Association Between Lycopene and Metabolic Disease Risk and Mortality: Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Protocol and Registration
2.2. Search Strategy and Data Collection
2.3. Eligibility Criteria
- Population: Adults (≥18 years) with or without metabolic dysfunction (e.g., MAFLD, obesity, diabetes, and insulin resistance).
- Intervention/Exposure: Serum lycopene levels or lycopene supplementation.
- Comparison: Healthy individuals or placebo/control groups.
- Outcomes: Metabolic markers (IGF-1, IGFBPs, glucose, insulin, and lipids), MAFLD risk, obesity, and diabetes status.
- Study Design: RCTs, cohort studies, case–control studies, and cross-sectional studies.
- Language: English.
- Publication Date: 2001 to 2025.
- Case reports, case series, reviews, and editorials were excluded, as were animal or in vitro studies.
2.4. Effect Estimates and Data Extraction for Meta-Analysis
2.5. Risk-of-Bias Assessment
2.6. Data Analysis
2.7. Planned Subgroup Analysis
3. Results
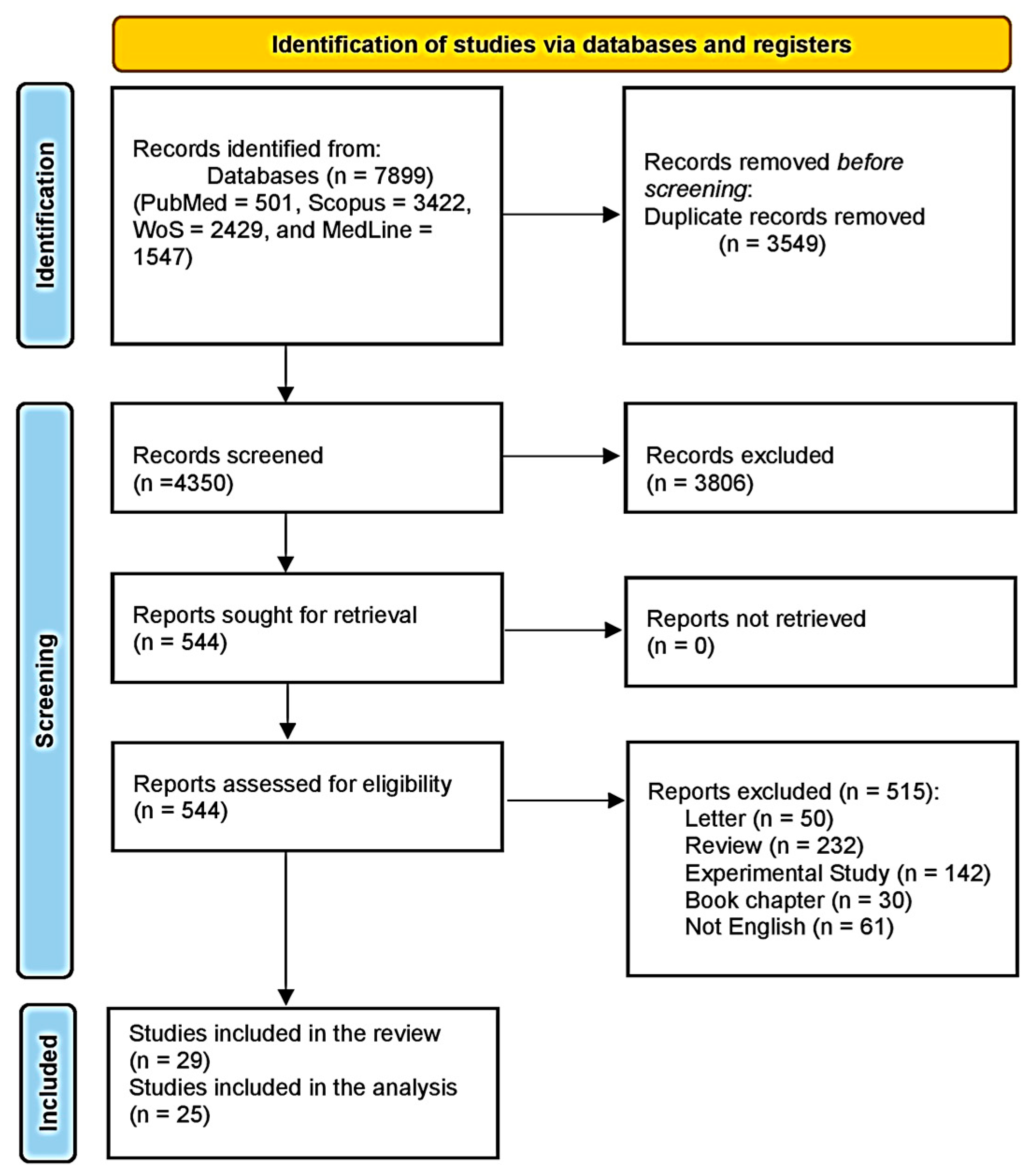
3.1. Study Selection
3.2. Characteristics of the Included Studies
3.3. Baseline Characteristics of the Included Patients
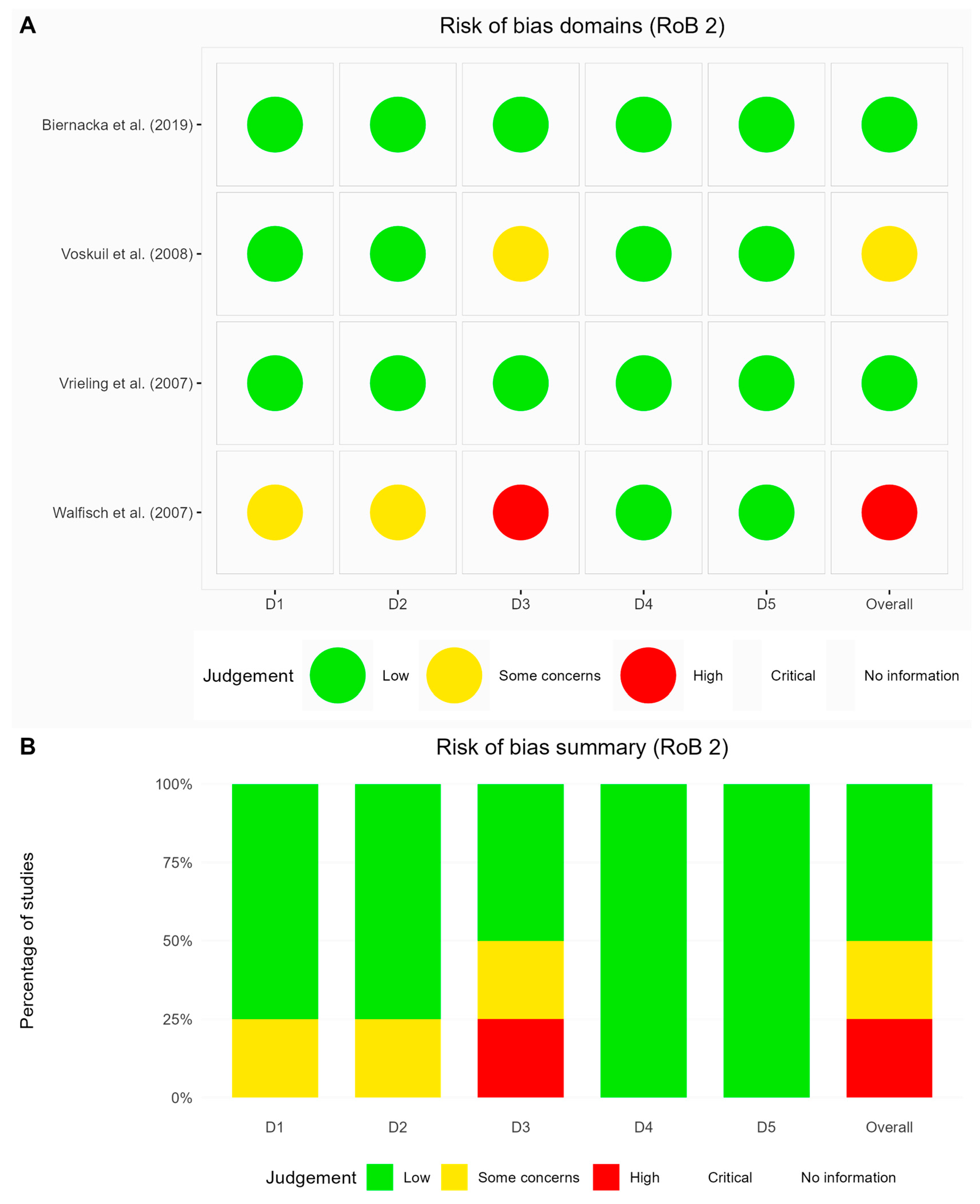
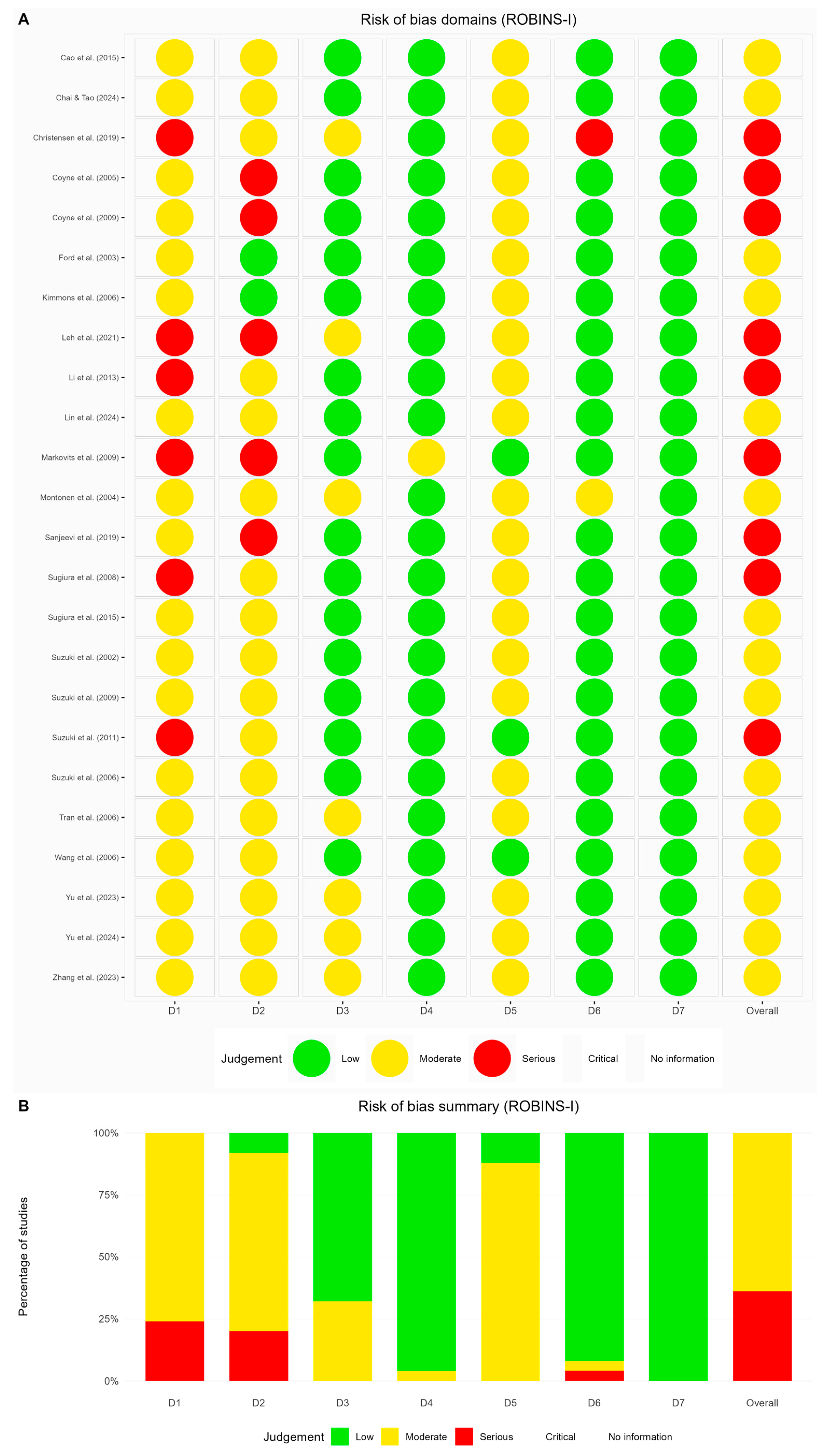
3.4. Risk of Bias
3.5. Primary and Secondary Outcomes
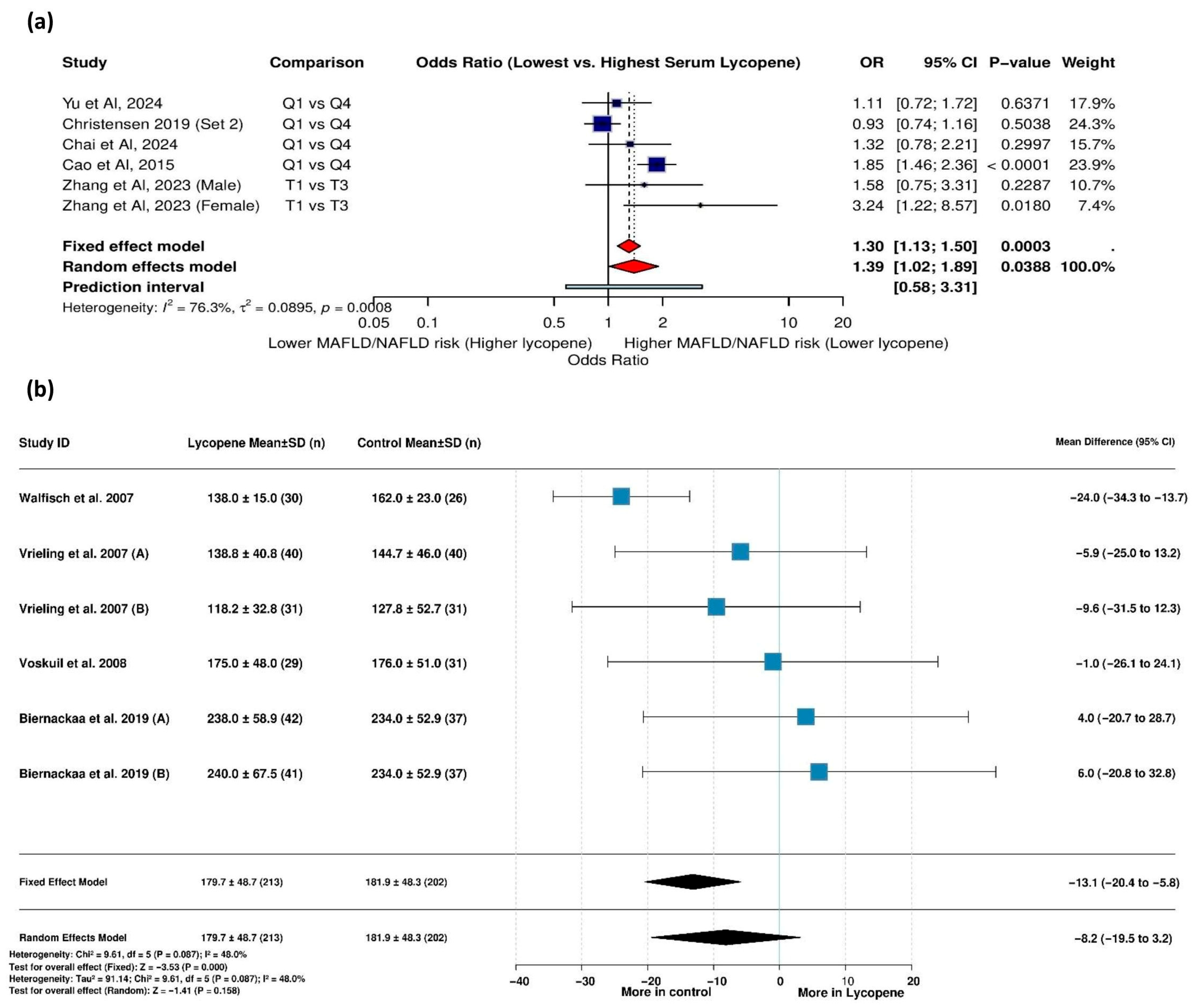
3.5.1. Serum Lycopene Levels and MAFLD Risk
3.5.2. Lycopene Supplementation and IGF-I Levels
3.5.3. IGF-I Levels Across Lycopene Quantiles
3.5.4. Lycopene Supplementation and IGF-II Levels
3.5.5. Lycopene Supplementation and IGFBP-1 Levels
3.5.6. Lycopene Supplementation and IGFBP-2 Levels
3.5.7. Lycopene Supplementation and IGFBP-3
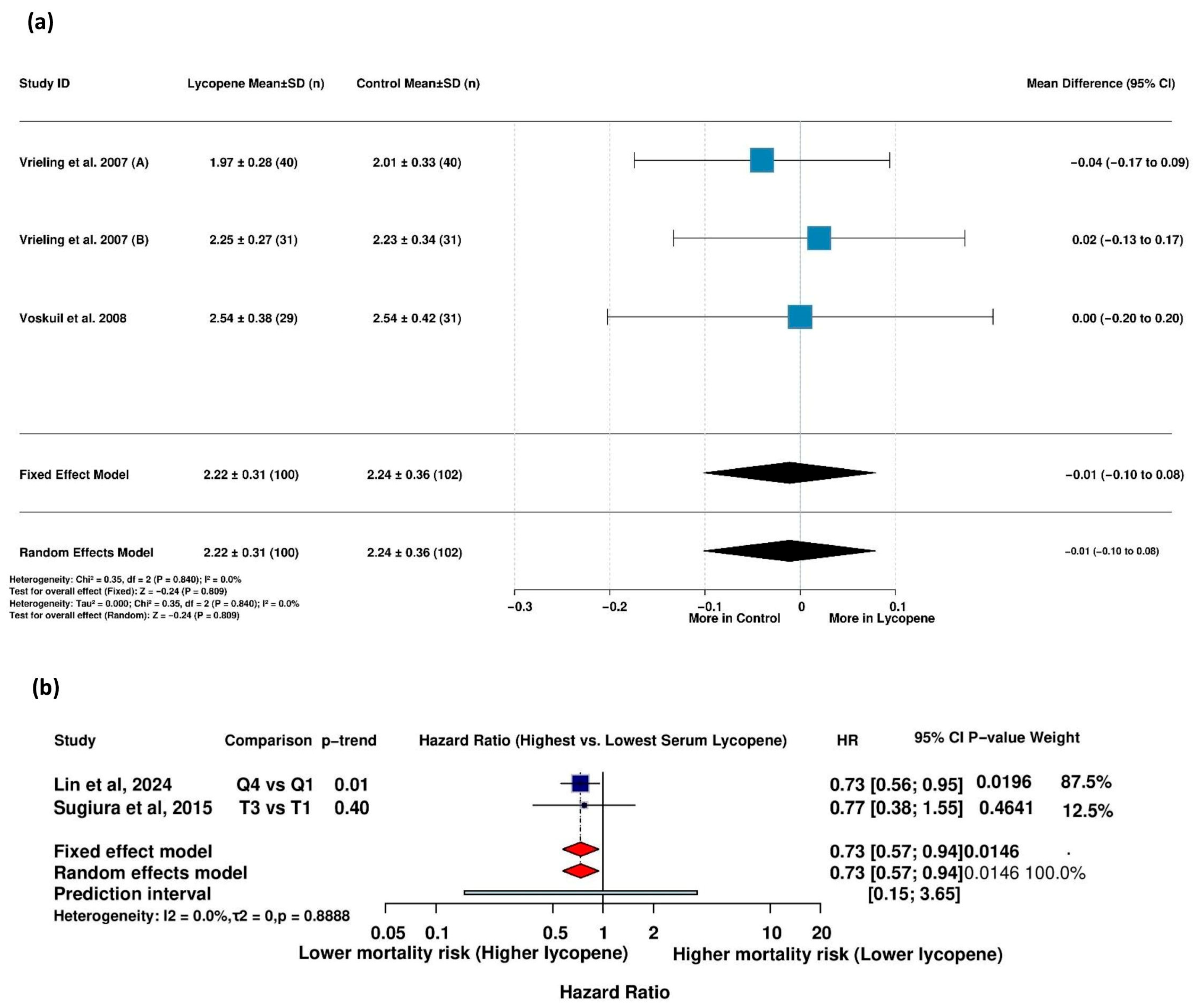
3.5.8. Association Between Serum Lycopene and Mortality in Patients with Metabolic Diseases
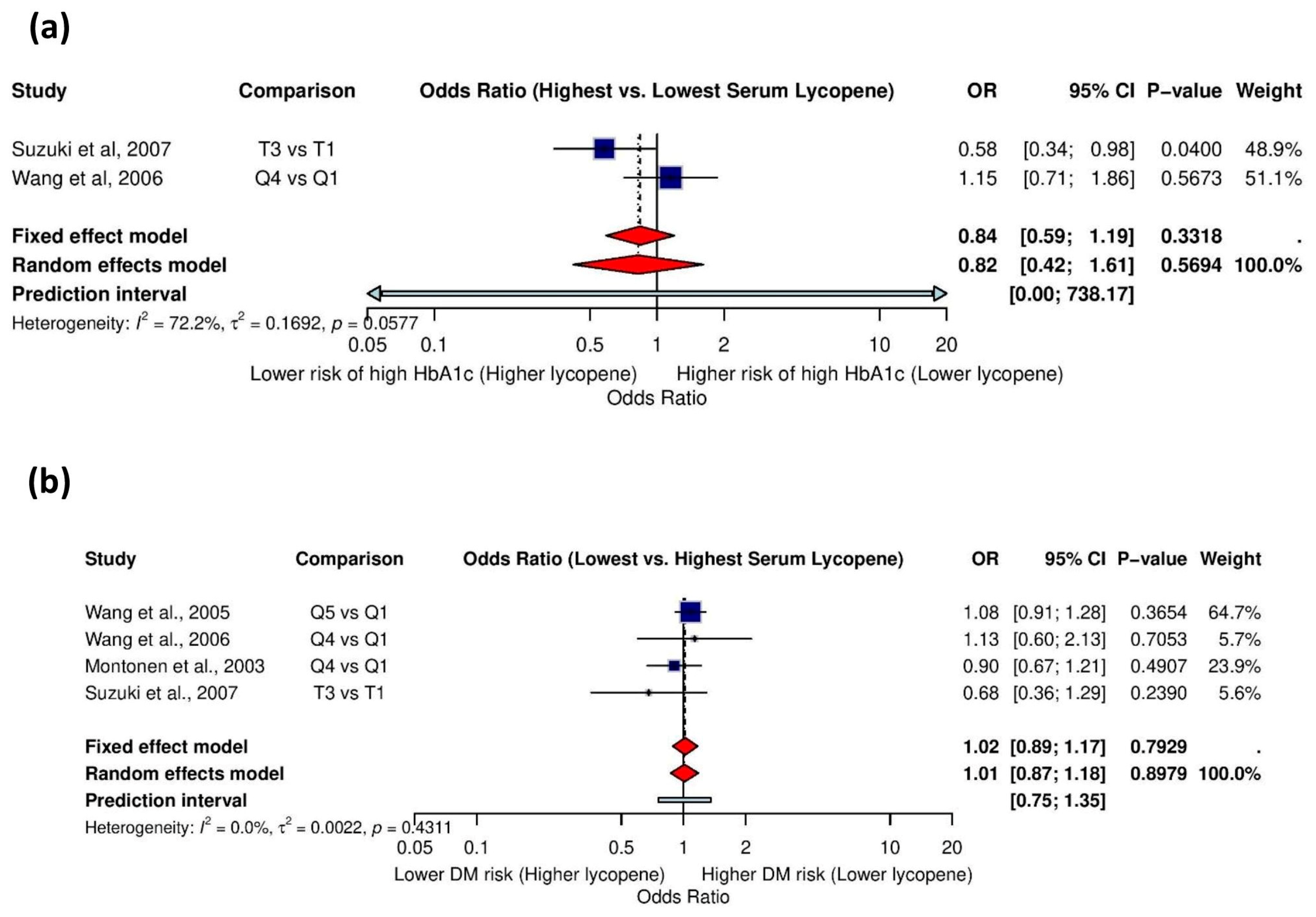
3.5.9. Association Between Serum Lycopene and High HbA1c Levels
3.5.10. Association Between Serum Lycopene and History of Diabetes Mellitus
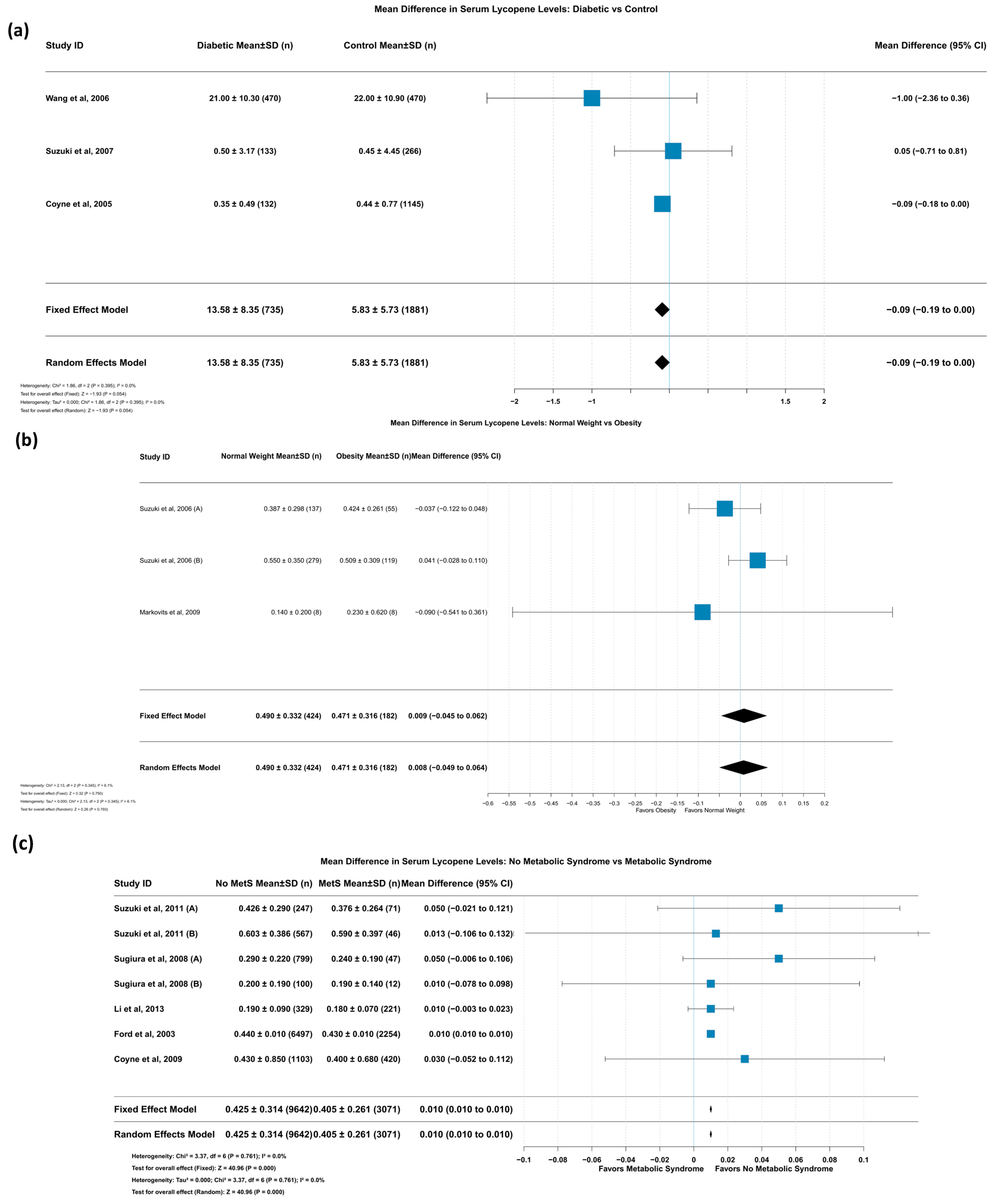
3.5.11. Mean Difference in Serum Lycopene Levels: Diabetic vs. Control
3.5.12. Mean Difference in Serum Lycopene Levels: Normal Weight vs. Obesity
3.5.13. Mean Difference in Serum Lycopene Levels: No Metabolic Syndrome vs. Metabolic Syndrome
3.6. Publication Bias
4. Discussion
4.1. Association Between Serum Lycopene and MAFLD Risk
4.2. Association Between Serum Lycopene and IGF Levels
4.3. Association Between Serum Lycopene and Mortality Risk
4.4. Implications for Future Directions
4.5. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BMI | Body mass index |
| CI | Confidence intervals |
| HR | Hazard ratio |
| IGF | Insulin-like growth factor |
| IGFBPs | Insulin-growth-factor-binding proteins |
| MAFLD | Metabolic fatty liver disease |
| MD | Mean difference |
| MDA-LDL | Malondialdehyde-modified LDL |
| NAFLD | Nonalcoholic fatty liver disease |
| OR | Odds ratio |
| RCT | Randomized controlled trial |
| ROS | Reactive oxygen species |
| T2DM | Type 2 Diabetes Mellitus |
References
- Masenga, S.K.; Kabwe, L.S.; Chakulya, M.; Kirabo, A. Mechanisms of oxidative stress in metabolic syndrome. Int. J. Mol. Sci. 2023, 24, 7898. [Google Scholar] [CrossRef] [PubMed]
- Micha, R.; Peñalvo, J.L.; Cudhea, F.; Imamura, F.; Rehm, C.D.; Mozaffarian, D. Association between dietary factors and mortality from heart disease, stroke, and type 2 diabetes in the United States. JAMA 2017, 317, 912–924. [Google Scholar] [CrossRef]
- Manna, P.; Jain, S.K. Obesity, oxidative stress, adipose tissue dysfunction, and the associated health risks: Causes and therapeutic strategies. Metab. Syndr. Relat. Disord. 2015, 13, 423–444. [Google Scholar] [CrossRef]
- Rodriguez-Concepcion, M.; Avalos, J.; Bonet, M.L.; Boronat, A.; Gomez-Gomez, L.; Hornero-Mendez, D.; Limon, M.C.; Meléndez-Martínez, A.J.; Medill-Alonso, B.; Palou, A.; et al. A global perspective on carotenoids: Metabolism, biotechnology, and benefits for nutrition and health. Prog. Lipid Res. 2018, 70, 62–93. [Google Scholar] [CrossRef]
- Wu, S.; Guo, X.; Shang, J.; Li, Y.; Dong, W.; Peng, Q.; Xie, Z.; Chen, C. Effects of lycopene attenuating injuries in ischemia and reperfusion. Oxidative Med. Cell. Longev. 2022, 2022, 9309327. [Google Scholar] [CrossRef] [PubMed]
- Eggersdorfer, M.; Wyss, A. Carotenoids in human nutrition and health. Arch. Biochem. Biophys. 2018, 652, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Roust, L.R.; DiBaise, J.K. Nutrient deficiencies prior to bariatric surgery. Curr. Opin. Clin. Nutr. Metab. Care. 2017, 20, 138–144. [Google Scholar] [CrossRef]
- Wang, Y.H.; Zhang, R.R.; Yin, Y.; Tan, G.F.; Wang, G.L.; Liu, H.; Zhuang, J.; Zhang, J.; Zhuang, F.-Y.; Xiong, A.-S. Advances in engineering the production of the natural red pigment lycopene: A systematic review from a biotechnology perspective. J. Adv. Res. 2023, 46, 31–47. [Google Scholar] [CrossRef]
- Woodside, J.V.; McGrath, A.J.; Lyner, N.; McKinley, M.C. Carotenoids and health in older people. Maturitas 2015, 80, 63–68. [Google Scholar] [CrossRef]
- Kulawik, A.; Rosiak, N.; Miklaszewski, A.; Cielecka-Piontek, J.; Zalewski, P. Investigation of cyclodextrin as potential carrier for lycopene. Arch. Pharm. 2024, 74, 178–205. [Google Scholar] [CrossRef]
- Imran, M.; Ghorat, F.; Ul-Haq, I.; Ur-Rehman, H.; Aslam, F.; Heydari, M.; Shariati, M.A.; Okuskhanova, E.; Yessimbekov, Z.; Thiruvengadam, M.; et al. Lycopene as a natural antioxidant used to prevent human health disorders. Antioxidants 2020, 9, 706. [Google Scholar] [CrossRef]
- Cervantes-Paz, B.; de Jesús Ornelas-Paz, J.; Ruiz-Cruz, S.; Rios-Velasco, C.; Ibarra-Junquera, V.; Yahia, E.M.; Gardea-Béjar, A.A. Effects of pectin on lipid digestion and possible implications for carotenoid bioavailability during pre-absorptive stages: A review. Food Res. Int. 2017, 99, 917–927. [Google Scholar] [CrossRef]
- Van Steenwijk, H.P.; Bast, A.; de Boer, A. The role of circulating lycopene in low-grade chronic inflammation: A systematic review of the literature. Molecules 2020, 25, 4378. [Google Scholar] [CrossRef]
- Abenavoli, L.; Procopio, A.C.; Paravati, M.R.; Costa, G.; Milić, N.; Alcaro, S.; Luzza, F. Mediterranean diet: The beneficial effects of lycopene in non-alcoholic fatty liver disease. J. Clin. Med. 2022, 11, 3477. [Google Scholar] [CrossRef] [PubMed]
- Charlton, A.; Garzarella, J.; Jandeleit-Dahm, K.A.M.; Jha, J.C. Oxidative Stress and Inflammation in Renal and Cardiovascular Complications of Diabetes. Biology 2021, 10, 18. [Google Scholar] [CrossRef]
- Senkus, K.E.; Tan, L.; Crowe-White, K.M. Lycopene and Metabolic Syndrome: A Systematic Review of the Literature. Adv. Nutr. 2019, 10, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Qian, F.; Huo, D. Circulating Insulin-Like Growth Factor-1 and Risk of Total and 19 Site-Specific Cancers: Cohort Study Analyses from the UK Biobank. Cancer Epidemiol. Biomark. Prev. 2020, 29, 2332–2342. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Yang, F. The effects of lycopene supplementation on serum insulin-like growth factor 1 (IGF-1) levels and cardiovascular disease: A dose-response meta-analysis of clinical trials. Complement. Ther. Med. 2021, 56, 102632. [Google Scholar] [CrossRef]
- Meshkini, F.; Ramezani-Jolfaie, N.; Sargazi, S.; Clark, C.C.T.; Soltani, S. The effects of lycopene supplementation on insulin-like growth factor-1 and insulin-like growth factor binding proteins: A systematic review of randomized controlled trials. Phytother. Res. 2022, 36, 1633–1643. [Google Scholar] [CrossRef]
- Kulawik, A.; Cielecka-Piontek, J.; Czerny, B.; Kamiński, A.; Zalewski, P. Relationship Between Lycopene and Metabolic Diseases. Nutrients 2024, 16, 3708. [Google Scholar] [CrossRef]
- Parums, D.V. Editorial: Review Articles, Systematic Reviews, Meta-Analysis, and the Updated Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 Guidelines. Med. Sci. Monit. 2021, 27, e934475. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions, 2nd ed.; John Wiley & Sons: Chichester, UK, 2019. [Google Scholar]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Savović, J.; Page, M.J.; Sterne, J.A.C. Revised Cochrane risk-of-bias tool for randomized trials (RoB 2). Cochrane Database Syst. Rev. 2022. Available online: https://methods.cochrane.org/bias/resources/rob-2-revised-cochrane-risk-bias-tool-randomized-trials (accessed on 2 June 2025).
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, 4919. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Boutron, I.; Page, M.J.; Higgins, J.P.T.; Altman, D.G.; Lundh, A.; Hróbjartsson, A. Considering bias and conflicts of interest among the included studies. In Cochrane Handbook for Systematic Reviews of Interventions, 2nd ed.; Higgins, J.P.T., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M.J., Welch, V.A., Eds.; John Wiley & Sons: Chichester, UK, 2019; pp. 177–204. [Google Scholar]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [PubMed]
- Schwarzer, G. Meta: An R package for meta-analysis. R News 2007, 7, 40–45. [Google Scholar]
- Viechtbauer, W. Conducting meta-analyses in R with the metafor package. J. Stat. Softw. 2010, 36, 1–48. [Google Scholar] [CrossRef]
- Gordon, M.; Lumley, T. Forestplot, Advanced Forest Plot Using ‘Grid’ Graphics, package version. 2017. Available online: https://cran.r-project.org/web/packages/forestplot/index.html (accessed on 2 June 2025).
- Neuwirth, E. RColorBrewer: Colorbrewer Palettes. V. 1.1-3. 2022. Available online: https://CRAN.R-project.org/package=RColorBrewer (accessed on 2 June 2025).
- Chai, W.; Tao, M.H. Overall and Sex-Specific Associations of Serum Lipid-Soluble Micronutrients with Metabolic Dysfunction-Associated Steatotic Liver Disease among Adults in the United States. Nutrients 2024, 16, 1242. [Google Scholar] [CrossRef]
- Lin, B.; Liu, Z.; Li, D.; Zhang, T.; Yu, C. Associations of serum carotenoids with all-cause and cardiovascular mortality in adults with MAFLD. Nutr. Metab. Cardiovasc. Dis. 2024, 34, 2315–2324. [Google Scholar] [CrossRef]
- Yu, J.; Guo, P. Association between dietary intake of carotenoids and metabolic dysfunction-associated fatty liver disease in US adults: National Health and Nutrition Examination Survey 2017–March 2020. Public Health Nutr. 2024, 27, e168. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Yuan, C.; Song, X.; Zhang, H. Association of Dietary Carotenoids Intakes with Obesity in Adults: NHANES 2007–2018. J. Nutr. Sci. Vitaminol. 2023, 69, 402–411. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, L.; Jia, L.; Liu, J. Association between carotenoid intake and metabolic dysfunction-associated fatty liver disease among US adults: A cross-sectional study. Medicine 2023, 102, e36658. [Google Scholar] [CrossRef]
- Leh, H.E.; Mohd Sopian, M.; Abu Bakar, M.H.; Lee, L.K. The role of lycopene for the amelioration of glycaemic status and peripheral antioxidant capacity among the Type II diabetes mellitus patients: A case–control study. Ann. Med. 2021, 53, 1060–1066. [Google Scholar] [CrossRef]
- Biernacka, K.M.; Holly, J.M.; Martin, R.M.; Frankow, A.; Bull, C.J.; Hamdy, F.C.; Donovan, J.L.; Neal, D.E.; Metcalfe, C.; Lane, A. Effect of green tea and lycopene on the insulin-like growth factor system: The ProDiet randomized controlled trial. Eur. J. Cancer Prev. 2019, 28, 569–575. [Google Scholar] [CrossRef]
- Christensen, K.; Lawler, T.; Mares, J. Dietary carotenoids and non-alcoholic fatty liver disease among US adults, NHANES 2003–2014. Nutrients 2019, 11, 1101. [Google Scholar] [CrossRef]
- Sanjeevi, N.; Lipsky, L.M.; Nansel, T.R. Hyperglycemia and carotenoid intake are associated with serum carotenoids in youth with type 1 diabetes. J. Acad. Nutr. Diet. 2019, 119, 1340–1348. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Wang, C.; Liu, J.; Liu, Z.M.; Ling, W.H.; Chen, Y.M. Greater serum carotenoid levels associated with lower prevalence of nonalcoholic fatty liver disease in Chinese adults. Sci. Rep. 2015, 5, 12951. [Google Scholar] [CrossRef]
- Sugiura, M.; Nakamura, M.; Ogawa, K.; Ikoma, Y.; Yano, M. High-serum carotenoids associated with lower risk for developing type 2 diabetes among Japanese subjects: Mikkabi cohort study. BMJ Open Diabetes Res. Care 2015, 3, e000147. [Google Scholar] [CrossRef]
- Li, Y.; Guo, H.; Wu, M.; Liu, M. Serum and dietary antioxidant status is associated with lower prevalence of the metabolic syndrome in a study in Shanghai, China. Asia Pac. J. Clin. Nutr. 2013, 22, 60–68. [Google Scholar]
- Suzuki, K.; Ito, Y.; Inoue, T.; Hamajima, N. Inverse association of serum carotenoids with prevalence of metabolic syndrome among Japanese. Clin. Nutr. 2011, 30, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Coyne, T.; Ibiebele, T.I.; Baade, P.D.; McClintock, C.S.; Shaw, J.E. Metabolic syndrome and serum carotenoids: Findings of a cross-sectional study in Queensland, Australia. Br. J. Nutr. 2009, 102, 1668–1677. [Google Scholar] [CrossRef] [PubMed]
- Markovits, M.N.; Levy, M.Y. The effect of tomato-derived lycopene on low carotenoids and enhanced systemic inflammation and oxidation in severe obesity. Wiley Online Libr. 2009, 11, 598–601. [Google Scholar]
- Suzuki, K.; Ito, Y.; Hashimoto, S.; Kawado, M.; Inoue, T.; Ando, M.; Watanabe, Y.; Inaba, Y.; Tajima, K.; Nakachi, K.; et al. Association of Serum Retinol and Carotenoids with Insulin-like Growth Factors and Insulin-like Growth Factor Binding Protein-3 among Control Subjects of a Nested Case-control Study in the JACC study. Age 2009, 1, 5. [Google Scholar]
- Sugiura, M.; Nakamura, M.; Ogawa, K.; Ikoma, Y.; Matsumoto, H.; Ando, F.; Shimokata, H.; Yano, M. Associations of serum carotenoid concentrations with the metabolic syndrome: Interaction with smoking. Br. J. Nutr. 2008, 100, 1297–1306. [Google Scholar] [CrossRef] [PubMed]
- Voskuil, D.W.; Vrieling, A.; Korse, C.M.; Beijnen, J.H.; Bonfrer, J.M.; van Doorn, J.; Kaas, R.; Oldenburg, H.S.A.; Russell, N.S.; Rutgers, E.J.T.; et al. Effects of lycopene on the insulin-like growth factor (IGF) system in premenopausal breast cancer survivors and women at high familial breast cancer risk. Nutr. Cancer 2008, 60, 342–353. [Google Scholar] [CrossRef]
- Vrieling, A.; Voskuil, D.W.; Bonfrer, J.M.; Korse, C.M.; van Doorn, J.; Cats, A.; Depla, A.C.; Timmer, R.; Witteman, B.J.; Van Leeuwen, F.E.; et al. Lycopene supplementation elevates circulating insulin-like growth factor–binding protein-1 and-2 concentrations in persons at greater risk of colorectal cancer. Am. J. Clin. Nutr. 2007, 86, 1456–1462. [Google Scholar] [CrossRef]
- Walfisch, S.; Walfisch, Y.; Kirilov, E.; Linde, N.; Mnitentag, H.; Agbaria, R.; Sharoni, Y.; Levy, J. Tomato lycopene extract supplementation decreases insulin-like growth factor-I levels in colon cancer patients. Eur. J. Cancer Prev. 2007, 16, 298–303. [Google Scholar] [CrossRef]
- Kimmons, J.E.; Blanck, H.M.; Tohill, B.C.; Zhang, J.; Khan, L.K. Associations between body mass index and the prevalence of low micronutrient levels among US adults. Medscape Gen. Med. 2006, 8, 59. [Google Scholar]
- Suzuki, K.I.T.; Hioki, R.; Ochiai, J.; Kusuhara, Y.; Ichino, N.; Osakabe, K.; Hamajima, N.; Ito, Y. Association of abdominal obesity with decreased serum levels of carotenoids in a healthy Japanese population. Clin. Nutr. 2006, 25, 780–789. [Google Scholar] [CrossRef]
- Tran, C.D.D.; Bérubé, S.; Pollak, M.; Brisson, J. Relation of insulin-like growth factor (IGF) I and IGF-binding protein 3 concentrations with intakes of fruit, vegetables, and antioxidants. Am. J. Clin. Nutr. 2006, 84, 1518–1526. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, L.L.; Pradhan, A.D.; Manson, J.E.; Buring, J.E.; Gaziano, J.M.; Sesso, H.D. Plasma lycopene, other carotenoids, and the risk of type 2 diabetes in women. Am. J. Epidemiol. 2006, 164, 576–585. [Google Scholar] [CrossRef]
- Wang, L.L.S.; Manson, J.E.; Gaziano, J.M.; Buring, J.E.; Sesso, H.D. The consumption of lycopene and tomato-based food products is not associated with the risk of type 2 diabetes in women. J. Nutr. 2006, 136, 620–625. [Google Scholar] [CrossRef]
- Coyne, T.I.T.I.; Baade, P.D.; Dobson, A.; McClintock, C.; Dunn, S.; Leonard, D.; Shaw, J. Diabetes mellitus and serum carotenoids: Findings of a population-based study in Queensland, Australia. Am. J. Clin. Nutr. 2005, 82, 685–693. [Google Scholar] [CrossRef] [PubMed]
- Montonen, J.; Knekt, P.; Jarvinen, R.; Reunanen, A. Dietary Antioxidant Intake and Risk of Type 2 Diabetes. Diabetes Care 2004, 27, 362–366. [Google Scholar] [CrossRef] [PubMed]
- Ford, A.H.; Giles, W.H.; Brown, D.W. The metabolic syndrome and antioxidant concentrations: Findings from the Third National Health and Nutrition Examination Survey. Diabetes 2003, 52, 2346–2352. [Google Scholar] [CrossRef]
- Suzuki, Y.; Nakamura, S.; Ochiai, J.; Aoki, K. Relationship between serum carotenoids and hyperglycemia: A population-based cross-sectional study. J. Epidemiol. 2002, 12, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Chai, W.; Conroy, S.M.; Maskarinec, G.; Franke, A.A.; Pagano, I.S.; Cooney, R.V. Associations between obesity and serum lipid-soluble micronutrients among premenopausal women. Nutr. Res. 2010, 30, 227–232. [Google Scholar] [CrossRef]
- Suzuki, K.; Ito, Y.; Ochiai, J.; Kusuhara, Y.; Hashimoto, S.; Tokudome, S.; Kojima, M.; Wakai, K.; Toyoshima, H.; Tamakoshi, K.; et al. Relationship between obesity and serum markers of oxidative stress and inflammation in Japanese. Asian Pac. J. Cancer Prev. 2003, 4, 259–266. [Google Scholar]
- Chai, W.; Eaton, S.; Rasmussen, H.E.; Tao, M.H. Associations of dietary lipid-soluble micronutrients with hepatic steatosis among adults in the United States. Biomedicines 2021, 9, 1093. [Google Scholar] [CrossRef]
- Rinella, M.E.; Lazarus, J.V.; Ratziu, V.; Francque, S.M.; Sanyal, A.J.; Kanwal, F.; Romero, D.; Abdelmalek, M.F.; Anstee, Q.M.; Pablo Arab, J.P.; et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. Hepatology 2023, 78, 1966–1986. [Google Scholar] [CrossRef] [PubMed]
- Kucuk, O.; Sarkar, F.H.; Sakr, W.; Djuric, Z.; Pollak, M.N.; Khachik, F.; Li, Y.W.; Banerjee, M.; Grignon, D.; Bertram, J.S.; et al. Phase II randomized clinical trial of lycopene supplementation before radical prostatectomy. Cancer Epidemiol. Biomark. Prev. 2001, 10, 861–868. [Google Scholar]
- Signorello, L.; Kuper, H.; Lagiou, P.; Wuu, J.; Mucci, L.; Trichopoulos, D.; Adami, H.-O. Lifestyle factors and insulin-like growth factor 1 levels among elderly men. Eur. J. Cancer Prev. 2000, 9, 173–178. [Google Scholar] [CrossRef]
- Elmlinger, M.W.; Kühnel, W.; Weber, M.M.; Ranke, M.B. Reference ranges for two automated chemiluminescent assays for serum insulin-like growth factor I (IGF-I) and IGF-binding protein 3 (IGFBP-3). Clin. Chem. Lab. Med. 2004, 42, 654–664. [Google Scholar] [CrossRef]
- D’Ercole, A.J.; Applewhite, G.T.; Underwood, L.E. Evidence that somatomedin is synthesized by multiple tissues in the fetus. Dev. Biol. 1980, 75, 315–328. [Google Scholar] [CrossRef] [PubMed]
- Delafontaine, P.; Song, Y.H.; Li, Y. Expression, regulation, and function of IGF-1, IGF-1R, and IGF-1 binding proteins in blood vessels. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Lewitt, M.S.; Saunders, H.; Phuyal, J.L.; Baxter, R.C. Complex formation by human insulin-like growth factor-binding protein-3 and human acid-labile subunit in growth hormone-deficient rats. Endocrinology 1994, 134, 2404–2409. [Google Scholar] [CrossRef]
- Jones, J.I.; Clemmons, D.R. Insulin-like growth factors and their binding proteins: Biological actions. Endocr. Rev. 1995, 16, 3–34. [Google Scholar]
- Peruzzi, F.; Prisco, M.; Dews, M.; Salomoni, P.; Grassilli, E.; Romano, G.; Calabretta, B.; Baserga, R. Multiple signaling pathways of the insulin-like growth factor 1 receptor in protection from apoptosis. Mol. Cell. Biol. 1999, 19, 7203–7215. [Google Scholar] [CrossRef]
- Li, L.; Yu, H.; Schumacher, F.; Casey, G.; Witte, J.S. Relation of serum insulin-like growth factor-I (IGF-I) and IGF binding protein-3 to risk of prostate cancer (United States). Cancer Causes Control 2003, 14, 721–726. [Google Scholar] [CrossRef]
- Morton, R.W.; Sato, K.; Gallaugher, M.P.; Oikawa, S.Y.; McNicholas, P.D.; Fujita, S.; Phillips, S.M. Muscle androgen receptor content but not systemic hormones is associated with resistance training-induced skeletal muscle hypertrophy in healthy, young men. Front. Physiol. 2018, 9, 1373. [Google Scholar] [CrossRef] [PubMed]
- Zainuddin, A.A.; Nor, N.M.; Yusof, S.M.; Ibrahim, A.I.N.; Aris, T.; Huat, F.L. Changes in energy and nutrient intakes among Malaysian adults: Findings from the Malaysian Adult Nutrition Survey (MANS) 2003 and 2014. Malays. J. Nutr. 2019, 25, 273–285. [Google Scholar] [CrossRef]
- Ford, E.S.; Will, J.C.; Bowman, B.A.; Narayan, K.V. Diabetes mellitus and serum carotenoids: Findings from the Third National Health and Nutrition Examination Survey. Am. J. Epidemiol. 1999, 149, 168–176. [Google Scholar] [CrossRef]
- Ylönen, K.; Alfthan, G.; Groop, L.; Saloranta, C.; Aro, A.; Virtanen, S.M. Dietary intakes and plasma concentrations of carotenoids and tocopherols in relation to glucose metabolism in subjects at high risk of type 2 diabetes: The Botnia Dietary Study. Am. J. Clin. Nutr. 2003, 77, 1434–1441. [Google Scholar] [CrossRef]
- Neyestani, T.; Shariatzadeh, N.; Gharavi, A.; Kalayi, A.; Khalaji, N. Physiological dose of lycopene suppressed oxidative stress and enhanced serum levels of immunoglobulin M in patients with Type 2 diabetes mellitus: A possible role in the prevention of long-term complications. J. Endocrinol. Investig. 2007, 30, 833–838. [Google Scholar] [CrossRef] [PubMed]
- Upritchard, J.E.; Sutherland, W.; Mann, J.I. Effect of supplementation with tomato juice, vitamin E, and vitamin C on LDL oxidation and products of inflammatory activity in type 2 diabetes. Diabetes Care 2000, 23, 733–738. [Google Scholar] [CrossRef]
- Yin, Y.; Zheng, Z.; Jiang, Z. Effects of lycopene on metabolism of glycolipid in type 2 diabetic rats. Biomed. Pharmacother. 2019, 109, 2070–2077. [Google Scholar] [CrossRef]
- Chen, D.; Huang, C.; Chen, Z. A review for the pharmacological effect of lycopene in central nervous system disorders. Biomed. Pharmacother. 2019, 111, 791–801. [Google Scholar] [CrossRef]
- Etienne, I.; Magalhães, L.V.B.; Cardoso, S.A.; de Freitas, R.B.; de Oliveira, G.P.; Palotás, A.; Lima, L.M. Oxidative stress markers in cognitively intact patients with diabetic neuropathy. Brain Res. Bull. 2019, 150, 196–200. [Google Scholar] [CrossRef]
- Park, B.; Lim, J.W.; Kim, H. Lycopene treatment inhibits activation of Jak1/Stat3 and Wnt/β-catenin signaling and attenuates hyperproliferation in gastric epithelial cells. Nutr. Res. 2019, 70, 70–81. [Google Scholar] [CrossRef]


| Study ID | Setting (Country) | Type of Study | Type of Metabolic Disease | Sample Size | |
|---|---|---|---|---|---|
| Study Group | Comparision Group | ||||
| Chai et al., 2024 [33] | US | Cross-sectional | MAFLD | 1148 | 2808 |
| Lin et al., 2024 [34] | US | Cross-sectional | MAFLD | 3040 | |
| Yu et al., 2024 [35] | US | Cross-sectional | MAFLD | 2925 | 2173 |
| Yu et al., 2023 [36] | China | Cross-sectional | Obesity | 10,192 | 15,676 |
| Zhang et al., 2023 [37] | US | Cross-sectional | MAFLD | 1476 | 1246 |
| Leh et al., 2021 [38] | Malaysia | Case–control | Hyperglycemia | 87 | 122 |
| Biernacka et al., 2019 [39] | UK | Randomized clinical trial | IGF Decline | Supplement: 41 Dietary Advice: 42 | 37 |
| Christensen et al., 2019 [40] | US | Cross-sectional | MAFLD | 30,295,373 | 61,456,846 |
| Sanjeevi et al., 2019 [41] | US | Secondary analysis of randomized clinical trial | Hyperglycemia | 136 | |
| Cao et al., 2015 [42] | China | Cross-sectional | MAFLD | 1486 | 1449 |
| Sugiura et al., 2015 [43] | Japan | Follow-up study on Mikkabi cohort study | Hyperglycemia | 864 | |
| Li et al., 2013 [44] | China | Cross-sectional | Metabolic Syndrome | 221 | 323 |
| Suzuki et al., 2011 [45] | Japan | Cross-sectional | Metabolic Syndrome | 117 | 814 |
| Coyne et al., 2009 [46] | Australia | Cross-sectional | Metabolic Syndrome | 424 | 1112 |
| Markovits et al., 2009 [47] | Israel | Clinical Trial | Obesity | 8 | 8 |
| Suzuki et al., 2009 [48] | Japan | Prospective cohort | IGF Decline | 924 | |
| Sugiura et al., 2008 [49] | Japan | Cross-sectional | Metabolic Syndrome | 59 | 899 |
| Voskuil et al., 2008 [50] | Netherlands | Randomized clinical trial | IGF Decline | 60 | |
| Vrieling et al., 2007 [51] | Netherlands | Randomized clinical trial | IGF Decline | 71 | |
| Walfisch et al., 2007 [52] | Israel | Randomized clinical trial | IGF Decline | 30 | 26 |
| Kimmons et al., 2006 [53] | US | Cross-sectional | Obesity | 16,191 | |
| Suzuki et al., 2007 [54] | Japan | Cross-sectional | Obesity | 174 | 416 |
| Tran et al., 2006 [55] | Canada | Cross-sectional | IGF Decline | 1542 | |
| Wang et al., 2006 [56] | US | Prospective case–control design | Hyperglycemia | 470 | 470 |
| Wang et al., 2005 [57] | US | Prospective cohort | Hyperglycemia | 35,783 | |
| Coyne et al., 2005 [58] | Australia | Cross-sectional | Hyperglycemia | 1597 | |
| Montonen et al., 2004 [59] | Finland | Prospective cohort | Hyperglycemia | 383 | 3921 |
| Ford et al., 2003 [60] | US | Cross-sectional | Metabolic Syndrome | 8808 | |
| Suzuki et al., 2002 [61] | Japan | Cross-sectional | Hyperglycemia | High HbA1c: 151 DM: 133 | Group 1: 302 Group 2: 266 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Viña, I.; Robles, A.; Viña, J.R. Association Between Lycopene and Metabolic Disease Risk and Mortality: Systematic Review and Meta-Analysis. Life 2025, 15, 944. https://doi.org/10.3390/life15060944
Viña I, Robles A, Viña JR. Association Between Lycopene and Metabolic Disease Risk and Mortality: Systematic Review and Meta-Analysis. Life. 2025; 15(6):944. https://doi.org/10.3390/life15060944
Chicago/Turabian StyleViña, Isabel, Alicia Robles, and Juan R. Viña. 2025. "Association Between Lycopene and Metabolic Disease Risk and Mortality: Systematic Review and Meta-Analysis" Life 15, no. 6: 944. https://doi.org/10.3390/life15060944
APA StyleViña, I., Robles, A., & Viña, J. R. (2025). Association Between Lycopene and Metabolic Disease Risk and Mortality: Systematic Review and Meta-Analysis. Life, 15(6), 944. https://doi.org/10.3390/life15060944







