The Effects of the Association Between a High-Fat Diet and Physical Exercise on BDNF Expression in the Hippocampus: A Comprehensive Review
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Hippocampus
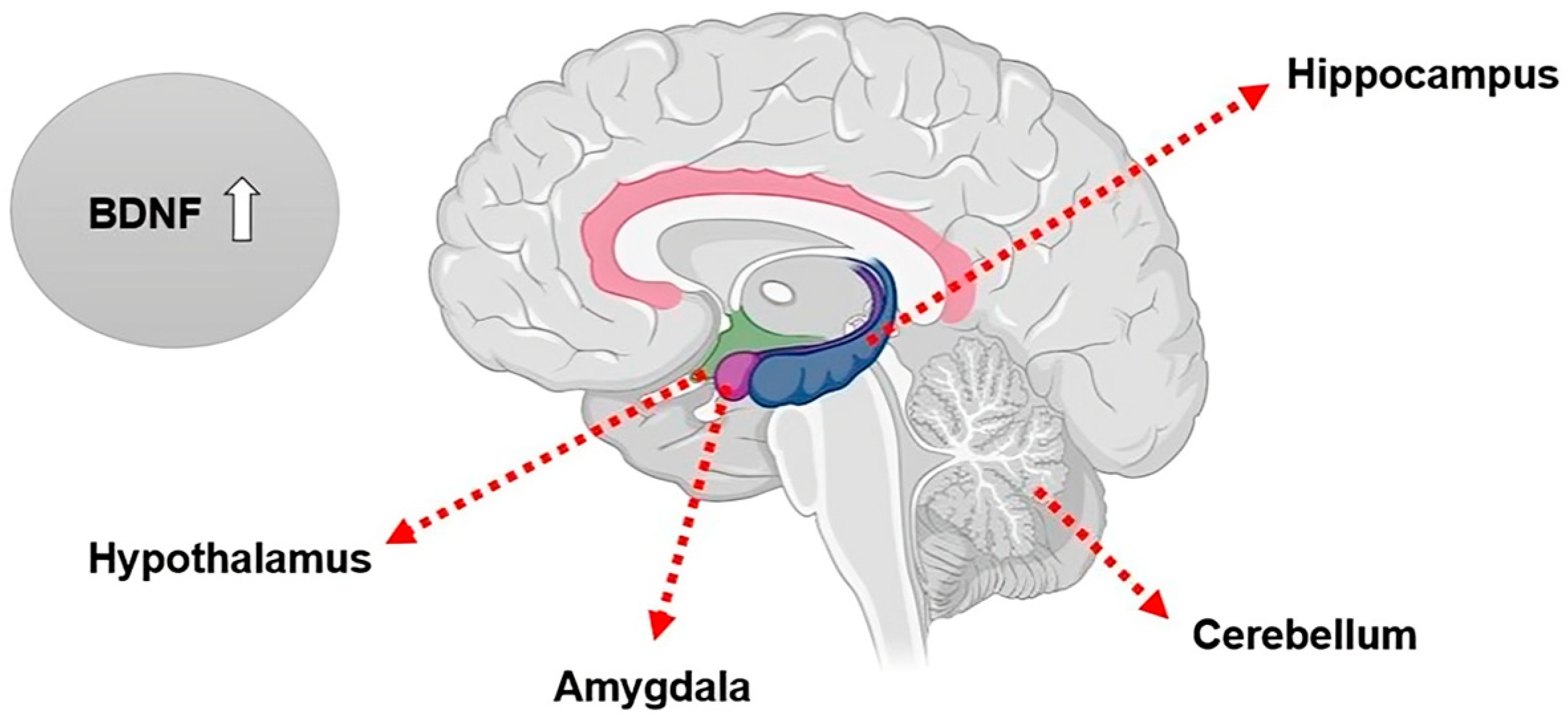
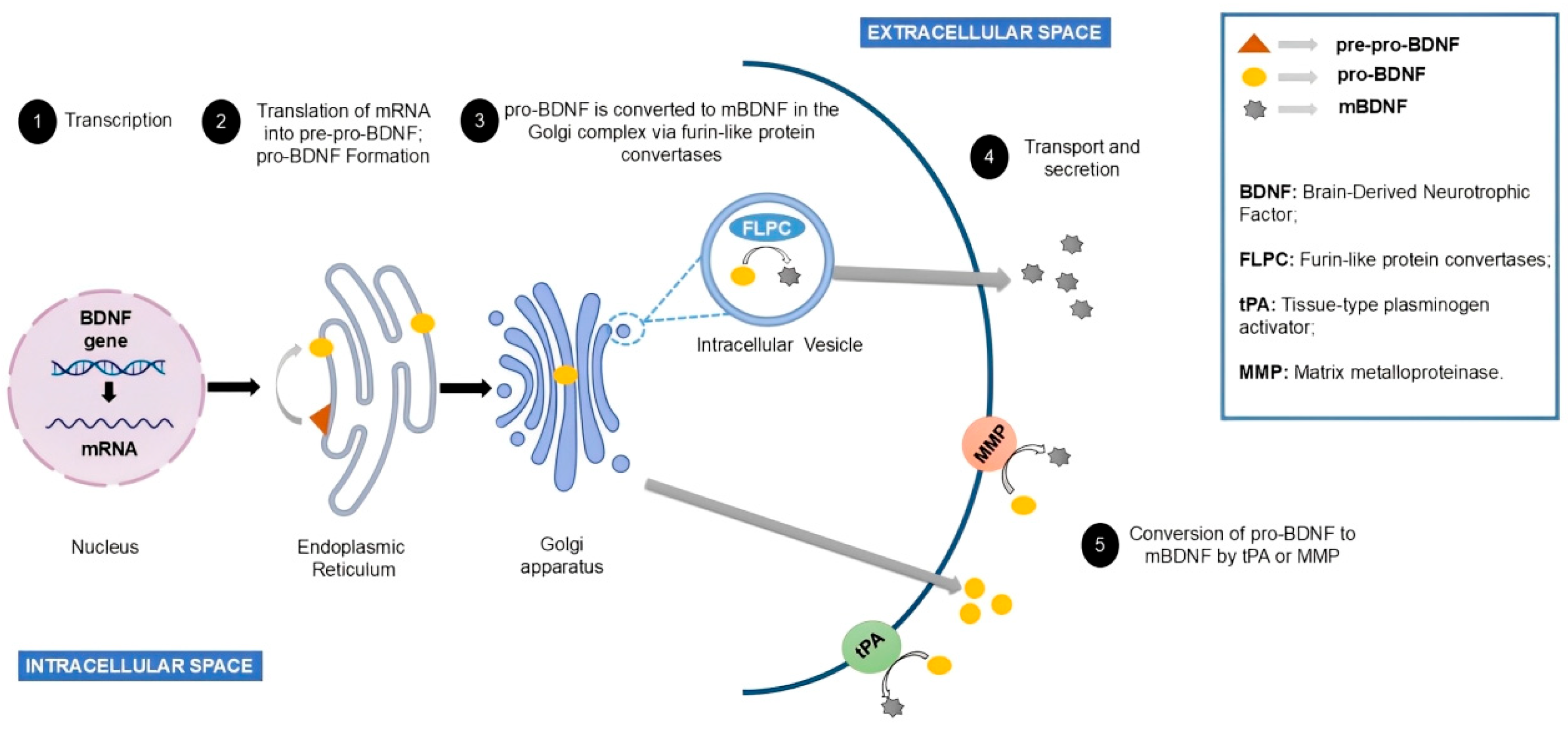
3.2. Brain-Derived Neurotrophic Factor (BDNF)
3.3. Pathophysiological Effects of High-Fat Diets
3.4. Neuroprotective Effects of Physical Exercise
3.5. The Combined Effects of High-Fat Diets and Exercise on the Hippocampus
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Park, H.S.; Park, S.S.; Kim, C.J.; Shin, M.S.; Kim, T.W. Exercise alleviates cognitive functions by enhancing hippocampal insulin signaling and neuroplasticity in high-fat diet-induced obesity. Nutrients 2019, 11, 1603. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Liu, B.B.; Cai, M.; Li, J.J.; Lou, S.J. Excessive endoplasmic reticulum stress and decreased neuroplasticity-associated proteins in prefrontal cortex of obese rats and the regulatory effects of aerobic exercise. Brain Res. Bull. 2018, 140, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Molteni, R.; Wu, U.M.; Vaynman, S.; Ying, Z.; Barnard, R.J.; Gómez-Pinilla, F. Exercise reverses the harmful effects of consumption of a high-fat diet on synaptic and behavioral plasticity associ-ated to the action of brain-derived neurotrophic factor. Neuroscience 2004, 123, 429–440. [Google Scholar] [CrossRef]
- Roussel, B.D.; Kruppa, A.J.; Miranda, E.; Crowther, D.C.; Lomas, D.A.; Marciniak, S.J. Endo-plasmic reticulum dysfunction in neurological disease. Lancet Neurol. 2013, 12, 105–118. [Google Scholar] [CrossRef] [PubMed]
- Cnop, M.; Foufelle, F.; Velloso, L.A. Endoplasmic reticulum stress, obesity and diabetes. Trends Mol. Med. 2012, 18, 59–68. [Google Scholar] [CrossRef]
- Mani, D.N.; Bawankule, D.; Saroj, B.K. Hyperlipidemic model: Studying lipid profile in small experimental animal. Int. J. Pharm. Pharm. Sci. 2012, 4, 337–340. [Google Scholar]
- Rosini, T.C.; Da Silva, A.S.R.; De Moraes, C. Diet-induced obesity: Rodent model for the study of obesity-related disorders. Rev. Assoc. Médica Bras. (Engl. Ed.) 2012, 58, 383–387. [Google Scholar]
- Tschöp, M.; Weyer, C.; Tataranni, P.A.; Devanarayan, V.; Ravussin, E.; Heiman, M.L. Circulating ghrelin levels are decreased in human obesity. Diabetes 2001, 4, 707–709. [Google Scholar] [CrossRef]
- Wirth, M.J.; Brun, A.; Grabert, J.; Patz, S.; Wahle, P. Accelerated dendritic development of rat cortical pyramidal cells and interneurons after biolistic transfection with BDNF and NT4/5. Development 2003, 130, 5827–5838. [Google Scholar] [CrossRef]
- Chae, C.H.; Jung, S.L.; An, S.H.; Park, B.Y.; Wang, S.W.; Cho, I.H.; Cho, J.Y.; Kim, H.T. Treadmill exercise im-proves cognitive function and facilitates nerve growth factor signaling by activating mito-gen-activated protein kinase/extracellular signal-regulated kinase1/2 in the streptozotocin-induced diabetic rat hippocampus. Neuroscience 2009, 164, 1665–1673. [Google Scholar] [CrossRef]
- Isacson, O.; Seo, H.; Lin, L.; Albeck, D.; Granholm, A.C. Alzheimer’s disease and Down’s syn-drome: Roles of APP, trophic factors and ACh. Trends Neurosci. 2002, 25, 79–84. [Google Scholar] [CrossRef]
- Bae, J.Y. Preventive Effects of Different Aerobic Exercise Intensities on the Decline of Cognitive Function in High-Fat Diet-Induced Obese Growing Mice. Medicina 2020, 56, 331. [Google Scholar] [CrossRef] [PubMed]
- Klein, C.; Jonas, W.; Iggena, D.; Empregar, L.; Rivalan, M.; Wiedmer, P.; Rivalan, M.; Steiner, B. Exercise prevents high-fat diet-induced impairment of flexible memory expression in the water maze and modulates adult hippocampal neurogenesis in mice. Neurobiol. Learn. Mem. 2016, 131, 26–35. [Google Scholar] [CrossRef]
- Cai, M.; Wang, H.; Li, J.J.; Zhang, Y.L.; Xin, L.; Li, F.; Lou, F. The signaling mechanisms of hippo-campal endoplasmic reticulum stress affecting neuronal plasticity-related protein levels in high fat diet-induced obese rats and the regulation of aerobic exercise. Brain Behav. Immun. 2016, 57, 347–359. [Google Scholar] [CrossRef]
- Kesner, R.P.; Lee, I.; Gilbert, P. A behavioral assessment of hippocampal function based on a subregional analysis. Rev. Neurosci. 2004, 15, 33–50. [Google Scholar] [CrossRef] [PubMed]
- Bear, M.F.; Connors, B.W.; Paradiso, M.A. Neuroscience: Unraveling the Nervous System, 4th ed.; Artmed: Porto Alegre, Brazil, 2017; p. 209. [Google Scholar]
- Deshmukh, S.S.; Knierim, J.J. Hippocampus. WIREs Cogn. Sci. 2012, 3, 231–251. [Google Scholar] [CrossRef] [PubMed]
- Knierim, J.J. The hippocampus. Curr. Biol. 2015, 23, R1116–R1121. [Google Scholar] [CrossRef]
- Moser, E.; Moser, M.B.; Andersen, P. Spatial learning impairment parallels the magnitude of dorsal hippocampal lesions, but is hardly present following ventral lesions. J. Neurosci. 1993, 9, 3916–3925. [Google Scholar] [CrossRef]
- Bannerman, M.; Rawlins, J.N.P.; McHugh, S.B.; Diácono, R.M.J.; Sim, B.K.; Bast, T. Regional dissociations within the hippocampus--memory and anxiety. Neurosci. Biobehav. Rev. 2004, 28, 273–283. [Google Scholar] [CrossRef]
- Hrybouski, S.; MacGillivray, M.; Huang, Y.; Madan, C.R.; Carter, R.; Seres, P.; Malykhin, N.V. Involvement of hippocampal subfields and anterior-posterior subregions in encoding and retrieval of item, spatial, and associative memories: Longitudinal versus transverse axis. Neuroimage 2019, 191, 568–586. [Google Scholar] [CrossRef]
- Fanselow, M.S.; Dong, H.W. Are the dorsal and ventral hippocampus functionally distinct structures? Neuron 2010, 65, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Amaral, D.G.; Scharfman, H.E.; Lavenex, P. The dentate gyrus: Fundamental neuroanatomical organization (dentate gyrus for dummies). Prog. Brain Res. 2007, 163, 3–22. [Google Scholar] [PubMed]
- Dremencov, E.; El Mansari, M.; Blier, P. Effects of sustained serotonin reuptake inhibition on the firing of dopamine neurons in the rat ventral tegmental area. J. Psychiatry Neurosci. 2009, 34, 223–229. [Google Scholar] [PubMed]
- Bartsch, T.; Wulff, P. The hippocampus in aging and disease: From plasticity to vulnerability. Neuroscience 2015, 309, 1–16. [Google Scholar] [CrossRef]
- Moscovitch, M.; Cabeza, R.; Winocur, G.; Nadel, L. Episodic Memory and Beyond: The Hip-pocampus and Neocortex in Transformation. Annu. Rev. Psychol. 2016, 67, 105–134. [Google Scholar] [CrossRef]
- Eichenbaum, H. The Cognitive Neuroscience of Memory: An Introduction, 4th ed.; Oxford University Press: Oxford, UK, 2011. [Google Scholar]
- Cefis, M.; Chaney, R.; Wirtz, J.; Méloux, A.; Quirié, A.; Leger, C.; Cefis, M. Molecular mechanisms underlying physical exercise-induced brain BDNF overproduction. Front. Mol. Neurosci. 2023, 16, 1275924. [Google Scholar] [CrossRef]
- Kim, T.W.; Choi, H.H.; Chung, Y.R. Treadmill exercise alleviates impairment of cognitive function by enhancing hippocampal neuroplasticity in the high-fat diet-induced obese mice. J. Exerc. Rehabil. 2016, 12, 156. [Google Scholar] [CrossRef]
- Oh, H.; Lewis, D.A.; Sibille, E. The Role of BDNF in Age-Dependent Changes of Excitatory and Inhibitory Synaptic Markers in the Human Prefrontal Cortex. Neuropsychopharmacology 2016, 41, 3080–3091. [Google Scholar] [CrossRef]
- Correia, A.S.; Cardoso, A.; Vale, N. BDNF unveiled: Exploring its role in major depression disorder serotonergic imbalance and associated stress conditions. Pharmaceutics 2023, 15, 2081. [Google Scholar] [CrossRef]
- Shi, W.; Chen, Y.; Gan, G.; Wang, D.; Ren, J.; Wang, Q.; Xu, Z.; Xie, W.; Zhang, Y.Q. Brain tumor regulates neuromuscular synapse growth and endocytosis in Drosophila by suppressing mad expression. J. Neurosci. 2013, 33, 12352–12363. [Google Scholar] [CrossRef]
- Kowiański, P.; Lietzau, G.; Czuba, E.; Waśkow, M.; Steliga, A.; Moryś, J. BDNF: A key factor with multipotent impact on brain signaling and synaptic plasticity. Cell Mol. Neurobiol. 2018, 38, 579–593. [Google Scholar] [CrossRef] [PubMed]
- Trombetta, I.C.; DeMoura, J.R.; Alves, C.R.; Carbonari-Brito, R.; Cepeda, F.X.; Lemos, J.R. Serum levels of BDNF in cardiovascular protection and in response to exercise. Arq. Bras. Cardiol. 2020, 115, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, K.; Poo, M. Activity-dependent neural plasticity from bench to bedside. Neuron 2013, 80, 729–741. [Google Scholar] [CrossRef]
- Gao, J.; Lai, M.Y.; Mai, T.T.; Fu, W.; Wang, M.Y.; Ning, B.L.; Fu, W.B. Effects of electroacu-puncture on BNDF/mTORC1 signaling pathway and synaptic plasticity in prefrontal cortex of rats exposed to chronic unpredictable mild stress. Zhen Ci Yan Jiu 2022, 47, 15–20. [Google Scholar] [PubMed]
- Zhang, X.L.; Wang, L.; Xiong, L.; Huang, F.H.; Xue, H. Timosaponin B-III exhibits antidepressive activity in a mouse model of postpartum depression by the regulation of inflammatory cytokines, BNDF signaling and synaptic plasticity. Exp. Ther. Med. 2017, 14, 3856–3861. [Google Scholar] [CrossRef]
- Kim, T.W.; Baek, K.W.; Yu, H.S.; Ko, G.; Hwang, L.; Jung-Jun, P. High-intensity exercise improves cognitive function and hippocampal brain-derived neurotrophic factor expression in obese mice maintained on high-fat diet. J. Exerc. Rehabil. 2020, 16, 124–131. [Google Scholar] [CrossRef]
- Feng, Y.; Tian, X.; Zhang, M.; Lou, S. Treadmill Exercise Reverses the Change of Dendritic Morphology and Activates BNDF-mTOR Signaling Pathway in the Hippocampus and Cerebral Cortex of Ovariectomized Mice. J. Mol. Neurosci. 2021, 71, 1849–1862. [Google Scholar] [CrossRef]
- Liu, W.F.; Liu, S.P.; Fu, R.; Wang, Z.Y.; Kuang, H.Y.; Xia, Y.; Tang, C.F. Effects of endurance exercise on synaptic plasticity in cerebral cortex of aged rats and related regulatory mechanism. Zhongguo Ying Yong Sheng Li Xue Za Zhi 2019, 35, 339–345. [Google Scholar]
- Huang, T.; Larsen, K.T.; Ried-Larsen, M.; Moller, N.C.; Andersen, L.B. The effects of physical activity and exercise on brain-derived neurotrophic factor in healthy humans: A review. Scand. J. Med. Sci. Sports 2014, 24, 1–10. [Google Scholar] [CrossRef]
- Murawska-Ciałowicz, E.; Wiatr, M.; Ciałowicz, M.; Gomes, A.G.; Borowicz, W.; Ro-cha-Rodrigues, S.; Borowicz, W.; Wiatr, M. BDNF impact on biological markers of depression—Role of physical exercise and training. Int. J. Environ. Res. Public Health 2021, 18, 7553. [Google Scholar] [CrossRef]
- Murray, R.K.; Bender, D.A.; Botham, K.M.; Kennelly, P.J.; Rodwell, V.W.; Weil, P.A. Harper: Illustrated Biochemistry; AMGH LTDA: São Paulo, Brazil, 2017; 211p. [Google Scholar]
- Remesar, X.; Rafecas, I.; Fernández-López, J.A.; Alemany, M. Metabolic aspects of the con-sumption of edible oils in rats. Food Chem. Toxicol. 2000, 38, 425–431. [Google Scholar]
- Spencer, S.J.; D’Angelo, H.; Soch, A.; Watkins, L.R.; Maier, S.F. High-fat diet and palmitic acid increase microglial IL-1β expression and caspase signaling in the absence of an active Toll-like receptor 4 pathway. Brain Behav. Immun. 2017, 60, 1–8. [Google Scholar] [CrossRef]
- Han, T.K.; Leem, Y.H.; Kim, H.S. Treadmill exercise restores high fat diet-induced disturbance of hippocampal neurogenesis through β2-adrenergic receptor-dependent induction of thioredoxin-1 and brain-derived neurotrophic factor. Brain Res. 2019, 1707, 154–163. [Google Scholar] [CrossRef] [PubMed]
- Fabunmi, T.M.; Raji, R.O.; Odetokun, I.A. High-fat diets and human health: Balancing risks and benefits. Nutr. Rev. 2024, 82, 19–32. [Google Scholar]
- Stranahan, A.M.; Norman, E.D.; Lee, K.; Cutler, R.G.; Telljohann, R.S.; Egan, J.M.; Mattson, M.P. Di-et-induced insulin resistance impairs hippocampal synaptic plasticity and cognition in mid-dle-aged rats. Hippocampus 2008, 18, 1085–1088. [Google Scholar] [CrossRef] [PubMed]
- Valladolid-Acebes, I.; Stucchi, P.; Cano, V.; Fernández-Alfonso, M.S.; Merino, B.; Gil-Ortega, M.; Ruiz-Gayo, M.; Del Olmo, N. High-fat diets impair spatial learning in the radial-arm maze in mice. Neurobiol. Learn. Mem. 2011, 95, 80–85. [Google Scholar] [CrossRef]
- Sharma, S.; Fernandes, M.F.; Fulton, S. Adaptations in brain reward circuitry underlie palatable food cravings and anxiety induced by high-fat diet withdrawal. Int. J. Obes. 2013, 37, 1183–1191. [Google Scholar] [CrossRef]
- Lindqvist, A.; Mohapel, P.; Bouter, B.; Frielingsdorf, H.; Pizzo, D.; Brundin, P.; Charlotte, E.-A. High-fat diet impairs hippocampal neurogenesis in male rats. Eur. J. Neurol. 2006, 13, 1385–1388. [Google Scholar] [CrossRef]
- Pistell, P.J.; Morrison, C.D.; Gupta, S.; Knight, A.G.; Keller, J.N.; Ingram, D.K.; Bruce-Keller, A.J. Cognitive impairment following high fat diet consumption is associated with brain inflammation. J. Neuroimmunol. 2010, 219, 25–32. [Google Scholar] [CrossRef]
- Spinelli, M.; Fusco, S.; Mainardi, M.; Scala, F.; Natale, F.; Lapenta, R.; Mattera, A. Brain insulin resistance impairs hippocampal synaptic plasticity and memory by increasing GluA1 palmitoylation through FoxO3a. Nat. Commun. 2017, 8, 2009. [Google Scholar] [CrossRef]
- Khoramipour, K.; Chamari, K.; Hekmatikar, A.A.; Ziyaiyan, A.; Taherkhani, S.; Elguindy, N.M.; Bragazzi, N.L. Adiponectin: Structure, physiological functions, role in diseases, and effects of nutrition. Nutrients 2021, 4, 1180. [Google Scholar] [CrossRef] [PubMed]
- Rasouli, N.; Kern, P.A. Adipocytokines and the metabolic complications of obesity. J. Clin. Endocrinol. Metab. 2008, 93, s64–s73. [Google Scholar] [CrossRef] [PubMed]
- Shen, R.; Ardianto, C.; Celia, C.; Sidharta, V.M.; Sasmita, P.K.; Satriotomo, I.; Turana, Y. Brain-derived neurotrophic factor interplay with oxidative stress: Neuropathology approach in potential biomarker of Alzheimer’s disease. Dement. Neuropsychol. 2023, 17, e20230012. [Google Scholar] [CrossRef]
- Woo, J.; Shin, K.O.; Park, S.Y.; Jang, K.S.; Kang, S. The effects of treadmill exercise on spatial learning ability and hippocampal neurogenesis in high-fat diet-induced obese mice. J. Exerc. Nutr. Biochem. 2013, 17, 11–18. [Google Scholar]
- Arida, R.M.; Scorza, F.A.; Da Silva, S.G.; Cysneiros, R.M.; Cavalheiro, E.A. Exercise paradigms to study brain injury recovery in rodents. Am. J. Phys. Med. Rehabil. 2011, 90, 452–465. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, A.M.; Pase, C.S.; Boufleur, N.; Roversi, K.; Barcelos, R.C.S.; Benvegnú, D.M.; Segat, H.J. Exercise affects memory acquisition, anxiety-like symptoms and activity of membrane-bound enzyme in brain of rats fed with different dietary fats: Impairments of trans fat. Neuroscience 2011, 195, 80–88. [Google Scholar] [CrossRef]
- Tari, A.R.; Walker, T.L.; Huuha, A.M.; Sando, S.B.; Wisloff, U. Neuroprotective mechanisms of exercise and the importance of fitness for healthy brain ageing. Lancet 2025, 405, 1093–1118. [Google Scholar] [CrossRef]
- Mahalakshmi, B.; Maurya, N.; Lee, S.D.; Bharath Kumar, V. Possible Neuroprotective Mecha-nisms of Physical Exercise in Neurodegeneration. Int. J. Mol. Sci. 2020, 21, 5895. [Google Scholar] [CrossRef]
- Tuon, T.; Souza, P.S.; Santos, M.F.; Pereira, F.T.; Pedroso, G.S.; Luciano, T.F.; De Souza, C.T.; Dutra, R.C.; Silveira, P.C.; Pinho, R.A. Physical Training Regulates Mitochondrial Parameters and Neuroinflammatory Mechanisms in an Experimental Model of Parkinson’s Disease. Oxid. Med. Cell Longev. 2015, 26, 1809. [Google Scholar] [CrossRef]
- Fonseca, I.A.; Passos, R.L.; Araújo, F.A.; Lima, M.R.; Lacerda, D.R.; Pires, W.; Soares, D.D. Exercising for food: Bringing the laboratory closer to nature. J. Exp. Biol. 2014, 217, 3274–3282. [Google Scholar] [CrossRef]
- Noble, E.E.; Mavanji, V.; Little, M.R.; Billington, C.J.; Kotz, C.M.; Wang, C. Exercise reduces diet-induced cognitive decline and increases hippocampal brain-derived neurotrophic factor in CA3 neurons. Neurobiol. Learn. Mem. 2014, 114, 40–50. [Google Scholar] [CrossRef]
- Maniam, J.; Morris, M.J. Voluntary exercise and palatable high-fat diet both improve behav-ioural profile and stress responses in male rats exposed to early life stress: Role of hippocampus. Psychoneuroendocrinology 2010, 35, 1553–1564. [Google Scholar] [CrossRef]
- Paiva, K.M.; Oliveira, R.F.; De Freitas, L.H.M.; Da Rocha, G.S.; Freire, K.F.; De Souza Cavalcante, J.; de Gois Morais, P.L.A. Physical exercise and flaxseed oil supplementation influence the glial plasticity in the rat hippocampus. Acta Neurobiol. Exp. 2022, 82, 448–461. [Google Scholar] [CrossRef] [PubMed]
- Borba, E.M.; Duarte, J.A.; Bristot, G.; Scotton, E.; Camozzato, A.L.; Chaves, M.L.F. Brain-derived neurotrophic factor serum levels and hippocampal volume in mild cognitive impairment and dementia due to Alzheimer disease. Dement. Geriatr. Cogn. Disord. Extra 2017, 6, 559–567. [Google Scholar] [CrossRef]
- El Hayek, L.; Khalifeh, M.; Zibara, V.; Abi Assaad, R.; Emmanuel, N.; Karnib, N.; El-Ghandour, R. Lactate mediates the effects of exercise on learning and memory through SIRT1-dependent activation of hippocampal brain-derived neurotrophic factor (BDNF). J. Neurosci. 2019, 39, 2369–2382. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Deng, F.; Wang, J.; Liu, Y.; Zhou, W.; Qu, L.; Cheng, M. Intensity-dependent effects of con-secutive treadmill exercise on spatial learning and memory through the p-CREB/BDNF/NMDAR signaling in hippocampus. Behav. Brain Res. 2020, 386, 112599. [Google Scholar] [CrossRef] [PubMed]
- Rosa, E.F.; Takahashi, S.; Aboulafia, J.; Nouailhetas, V.L.; Oliveira, M.G. Oxidative stress in-duced by intense and exhaustive exercise impairs murine cognitive function. J. Neurophysiol. 2007, 98, 1820–1826. [Google Scholar] [CrossRef]
- Sun, L.N.; Li, X.L.; Wang, F.; Zhang, J.; Wang, D.D.; Yuan, L.; Wu, M. High-intensity treadmill running impairs cognitive behavior and hippocampal synaptic plasticity of rats via activation of inflammatory response. J. Neurosci. Res. 2017, 95, 1611–1620. [Google Scholar] [CrossRef]
- Lan, Y.; Huang, Z.; Jiang, Y.; Zhou, X.; Zhang, J.; Zhang, D.; Wang, B. Strength exercise weakens aerobic exercise-induced cognitive improvements in rats. PLoS ONE 2018, 13, 0205562. [Google Scholar] [CrossRef]
- Scheffer, D.D.L.; Latini, A. Exercise-induced immune system response: Anti-inflammatory status on peripheral and central organs. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165823. [Google Scholar] [CrossRef]
- Loprinzi, P.D.; Frith, E. A brief primer on the mediational role of BDNF in the exercise-memory link. Clin. Physiol. Funct. Imaging 2019, 39, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Woo, J.; Shin, K.O.; Park, S.Y.; Jang, K.S.; Kang, S. Effects of exercise and diet change on cognition function and synaptic plasticity in high fat diet induced obese rats. Lipids Health Dis. 2013, 12, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Yi, S.S.; Hwang, I.K.; Yoo, K.Y.; Park, O.K.; Yu, J.; Yan, B.; Kim, I.Y.; Kim, Y.N.; Pai, T.; Song, W.; et al. Effects of treadmill exercise on cell proliferation and differentiation in the subgranular zone of the dentate gyrus in a rat model of type II diabetes. Neurochem. Res. 2009, 34, 1039–1046. [Google Scholar] [CrossRef] [PubMed]
- Sleiman, S.F.; Henry, J.; Al-Haddad, R.; El Hayek, L.; Abou Haidar, E.; Stringer, T.; Ulja, D. Exercise promotes the expression of brain derived neurotrophic factor (BDNF) through the action of the ketone body β-hydroxybutyrate. Elife 2016, 5, 15092. [Google Scholar] [CrossRef]
- Rossi, C.; Angelucci, A.; Costantin, L.; Braschi, C.; Mazzantini, M.; Babbini, F.; Fabbri, M.E. Brain-derived neurotrophic factor (BDNF) is required for the enhancement of hippocampal neurogenesis following environmental enrichment. Eur. J. Neurosci. 2006, 24, 1850–1856. [Google Scholar] [CrossRef]
| Search strategy |
| High-fat diet OR Hyperlipidic diet AND Exercise OR Physical activity AND BDNF OR Brain-derived neurotrophic factor AND Hippocampus |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gomes, F.T.d.S.; Andrade, A.V.D.d.; Moura Melo, P.K.; Júnior, R.R.d.S.; Souza, D.L.S.d.; Tavares, É.A.F.; Sena, I.G.d.; Fernandes, T.A.A.d.M.; Morais, P.L.A.d.G.; Fonseca, I.A.T.; et al. The Effects of the Association Between a High-Fat Diet and Physical Exercise on BDNF Expression in the Hippocampus: A Comprehensive Review. Life 2025, 15, 945. https://doi.org/10.3390/life15060945
Gomes FTdS, Andrade AVDd, Moura Melo PK, Júnior RRdS, Souza DLSd, Tavares ÉAF, Sena IGd, Fernandes TAAdM, Morais PLAdG, Fonseca IAT, et al. The Effects of the Association Between a High-Fat Diet and Physical Exercise on BDNF Expression in the Hippocampus: A Comprehensive Review. Life. 2025; 15(6):945. https://doi.org/10.3390/life15060945
Chicago/Turabian StyleGomes, Francisca Tayná da Silva, Antônio Vicente Dias de Andrade, Paloma Katlheen Moura Melo, Roque Ribeiro da Silva Júnior, Débora Lopes Silva de Souza, Élyssa Adriolly Freitas Tavares, Ingrid Garcia de Sena, Thales Allyrio Araújo de Medeiros Fernandes, Paulo Leonardo Araújo de Góis Morais, Ivana Alice Teixeira Fonseca, and et al. 2025. "The Effects of the Association Between a High-Fat Diet and Physical Exercise on BDNF Expression in the Hippocampus: A Comprehensive Review" Life 15, no. 6: 945. https://doi.org/10.3390/life15060945
APA StyleGomes, F. T. d. S., Andrade, A. V. D. d., Moura Melo, P. K., Júnior, R. R. d. S., Souza, D. L. S. d., Tavares, É. A. F., Sena, I. G. d., Fernandes, T. A. A. d. M., Morais, P. L. A. d. G., Fonseca, I. A. T., Borges, C. d. S., & Cavalcanti, J. R. L. d. P. (2025). The Effects of the Association Between a High-Fat Diet and Physical Exercise on BDNF Expression in the Hippocampus: A Comprehensive Review. Life, 15(6), 945. https://doi.org/10.3390/life15060945







