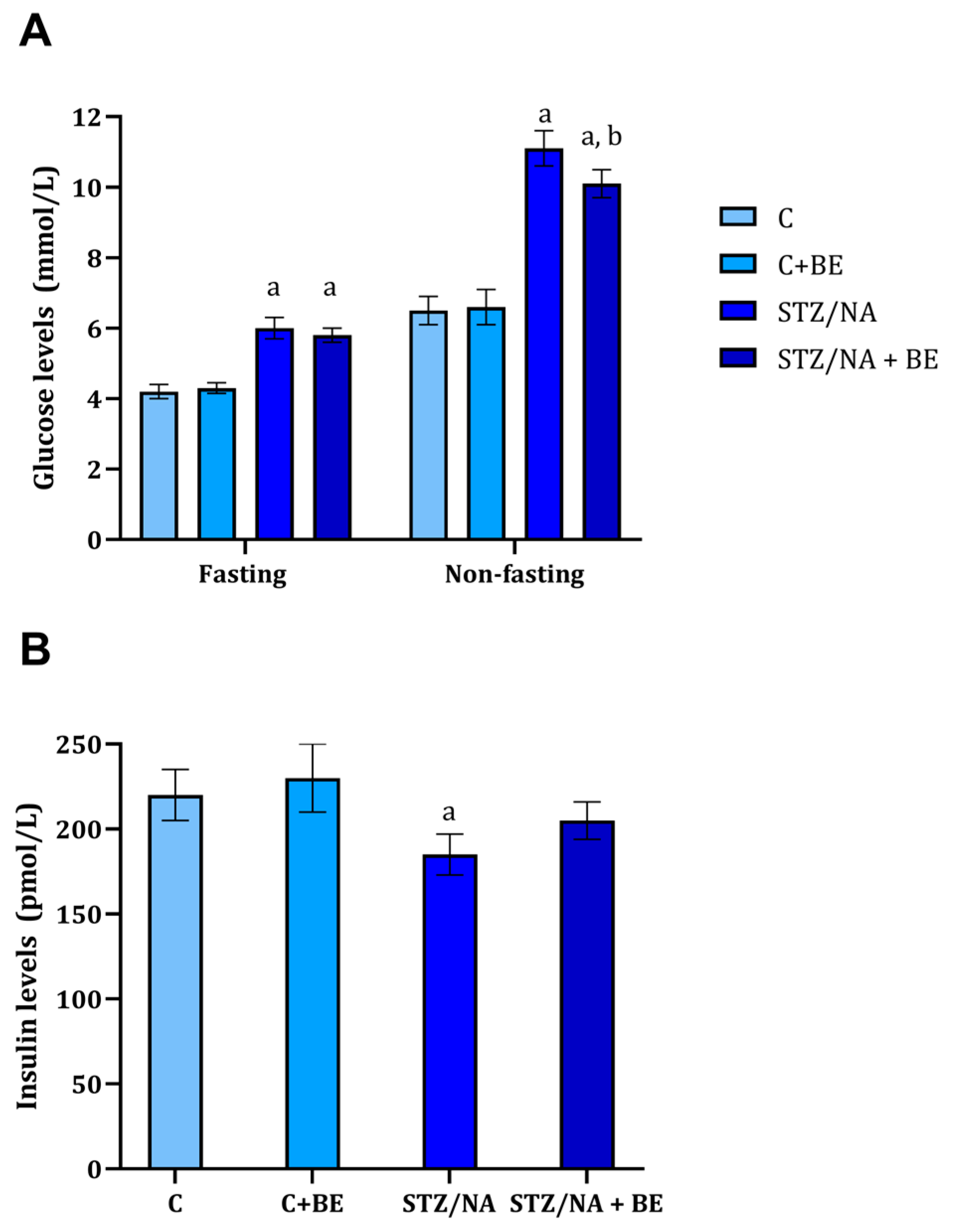
3.3. Effect of Bilberry Extract on Serum Glucose and Insulin
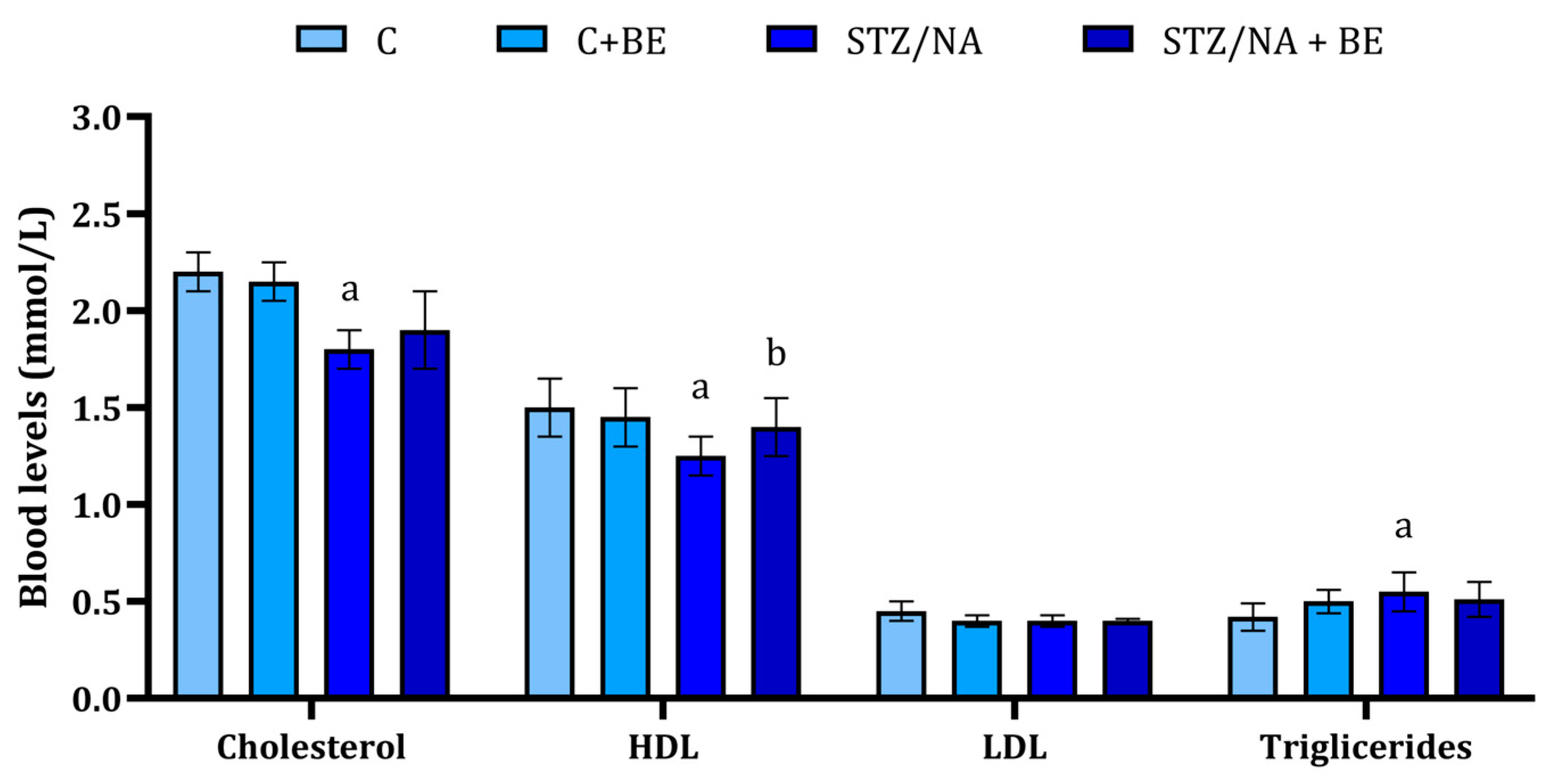
3.4. Effect of Bilberry Extract on Lipid Profile
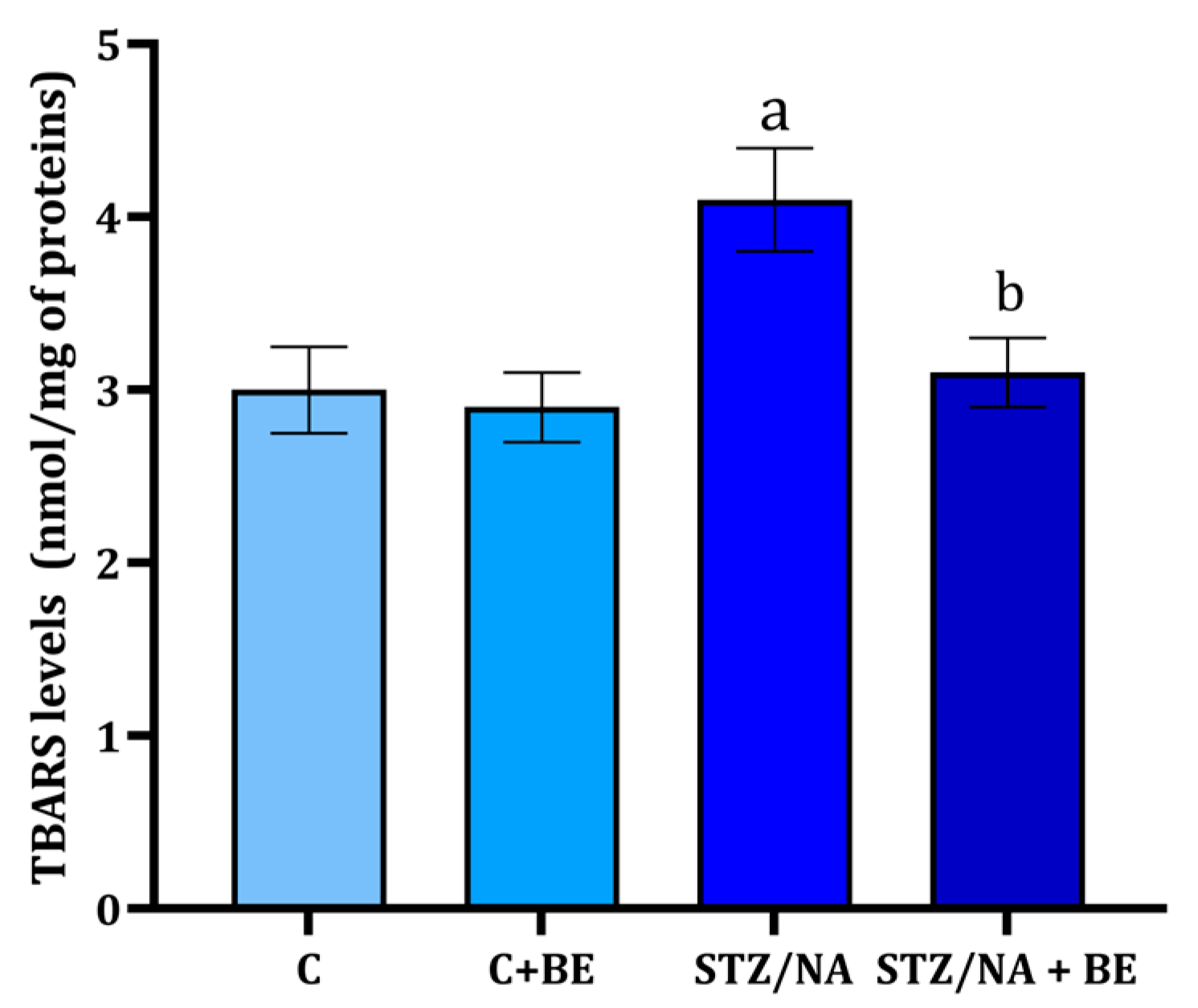
3.5. Effect of Bilberry Extract on TBARS Levels in Retinal Tissue
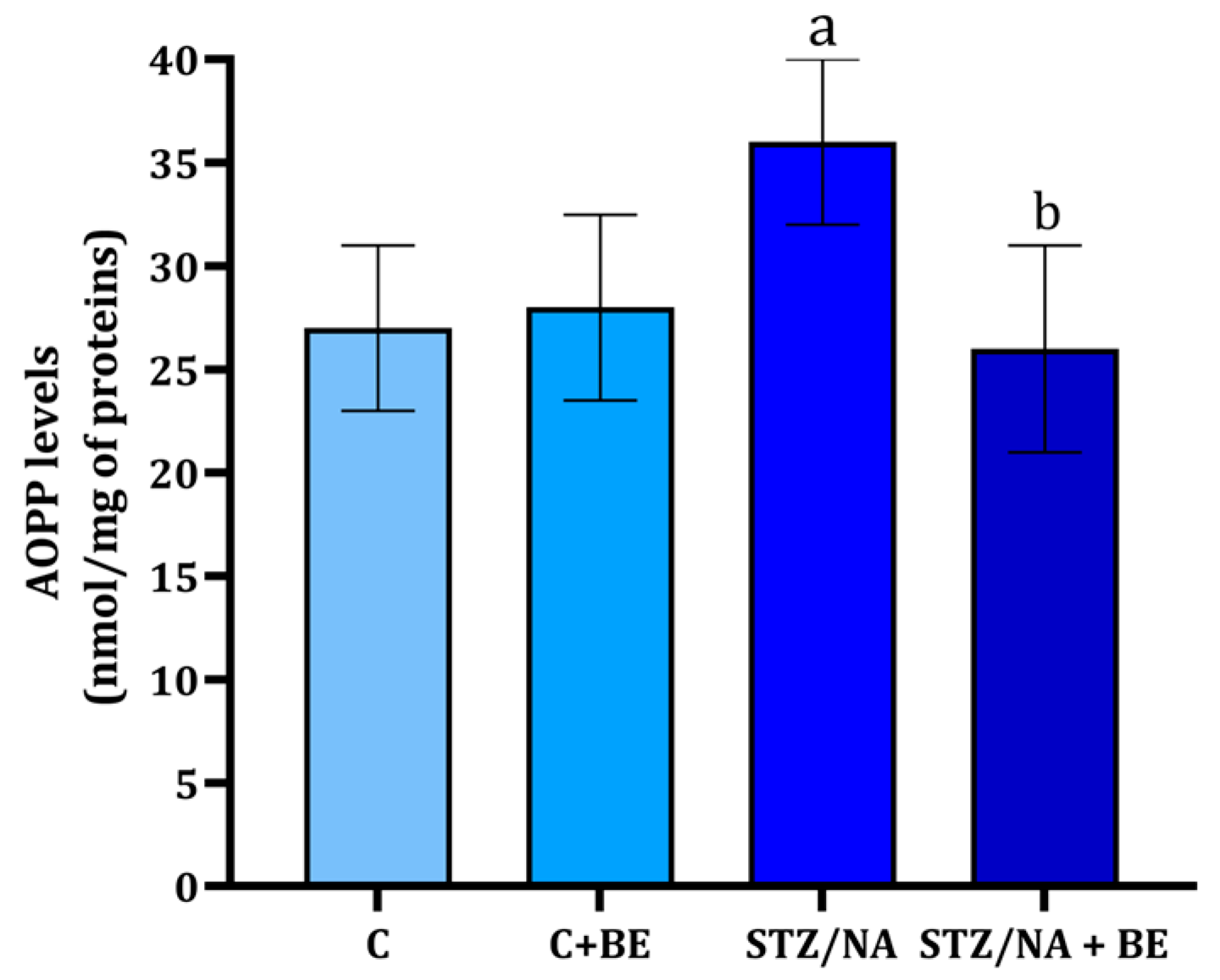
3.6. Effect of Bilberry Extract on AOPP in the Retinal Tissue
3.7. Effect of Bilberry Extract on iNOS in the Retinal Tissue
3.8. Effect of Bilberry Extract on VEGF in the Retinal Tissue
3.9. Effect of Bilberry Extract on MMP-9 in the Retinal Tissue
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shaw, J.E.; Sicree, R.A.; Zimmet, P.Z. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res. Clin. Pract. 2010, 87, 4–14. [Google Scholar] [CrossRef]
- Cheung, N.; Mitchell, P.; Wong, T.Y. Diabetic retinopathy. Lancet 2010, 376, 124–136. [Google Scholar] [CrossRef] [PubMed]
- Chew, E.Y.; Klein, M.L.; Ferris, F.L.; Remaley, N.A.; Murphy, R.P.; Chantry, K.; Hoogwerf, B.J.; Miller, D. Association of elevated serum lipid levels with retinal hard exudate in diabetic retinopathy. Early Treatment Diabetic Retinopathy Study (ETDRS) Report 22. Arch. Ophthalmol. 1996, 114, 1079–1084. [Google Scholar] [CrossRef]
- Tarr, J.M.; Kaul, K.; Chopra, M.; Kohner, E.M.; Chibber, R. Pathophysiology of diabetic retinopathy. ISRN Ophthalmol. 2013, 2013, 343560. [Google Scholar] [CrossRef]
- Salido, E.M.; de Zavalía, N.; Schreier, L.; De Laurentiis, A.; Rettori, V.; Chianelli, M.; Sarmiento, M.I.K.; Arias, P.; Rosenstein, R.E. Retinal changes in an experimental model of early type 2 diabetes in rats characterized by non-fasting hyperglycemia. Exp. Neurol. 2012, 236, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.; Hu, A.; Luo, Y.; Sun, W.; Hu, X.; Tang, S. Interleukin-4 and melatonin ameliorate high glucose and interleukin-1β stimulated inflammatory reaction in human retinal endothelial cells and retinal pigment epithelial cells. Mol. Vis. 2014, 20, 921–928. [Google Scholar]
- Chu, W.; Cheung, S.C.M.; Lau, R.A.W.; Benzie, I.F.F. Bilberry (Vaccinium myrtillus L.). In Herbal Medicine: Biomolecular and Clinical Aspects; Benzie, I.F.F., Wachtel-Galor, S., Eds.; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2011. [Google Scholar]
- Kim, J.; Kim, C.-S.; Lee, Y.M.; Sohn, E.; Jo, K.; Kim, J.S. Vaccinium myrtillus extract prevents or delays the onset of diabetes--induced blood–retinal barrier breakdown. Int. J. Food Sci. Nutr. 2015, 66, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Miyake, S.; Takahashi, N.; Sasaki, M.; Kobayashi, S.; Tsubota, K.; Ozawa, Y. Vision preservation during retinal inflammation by anthocyanin-rich bilberry extract: Cellular and molecular mechanism. Lab. Investig. 2012, 92, 102–109. [Google Scholar] [CrossRef]
- Asgary, S.; RafieianKopaei, M.; Sahebkar, A.; Shamsi, F.; Golimalekabadi, N. Anti-hyperglycemic and anti-hyperlipidemic effects of Vaccinium myrtillus fruit in experimentally induced diabetes (antidiabetic effect of Vaccinium myrtillus fruit). J. Sci. Food Agric. 2016, 96, 764–768. [Google Scholar] [CrossRef]
- Kowalska, K.; Olejnik, A.; Szwajgier, D.; Olkowicz, M. Inhibitory activity of chokeberry, bilberry, raspberry and cranberry polyphenol-rich extract towards adipogenesis and oxidative stress in differentiated 3T3-L1 adipose cells. PLoS ONE 2017, 12, e0188583. [Google Scholar] [CrossRef]
- Vaneková, Z.; Rollinger, J.M. Bilberries: Curative and Miraculous—A Review on Bioactive Constituents and Clinical Research. Front. Pharmacol. 2022, 13, 909914. [Google Scholar] [CrossRef]
- Može, Š.; Polak, T.; Gašperlin, L.; Koron, D.; Vanzo, A.; Ulrih, N.P.; Abram, V. Phenolics in Slovenian Bilberries (Vaccinium myrtillus L.) and Blueberries (Vaccinium corymbosum L.). J. Agric. Food Chem. 2011, 59, 6998–7004. [Google Scholar] [CrossRef] [PubMed]
- Masiello, P.; Broca, C.; Gross, R.; Roye, M.; Manteghetti, M.; Hillaire-Buys, D.; Novelli, M.; Ribes, G. Experimental NIDDM: Development of a new model in adult rats administered streptozotocin and nicotinamide. Diabetes 1998, 47, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Yao, N.; Lan, F.; He, R.-R.; Kurihara, H. Protective Effects of Bilberry (Vaccinium myrtillus L.) extract against endotoxin-induced uveitis in mice. J. Agric. Food Chem. 2010, 58, 4731–4736. [Google Scholar] [CrossRef] [PubMed]
- Skeie, J.M.; Tsang, S.H.; Mahajan, V.B. Evisceration of mouse vitreous and retina for proteomic analyses. J. Vis. Exp. 2011, 50, 2795. [Google Scholar]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Andreeva, I.; Kožemjakin, A.; Kiškun, A. Modification of the method of measurement of lipid peroxides in test with thiobarbituric acid. Lab. Delo 1988, 11, 41–43. [Google Scholar]
- Stojanović, N.M.; Maslovarić, A.; Mihajlović, I.; Marković, A.; Randjelović, P.J.; Sokolović, D. Melatonin treatment prevents carbon-tetrachloride induced rat brain injury. Toxicol Res 2023, 12, 895–901. [Google Scholar] [CrossRef]
- Lee, R.; Wong, T.Y.; Sabanayagam, C. Epidemiology of diabetic retinopathy, diabetic macular edema and related vision loss. Eye Vis. 2015, 2, 17. [Google Scholar] [CrossRef]
- Kianbakht, S.; Abasi, B.; Dabaghian, F.H. Anti-Hyperglycemic Effect of Vaccinium arctostaphylos in Type 2 Diabetic Patients: A Randomized Controlled Trial. Res. Complement. Med. 2013, 20, 17–22. [Google Scholar] [CrossRef]
- Takahashi, A.; Shimizu, H.; Okazaki, Y.; Sakaguchi, H.; Taira, T.; Suzuki, T.; Chiji, H. Anthocyanin-rich phytochemicals from aronia fruits inhibit visceral fat accumulation and hyperglycemia in high-fat diet-induced dietary obese rats. J. Oleo Sci. 2015, 64, 1243–1250. [Google Scholar] [CrossRef] [PubMed]
- Jayaprakasam, B.; Vareed, S.K.; Olson, L.K.; Nair, M.G. Insulin secretion by bioactive anthocyanins and anthocyanidins present in fruits. J. Agric. Food Chem. 2005, 53, 28–31. [Google Scholar] [CrossRef] [PubMed]
- Martineau, L.C.; Couture, A.; Spoor, D.; Benhaddou-Andaloussi, A.; Harris, C.; Meddah, B.; Leduc, C.; Burt, A.; Vuong, T.; Le, P.M.; et al. Anti-diabetic properties of the Canadian lowbush blueberry Vaccinium angustifolium Ait. Phytomedicine 2006, 13, 612–623. [Google Scholar] [CrossRef]
- Popović, D.; Đukić, D.; Katić, V.; Jović, Z.; Jović, M.; Lalić, J.; Golubović, I.; Stojanović, S.; Ulrih, N.P.; Stanković, M.; et al. Antioxidant and proapoptotic effects of anthocyanins from bilberry extract in rats exposed to hepatotoxic effects of carbon tetrachloride. Life Sci. 2016, 157, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, L.; Lu, F.; Yang, X.; Deng, Q.; Ji, B.; Huang, F. Retinoprotective effects of bilberry anthocyanins via antioxidant, anti-inflammatory, and anti-apoptotic mechanisms in a visible light-induced retinal degeneration model in pigmented rabbits. Molecules 2015, 20, 22395–22410. [Google Scholar] [CrossRef]
- Opatrilova, R.; Kubatka, P.; Caprnda, M.; Büsselberg, D.; Krasnik, V.; Vesely, P.; Saxena, S.; Ruia, S.; Mozos, I.; Rodrigo, L.; et al. Nitric oxide in the pathophysiology of retinopathy: Evidences from preclinical and clinical researches. Acta Ophthalmol. 2018, 96, 222–231. [Google Scholar] [CrossRef]
- Hu, W.; Wang, W.; Ma, Q.; Liu, T.; Zhang, J.; Zhang, J. Blueberry anthocyanin-enriched extract ameliorates transverse aortic constriction-induced myocardial dysfunction via the DDAH1/ADMA/NO signaling pathway in mice. Mol. Med. Rep. 2020, 21, 454–462. [Google Scholar] [CrossRef]
- Qazi, Y.; Maddula, S.; Ambati, B.K. Mediators of ocular angiogenesis. J. Genet. 2009, 88, 495–515. [Google Scholar] [CrossRef] [PubMed]
- Larsson, P.; Syed Khaja, A.S.; Semenas, J.; Wang, T.; Sarwar, M.; Dizeyi, N.; Simoulis, A.; Hedblom, A.; Wai, S.N.; Ødum, N.; et al. The functional interlink between AR and MMP9/VEGF signaling axis is mediated through PIP5K1α/pAKT in prostate cancer. Int. J. Cancer 2020, 146, 1686–1699. [Google Scholar] [CrossRef]
- Özduran, G.; Yücecan, S. Effects of Bilberry (Vaccinium myrtillus L.) on Cancer: A Narrative Review. Akad. Gıda 2023, 21, 375–387. [Google Scholar] [CrossRef]
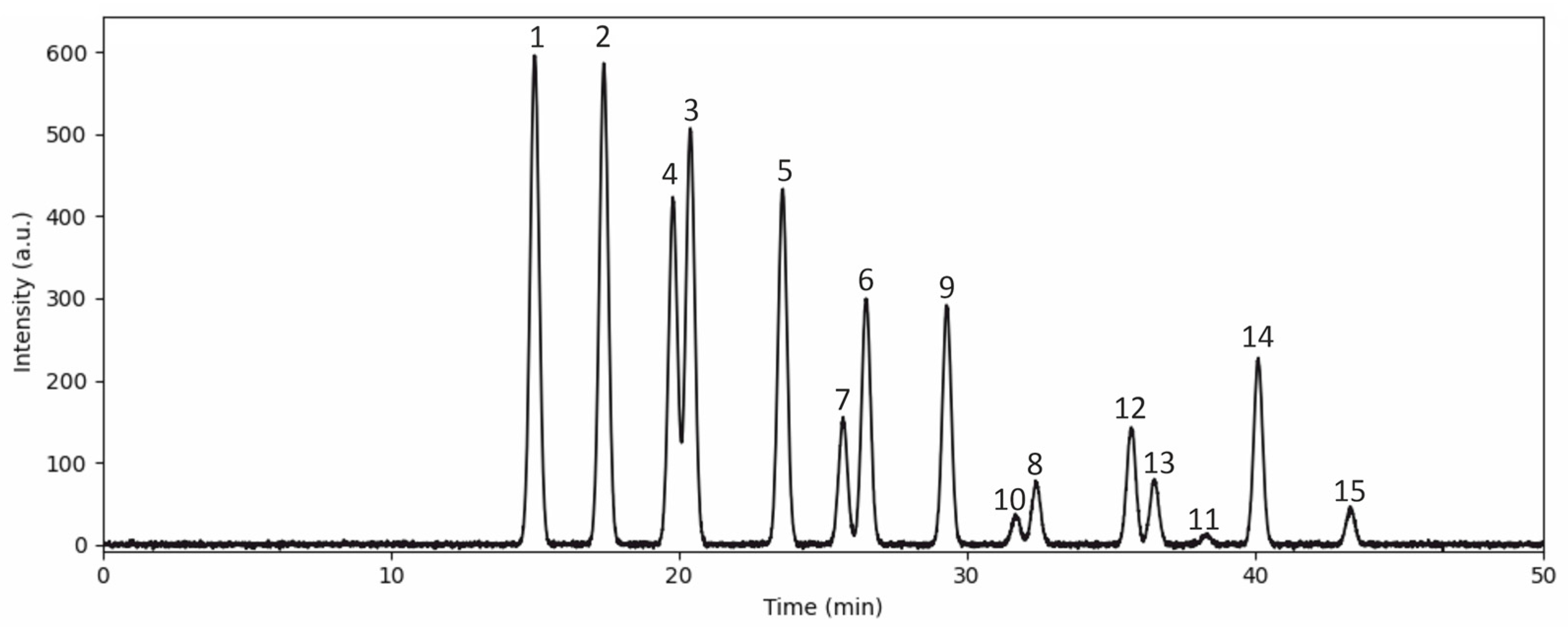
| No. | Anthocyanins | mg/L | mg/100 g | % | % |
|---|---|---|---|---|---|
| 1 | Delphinidin 3-galactoside | 595.98 ± 0.9 | 132.05 ± 0.2 | 15.3 | 43.3 |
| 2 | Delphinidin 3-glucoside | 584.29 ± 1.0 | 129.46 ± 0.2 | 15.0 | |
| 3 | Delphinidin 3-arabinoside | 506.38 ± 1.3 | 112.20 ± 0.4 | 13.0 | |
| 4 | Cyanidin 3-galactoside | 420.69 ± 1.2 | 93.21 ± 0.3 | 10.8 | 29.6 |
| 5 | Cyanidin 3-glucoside | 432.37 ± 0.5 | 95.80 ± 0.20 | 11.1 | |
| 6 | Cyanidin 3-arabinoside | 299.93 ± 0.3 | 66.45 ± 0.1 | 7.7 | |
| 7 | Petunidin 3-galactoside | 151.91 ± 0.8 | 33.66 ± 0.2 | 3.9 | 13.5 |
| 8 | Petunidin 3-arabinoside | 74.01 ± 0.3 | 16.39 ± 0.1 | 1.9 | |
| 9 | Petunidin 3-glucoside | 292.14 ± 6.4 | 64.73 ± 1.4 | 7.5 | |
| 10 | Peonidin 3-galactoside | 35.05 ± 0.2 | 7.76 ± 0.1 | 0.9 | 4.9 |
| 11 | Peonidin 3-arabinoside | 11.68 ± 0.2 | 2.58 ± 0.1 | 0.3 | |
| 12 | Peonidin 3-glucoside | 144.12 ± 0.3 | 31.93 ± 0.1 | 3.7 | |
| 13 | Malvidin 3-galactoside | 77.90 ± 0.2 | 17.26 ± 0.3 | 2.0 | 8.9 |
| 14 | Malvidin 3-glucoside | 225.92 ± 0.8 | 50.05 ± 0.1 | 5.8 | |
| 15 | Malvidin 3-arabinoside | 42.84 ± 1.2 | 9.49 ± 0.3 | 1.1 | |
| Total | 3895.2 ± 53.7 | 863.4 ± 11.5 | 100 | 100 |
| Parameters/Group | I (C) | II (C + BE) | III (STZ/NA) | IV (STZ/NA + BE) |
|---|---|---|---|---|
| Initial body weight (g) | 351 ± 7 | 365 ± 15 | 352 ± 14 | 351 ± 10 |
| Final body weight (g) | 395 ± 12 | 400 ± 12 | 413 ± 19 | 401 ± 21 |
| Food consumption (g/day) | 30 ± 2 | 31 ± 2 | 36 ± 3 a | 30 ± 4 b |
| Water consumption (mL/day) | 41 ± 4 | 41 ± 6 | 56 ± 6 a | 45 ± 7 b |
| Parameter/Group | I (C) | II (C + BE) | III (STZ/NA) | IV (STZ/NA + BE) |
|---|---|---|---|---|
| iNOS (IU/mg) | 13.9 ± 0.9 | 14.1 ± 1 | 17.2 ± 0.9 a | 14.9 ± 0.8 |
| VEGF (pg/mg) | 22.2 ± 3.6 | 20.9 ± 1.9 | 27.5 ± 2.5 a | 23.9 ± 1.7 b |
| MMP-9 (pg/mg) | 47.4 ± 15.1 | 60.3 ± 7.6 | 86.4 ± 8.6 a | 41.2 ± 8.2 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petrović, M.; Trenkić, M.; Veselinović, M.; Smiljković, A.; Sokolović, D. Biochemical Study of Bilberry Extract Potential in Preventing Retinal Damage in Rat Model of Diabetes Induced by Streptozotocin/Nicotinamide. Life 2025, 15, 1006. https://doi.org/10.3390/life15071006
Petrović M, Trenkić M, Veselinović M, Smiljković A, Sokolović D. Biochemical Study of Bilberry Extract Potential in Preventing Retinal Damage in Rat Model of Diabetes Induced by Streptozotocin/Nicotinamide. Life. 2025; 15(7):1006. https://doi.org/10.3390/life15071006
Chicago/Turabian StylePetrović, Maja, Marija Trenkić, Marija Veselinović, Aleksandra Smiljković, and Dušan Sokolović. 2025. "Biochemical Study of Bilberry Extract Potential in Preventing Retinal Damage in Rat Model of Diabetes Induced by Streptozotocin/Nicotinamide" Life 15, no. 7: 1006. https://doi.org/10.3390/life15071006
APA StylePetrović, M., Trenkić, M., Veselinović, M., Smiljković, A., & Sokolović, D. (2025). Biochemical Study of Bilberry Extract Potential in Preventing Retinal Damage in Rat Model of Diabetes Induced by Streptozotocin/Nicotinamide. Life, 15(7), 1006. https://doi.org/10.3390/life15071006






