Associations Between Shift Work, Sociodemographic and Lifestyle Characteristics, Body Measurements, and MASLD
Abstract
1. Introduction
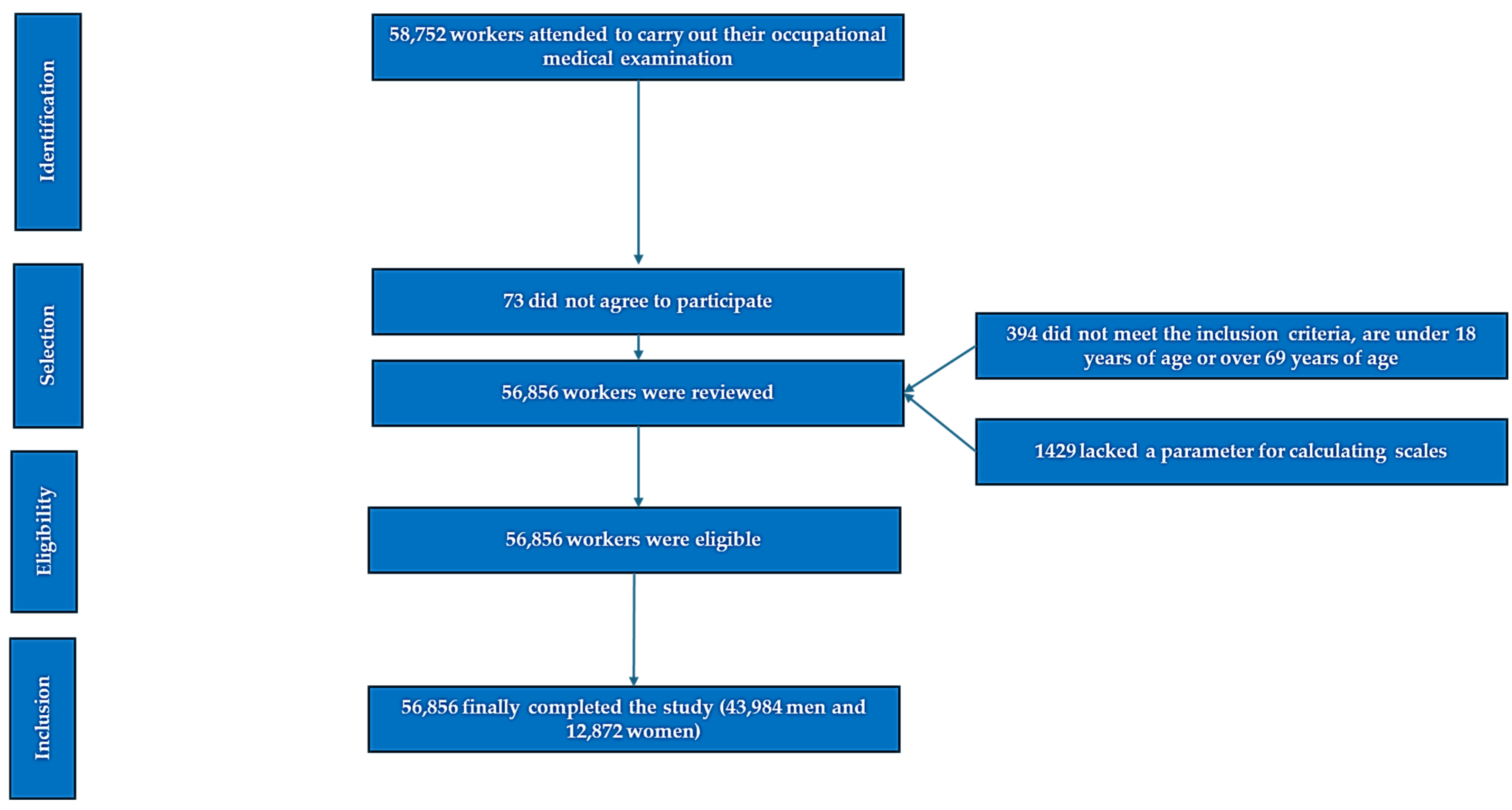
2. Methods
2.1. Participants
- Age between 18 and 69 years.
- Active employment under contract with one of the participating organizations.
- Provision of informed consent for study participation.
- Authorization for the use of personal health data for epidemiological research.
2.2. Variable Assessment
2.3. Anthropometric Assessment
2.4. Clinical Measurements
2.5. Laboratory Analysis
2.6. Non Alcoholic Fatty Liver Disease Scales (Table 1)
- Sex was recorded as male or female.
- Age was determined from the date of birth to the examination date.
- Educational level was categorized as primary, secondary, or tertiary (university) education.
- Social class was assigned based on the 2011 Spanish National Classification of Occupations (CNO-11) [26] following the Spanish Society of Epidemiology framework:
- Class I: University professionals, executives, elite athletes, and artists.
- Class II: Technicians and skilled self-employed workers.
- Class III: Manual laborers and less qualified workers.
| Index | Formula/Components | High-Risk Threshold |
|---|---|---|
| Fatty Liver Index (FLI) | FLI = [(e^(0.953 × ln(triglycerides) + 0.139 × BMI + 0.718 × ln(γ-GTP) + 0.053 × waist circumference − 15.745))/(1 + e^(0.953 × ln(triglycerides) + 0.139 × BMI + 0.718 × ln(γ-GTP) + 0.053 × waist circumference − 15.745))] × 100 | ≥60 |
| Hepatic Steatosis Index (HSI) | HSI = 8 × (ALT/AST) + BMI + 2 (if diabetic) + 2 (if woman) | ≥36 |
| Zhejliang University Index (ZJU) | ZJU Index = BMI (kg/m2) + fasting plasma glucose (mmol/L) + triglyceride (mmol/L) + 3 × [AST(IU/L)/ALT(IU/L)] + 2 (if woman) | ≥38 |
| Faty Liver Disease Index (FLD) | FLD index = BMI + TG + 3 × (ALT/AST ratio) + 2 × HG (yes = 1, no = 0) | ≥37 |
| Framingham Steatosis Index (FSI) | FSI = −7.981 + 0.011 × Age − 0.146 × Sex (female = 1, male = 0) + 0.173 × BMI + 0.007 × Triglycerides + 0.593 × Hypertension (yes = 1, no = 0) + 0.789 × Diabetes (yes = 1, no = 0) + 1.1 × (ALT/AST ratio > = 1.33, yes = 1, no = 0) | Continuos |
| Lipid Accumulation Product (LAP) | Men:(WC (cm) − 65) × TG (mmol/L)); Women:(WC (cm) − 58) × TG (mmol/L)) | ≥42.7 |
| BARD Score | BMI ≥ 28 = 1 point, (AST/ALT) ratio ≥ 0.8 = 2 points, type 2 diabetes mellitus = 1 point. | 2–4 points |
2.7. Statistical Analysis
3. Results
4. Discussion
4.1. Strengths and Limitations
4.2. Public Health and Occupational Implications
4.3. Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Koshy, A. Evolving Global Etiology of Hepatocellular Carcinoma (HCC): Insights and Trends for 2024. J. Clin. Exp. Hepatol. 2025, 15, 102406. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Younossi, Z.M.; Wong, G.; Anstee, Q.M.; Henry, L. The Global Burden of Liver Disease. Clin. Gastroenterol. Hepatol. 2023, 21, 1978–1991, Erratum in BMC Cardiovasc Disord. 2006, 6, 5. [Google Scholar] [CrossRef] [PubMed]
- Boccatonda, A.; Andreetto, L.; D’ardes, D.; Cocco, G.; Rossi, I.; Vicari, S.; Schiavone, C.; Cipollone, F.; Guagnano, M.T. From NAFLD to MAFLD: Definition, Pathophysiological Basis and Cardiovascular Implications. Biomedicines 2023, 11, 883. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Han, S.K.; Baik, S.K.; Kim, M.Y. Non-alcoholic fatty liver disease: Definition and subtypes. Clin. Mol. Hepatol. 2023, 29 (Suppl.), S5–S16. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Friedman, S.L.; Pinzani, M. Hepatic fibrosis 2022: Unmet needs and a blueprint for the future. Hepatology. 2022, 75, 473–488. [Google Scholar] [CrossRef] [PubMed]
- Ginès, P.; Krag, A.; Abraldes, J.G.; Solà, E.; Fabrellas, N.; Kamath, P.S. Liver cirrhosis. Lancet 2021, 398, 1359–1376. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, P.; Kulik, L.M. Hepatocellular Carcinoma: New Developments. Clin. Liver Dis. 2023, 27, 85–102. [Google Scholar] [CrossRef] [PubMed]
- Martínez Jover, A.; López González, A.A.; Tomás Gil, P.; Coll Villalonga, J.L.; Martí Lliteras, P.; Ramírez Manent, J.I. Association between nonalcoholic fatty liver disease risk scales and metabolic syndrome scales in 418.343 spanish workers. Acad. J. Health Sci. 2023, 38, 130–136. [Google Scholar] [CrossRef]
- Rinella, M.E.; Lazarus, J.V.; Ratziu, V.; Francque, S.M.; Sanyal, A.J.; Kanwal, F.; Romero, D.; Abdelmalek, M.F.; Anstee, Q.M.; Arab, J.P.; et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. J. Hepatol. 2023, 79, 1542–1556. [Google Scholar] [CrossRef] [PubMed]
- Hohor, S.; Mandanach, C.; Maftei, A.; Zugravu, C.A.; Oțelea, M.R. Impaired Melatonin Secretion, Oxidative Stress and Metabolic Syndrome in Night Shift Work. Antioxidants 2023, 12, 959. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hong, H.C.; Kim, Y.M. Multimorbidity and its Associated Factors in Korean Shift Workers: Population-Based Cross-Sectional Study. JMIR Public Health Surveill. 2024, 10, e55014. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chaput, J.P.; McHill, A.W.; Cox, R.C.; Broussard, J.L.; Dutil, C.; da Costa, B.G.G.; Sampasa-Kanyinga, H.; Wright, K.P., Jr. The role of insufficient sleep and circadian misalignment in obesity. Nat. Rev. Endocrinol. 2023, 19, 82–97. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lee, S.Y. Sleep Strategies for Shift Work Nurses. Hu Li Za Zhi 2024, 71, 22–28. (In Chinese) [Google Scholar] [CrossRef] [PubMed]
- Boivin, D.B.; Boudreau, P.; Kosmadopoulos, A. Disturbance of the Circadian System in Shift Work and Its Health Impact. J. Biol. Rhythms 2022, 37, 3–28. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kervezee, L.; Kosmadopoulos, A.; Boivin, D.B. Metabolic and cardiovascular consequences of shift work: The role of circadian disruption and sleep disturbances. Eur. J. Neurosci. 2020, 51, 396–412. [Google Scholar] [CrossRef]
- Bayon, V.; Berger, M.; Solelhac, G.; Haba-Rubio, J.; Marques-Vidal, P.; Strippoli, M.P.; Preisig, M.; Leger, D.; Heinzer, R. Impact of night and shift work on metabolic syndrome and its components: A cross-sectional study in an active middle-to-older-aged population-based sample. BMJ Open 2022, 12, e053591. [Google Scholar] [CrossRef] [PubMed]
- Tosoratto, J.; Tárraga López, P.J.; López-González, Á.A.; Vallejos, D.; Martínez-Almoyna Rifá, E.; Ramirez-Manent, J.I. Association of Shift Work, Sociodemographic Variables and Healthy Habits with Obesity Scales. Life 2024, 14, 1503. [Google Scholar] [CrossRef] [PubMed]
- Hamieh, N.; Airagnes, G.; Descatha, A.; Goldberg, M.; Limosin, F.; Roquelaure, Y.; Lemogne, C.; Zins, M.; Matta, J. Atypical working hours are associated with tobacco, cannabis and alcohol use: Longitudinal analyses from the CONSTANCES cohort. BMC Public Health 2022, 22, 1834. [Google Scholar] [CrossRef]
- Martínez-Almoyna Rifá, E.; Tomás-Gil, P.; Coll Villalonga, J.L.; Ramírez-Manent, J.I.; Martí-Lliteras, P.; López-González, A.A. Relationship between values of 7 NAFLD scales and different RCV scales in 219,477 Spanish workers. Acad. J. Health Sci. 2023, 38, 52–59. [Google Scholar] [CrossRef]
- Lee, J.-H.; Kim, D.; Kim, H.J.; Lee, C.-H.; Yang, J.I.; Kim, W.; Kim, Y.J.; Yoon, J.-H.; Cho, S.-H.; Sung, M.-W.; et al. Hepatic steatosis index: A simple screening tool reflecting nonalcoholic fatty liver disease. Dig. Liver Dis. 2010, 42, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Zhang, C.; Zhang, Y.; Tang, F.; Li, H.; Zhang, Q.; Lin, H.; Wu, S.; Liu, Y.; Xue, F. Metabolic syndrome and its components as predictors of nonalcoholic fatty liver disease in a northern urban Han Chinese population: A prospective cohort study. Atherosclerosis 2015, 240, 144–148. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Almoyna Rifá, E.; Tomás-Gil, P.; Coll Villalonga, J.L.; Ramírez-Manent, J.I.; Riera Routon, K.; López-González, A.A. Variables that influence the values of 7 scales that determine the risk of nonalcoholic fatty liver disease and liver fibrosis in 219,477 spanish workers. Acad. J. Health Sci. 2023, 38, 9–16. [Google Scholar] [CrossRef]
- Kahn, H.S. The “lipid accumulation product” performs better than the body mass index for recognizing cardiovascular risk: A population-based comparison. BMC Cardiovasc. Disord. 2005, 5, 26. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Harrison, S.A.; Oliver, D.; Arnold, H.L.; Gogia, S.; Neuschwander-Tetri, B.A. Development and validation of a simple NAFLD clinical scoring system for identifying patients without advanced disease. Gut 2008, 57, 1441–1447. [Google Scholar] [CrossRef] [PubMed]
- Mestre Font, M.; Busquets-Cortés, C.; Ramírez-Manent, J.I.; Vallejos, D.; Sastre Alzamora, T.; López-González, A.A. Influence of sociodemographic variables and healthy habits on the values of cardiometabolic risk scales in 386,924 spanish workers. Acad. J. Health Sci. 2024, 39, 112–121. [Google Scholar] [CrossRef]
- Domingo-Salvany, A.; Bacigalupe, A.; Carrasco, J.M.; Espelt, A.; Ferrando, J.; Borrell, C.; del Grupo de Determinantes Sociales de Sociedad Española de Epidemiología. Propuestas de clase social neoweberiana y neomarxista a partir de la Clasificación Nacional de Ocupaciones. Gac. Sanit. 2013, 27, 263–272. (In Spanish) [Google Scholar] [CrossRef]
- Mestre-Font, M.; Busquets-Cortés, C.; Ramírez-Manent, J.I.; Tomás-Gil, P.; Paublini, H.; López-González, A.A. Influence of sociodemographic variables and healthy habits on the values of overweight and obesity scales in 386,924 Spanish workers. Acad. J. Health Sci. 2024, 39, 27–35. [Google Scholar] [CrossRef]
- Lee, P.H.; Macfarlane, D.J.; Lam, T.H.; Stewart, S.M. Validity of the International Physical Activity Questionnaire Short Form (IPAQ-SF): A systematic review. Int. J. Behav. Nutr. Phys. Act. 2011, 8, 115. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mestre-Font, M.; Busquets-Cortés, C.; Ramírez-Manent, J.I.; Tomás-Gil, P.; Paublini, H.; López-González, A.A. Influence of sociodemographic variables and healthy habits on the values of type 2 diabetes risk scales. Acad. J. Health Sci. 2024, 39, 99–106. [Google Scholar] [CrossRef]
- Vetter, C.; Devore, E.E.; Wegrzyn, L.R.; Massa, J.; Speizer, F.E.; Kawachi, I.; Rosner, B.; Stampfer, M.J.; Schernhammer, E.S. Association Between Rotating Night Shift Work and Risk of Coronary Heart Disease Among Women. JAMA 2016, 315, 1726–1734. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xu, J.; Ni, S.; Wang, Y.; Yan, M.; Yang, X.; Ge, H.; Jia, Z.; Yang, Z.; Shan, A.; Liu, H.; et al. Shift work and nonalcoholic fatty liver disease incidence among Chinese rail workers: A 4-year longitudinal cohort study. Int. Arch. Occup. Environ. Health. 2023, 96, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Fishbein, A.B.; Knutson, K.L.; Zee, P.C. Circadian disruption and human health. J. Clin. Investig. 2021, 131, e148286. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kecklund, G.; Axelsson, J. Health consequences of shift work and insufficient sleep. BMJ 2016, 355, i5210. [Google Scholar] [CrossRef] [PubMed]
- Sooriyaarachchi, P.; Jayawardena, R.; Pavey, T.; King, N.A. Shift work and the risk for metabolic syndrome among healthcare workers: A systematic review and meta-analysis. Obes. Rev. 2022, 23, e13489. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhao, I.; Bogossian, F.; Turner, C. The effects of shift work and interaction between shift work and overweight/obesity on low back pain in nurses: Results from a longitudinal study. J. Occup. Environ. Med. 2012, 54, 820–825. [Google Scholar] [CrossRef] [PubMed]
- Jouffe, C.; Weger, B.D.; Martin, E.; Atger, F.; Weger, M.; Gobet, C.; Ramnath, D.; Charpagne, A.; Morin-Rivron, D.; Powell, E.E.; et al. Disruption of the circadian clock component BMAL1 elicits an endocrine adaptation impacting on insulin sensitivity and liver disease. Proc. Natl. Acad. Sci. USA 2022, 119, e2200083119. [Google Scholar] [CrossRef]
- Ganesan, S.; Magee, M.; Stone, J.E.; Mulhall, M.D.; Collins, A.; Howard, M.E.; Lockley, S.W.; Rajaratnam, S.M.W.; Sletten, T.L. The Impact of Shift Work on Sleep, Alertness and Performance in Healthcare Workers. Sci. Rep. 2019, 9, 4635. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Costa, M.; Reddy, A.B. Circadian rhythms and the liver. Liver Int. 2023, 43, 1045–1057. [Google Scholar] [CrossRef]
- Lempesis, I.G. Illuminating the metabolic effects of circadian misalignment. Nat. Rev. Endocrinol. 2025, 21, 202. [Google Scholar] [CrossRef]
- Ferrell, J.M. Circadian rhythms and inflammatory diseases of the liver and gut. Liver Res. 2023, 7, 196–206. [Google Scholar] [CrossRef]
- Woodie, L.N.; Oral, K.T.; Krusen, B.M.; Lazar, M.A. The Circadian Regulation of Nutrient Metabolism in Diet-Induced Obesity and Metabolic Disease. Nutrients 2022, 14, 3136. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schettini, M.A.S.; Passos, R.F.D.N.; Koike, B.D.V. Shift Work and Metabolic Syndrome Updates: A Systematic Review. Sleep Sci. 2023, 16, 237–247. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cheng, W.J.; Liu, C.S.; Hu, K.C.; Cheng, Y.F.; Karhula, K.; Härmä, M. Night shift work and the risk of metabolic syndrome: Findings from an 8-year hospital cohort. PLoS ONE 2021, 16, e0261349. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bolshette, N.; Ibrahim, H.; Reinke, H.; Asher, G. Circadian regulation of liver function: From molecular mechanisms to disease pathophysiology. Nat. Rev. Gastroenterol. Hepatol. 2023, 20, 695–707. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.B. Noninvasive serum biomarkers for liver steatosis in nonalcoholic fatty liver disease: Current and future developments. Clin. Mol. Hepatol. 2023, 29 (Suppl.), S150–S156. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, S.; Wang, Y.; Wang, Z.; Wang, H.; Xue, C.; Li, Q.; Guan, W.; Yuan, J. Rotating night shift work and non-alcoholic fatty liver disease among steelworkers in China: A cross-sectional survey. Occup. Environ. Med. 2020, 77, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Zhao, E.; Tait, C.; Minacapelli, C.D.; Catalano, C.; Rustgi, V.K. Circadian Rhythms, the Gut Microbiome, and Metabolic Disorders. Gastro Hep. Adv. 2022, 1, 93–105. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mazzoccoli, G.; De Cosmo, S.; Mazza, T. The Biological Clock: A Pivotal Hub in Non-alcoholic Fatty Liver Disease Pathogenesis. Front. Physiol. 2018, 9, 193. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bu, L.-F.; Xiong, C.-Y.; Zhong, J.-Y.; Xiong, Y.; Li, D.-M.; Hong, F.-F.; Yang, S.-L. Non-alcoholic fatty liver disease and sleep disorders. World J. Hepatol. 2024, 16, 304–315. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Clark, A.B.; Coates, A.M.; Davidson, Z.E.; Bonham, M.P. Dietary Patterns under the Influence of Rotational Shift Work Schedules: A Systematic Review and Meta-Analysis. Adv. Nutr. 2023, 14, 295–316. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Flahr, H.; Brown, W.J.; Kolbe-Alexander, T.L. A systematic review of physical activity-based interventions in shift workers. Prev. Med. Rep. 2018, 10, 323–331. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Acabchuk, R.L.; Kamath, J.; Salamone, J.D.; Johnson, B.T. Stress and chronic illness: The inflammatory pathway. Soc. Sci. Med. 2017, 185, 166–170. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Khosravipour, M.; Khanlari, P.; Khazaie, S.; Khosravipour, H.; Khazaie, H. A systematic review and meta-analysis of the association between shift work and metabolic syndrome: The roles of sleep, gender, and type of shift work. Sleep Med. Rev. 2021, 57, 101427. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Lee, W. Shift work and non-alcoholic fatty liver disease in young, healthy workers. Sci. Rep. 2024, 14, 19367. [Google Scholar] [CrossRef]
- Zhang, J.W.; Zhang, N.; Lyu, Y.; Zhang, X.F. Influence of Sex in the Development of Liver Diseases. Semin. Liver Dis. 2025, 45, 15–32. [Google Scholar] [CrossRef] [PubMed]
- Bushnell, P.T.; Pana-Cryan, R.; Howard, J.; Quay, B.; Ray, T.K. Measuring the benefits of occupational safety and health research with economic metrics: Insights from the National Institute for Occupational Safety and Health. Am. J. Ind. Med. 2022, 65, 323–342. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- DeMaris, A. Combating unmeasured confounding in cross-sectional studies: Evaluating instrumental-variable and Heckman selection models. Psychol. Methods 2014, 19, 380–397. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
| No Shift Work | Shift Work | No Shift Work | Shift Work | |||
|---|---|---|---|---|---|---|
| Men n = 7444 | Men n = 5238 | Women n = 4422 | Women n = 6787 | |||
| Mean (SD) | Mean (SD) | p-Value | Mean (SD) | Mean (SD) | p-Value | |
| Age (years) | 44.6 (8.1) | 43.8 (10.9) | <0.001 | 42.4 (7.4) | 42.0 (10.0) | 0.011 |
| Height (cm) | 176.9 (5.7) | 175.1 (6.7) | <0.001 | 165.8 (5.1) | 162.5 (6.3) | <0.001 |
| Weight (kg) | 84.9 (14.9) | 85.2 (12.1) | 0.328 | 66.2 (11.6) | 66.7 (11.0) | 0.025 |
| Waist (cm) | 88.7 (9.2) | 88.2 (10.8) | 0.004 | 73.5 (8.4) | 74.1 (9.1) | <0.001 |
| Systolic BP (mmHg) | 129.4 (14.1) | 130.3 (17.0) | 0.001 | 117.9 (15.1) | 119.1 (15.7) | <0.001 |
| Diastolic BP (mmHg) | 78.8 (9.8) | 79.2 (11.8) | 0.029 | 72.6 (9.9) | 72.7 (10.6) | 0.650 |
| Total cholesterol (mg(dL) | 195.0 (38.3) | 198.3 (35.3) | <0.001 | 190.8 (34.9) | 192.2 (36.2) | 0.031 |
| HDL-cholesterol (mg/dL) | 51.7 (11.4) | 48.5 (8.3) | <0.001 | 64.9 (16.1) | 56.0 (7.7) | <0.001 |
| LDL-cholesterol (mg/dL) | 120.4 (37.6) | 121.6 (32.6) | 0.050 | 110.2 (33.2) | 115.3 (34.1) | <0.001 |
| Triglycerides (mmHg) | 128.5 (81.7) | 133.2 (78.4) | 0.001 | 85.7 (41.0) | 97.9 (58.0) | <0.001 |
| Glucose (mg/dL) | 91.1 (17.6) | 96.4 (25.4) | <0.001 | 86.6 (11.7) | 89.9 (13.8) | <0.001 |
| AST (U/I) | 24.1 (10.3) | 26.4 (14.9) | <0.001 | 19.8 (9.7) | 22.8 (12.0) | <0.001 |
| ALT (U/I) | 31.0 (19.1) | 35.9 (19.2) | 0.008 | 17.8 (6.6) | 20.2 (9.2) | <0.001 |
| GGT (U/I) | 35.7 (33.9) | 38.3 (36.5) | <0.001 | 21.4 (16.5) | 23.0 (25.5) | <0.001 |
| % | % | p-Value | % | % | p-Value | |
| 18–29 years old | 3.1 | 11.8 | <0.001 | 2.7 | 12.0 | <0.001 |
| 30–39 years old | 23.9 | 23.3 | 32.8 | 28.0 | ||
| 40–49 years old | 43.8 | 30.5 | 46.9 | 34.5 | ||
| 50–59 years old | 26.7 | 27.6 | 16.4 | 23.7 | ||
| 60–69 years old | 2.5 | 6.8 | 1.2 | 1.8 | ||
| White collar | 97.3 | 0 | <0.001 | 99.7 | 0.0 | <0.001 |
| Blue collar | 2.7 | 100 | 0.3 | 100.1 | ||
| Non-smokers | 69.2 | 68.5 | 0.203 | 65.7 | 64.7 | 0.129 |
| Smokers | 30.8 | 31.5 | 34.3 | 35.3 |
| Men | Women | |||||
|---|---|---|---|---|---|---|
| Non Shift Work n = 7444 | Shift Work n = 5238 | Non Shift Work n = 4422 | Shift Work n = 6787 | |||
| % | % | p-Value | % | % | p-Value | |
| Fatty liver index high | 28.2 | 32.2 | <0.001 | 5.4 | 6.2 | <0.001 |
| Hepatic steatosis index high | 58.6 | 65.6 | <0.001 | 45.2 | 54.5 | <0.001 |
| Zhejian University index high | 41.2 | 53.2 | <0.001 | 27.3 | 50.1 | <0.001 |
| Fatty liver disease index high | 65.6 | 73.4 | <0.001 | 52.3 | 60.1 | <0.001 |
| Lipid accumulation product high | 43.7 | 46.4 | <0.001 | 20.4 | 27.1 | <0.001 |
| BARD score high | 43.5 | 71.5 | <0.001 | 49.9 | 85.7 | <0.001 |
| FLI High | HSI High | ZJU High | FLD High | LAP High | BARD High | |
|---|---|---|---|---|---|---|
| OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | |
| Female | 1 | 1 | 1 | 1 | 1 | 1 |
| Male | 6.56 (5.93–7.26) | 1.45 (1.20–1.75) | 1.47 (1.21–1.79) | 2.08 (1.91–2.25) | 2.51 (2.37–2.66) | 1.48 (1.39–1.57) |
| 18–29 years old | 1 | 1 | 1 | 1 | 1 | 1 |
| 30–39 years old | 1.13 (1.10–1.16) | 1.10 (1.06–1.15) | 1.12 (1.06–1.18) | 1.15 (1.08–1.22) | 1.17 (1.08–1.27) | 1.20 (1.09–1.29) |
| 40–49 years old | 1.44 (1.19–1.74) | 1.51 (1.39–1.63) | 1.29 (1.20–1.39) | 1.26 (1.17–1.35) | 1.51 (1.39–1.63) | 2.39 (2.21–2.57) |
| 50–59 years old | 2.88 (2.35–3.53) | 1.79 (1.62–1.97) | 1.81 (1.68–1.94) | 1.60 (1.43–1.77) | 2.30 (1.95–2.70) | 2.66 (2.41–2.91) |
| 60–69 years old | 4.61 (3.45–6.16) | 2.09 (1.89–2.29) | 2.48 (2.23–2.73) | 1.99 (1.80–2.18) | 3.16 (2.61–3.83) | 3.13 (2.88–3.38) |
| White collar | 1 | 1 | 1 | 1 | 1 | 1 |
| Blue collar | 3.06 (2.30–4.06) | 6.88 (6.50–7.16) | 8.12 (7.60–8.64) | 2.18 (2.05–2.31) | 2.07 (1.88–2.26) | 1.45 (1.30–1.6) |
| Non-shift work | 1 | 1 | 1 | 1 | 1 | 1 |
| Shift work | 2.45 (1.84–3.26) | 7.83 (7.40–8.26) | 5.91 (5.60–6.22) | 2.53 (2.31–2.75) | 1.57 (1.39–1.75) | 3.83 (3.60–4.06) |
| Non-smokers | 1 | 1 | 1 | 1 | 1 | 1 |
| Smokers | 1.10 (1.04–1.17) | 1.08 (1.05–1.12) | 1.09 (1.05–1.13) | 1.18 (1.09–1.27) | 1.05 (1.01–1.12) | 1.23 (1.16–1.30) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tosoratto, J.; Tárraga López, P.J.; López-González, Á.A.; Busquets-Cortes, C.; Obrador de Hevia, J.; Ramirez-Manent, J.I. Associations Between Shift Work, Sociodemographic and Lifestyle Characteristics, Body Measurements, and MASLD. Life 2025, 15, 961. https://doi.org/10.3390/life15060961
Tosoratto J, Tárraga López PJ, López-González ÁA, Busquets-Cortes C, Obrador de Hevia J, Ramirez-Manent JI. Associations Between Shift Work, Sociodemographic and Lifestyle Characteristics, Body Measurements, and MASLD. Life. 2025; 15(6):961. https://doi.org/10.3390/life15060961
Chicago/Turabian StyleTosoratto, Javier, Pedro Juan Tárraga López, Ángel Arturo López-González, Carla Busquets-Cortes, Joan Obrador de Hevia, and José Ignacio Ramirez-Manent. 2025. "Associations Between Shift Work, Sociodemographic and Lifestyle Characteristics, Body Measurements, and MASLD" Life 15, no. 6: 961. https://doi.org/10.3390/life15060961
APA StyleTosoratto, J., Tárraga López, P. J., López-González, Á. A., Busquets-Cortes, C., Obrador de Hevia, J., & Ramirez-Manent, J. I. (2025). Associations Between Shift Work, Sociodemographic and Lifestyle Characteristics, Body Measurements, and MASLD. Life, 15(6), 961. https://doi.org/10.3390/life15060961







