Exceptional Heme Tolerance in Serratia plymuthica: Proteomic Insights into Oxidative Stress Adaptation in the Aedes aegypti Midgut
Abstract
1. Introduction
2. Material and Methods
2.1. Microorganism and Culture Conditions
2.2. Proteomic Analysis
2.2.1. Obtaining of Bacterial Biomass
2.2.2. Protein Extraction
2.2.3. Protein Digestion
2.2.4. LC-MS/MS Analysis
2.2.5. Proteomics Data Analysis
3. Results
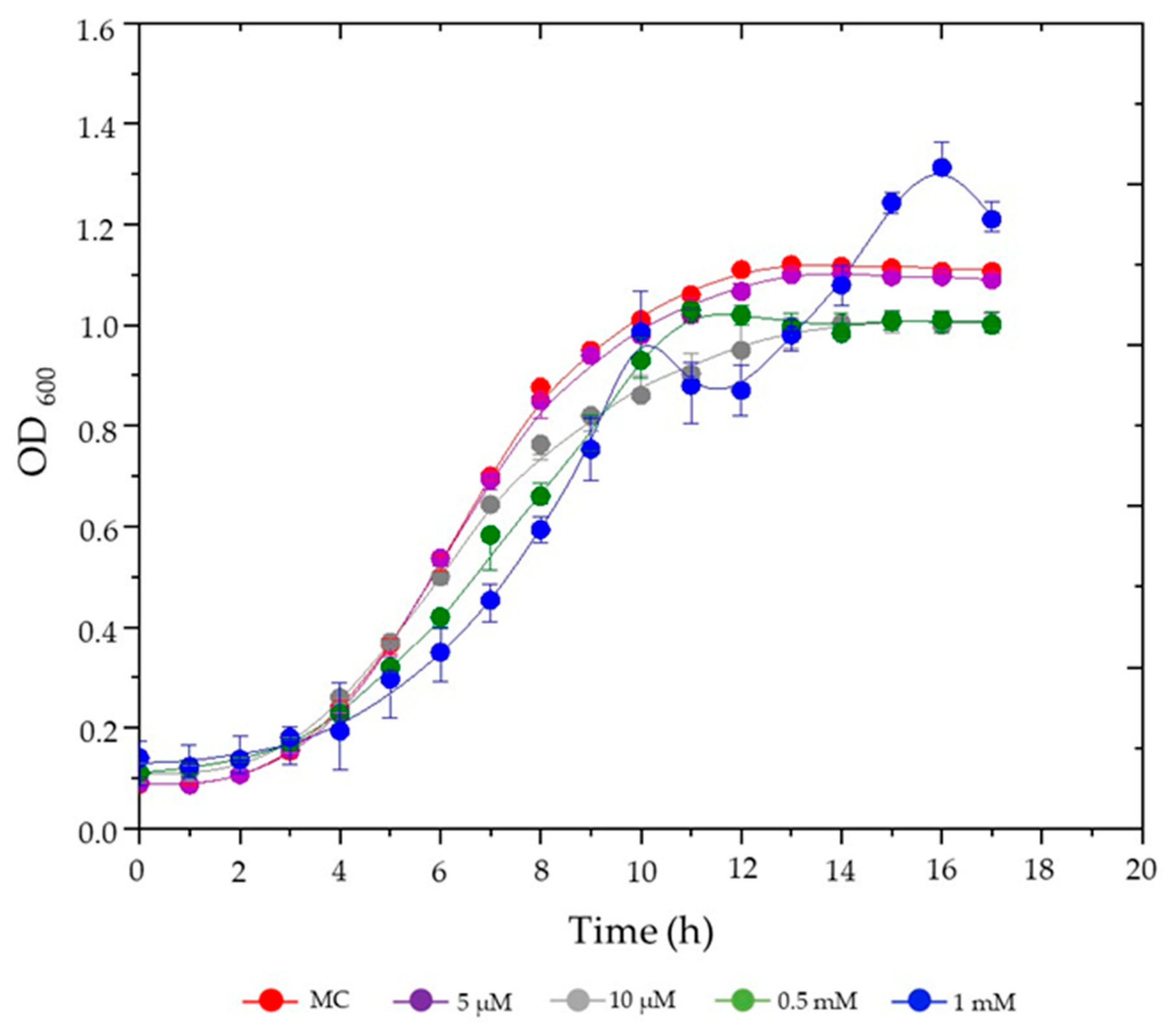
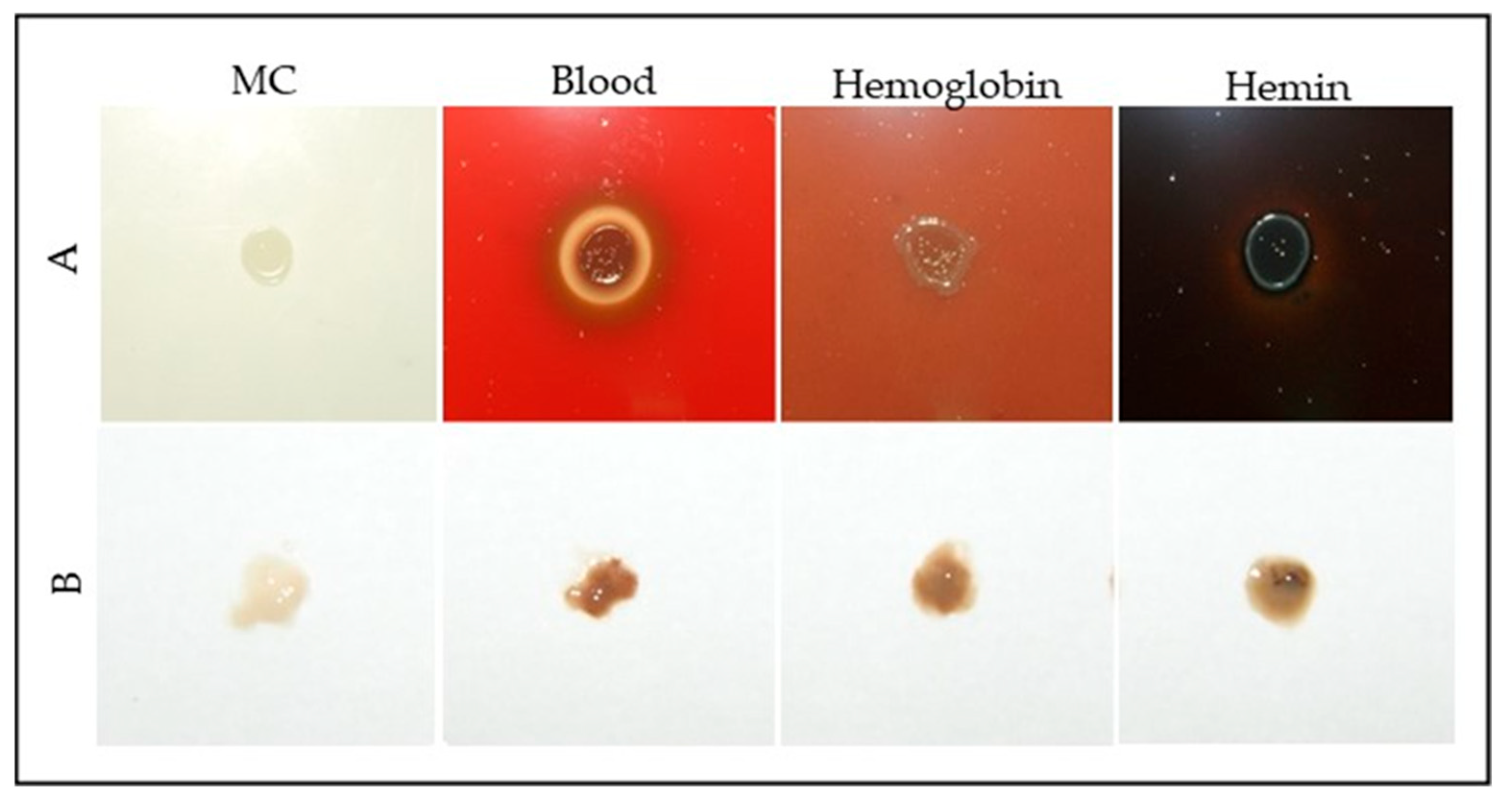
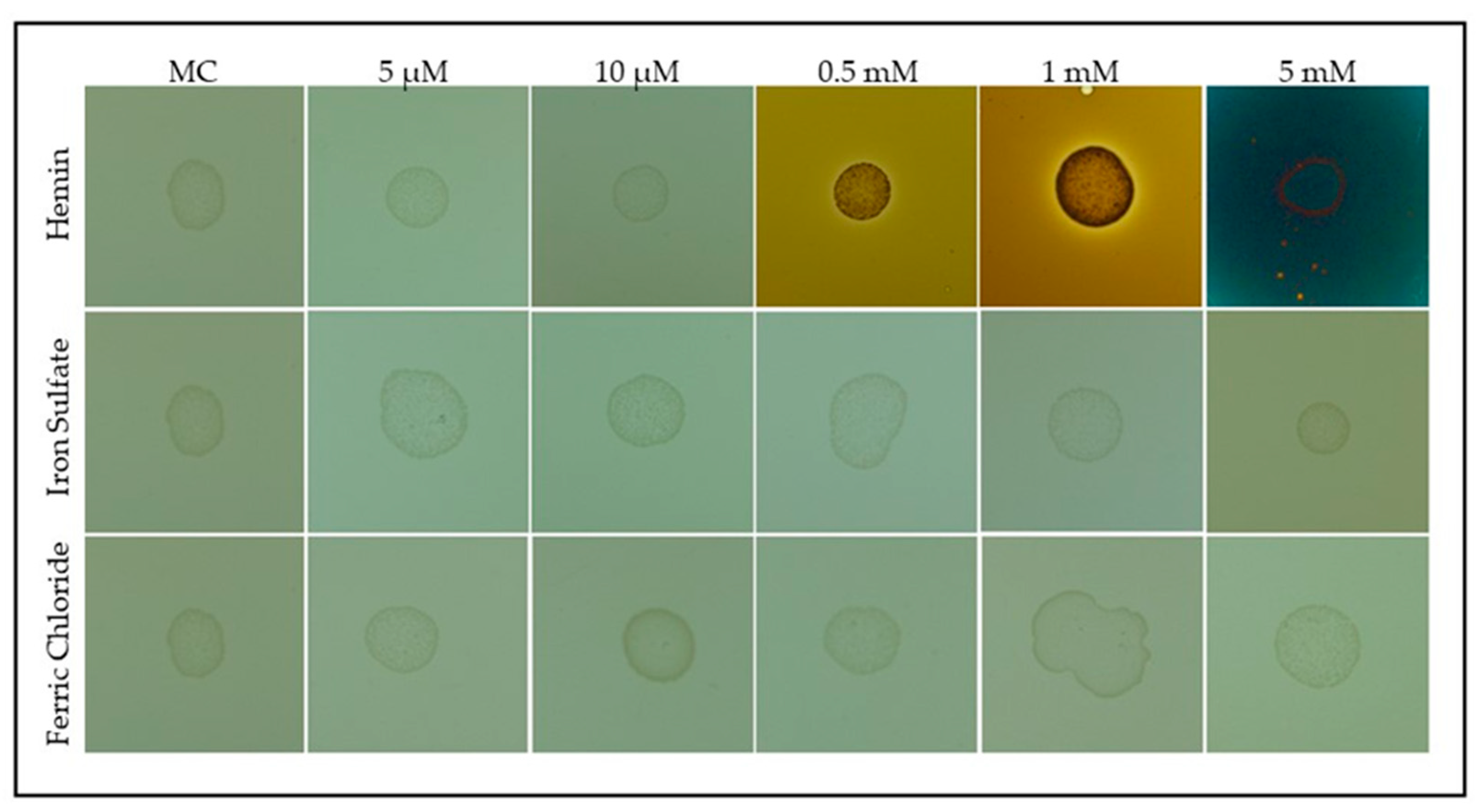
3.1. Effects of Hemin Supplementation on S. plymuthica Growth
3.2. Effects of Hemin on Proteomic Profiles
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gómez, M.; Martinez, D.; Muñoz, M.; Ramírez, J.D. Aedes aegypti and Aedes albopictus microbiome/virome: New strategies for controlling arboviral transmission? Parasit. Vectors 2022, 15, 287. [Google Scholar] [CrossRef] [PubMed]
- Talyuli, O.A.C.; Bottino-Rojas, V.; Polycarpo, C.R.; Oliveira, P.L.; Paiva-Silva, G.O. Non-immune traits triggered by blood intake impact vectorial competence. Front. Physiol. 2021, 12, 638033. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, J.H.; Gonçalves, R.L.; Lara, F.A.; Dias, F.A.; Gandara, A.C.; Menna-Barreto, R.F.; Edwards, M.C.; Laurindo, F.R.; Silva-Neto, M.A.; Sorgine, M.H.; et al. Blood meal-derived heme decreases ROS levels in the midgut of Aedes aegypti and allows proliferation of intestinal microbiota. PLoS Pathog. 2011, 7, e1001320. [Google Scholar] [CrossRef]
- Oliveira, J.H.M.; Talyuli, O.A.C.; Goncalves, R.L.S.; Paiva-Silva, G.O.; Sorgine, M.H.F.; Alvarenga, P.H.; Oliveira, P.L. Catalase protects Aedes aegypti from oxidative stress and increases midgut infection prevalence of Dengue but not Zika. PLoS Negl. Trop. Dis. 2017, 11, e0005525. [Google Scholar] [CrossRef]
- Orozimbo, K.B.D.S.; Tauil, D.D.S.G.; Licurgo, A.M.; Moreira, F.F.; Araújo, J.D.S.; Bertonceli, M.A.A.; Seabra, S.H.; Machado, O.L.T.; Lemos, F.J.A. Structural and functional analysis of hemoglobin binding to the peritrophic matrix during blood digestion in Aedes aegypti. Insects 2025, 16, 116. [Google Scholar] [CrossRef] [PubMed]
- Bottino-Rojas, V.; Talyuli, O.A.; Jupatanakul, N.; Sim, S.; Dimopoulos, G.; Venancio, T.M.; Bahia, A.C.; Sorgine, M.H.; Oliveira, P.L.; Paiva-Silva, G.O. Heme signaling impacts global gene expression, immunity and dengue virus infectivity in Aedes aegypti. PLoS ONE 2015, 10, e0135985. [Google Scholar] [CrossRef]
- Pascoa, V.; Oliveira, P.L.; Dansa-Petretski, M.; Silva, J.R.; Alvarenga, P.H.; Jacobs-Lorena, M.; Lemos, F.J. Aedes aegypti peritrophic matrix and its interaction with heme during blood digestion. Insect Biochem. Mol. Biol. 2002, 32, 517–523. [Google Scholar] [CrossRef]
- Eggleston, H.; Adelman, Z.N. Transcriptomic analyses of Aedes aegypti cultured cells and ex vivo midguts in response to an excess or deficiency of heme: A quest for transcriptionally-regulated heme transporters. BMC Genom. 2020, 21, 604. [Google Scholar] [CrossRef]
- Liu, H.; Yin, J.; Huang, X.; Zang, C.; Zhang, Y.; Cao, J.; Gong, M. Mosquito gut microbiota: A review. Pathogens 2024, 13, 691. [Google Scholar] [CrossRef]
- Gaio, A.O.; Gusmão, D.S.; Santos, A.V.; Berbert-Molina, M.A.; Pimenta, P.F.; Lemos, F.J. Contribution of midgut bacteria to blood digestion and egg production in Aedes aegypti (Diptera: Culicidae) (L.). Parasit. Vectors 2011, 4, 105. [Google Scholar] [CrossRef]
- Gusmão, D.S.; Santos, A.V.; Marini, D.C.; Bacci, M.; Berbert-Molina, M.A.; Lemos, F.J. Culture-dependent and culture-independent characterization of microorganisms associated with Aedes aegypti (Diptera: Culicidae) (L.) and dynamics of bacterial colonization in the midgut. Acta Trop. 2010, 115, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Kulkova, I.; Wróbel, B.; Dobrzyński, J. Serratia spp. as plant growth-promoting bacteria alleviating salinity, drought, and nutrient imbalance stresses. Front. Microbiol. 2024, 15, 1342331. [Google Scholar] [CrossRef]
- Nordstedt, N.P.; Jones, M.L. Genomic analysis of Serratia plymuthica MBSA-MJ1: A plant growth promoting rhizobacteria that improves water stress tolerance in greenhouse ornamentals. Front. Microbiol. 2021, 12, 653556. [Google Scholar] [CrossRef]
- Kakani, P.; Gupta, L.; Kumar, S. Heme-Peroxidase 2, a peroxinectin-like gene, regulates bacterial homeostasis in Anopheles stephensi midgut. Front. Physiol. 2020, 11, 572340. [Google Scholar] [CrossRef]
- Vicente, C.S.; Nascimento, F.X.; Ikuyo, Y.; Cock, P.J.; Mota, M.; Hasegawa, K. The genome and genetics of a high oxidative stress tolerant Serratia sp. LCN16 isolated from the plant parasitic nematode Bursaphelenchus xylophilus. BMC Genom. 2016, 17, 301. [Google Scholar] [CrossRef]
- Wang, H.; Liu, H.; Peng, H.; Wang, Y.; Zhang, C.; Guo, X.; Wang, H.; Liu, L.; Lv, W.; Cheng, P.; et al. A symbiotic gut bacterium enhances Aedes albopictus resistance to insecticide. PLoS Negl. Trop. Dis. 2022, 16, e0010208. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ramirez, J.L.; Souza-Neto, J.; Torres Cosme, R.; Rovira, J.; Ortiz, A.; Pascale, J.M.; Dimopoulos, G. Reciprocal tripartite interactions between the Aedes aegypti midgut microbiota, innate immune system and dengue virus influences vector competence. PLoS Negl. Trop. Dis. 2012, 6, e1561. [Google Scholar] [CrossRef] [PubMed]
- Damerval, C.; De Vienne, D.; Zivy, M.; Thiellement, H. Technical improvements in two-dimensional electrophoresis increase the level of genetic variation detected in wheat-seedling proteins. Electrophoresis 1986, 7, 52–54. [Google Scholar] [CrossRef]
- Reis, R.S.; Vale, E.M.; Heringer, A.S.; Santa-Catarina, C.; Silveira, V. Putrescine induces somatic embryo development and proteomic changes in embryogenic callus of sugarcane. J. Proteom. 2016, 130, 170–179. [Google Scholar] [CrossRef]
- Perez-Riverol, Y.; Bandla, C.; Kundu, D.J.; Kamatchinathan, S.; Bai, J.; Hewapathirana, S.; John, N.S.; Prakash, A.; Walzer, M.; Wang, S.; et al. The PRIDE database at 20 years: 2025 update. Nucleic Acids Res. 2025, 53, D543–D553. [Google Scholar] [CrossRef]
- Conesa, A.; Götz, S.; García-Gómez, J.M.; Terol, J.; Talón, M.; Robles, M. Blast2GO: A universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics 2005, 21, 3674–3676. [Google Scholar] [CrossRef] [PubMed]
- Wan, S.; Mak, M.W.; Kung, S.Y. FUEL-mLoc: Feature-unified prediction and explanation of multi-localization of cellular proteins in multiple organisms. Bioinformatics 2017, 33, 749–750. [Google Scholar] [CrossRef]
- Ge, S.X.; Jung, D.; Yao, R. ShinyGO: A graphical enrichment tool for animals and plants. Bioinformatics 2020, 36, 2628–2629. [Google Scholar] [CrossRef] [PubMed]
- Fox, D.; Asadollahi, K.; Samuels, I.; Spicer, B.; Kropp, A.; Lupton, C.; Lim, K.; Wang, C.; Venugopal, H.; Dramicanin, M.; et al. Inhibiting heme-piracy by pathogenic Escherichia coli using de novo-designed proteins. bioRxiv, 2024. [Google Scholar] [CrossRef]
- Nitzan, Y.; Ladan, H.; Malik, Z. Growth-inhibitory effect of hemin on staphylococci. Curr. Microbiol. 1987, 14, 279–284. [Google Scholar] [CrossRef]
- Akhter, F.; Womack, E.; Vidal, J.E.; Le Breton, Y.; McIver, K.S.; Pawar, S.; Eichenbaum, Z. Hemoglobin stimulates vigorous growth of Streptococcus pneumoniae and shapes the pathogen’s global transcriptome. Sci. Rep. 2020, 10, 15202. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lan, C.Q.; Oddone, G.; Mills, D.A.; Block, D.E. Kinetics of Lactococcus lactis growth and metabolite formation under aerobic and anaerobic conditions in the presence or absence of hemin. Biotechnol. Bioeng. 2006, 95, 1070–1080. [Google Scholar] [CrossRef] [PubMed]
- Torres, V.J.; Stauff, D.L.; Pishchany, G.; Bezbradica, J.S.; Gordy, L.E.; Iturregui, J.; Anderson, K.L.; Dunman, P.M.; Joyce, S.; Skaar, E.P. A Staphylococcus aureus regulatory system that responds to host heme and modulates virulence. Cell Host Microbe. 2007, 1, 109–119. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Létoffé, S.; Delepelaire, P.; Wandersman, C. Functional differences between heme permeases: Serratia marcescens HemTUV permease exhibits a narrower substrate specificity (restricted to heme) than the Escherichia coli DppABCDF peptide-heme permease. J. Bacteriol. 2008, 190, 1866–1870. [Google Scholar] [CrossRef]
- Perry, R.D.; Pendrak, M.L.; Schuetze, P. Identification and cloning of a hemin storage locus involved in the pigmentation phenotype of Yersinia pestis. J. Bacteriol. 1990, 172, 5929–5937. [Google Scholar] [CrossRef]
- Perry, R.D.; Lucier, T.S.; Sikkema, D.J.; Brubaker, R.R. Storage reservoirs of hemin and inorganic iron in Yersinia pestis. Infect. Immun. 1993, 61, 32–39. [Google Scholar] [CrossRef]
- Stojiljkovic, I.; Hantke, K. Transport of haemin across the cytoplasmic membrane through a haemin-specific periplasmic binding-protein-dependent transport system in Yersinia enterocolitica. Mol. Microbiol. 1994, 13, 719–732. [Google Scholar] [CrossRef] [PubMed]
- Cwerman, H.; Wandersman, C.; Biville, F. Heme and a five-amino-acid hemophore region form the bipartite stimulus triggering the has signaling cascade. J. Bacteriol. 2006, 188, 3357–3364. [Google Scholar] [CrossRef]
- Benevides-Matos, N.; Biville, F. The Hem and Has haem uptake systems in Serratia marcescens. Microbiology 2010, 156, 1749–1757. [Google Scholar] [CrossRef] [PubMed]
- Dent, A.T.; Wilks, A. Contributions of the heme coordinating ligands of the Pseudomonas aeruginosa outer membrane receptor HasR to extracellular heme sensing and transport. J. Biol. Chem. 2020, 295, 10456–10467. [Google Scholar] [CrossRef]
- Winterbourn, C.C. Toxicity of iron and hydrogen peroxide: The Fenton reaction. Toxicol. Lett. 1995, 82–83, 969–974. [Google Scholar] [CrossRef]
- Luo, D.; Wu, Z.; Bai, Q.; Zhang, Y.; Huang, M.; Huang, Y.; Li, X. Universal Stress Proteins: From Gene to Function. Int. J. Mol. Sci. 2023, 24, 4725. [Google Scholar] [CrossRef] [PubMed]
- Erdner, D.L.; Anderson, D.M. Ferredoxin and flavodoxin as biochemical indicators of iron limitation during open-ocean iron enrichment. Limnol. Oceanogr. 1999, 44, 1609–1615. [Google Scholar] [CrossRef]
- Tognetti, V.B.; Zurbriggen, M.D.; Morandi, E.N.; Fillat, M.F.; Valle, E.M.; Hajirezaei, M.R.; Carrillo, N. Enhanced plant tolerance to iron starvation by functional substitution of chloroplast ferredoxin with a bacterial flavodoxin. Proc. Natl. Acad. Sci. USA 2007, 104, 11495–11500. [Google Scholar] [CrossRef]
- Imlay, J.A. Pathways of oxidative damage. Annu. Rev. Microbiol. 2003, 57, 395–418. [Google Scholar] [CrossRef]
- Kruger, N.J.; von Schaewen, A. The oxidative pentose phosphate pathway: Structure and organisation. Curr. Opin. Plant Biol. 2003, 6, 236–246. [Google Scholar] [CrossRef]
- Martinson, V.G.; Strand, M.R. Diet–Microbiota Interactions Alter Mosquito Development. Front. Microbiol. 2021, 12, 650743. [Google Scholar] [CrossRef] [PubMed]
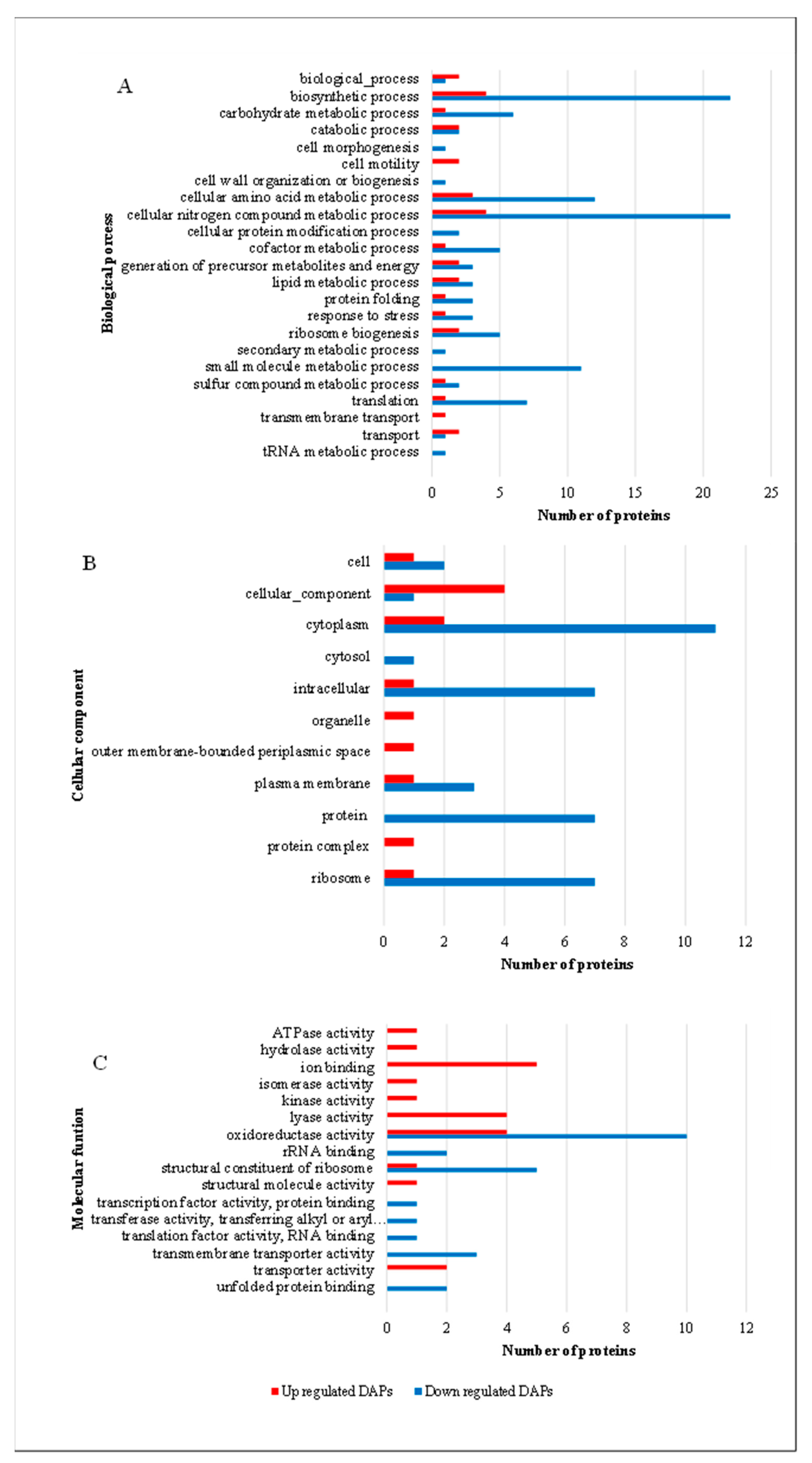

| UniProt ID | LC | Protein Name | ANOVA | Fold Change |
|---|---|---|---|---|
| Upregulated DAPs | ||||
| S4YPP8 | C | Universal stress protein | 0.0003 | 11.8 |
| S4YNE7 | IM | ABC transporter | 0.0008 | 4.7 |
| S4YIR5 | IM | Transcriptional regulator | 0.0000 | 4.0 |
| S4YJW6 | EX | Flagellin homolog p5 | 0.0099 | 3.9 |
| S4YUX6 | IM | Amino acid ABC transporter substrate-binding (PAAT family) | 0.0007 | 3.1 |
| S4YLR1 | C | Chorismate synthase | 0.0099 | 2.3 |
| S4YN26 | C | Transketolase | 0.0001 | 2.1 |
| S4YPB0 | P | Peptidylprolyl isomerase | 0.0017 | 2.0 |
| S4YKU6 | C | Acetate kinase | 0.0027 | 1.9 |
| S4YQI6 | C | Aspartate-semialdehyde dehydrogenase | 0.0078 | 1.9 |
| S4YF24 | P | Hypothetical protein | 0.0003 | 1.9 |
| S4YQU9 | C | Glycerol-3-phosphate dehydrogenase | 0.0132 | 1.9 |
| S4YKQ5 | FL | Flagellar biosynthesis | 0.0008 | 1.9 |
| S4YGS6 | P | ABC transporter substrate-binding | 0.0005 | 1.8 |
| S4YNH5 | C | Keto-deoxy-phosphogluconate aldolase | 0.0114 | 1.7 |
| S4YJK9 | P | ABC transporter substrate-binding | 0.0020 | 1.7 |
| S4YJR4 | IM | Enhanced serine sensitivity | 0.0018 | 1.7 |
| S4YKW2 | P | N-acetylglucosamine-6-phosphate deacetylase | 0.0063 | 1.7 |
| S4YIR6 | C | Ribosome-binding factor A | 0.0104 | 1.7 |
| S4YQ21 | P | D-ribose ABC transporter substrate-binding | 0.0047 | 1.7 |
| S4YPV1 | C | O-succinylhomoserine (thiol)-lyase | 0.0014 | 1.6 |
| S4YAT2 | C | Multifunctional fatty acid oxidation complex subunit alpha | 0.0054 | 1.6 |
| S4YDU9 | C | Succinate dehydrogenase iron-sulfur subunit | 0.0001 | 1.6 |
| S4YTM2 | IM | 50S ribosomal protein L4 | 0.0038 | 1.6 |
| S4YFI2 | P | Flavodoxin | 0.0133 | 1.6 |
| S4YCZ0 | IM | Lipo-related protein | 0.0015 | 1.5 |
| S4YGD8 | P | Amino acid ABC transporter substrate-binding | 0.0048 | 1.5 |
| S4YJ39 | C | Aconitate hydratase | 0.0174 | 1.5 |
| Downregulated DAPs | ||||
| S4YP79 | P | 50S ribosomal protein L30 | 0.0002 | 0.1 |
| S4YDE8 | C | Superoxide dismutase | 0.0000 | 0.2 |
| S4YJZ1 | C | Keto-deoxy-phosphogluconate aldolase | 0.0006 | 0.2 |
| S4YN10 | IM | F0F1 ATP synthase subunit delta | 0.0020 | 0.2 |
| S4YMN3 | P | Glycine betaine ABC transporter substrate-binding | 0.0265 | 0.3 |
| S4YTP0 | OM | cAMP regulatory protein | 0.0000 | 0.3 |
| S4YMN9 | IM | Multidrug export protein | 0.0008 | 0.4 |
| S4YEZ3 | IM | Cyclic di-GMP-binding family protein | 0.0000 | 0.4 |
| S4YNM7 | C | Family transcriptional regulator | 0.0002 | 0.4 |
| S4YPD4 | IM | 30S ribosomal protein S4 | 0.0032 | 0.4 |
| S4YLK3 | OM | Porin, Gram-negative type | 0.0000 | 0.4 |
| S4YRN2 | C | Sigma-24 factor | 0.0010 | 0.4 |
| S4YS69 | P | Agmatinase | 0.0002 | 0.4 |
| S4YLB8 | P | Ribosome hibernation promoting factor Hpf | 0.0012 | 0.4 |
| S4YJS5 | P | 30S ribosomal protein S16 | 0.0071 | 0.4 |
| S4YM12 | C | Cytosine deaminase | 0.0004 | 0.4 |
| S4YCV5 | C | Oxidoreductase | 0.0008 | 0.4 |
| S4YXW7 | IM | Glutamate racemase | 0.0195 | 0.4 |
| S4YB57 | C | Malate dehydrogenase | 0.0005 | 0.5 |
| S4YNW0 | C | Acetyl-coA carboxylase biotin carboxylase subunit | 0.0449 | 0.5 |
| S4YJI5 | C | Phosphoribosylglycinamide formyltransferase 2 | 0.0002 | 0.5 |
| S4YLD0 | EX | Peptidase M15 | 0.0348 | 0.5 |
| S4YD16 | C | Adenylate kinase | 0.0011 | 0.5 |
| S4YL10 | C | Aldehyde reductase | 0.0012 | 0.5 |
| S4YK92 | OM | Transcriptional regulator | 0.0165 | 0.5 |
| S4YE80 | C | GMP reductase | 0.0332 | 0.5 |
| S4YFE3 | C | Leucine–tRNA ligase | 0.0010 | 0.5 |
| S4YJJ1 | C | L-serine dehydratase 1 | 0.0017 | 0.5 |
| S4YK46 | C | Ribosome recycling factor | 0.0011 | 0.5 |
| S4YAD8 | C | Mannitol-1-phosphate 5-dehydrogenase | 0.0013 | 0.5 |
| S4YD37 | IM | Phosphatidylserine decarboxylase | 0.0204 | 0.6 |
| S4YE23 | C | Delta-aminolevulinic acid dehydratase | 0.0002 | 0.6 |
| S4YHN7 | C | Bifunctional UDP-glucuronic acid oxidase/UDP-4-amino-4-deoxy-L-arabinose formyltransferase | 0.0058 | 0.6 |
| S4YG49 | C | Molecular chaperone | 0.0039 | 0.6 |
| S4YNM9 | C | Elongation factor G | 0.0026 | 0.6 |
| S4YMR5 | C | Ferredoxin–NADP(+) reductase | 0.0401 | 0.6 |
| S4YAU6 | IM | 50S ribosomal protein L10 | 0.0000 | 0.6 |
| S4YDZ9 | P | Carbamoyl phosphate synthase large subunit | 0.0002 | 0.6 |
| S4YN87 | C | Pyruvate dehydrogenase (acetyl-transferring) homodimeric type | 0.0052 | 0.6 |
| S4YD04 | P | Phosphoribosylamine–glycine ligase | 0.0002 | 0.6 |
| S4YIF2 | P | 50S ribosomal protein L7/L12 | 0.0015 | 0.6 |
| S4YFK9 | IM | Succinate dehydrogenase flavoprotein subunit | 0.0000 | 0.6 |
| S4YDV0 | C | Glucose-1-phosphate thymidylyltransferase | 0.0404 | 0.6 |
| S4YC66 | C | Urocanate hydratase | 0.0003 | 0.6 |
| S4YN76 | C | Glutathione S-transferase | 0.0001 | 0.6 |
| S4YJV4 | P | Serine/threonine kinase | 0.0295 | 0.6 |
| S4YH64 | C | Transcriptional regulator | 0.0302 | 0.7 |
| S4YJJ2 | C | Acetolactate synthase isozyme 3 small subunit | 0.0168 | 0.7 |
| S4YF25 | C | Threonine synthase | 0.0000 | 0.7 |
| S4YG26 | C | Peptidylprolyl isomerase | 0.0003 | 0.7 |
| S4YGX9 | F | Phenazine biosynthesis family protein | 0.0076 | 0.7 |
| S4YV94 | IM | Hypothetical protein | 0.0216 | 0.7 |
| S4YMD1 | C | Universal stress protein | 0.0073 | 0.7 |
| S4YDU1 | C | 60 kDa chaperonin | 0.0015 | 0.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Machado, S.d.H.; Rodrigues, R.C.C.; Bertonceli, M.A.A.; Gaio, A.d.O.; Petroceli-Mota, G.; Reis, R.d.S.; Berbert-Molina, M.A.; Silveira, V.; Lemos, F.J.A. Exceptional Heme Tolerance in Serratia plymuthica: Proteomic Insights into Oxidative Stress Adaptation in the Aedes aegypti Midgut. Life 2025, 15, 950. https://doi.org/10.3390/life15060950
Machado SdH, Rodrigues RCC, Bertonceli MAA, Gaio AdO, Petroceli-Mota G, Reis RdS, Berbert-Molina MA, Silveira V, Lemos FJA. Exceptional Heme Tolerance in Serratia plymuthica: Proteomic Insights into Oxidative Stress Adaptation in the Aedes aegypti Midgut. Life. 2025; 15(6):950. https://doi.org/10.3390/life15060950
Chicago/Turabian StyleMachado, Sâmella da Hora, Rívea Cristina Custódio Rodrigues, Maria Aparecida Aride Bertonceli, Analiz de Oliveira Gaio, Gabriela Petroceli-Mota, Ricardo de Souza Reis, Marília Amorim Berbert-Molina, Vanildo Silveira, and Francisco José Alves Lemos. 2025. "Exceptional Heme Tolerance in Serratia plymuthica: Proteomic Insights into Oxidative Stress Adaptation in the Aedes aegypti Midgut" Life 15, no. 6: 950. https://doi.org/10.3390/life15060950
APA StyleMachado, S. d. H., Rodrigues, R. C. C., Bertonceli, M. A. A., Gaio, A. d. O., Petroceli-Mota, G., Reis, R. d. S., Berbert-Molina, M. A., Silveira, V., & Lemos, F. J. A. (2025). Exceptional Heme Tolerance in Serratia plymuthica: Proteomic Insights into Oxidative Stress Adaptation in the Aedes aegypti Midgut. Life, 15(6), 950. https://doi.org/10.3390/life15060950






