The Role of Neutrophil-to-Lymphocyte Ratio and Platelet-to-Lymphocyte Ratio in Predicting Atrial Fibrillation and Its Comorbidities
Abstract
1. Introduction
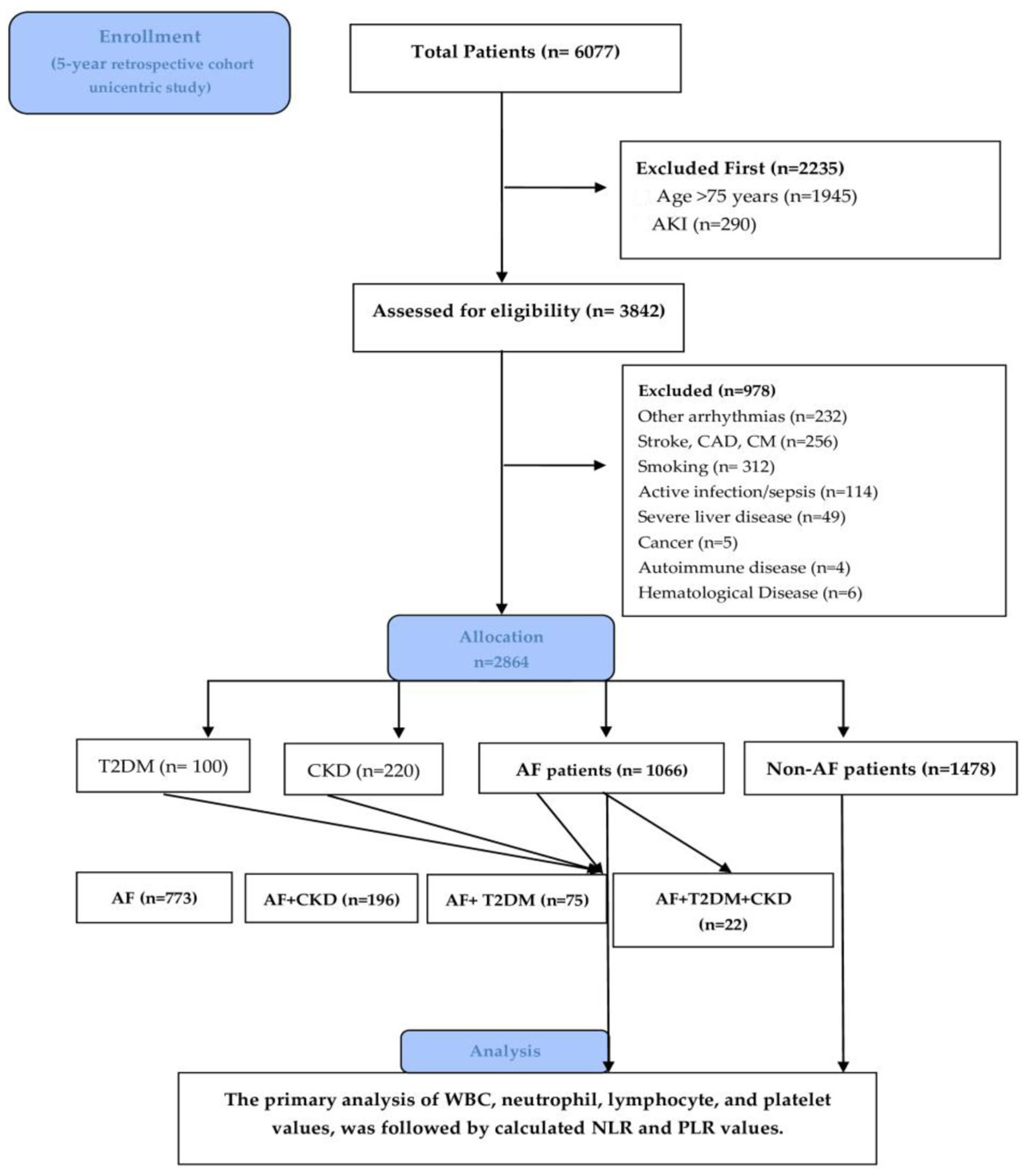
2. Materials and Methods
2.1. Study Design and Extraction Data
2.2. Statistical Analysis
2.3. Ethics Statement
3. Results
3.1. Baseline Characteristic
3.2. Association Between AF, Obesity, and Dyslipidemia
3.3. NLR Value, AF Association, and Comorbidities
3.4. PLR, AF, and Comorbidities
3.5. Neutrophils, Lymphocytes, and Platelet Values in AF Patients
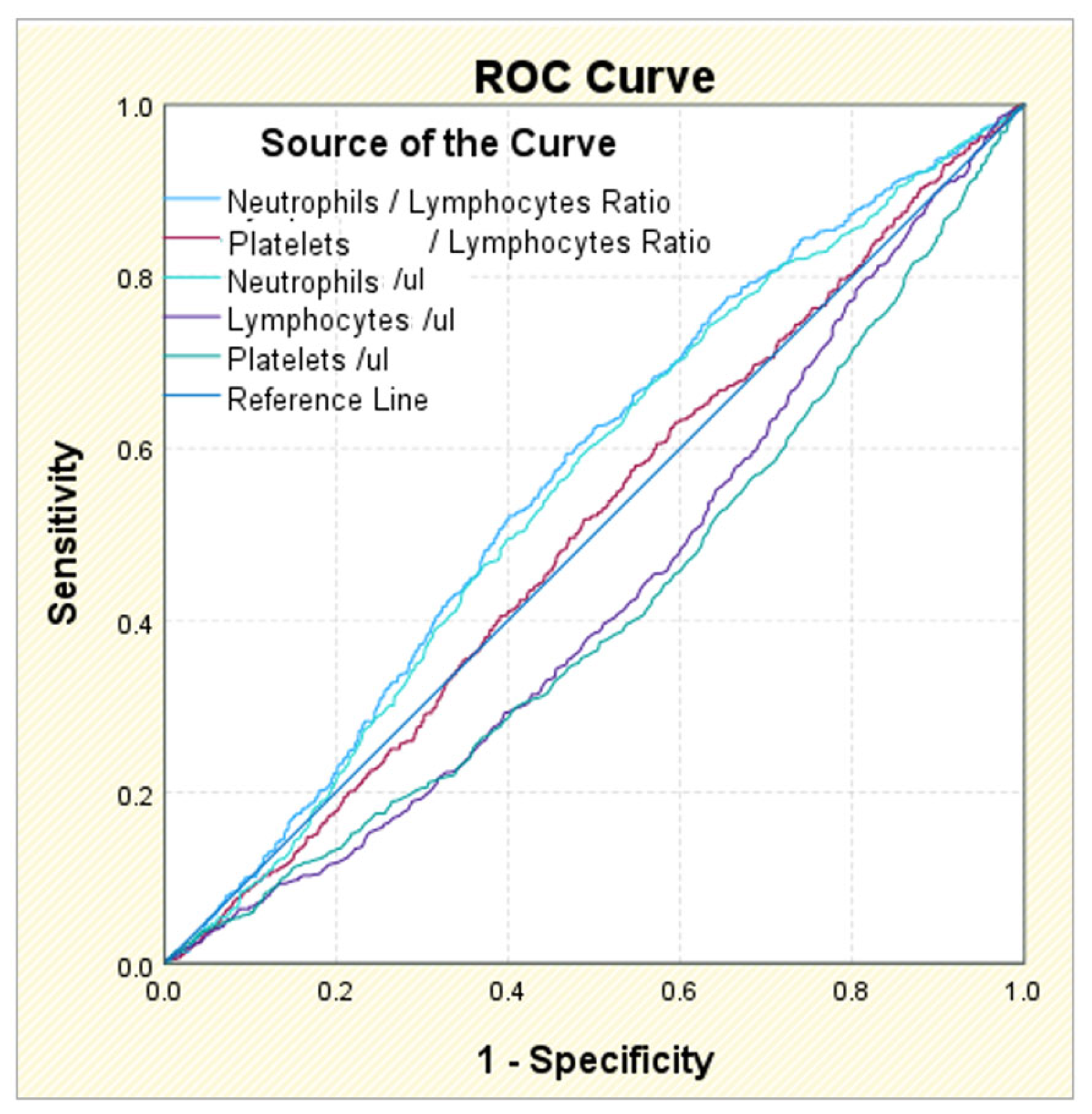
3.6. ROC Analysis in AF Patients and Comorbidities
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Van Gelder, I.C.; Rienstra, M.; Bunting, K.V.; Casado-Arroyo, R.; Caso, V.; Crijns, H.J.G.M.; De Potter, T.J.R.; Dwight, J.; Guasti, L.; Hanke, T.; et al. 2024 ESC Guidelines for the management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): Developed by the task force for the management of atrial fibrillation of the European Society of Cardiology (ESC), with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Endorsed by the European Stroke Organisation (ESO). Eur. Heart J. 2024, 45, 3314–3414. [Google Scholar] [CrossRef] [PubMed]
- Joglar, J.A.; Chung, M.K.; Armbruster, A.L.; Benjamin, E.J.; Chyou, J.Y.; Cronin, E.M.; Deswal, A.; Eckhardt, L.L.; Goldberger, Z.D.; Gopinathannair, R.; et al. 2023 ACC/AHA/ACCP/HRS Guideline for the Diagnosis and Management of Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2024, 149, e1–e156. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; An, G.; Wang, B.; Chen, Y.; Liu, G.; Wang, X.; Liu, S.; Zhang, D.; Sun, D.; Zhang, Y.; et al. Integrated analysis of the lncRNA-miRNA-mRNA network based on competing endogenous RNA in atrial fibrillation. Front. Cardiovasc. Med. 2023, 10, 1099124. [Google Scholar] [CrossRef] [PubMed]
- Mukai, Y. Inflammation and atrial fibrillation. J. Arrhythm. 2024, 40, 26–27. [Google Scholar] [CrossRef]
- Keefe, J.A.; Zhao, S.; Wehrens, X.H.T. A mechanistic LNK between inflammation and atrial fibrillation? Cardiovasc. Res. 2024, 120, 814–816. [Google Scholar] [CrossRef]
- Dereli, S.; Bayramoğlu, A.; Yontar, O.C. Usefulness of platelet to lymphocyte ratio for predicting recurrence of atrial fibrillation after direct current cardioversion. Ann. Noninvasive Electrocardiol. 2019, 24, e12616. [Google Scholar] [CrossRef]
- Shi, Y.; Xuan, C.; Ji, W.; Wang, F.; Huang, J.; Li, L.; Wang, H.; Deng, J.; Shao, J.; Chen, K.; et al. Combination of platelet-to-lymphocyte ratio and D-dimer for the identification of cardiogenic cerebral embolism in non-valvular atrial fibrillation. Front. Neurol. 2023, 14, 1069261. [Google Scholar] [CrossRef]
- Wu, C.C.; Wu, C.H.; Lee, C.H.; Cheng, C.I. Association between neutrophil percentage-to-albumin ratio (NPAR), neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR) and long-term mortality in community-dwelling adults with heart failure: Evidence from US NHANES 2005–2016. BMC Cardiovasc. Disord. 2023, 23, 312. [Google Scholar] [CrossRef]
- Omrani-Nava, V.; Moosazadeh, M.; Bahar, A.; Hedayatizadeh-Omran, A.; Ahmadi, A.; Alizadeh-Navaei, R. Neutrophil-to-lymphocyte, platelet-to-lymphocyte and lymphocyte-to-monocyte ratios, any association with metabolic syndrome? Casp. J. Intern. Med. 2023, 14, 567–571. [Google Scholar]
- Dascalu, A.M.; Georgescu, A.; Costea, A.C.; Tribus, L.; El Youssoufi, A.; Serban, D.; Arsene, A.L.; Stana, D.; Alexandrescu, C.; Cristea, B.M.; et al. Association Between Neutrophil-to-Lymphocyte Ratio (NLR) and Platelet-to-Lymphocyte Ratio (PLR) With Diabetic Retinopathy in Type 2 Diabetic Patients. Cureus 2023, 15, e48581. [Google Scholar] [CrossRef]
- Brito, G.M.C.; Fontenele, A.M.M.; Carneiro, E.; Nogueira, I.A.L.; Cavalcante, T.B.; Vale, A.A.M.; Monteiro, S.C.M.; Salgado Filho, N. Neutrophil-to-Lymphocyte and Platelet-to-Lymphocyte Ratios in Nondialysis Chronic Kidney Patients. Int. J. Inflam. 2021, 2021, 6678960. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Wang, D.; Jia, J.; Zhang, J.; Liu, Y.; Lu, J.; Zhao, X.; Yan, J. Neutrophil-to-Lymphocyte Ratio, Lymphocyte-to-Monocyte Ratio and Platelet-to-Lymphocyte Ratio as Predictors of Short- and Long-Term Outcomes in Ischemic Stroke Patients with Atrial Fibrillation. J. Inflamm. Res. 2024, 17, 6661–6672. [Google Scholar] [CrossRef] [PubMed]
- Stevens, P.E.; Ahmed, S.B.; Carrero, J.J.; Foster, B.; Francis, A.; Hall, R.K.; Herrington, W.G.; Hill, G.; Inker, L.A.; Kazancıoğlu, R.; et al. KDIGO 2024 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int. 2024, 105, S117–S314. [Google Scholar] [CrossRef]
- American Diabetes Association Professional Practice Committee. 2. Diagnosis and Classification of Diabetes: Standards of Care in Diabetes—2025. Diabetes Care 2024, 48 (Suppl. S1), S27–S49. [Google Scholar] [CrossRef]
- Howard, R.; Scheiner, A.; Kanetsky, P.A.; Egan, K.M. Sociodemographic and lifestyle factors associated with the neutrophil-to-lymphocyte ratio. Ann. Epidemiol. 2019, 38, 11–21.e6. [Google Scholar] [CrossRef]
- Wu, L.; Yuan, Z.; Zeng, Y.; Yang, L.; Hu, Q.; Zhang, H.; Li, C.; Chen, Y.; Zhang, Z.; Zhong, L.; et al. Routinely available inflammation biomarkers to predict stroke and mortality in atrial fibrillation. Clinics 2025, 80, 100610. [Google Scholar] [CrossRef]
- Peng, L.; Liu, L.; Chai, M.; Cai, Z.; Wang, D. Predictive value of neutrophil to lymphocyte ratio for clinical outcome in patients with atrial fibrillation: A systematic review and meta-analysis. Front. Cardiovasc. Med. 2024, 11, 1461923. [Google Scholar] [CrossRef]
- Mureșan, A.V.; Russu, E.; Arbănași, E.M.; Kaller, R.; Hosu, I.; Arbănași, E.M.; Voidăzan, S.T. The Predictive Value of NLR, MLR, and PLR in the Outcome of End-Stage Kidney Disease Patients. Biomedicines 2022, 10, 1272. [Google Scholar] [CrossRef]
- Zhou, X.; Dudley, S.C. Evidence for Inflammation as a Driver of Atrial Fibrillation. Front. Cardiovasc. Med. 2020, 7, 62. [Google Scholar] [CrossRef]
- Geng, X.; Wang, D.W.; Li, H. The pivotal role of neutrophil extracellular traps in cardiovascular diseases: Mechanisms and therapeutic implications. Biomed. Pharmacother. 2024, 179, 117289. [Google Scholar] [CrossRef]
- Ajoolabady, A.; Nattel, S.; Lip, G.Y.H.; Ren, J. Inflammasome Signaling in Atrial Fibrillation. J. Am. Coll. Cardiol. 2022, 79, 2349–2366. [Google Scholar] [CrossRef] [PubMed]
- Rienstra, M.; Sun, J.X.; Magnani, J.W.; Sinner, M.F.; Lubitz, S.A.; Sullivan, L.M.; Ellinor, P.T.; Benjamin, E.J. White blood cell count and risk of incident atrial fibrillation (from the Framingham Heart Study). Am. J. Cardiol. 2012, 109, 533–537. [Google Scholar] [CrossRef] [PubMed]
- Nyrnes, A.; Njølstad, I.; Mathiesen, E.B.; Wilsgaard, T.; Hansen, J.B.; Skjelbakken, T.; Jørgensen, L.; Løchen, M.L. Inflammatory biomarkers as risk factors for future atrial fibrillation. An eleven-year follow-up of 6315 men and women: The Tromsø study. Gend. Med. 2012, 9, 536–547.e2. [Google Scholar] [CrossRef] [PubMed]
- Weymann, A.; Ali-Hasan-Al-Saegh, S.; Sabashnikov, A.; Popov, A.F.; Mirhosseini, S.J.; Liu, T.; Lotfaliani, M.; Sa, M.; Baker, W.L.L.; Yavuz, S.; et al. Prediction of New-Onset and Recurrent Atrial Fibrillation by Complete Blood Count Tests: A Comprehensive Systematic Review with Meta-Analysis. Med. Sci. Monit. Basic. Res. 2017, 23, 179–222. [Google Scholar] [CrossRef]
- Li, Q.; Nie, J.; Cao, M.; Luo, C.; Sun, C. Association between inflammation markers and all-cause mortality in critical ill patients with atrial fibrillation: Analysis of the Multi-Parameter Intelligent Monitoring in Intensive Care (MIMIC-IV) database. Int. J. Cardiol. Heart Vasc. 2024, 51, 101372. [Google Scholar] [CrossRef]
- Bell, D.S.H.; Goncalves, E. Atrial fibrillation and type 2 diabetes: Prevalence, etiology, pathophysiology and effect of anti-diabetic therapies. Diabetes Obes. Metab. 2019, 21, 210–217. [Google Scholar] [CrossRef]
- Sidhu, B.; Mavilakandy, A.; Hull, K.L.; Koev, I.; Vali, Z.; Burton, J.O.; Ng, G.A. Atrial Fibrillation and Chronic Kidney Disease: Aetiology and Management. Rev. Cardiovasc. Med. 2024, 25, 143. [Google Scholar] [CrossRef]
- Chen, H.L.; Wu, C.; Cao, L.; Wang, R.; Zhang, T.Y.; He, Z. The association between the neutrophil-to-lymphocyte ratio and type 2 diabetes mellitus: A cross-sectional study. BMC Endocr. Disord. 2024, 24, 107. [Google Scholar] [CrossRef]
- Linz, D.; Gawalko, M.; Betz, K.; Hendriks, J.M.; Lip, G.Y.H.; Vinter, N.; Guo, Y.; Johnsen, S. Atrial fibrillation: Epidemiology, screening and digital health. Lancet Reg. Health–Eur. 2024, 37, 100786. [Google Scholar] [CrossRef]
- Trtica Majnaric, L.; Guljas, S.; Bosnic, Z.; Seric, V.; Wittlinger, T. Neutrophil-to-Lymphocyte Ratio as a Cardiovascular Risk Marker May Be Less Efficient in Women Than in Men. Biomolecules 2021, 11, 528. [Google Scholar] [CrossRef]
- Scurt, F.G.; Ganz, M.J.; Herzog, C.; Bose, K.; Mertens, P.R.; Chatzikyrkou, C. Association of metabolic syndrome and chronic kidney disease. Obes. Rev. 2024, 25, e13649. [Google Scholar] [CrossRef] [PubMed]
- Soler-Espejo, E.; Marín, F.; López-Gálvez, R.; Ramos-Bratos, M.P.; Sánchez-Villalobos, M.; Esteve-Pastor, M.A.; Lip, G.Y.H.; Rivera-Caravaca, J.M.; Roldán, V. The Neutrophil-to-Lymphocyte Ratio Is an Independent Inflammatory Biomarker for Adverse Events in Patients With Atrial Fibrillation: Insights From the Murcia AF Project II (MAFP-II) Cohort Study. Clin. Cardiol. 2025, 48, e70102. [Google Scholar] [CrossRef] [PubMed]
- Kundnani, N.R.; Sharma, A.; Lighezan, D.F.; Georgescu, D.; Morariu, S.I.; Nisulescu, D.D.; Bita, R.G.; Rosca, C.I. Use of Neutrophil-to-Lymphocyte Ratio to Predict In-Hospital Mortality in Patients Admitted with Acute Decompensation of Atrial Fibrillation. J. Clin. Med. 2024, 13, 4719. [Google Scholar] [CrossRef] [PubMed]
- Calixte, R.; Ye, Z.; Haq, R.; Aladhamy, S.; Camacho-Rivera, M. Demographic and Social Patterns of the Mean Values of Inflammatory Markers in U.S. Adults: A 2009-2016 NHANES Analysis. Diseases 2023, 11, 14. [Google Scholar] [CrossRef]
- Tonyali, S.; Ceylan, C.; Yahsi, S.; Karakan, M.S. Does neutrophil to lymphocyte ratio demonstrate deterioration in renal function? Ren. Fail. 2018, 40, 209–212. [Google Scholar] [CrossRef]
- Aneez, F.A.; Shariffdeen, N.; Haleem, F.A.; Thangarajah, B.R.; Rasaratnam, K. Correlation between neutrophil to lymphocyte ratio and platelet to lymphocyte ratio with proteinuria in different stages of chronic kidney disease. Egypt. J. Intern. Med. 2024, 36, 6. [Google Scholar] [CrossRef]
- Altunoren, O.; Akkus, G.; Sezal, D.T.; Ciftcioglu, M.; Guzel, F.B.; Isiktas, S.; Torun, G.I.; Uyan, M.; Sokmen, M.F.; Sevim, H.A.; et al. Does neutrophyl to lymphocyte ratio really predict chronic kidney disease progression? Int. Urol. Nephrol. 2019, 51, 129–137. [Google Scholar] [CrossRef]
- Lin, C.H.; Li, Y.H.; Wang, Y.Y.; Chang, W.D. Higher Neutrophil-To-Lymphocyte Ratio Was Associated with Increased Risk of Chronic Kidney Disease in Overweight/Obese but Not Normal-Weight Individuals. Int. J. Environ. Res. Public. Health 2022, 19, 8077. [Google Scholar] [CrossRef]
- Rustiasari, U.J.; Roelofs, J.J. The Role of Platelets in Diabetic Kidney Disease. Int. J. Mol. Sci. 2022, 23, 8270. [Google Scholar] [CrossRef]
- Kawahito, K.; Kobayashi, E.; Ohmori, M.; Harada, K.; Kitoh, Y.; Fujimura, A.; Fuse, K. Enhanced responsiveness of circulatory neutrophils after cardiopulmonary bypass: Increased aggregability and superoxide producing capacity. Artif. Organs 2000, 24, 37–42. [Google Scholar] [CrossRef]
- Lo, B.; Fijnheer, R.; Nierich, A.P.; Bruins, P.; Kalkman, C.J. C-reactive protein is a risk indicator for atrial fibrillation after myocardial revascularization. Ann. Thorac. Surg. 2005, 79, 1530–1535. [Google Scholar] [CrossRef] [PubMed]
- Gungor, H.; Babu, A.S.; Zencir, C.; Akpek, M.; Selvi, M.; Erkan, M.H.; Durmaz, S. Association of Preoperative Platelet-to-Lymphocyte Ratio with Atrial Fibrillation after Coronary Artery Bypass Graft Surgery. Med. Princ. Pract. 2017, 26, 164–168. [Google Scholar] [CrossRef] [PubMed]
- Fan, Q.; Gao, L.; Wang, Z.; Ndjana Lessomo, F.Y.; Wang, G. Prognostic Value of Platelet-to-Lymphocyte Ratio Combined with CHA(2)DS(2)-VAS(c) Score for Nonvalvular Atrial Fibrillation Induced Cardiogenic Cerebral Embolism. J. Inflamm. Res. 2023, 16, 5937–5947. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Sun, H.; Tang, Y.; Luo, Y.; Liu, H. Platelet-to-Lymphocyte Ratio Improves the Predictive Ability of the Risk Score for Atrial Fibrillation Recurrence After Radiofrequency Ablation. J. Inflamm. Res. 2023, 16, 6023–6038. [Google Scholar] [CrossRef]
- Chen, C.; Tang, X.; Fan, P. Platelet-to-Lymphocyte Ratio as an Independent Factor Associated With Atrial Tachyarrhythmia. Cureus 2023, 15, e46775. [Google Scholar] [CrossRef]
- He, L.; Liu, R.; Yue, H.; Zhang, X.; Pan, X.; Sun, Y.; Shi, J.; Zhu, G.; Qin, C.; Guo, Y. Interaction between neutrophil extracellular traps and cardiomyocytes contributes to atrial fibrillation progression. Signal Transduct. Target. Ther. 2023, 8, 279. [Google Scholar] [CrossRef]
- Li, T.; Wang, Y.; Wan, J.; Duan, Y.; Xu, Y.; Yang, M. The interaction between neutrophils and atrial myocytes in the occurrence and development of atrial fibrillation. BMC Cardiovasc. Disord. 2024, 24, 519. [Google Scholar] [CrossRef]
- Wang, X.; Pan, Y.; Zhang, R.; Wang, M.; Meng, X.; Li, Z.; Li, H.; Wang, Y.; Zhao, X.; Wang, Y.; et al. Inflammation and Adverse Outcomes in Patients With Acute Ischemic Stroke With and Without Chronic Kidney Disease. J. Am. Heart Assoc. 2024, 13, e033450. [Google Scholar] [CrossRef]
- Feng, Y.; Liu, X.; Tan, H. Causal association of peripheral immune cell counts and atrial fibrillation: A Mendelian randomization study. Front. Cardiovasc. Med. 2022, 9, 1042938. [Google Scholar] [CrossRef]
- Yen, C.H.; Wu, I.W.; Lee, C.C.; Hsu, K.H.; Sun, C.Y.; Chen, C.Y.; Pan, H.C.; Hsu, H.J. The prognostic value of peripheral total and differential leukocyte count in renal progression: A community-based study. PLoS ONE 2021, 16, e0258210. [Google Scholar] [CrossRef]
- Iijima, R.; Ndrepepa, G.; Mehilli, J.; Bruskina, O.; Schulz, S.; Schömig, A.; Kastrati, A. Relationship between platelet count and 30-day clinical outcomes after percutaneous coronary interventions. Pooled analysis of four ISAR trials. Thromb. Haemost. 2007, 98, 852–857. [Google Scholar] [PubMed]
- Li, X.; Wang, L.; Liu, M.; Zhou, H.; Xu, H. Association between neutrophil-to-lymphocyte ratio and diabetic kidney disease in type 2 diabetes mellitus patients: A cross-sectional study. Front. Endocrinol. 2023, 14, 1285509. [Google Scholar] [CrossRef] [PubMed]
- Shao, Q.; Chen, K.; Rha, S.W.; Lim, H.E.; Li, G.; Liu, T. Usefulness of Neutrophil/Lymphocyte Ratio as a Predictor of Atrial Fibrillation: A Meta-analysis. Arch. Med. Res. 2015, 46, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.; Zhou, J.; Chou, O.H.I.; Chan, J.; Leung, K.S.K.; Lee, T.T.L.; Wong, W.T.; Wai, A.K.C.; Liu, T.; Zhang, Q.; et al. Predictive value of neutrophil-to-lymphocyte ratio for atrial fibrillation and stroke in type 2 diabetes mellitus: The Hong Kong Diabetes Study. Endocrinol. Diabetes Metab. 2023, 6, e397. [Google Scholar] [CrossRef]
- Li, L.; Shen, Q.; Rao, S. Association of Neutrophil-to-Lymphocyte Ratio and Platelet-to-Lymphocyte Ratio with Diabetic Kidney Disease in Chinese Patients with Type 2 Diabetes: A Cross-Sectional Study. Ther. Clin. Risk Manag. 2022, 18, 1157–1166. [Google Scholar] [CrossRef]

| N | Mean | Std. Deviation | Min | Max | Median | IQR | |
|---|---|---|---|---|---|---|---|
| 25th–75th | |||||||
| Days of hospital | 1084 | 6.25 | 3.00 | 0.00 | 30.00 | 6.00 | 4.00–7.00 |
| NLR | 1066 | 4.95 | 4.03 | 0.19 | 38.25 | 3.74 | 2.44–5.95 |
| PLR | 1066 | 15.66 | 13.79 | 1.74 | 172.97 | 11.64 | 7.94–18.12 |
| Neutrophils ×103/µL | 1066 | 69.40 | 10.92 | 15.30 | 93.00 | 70.10 | 62.50–77.10 |
| Lymphocytes ×103/µL | 1066 | 19.79 | 9.44 | 2.40 | 80.0 | 18.70 | 12.90–25.83 |
| Platelet ×103/µL | 1067 | 234.10 | 88.65 | 32.00 | 780.00 | 223.00 | 180.00–274.00 |
| Hb (g/dL) | 960 | 12.90 | 2.40 | 3.40 | 19.60 | 13.20 | 11.80–14.40 |
| Leucocytes ×103/µL | 1066 | 12.45 | 55.92 | 0.00 | 1406.60 | 831 | 6.42–10.42 |
| CRP (mg/dL) | 938 | 2.62 | 4.71 | 0.02 | 42.84 | 0.92 | 0.36–2.66 |
| Fibrinogen (mg/dL) | 644 | 361.87 | 91.23 | 140.00 | 816.00 | 356.50 | 303.00–415.00 |
| Na (mmol/L) | 1078 | 138.43 | 5.10 | 104.00 | 149.00 | 140.00 | 137.00–141.00 |
| K (mmol/L) | 1016 | 4.45 | 0.61 | 2.30 | 7.40 | 4.40 | 4.10–4.80 |
| Urea (mg/dL) | 1080 | 48.83 | 25.50 | 11.00 | 189.00 | 42.00 | 33.00–57.00 |
| Creatinine (mg/dL) | 1079 | 1.02 | 0.42 | 0.38 | 5.49 | 0.91 | 0.76–1.14 |
| HbA1c (%) | 558 | 6.66 | 1.49 | 3.90 | 14.50 | 6.20 | 5.70–7.20 |
| CK (mg/dL) | 772 | 150.27 | 508.46 | 15.00 | 11,087.00 | 78.00 | 51.00–133.75 |
| CK-MB (mg/dL) | 655 | 25.64 | 31.44 | 6.00 | 431.00 | 20.00 | 15.00–27.00 |
| NT-proBNP (pg/mL) | 170 | 5081.77 | 5879.78 | 93.10 | 28,839.00 | 2718.00 | 1191.75–6527.25 |
| hsTnI (ng/mL) | 136 | 40.99 | 117.55 | 0.00 | 1009.00 | 10.05 | 6.29–25.55 |
| Total cholesterol (mg/dL) | 996 | 152.48 | 46.81 | 40.00 | 382.00 | 148.00 | 119.00–180.75 |
| LDL (mg/dL) | 309 | 94.19 | 37.73 | 17.00 | 209.00 | 89.00 | 66.00–118.00 |
| HDL (mg/dL) | 985 | 40.14 | 15.80 | 5.00 | 146.00 | 38.00 | 29.00–49.00 |
| TG (mg/dL) | 987 | 106.79 | 57.97 | 28.00 | 679.00 | 93.00 | 71.00–126.00 |
| Ferritin (mg/dL) | 713 | 253.64 | 1387.84 | 5.00 | 35,441.00 | 111.00 | 59.00–216.00 |
| Iron (mg/dL) | 770 | 65.37 | 37.39 | 6.00 | 253.00 | 57.50 | 38.00–83.25 |
| AST (U/L) | 983 | 44.76 | 116.53 | 7.00 | 1573.00 | 24.00 | 19.00–33.00 |
| ALT (U/L) | 1061 | 39.34 | 88.53 | 4.00 | 1829.00 | 22.00 | 16.00–34.00 |
| NLR | IQR | Kruskal–Wallis H Test | |||||||
|---|---|---|---|---|---|---|---|---|---|
| N | Mean | Std. Deviation | Min | Max | Median | 25th | 75th | ||
| Score AF + Comorbidities | |||||||||
| Absent | 1478 | 4.52 | 4.77 | 0.34 | 68.50 | 2.89 | 2.00 | 5.05 | H = 70.627 |
| AF | 773 | 4.80 | 4.06 | 0.21 | 38.25 | 3.62 | 2.38 | 5.53 | p < 0.001 ** |
| T2DM+AF | 75 | 4.45 | 3.13 | 0.95 | 15.64 | 3.44 | 2.32 | 5.95 | |
| CKD+AF | 196 | 5.51 | 4.08 | 0.19 | 30.07 | 4.56 | 2.83 | 6.71 | |
| T2DM+CKD+AF | 22 | 6.95 | 4.31 | 1.99 | 17.06 | 6.43 | 3.04 | 9.04 | |
| NLR | IQR | Kruskal–Wallis H Test | |||||||
|---|---|---|---|---|---|---|---|---|---|
| N | Mean | Std. Deviation | Min | Max | Median | 25th | 75th | ||
| AF + Comorbidities | |||||||||
| Absent | 1478 | 15.61 | 14.65 | 0.24 | 173.68 | 11.05 | 7.68 | 17.51 | H = 15.478 |
| AF | 773 | 14.92 | 13.13 | 1.74 | 172.97 | 11.41 | 7.74 | 16.70 | p = 0.004 ** |
| T2DM+AF | 75 | 14.89 | 10.76 | 3.04 | 64.14 | 11.02 | 7.89 | 19.27 | |
| CKD+AF | 196 | 18.55 | 16.89 | 3.14 | 135.52 | 13.37 | 8.90 | 22.00 | |
| T2DM+CKD+AF | 22 | 18.17 | 11.72 | 5.98 | 46.11 | 13.46 | 8.66 | 26.25 | |
| Diagnostic: AF | AUC | p-Value | 95% CI | Gini Index | Cut-Off Value | Sensitivity | Specificity | |
|---|---|---|---|---|---|---|---|---|
| L.inf | L.sup | |||||||
| Neutrophil/Lymphocyte Ratio | 0.565 | 0.000 ** | 0.541 | 0.590 | 0.131 | 3.06 | 0.594 | 0.468 |
| Platelet/Lymphocyte Ratio | 0.503 | 0.801 | 0.478 | 0.528 | 0.006 | 9.60 | 0.630 | 0.406 |
| Neutrophils/μL | 0.554 | 0.000 ** | 0.530 | 0.579 | 0.108 | 64.25 | 0.670 | 0.439 |
| Lymphocytes/μL | 0.432 | 0.000 ** | 0.408 | 0.457 | −0.136 | 4.95 | 0.986 | 0.029 |
| Platelets/μL | 0.416 | 0.000 ** | 0.391 | 0.440 | −0.169 | 73.00 | 0.992 | 0.011 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gosav, E.M.; Tanase, D.M.; Ouatu, A.; Buliga-Finis, O.N.; Popescu, D.; Dascalu, C.G.; Dima, N.; Badescu, M.C.; Rezus, C. The Role of Neutrophil-to-Lymphocyte Ratio and Platelet-to-Lymphocyte Ratio in Predicting Atrial Fibrillation and Its Comorbidities. Life 2025, 15, 960. https://doi.org/10.3390/life15060960
Gosav EM, Tanase DM, Ouatu A, Buliga-Finis ON, Popescu D, Dascalu CG, Dima N, Badescu MC, Rezus C. The Role of Neutrophil-to-Lymphocyte Ratio and Platelet-to-Lymphocyte Ratio in Predicting Atrial Fibrillation and Its Comorbidities. Life. 2025; 15(6):960. https://doi.org/10.3390/life15060960
Chicago/Turabian StyleGosav, Evelina Maria, Daniela Maria Tanase, Anca Ouatu, Oana Nicoleta Buliga-Finis, Diana Popescu, Cristina Gena Dascalu, Nicoleta Dima, Minerva Codruta Badescu, and Ciprian Rezus. 2025. "The Role of Neutrophil-to-Lymphocyte Ratio and Platelet-to-Lymphocyte Ratio in Predicting Atrial Fibrillation and Its Comorbidities" Life 15, no. 6: 960. https://doi.org/10.3390/life15060960
APA StyleGosav, E. M., Tanase, D. M., Ouatu, A., Buliga-Finis, O. N., Popescu, D., Dascalu, C. G., Dima, N., Badescu, M. C., & Rezus, C. (2025). The Role of Neutrophil-to-Lymphocyte Ratio and Platelet-to-Lymphocyte Ratio in Predicting Atrial Fibrillation and Its Comorbidities. Life, 15(6), 960. https://doi.org/10.3390/life15060960












