The Efficacy of Curcumin-Mediated Photodynamic Therapy in the Treatment of Oral Squamous Cell Carcinoma: A Systematic Review of In Vitro Studies
Abstract
1. Introduction
2. Materials and Methods
2.1. Focused Question
2.2. Information Sources and Search Strategy
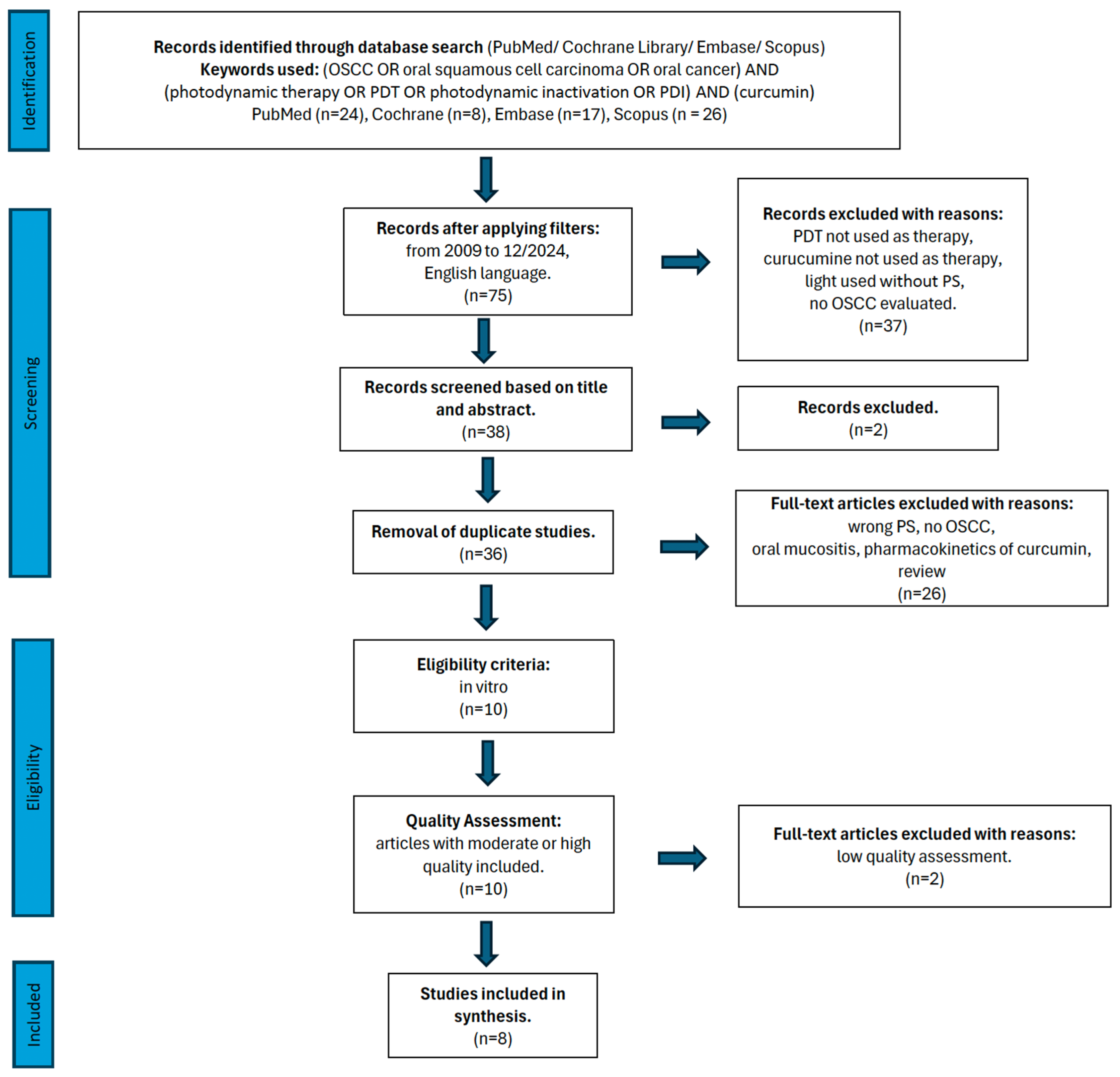
2.3. Study Selection
2.4. Risk of Bias in Individual Studies
2.5. Quality Assessment and Risk of Bias Across Studies
2.6. Data Extraction
3. Results
3.1. Primary Outcome
3.2. Study Selection During Full-Text Analysis
3.3. Data Presentation
3.4. General Characteristics of the Included Studies
3.5. Characteristics of Light Sources Used in PDT
3.6. Characteristics of Curcumin Used as a Photosensitizer in PDT
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Schiavoni, V.; Emanuelli, M.; Sartini, D.; Salvolini, E.; Pozzi, V.; Campagna, R. Curcumin and its Analogues in Oral Squamous Cell Carcinoma: State-of-the-art and Therapeutic Potential. Anticancer Agents Med. Chem. 2024, 25, 313–329. [Google Scholar] [CrossRef] [PubMed]
- Ravera, S.; Pasquale, C.; Panfoli, I.; Bozzo, M.; Agas, D.; Bruno, S.; Hamblin, M.R.; Amaroli, A. Assessing the Effects of Curcumin and 450 nm Photodynamic Therapy on Oxidative Metabolism and Cell Cycle in Head and Neck Squamous Cell Carcinoma: An In Vitro Study. Cancers 2024, 16, 1642. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ghanem, A.S.; Memon, H.A.; Nagy, A.C. Evolving trends in oral cancer burden in Europe: A systematic review. Front. Oncol. 2024, 14, 1444326. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Beyer, K.; Nikfarjam, F.; Butting, M.; Meissner, M.; König, A.; Bosca, A.R.; Kaufmann, R.; Heidemann, D.; Bernd, A.; Kippenberger, S.; et al. Photodynamic Treatment of Oral Squamous Cell Carcinoma Cells with Low Curcumin Concentrations. J. Cancer 2017, 8, 1271–1283. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nokovitch, L.; Maquet, C.; Crampon, F.; Taihi, I.; Roussel, L.-M.; Obongo, R.; Virard, F.; Fervers, B.; Deneuve, S. Oral Cavity Squamous Cell Carcinoma Risk Factors: State of the Art. J. Clin. Med. 2023, 12, 3264. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rezazadeh, F.; Andisheh-Tadbir, A.; Malek Mansouri, Z.; Khademi, B.; Bayat, P.; Sedarat, H.; Tabesh, A.; Tayebi Khorami, E. Evaluation of recurrence, mortality and treatment complications of oral squamous cell carcinoma in public health centers in Shiraz during 2010 to 2020. BMC Oral Health 2023, 23, 341. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Łopaciński, M.; Fiegler-Rudol, J.; Niemczyk, W.; Skaba, D.; Wiench, R. Riboflavin- and Hypericin-Mediated Antimicrobial Photodynamic Therapy as Alternative Treatments for Oral Candidiasis: A Systematic Review. Pharmaceutics 2025, 17, 33. [Google Scholar] [CrossRef]
- Wiench, R.; Nowicka, J.; Pajączkowska, M.; Kuropka, P.; Skaba, D.; Kruczek-Kazibudzka, A.; Kuśka-Kiełbratowska, A.; Grzech-Leśniak, K. Influence of Incubation Time on Ortho-Toluidine Blue Mediated Antimicrobial Photodynamic Therapy Directed against Selected Candida Strains—An In Vitro Study. Int. J. Mol. Sci. 2021, 22, 10971. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zembala, M.; Pakosz, K.; Zakliczyński, M.; Król, W.; Pyka, Ł.; Zakliczyńska, H.; Trybunia, D.; Wiench, R.; Ilewicz, L.; Skrzep-Poloczek, B.; et al. Association of transforming growth factor β1 (TGF-β1) with gingival hyperplasia in heart transplant patients undergoing cyclosporine-A treatment. Ann. Transplant. 2012, 17, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Fiegler-Rudol, J.; Zięba, N.; Turski, R.; Misiołek, M.; Wiench, R. Hypericin-Mediated Photodynamic Therapy for Head and Neck Cancers: A Systematic Review. Biomedicines 2025, 13, 181. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fiegler-Rudol, J.; Łopaciński, M.; Los, A.; Skaba, D.; Wiench, R. Riboflavin-Mediated Photodynamic Therapy in Periodontology: A Systematic Review of Applications and Outcomes. Pharmaceutics 2025, 17, 217. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kruczek-Kazibudzka, A.; Lipka, B.; Fiegler-Rudol, J.; Tkaczyk, M.; Skaba, D.; Wiench, R. Toluidine Blue and Chlorin-e6 Mediated Photodynamic Therapy in the Treatment of Oral Potentially Malignant Disorders: A Systematic Review. Int. J. Mol. Sci. 2025, 26, 2528. [Google Scholar] [CrossRef]
- Gilowski, Ł.; Wiench, R.; Polakiewicz-Gilowska, A.; Dwornicka, K. Necrotizing sialometaplasia of the palatal mucosa in patient with history of anorexia: Review and case report. Am. J. Otolaryngol. 2014, 35, 400–401. [Google Scholar] [CrossRef] [PubMed]
- Kawczyk-Krupka, A.; Bartusik-Aebisher, D.; Latos, W.; Cieślar, G.; Sieroń, K.; Kwiatek, S.; Oleś, P.; Kwiatek, B.; Aebisher, D.; Krupka, M.; et al. Clinical Trials and Basic Research in Photodynamic Diagnostics and Therapies from the Center for Laser Diagnostics and Therapy in Poland. Photochem. Photobiol. 2020, 96, 539–549. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Ji, X.; Zhang, Q.; Wei, Y. Curcumin combined with photodynamic therapy, promising therapies for the treatment of cancer. Biomed. Pharmacother. 2022, 146, 112567. [Google Scholar] [CrossRef] [PubMed]
- Amalraj, A.; Pius, A.; Gopi, S.; Gopi, S. Biological activities of curcuminoids, other biomolecules from turmeric and their derivatives—A review. J. Tradit. Complement. Med. 2016, 7, 205–233. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kubizna, M.; Dawiec, G.; Wiench, R. Efficacy of Curcumin-Mediated Antimicrobial Photodynamic Therapy on Candida spp.—A Systematic Review. Int. J. Mol. Sci. 2024, 25, 8136. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Akbik, D.; Ghadiri, M.; Chrzanowski, W.; Rohanizadeh, R. Curcumin as a wound healing agent. Life Sci. 2014, 116, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Hussain, Z.; Thu, H.E.; Ng, S.-F.; Khan, S.; Katas, H. Nanoencapsulation, an efficient and promising approach to maximize wound healing efficacy of curcumin: A review of new trends and state-of-the-art. Colloids Surf. B Biointerfaces 2017, 150, 223–241. [Google Scholar] [CrossRef] [PubMed]
- Schamberger, B.; Plaetzer, K. Photofungizides Based on Curcumin and Derivates Thereof against Candida albicans and Aspergillus niger. Antibiotics 2021, 10, 1315. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bernd, A. Visible light and/or UVA offer a strong amplification of the anti-tumor effect of curcumin. Phytochem. Rev. 2014, 13, 183–189. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Watson, P.F.; Petrie, A. Method agreement analysis: A review of correct methodology. Theriogenology 2010, 73, 1167–1179. [Google Scholar] [CrossRef] [PubMed]
- Wolnicka-Glubisz, A.; Wisniewska-Becker, A. Dual Action of Curcumin as an Anti- and Pro-Oxidant from a Biophysical Perspective. Antioxidants 2023, 12, 1725. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Higgins, J.; Savović, J.; Page, M.; Elbers, R.; Sterne, J. Assessing risk of bias in a randomized trial. In Cochrane Handbook for Systematic Reviews of Interventions, 2nd ed.; Higgins, J., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M., Welch, V., Eds.; John Wiley & Sons: Chichester, UK, 2019; pp. 205–228. [Google Scholar]
- Zhu, F.; Tan, G.; Jiang, Y.; Yu, Z.; Ren, F. Rational design of multi-stimuli-responsive gold nanorod-curcumin conjugates for chemo-photothermal synergistic cancer therapy. Biomater. Sci. 2018, 6, 2905–2917. [Google Scholar] [CrossRef] [PubMed]
- Silvestre, F.; Santos, C.; Silva, V.; Ombredane, A.; Pinheiro, W.; Andrade, L.; Garcia, M.; Pacheco, T.; Joanitti, G.; Luz, G.; et al. Pharmacokinetics of Curcumin Delivered by Nanoparticles and the Relationship with Antitumor Efficacy: A Systematic Review. Pharmaceuticals 2023, 16, 943. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pires Marques, E.C.; Piccolo Lopes, F.; Nascimento, I.C.; Morelli, J.; Pereira, M.V.; Meiken, V.M.M.; Pinheiro, S.L. Photobiomodulation and photodynamic therapy for the treatment of oral mucositis in patients with cancer. Photodiagnosis Photodyn. Ther. 2020, 29, 101621. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, S.L.; Bonadiman, A.C.; Borges Lemos, A.L.d.A.; Annicchino, B.M.; Segatti, B.; Pucca, D.S.; Dutra, P.T.; Silva, R.M.d.C.e.; Leal, F. Photobiomodulation Therapy in Cancer Patients with Mucositis: A Clinical Evaluation. Photobiomodulation Photomed. Laser Surg. 2019, 37, 142–150. [Google Scholar] [CrossRef] [PubMed]
- de Cássia Dias Viana Andrade, R.; Azevedo Reis, T.; Rosa, L.P.; de Oliveira Santos, G.P.; da CristinaSilva, F. Comparative randomized trial study about the efficacy of photobiomodulation and curcumin antimicrobial photodynamic therapy as a coadjuvant treatment of oral mucositis in oncologic patients: Antimicrobial, analgesic, and degree alteration effect. Support. Care Cancer 2022, 30, 7365–7371. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, L.L.; Durães, C.P.; Menezes, A.S.d.S.; Tabosa, A.T.L.; Barbosa, C.U.; Filho, A.d.P.S.; Souza, D.P.S.d.P.; Guimarães, V.H.D.; Santos, S.H.S.; de Paula, A.M.B.; et al. Comparison between two antimicrobial photodynamic therapy protocols for oral candidiasis in patients undergoing treatment for head and neck cancer: A two-arm, single-blind clinical trial. Photodiagnosis Photodyn. Ther. 2022, 39, 102983. [Google Scholar] [CrossRef] [PubMed]
- da Silva, L.A.; Pinheiro, S.L. Clinical Evaluation of Intravascular Blood Irradiation with Laser, Photobiomodulation, and Photodynamic Therapy in Cancer Patients with Mucositis. Photobiomodulation Photomed. Laser Surg. 2021, 39, 687–695. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Zhang, Y.; He, Y.; Xiong, M.; Huang, H.; Pei, S.; Liao, J.; Wang, Y.; Shao, D. Green synthesis of carrier-free curcumin nanodrugs for light-activated breast cancer photodynamic therapy. Colloids Surf. B Biointerfaces 2019, 180, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Mohammad, S.; Pant, A.B.; Mishra, P.R.; Pandey, G.; Gupta, S.; Farooqui, S. Co-delivery of 5-Fluorouracil and Curcumin Nanohybrid Formulations for Improved Chemotherapy Against Oral Squamous Cell Carcinoma. J. Maxillofac. Oral Surg. 2018, 17, 597–610. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ortega, A.; da Silva, A.B.; da Costa, L.M.; Zatta, K.C.; Onzi, G.R.; da Fonseca, F.N.; Guterres, S.S.; Paese, K. Thermosensitive and mucoadhesive hydrogel containing curcumin-loaded lipid-core nanocapsules coated with chitosan for the treatment of oral squamous cell carcinoma. Drug Deliv. Transl. Res. 2023, 13, 642–657. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Mohammad, S.; Gupta, S.; Mahdi, A.A.; Dixit, R.K.; Singh, V.; Samadi, F.M. Chemoprotective effect of nanocurcumin on 5-fluorouracil-induced-toxicity toward oral cancer treatment. Natl. J. Maxillofac. Surg. 2018, 9, 160–166. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hoornstra, D.; Vesterlin, J.; Pärnänen, P.; Al-Samadi, A.; Zlotogorski-Hurvitz, A.; Vered, M.; Salo, T. Fermented Lingonberry Juice Inhibits Oral Tongue Squamous Cell Carcinoma Invasion In Vitro Similarly to Curcumin. In Vivo 2018, 32, 1089–1095. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- M, D.; T.N, U.; Eswaramoorthy, R. In Vitro Exploration of Dark Cytotoxicity of Anthocyanin-Curcumin Combination, A Herbal Photosensitizer. Cureus 2024, 16, e56714. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hatamipour, M.; Ramezani, M.; Tabassi, S.A.S.; Johnston, T.P.; Ramezani, M.; Sahebkar, A. Demethoxycurcumin: A naturally occurring curcumin analogue with antitumor properties. J. Cell. Physiol. 2018, 233, 9247–9260. [Google Scholar] [CrossRef]
- Yang, H.; Wei, Y.-C.; Li, W.-C.; Chen, H.-Y.; Lin, H.-Y.; Chiang, C.-P.; Chen, H.-M. Natural Compounds Modulate Drug Transporter Mediated Oral Cancer Treatment. Biomolecules 2020, 10, 1335. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Olek, M.; Kasperski, J.; Skaba, D.; Wiench, R.; Cieślar, G.; Kawczyk-Krupka, A. Photodynamic therapy for the treatment of oral squamous carcinoma-Clinical implications resulting from in vitro research. Photodiagnosis Photodyn. Ther. 2019, 27, 255–267. [Google Scholar] [CrossRef] [PubMed]
- Saberi, S.; Hakimiha, N.; Alaeddini, M.; Etemad-Moghadam, S.; Roudbari, P.; Shahabi, S. In Vitro Anti-tumor Effects of Photodynamic Therapy on Oral Squamous Cell Carcinoma: A Review. J. Lasers Med. Sci. 2022, 13, e49. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xu, C.; Wang, M.; Guo, W.; Sun, W.; Liu, Y. Curcumin in Osteosarcoma Therapy: Combining with Immunotherapy, Chemotherapeutics, Bone Tissue Engineering Materials and Potential Synergism with Photodynamic Therapy. Front. Oncol. 2021, 11, 672490. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nasrin, A.; Hassan, M.; Gomes, V.G. Two-photon active nucleus-targeting carbon dots: Enhanced ROS generation and photodynamic therapy for oral cancer. Nanoscale 2020, 12, 20598–20603. [Google Scholar] [CrossRef] [PubMed]
- Kah, G.; Chandran, R.; Abrahamse, H. Curcumin a Natural Phenol and Its Therapeutic Role in Cancer and Photodynamic Therapy: A Review. Pharmaceutics 2023, 15, 639. [Google Scholar] [CrossRef]
- Leman, E. Elucidating the Effect of Photodynamic Treatment and Pro-Apoptopic Factors on the Growth of Human Head and Neck Cancer Cells. Ph.D. Thesis, University of Haifa, Haifa, Israel, 2016; p. 28748740. [Google Scholar]
- Duse, L.; Pinnapireddy, S.R.; Strehlow, B.; Jedelská, J.; Bakowsky, U. Low level LED photodynamic therapy using curcumin loaded tetraether liposomes. Eur. J. Pharm. Biopharm. 2018, 126, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Zholobak, N.; Shcherbakov, A.; Ivanova, O.; Reukov, V.; Baranchikov, A.; Ivanov, V. Nanoceria-curcumin conjugate: Synthesis and selective cytotoxicity against cancer cells under oxidative stress conditions. J. Photochem. Photobiol. B Biol. 2020, 209, 111921. [Google Scholar] [CrossRef]
- Wu, Q.; Ning, H.; Wang, H.; Hua, H.; Li, W.; Xu, B. Cancer cell membrane camouflaging mesoporous nanoplatform interfering with cellular redox homeostasis to amplify photodynamic therapy on oral carcinoma. J. Drug Target. 2023, 31, 511–520. [Google Scholar] [CrossRef] [PubMed]
- Pavarina, A.C.; Ribeiro, A.P.D.; Dovigo, L.N.; de Andrade, C.R.; de Souza Costa, C.A.; Vergani, C.E. Photodynamic Therapy to Eradicate Tumor Cells. In Cell Metabolism—Cell Homeostasis and Stress Response; IntechOpen: London, UK, 2012. [Google Scholar] [CrossRef]
- Roschenko, V.; Ayoub, A.M.; Engelhardt, K.; Schäfer, J.; Amin, M.U.; Preis, E.; Mandic, R.; Bakowsky, U. Lipid-Coated Polymeric Nanoparticles for the Photodynamic Therapy of Head and Neck Squamous Cell Carcinomas. Pharmaceutics 2023, 15, 2412. [Google Scholar] [CrossRef]
- Singh, S.P.; Sharma, M.; Gupta, P.K. Enhancement of phototoxicity of curcumin in human oral cancer cells using silica nanoparticles as delivery vehicle. Lasers Med. Sci. 2014, 29, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Dujic, J.; Kippenberger, S.; Ramirez-Bosca, A.; Diaz-Alperi, J.; Bereiter-Hahn, J.; Kaufmann, R.; Bernd, A.; Hofmann, M. Curcumin in combination with visible light inhibits tumor growth in a xenograft tumor model. Int. J. Cancer 2009, 124, 1422–1428. [Google Scholar] [CrossRef] [PubMed]
- Fiegler-Rudol, J.; Kapłon, K.; Kotucha, K.; Moś, M.; Skaba, D.; Kawczyk-Krupka, A.; Wiench, R. Hypocrellin-Mediated PDT: A Systematic Review of Its Efficacy, Applications, and Outcomes. Int. J. Mol. Sci. 2025, 26, 4038. [Google Scholar] [CrossRef] [PubMed]
- M, D.; Tn, U.; Eeswaramoorthy, R. In Vitro Evaluation of Light-Induced Cytotoxic Property: Synergistic Effects of Anthocyanin/Curcumin as a Photosensitizer. Cureus 2023, 15, e48537. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ambreen, G.; Duse, L.; Tariq, I.; Ali, U.; Ali, S.; Pinnapireddy, S.R.; Bette, M.; Bakowsky, U.; Mandic, R. Sensitivity of Papilloma Virus-Associated Cell Lines to Photodynamic Therapy with Curcumin-Loaded Liposomes. Cancers 2020, 12, 3278. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Warakomska, A.; Fiegler-Rudol, J.; Kubizna, M.; Skaba, D.; Wiench, R. The Role of Photodynamic Therapy Mediated by Natural Photosensitisers in the Management of Peri-Implantitis: A Systematic Review. Pharmaceutics 2025, 17, 443. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Feng, Y.; Xiong, Y.; Qiao, T.; Li, X.; Jia, L.; Han, Y. Lactate dehydrogenase A: A key player in carcinogenesis and potential target in cancer therapy. Cancer Med. 2018, 7, 6124–6136. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Buss, S.; Dobra, J.; Goerg, K.; Hoffmann, S.; Kippenberger, S.; Kaufmann, R.; Hofmann, M.; Bernd, A. Visible light is a better co-inducer of apoptosis for curcumin-treated human melanoma cells than UVA. PLoS ONE 2013, 8, e79748. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rutz, J.; Maxeiner, S.; Juengel, E.; Bernd, A.; Kippenberger, S.; Zöller, N.; Chun, F.K.-H.; Blaheta, R.A. Growth and Proliferation of Renal Cell Carcinoma Cells Is Blocked by Low Curcumin Concentrations Combined with Visible Light Irradiation. Int. J. Mol. Sci. 2019, 20, 1464. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Agostinis, P.; Berg, K.; Cengel, K.A.; Foster, T.H.; Girotti, A.W.; Gollnick, S.O.; Hahn, S.M.; Hamblin, M.R.; Juzeniene, A.; Kessel, D.; et al. Photodynamic therapy of cancer: An update. CA Cancer J. Clin. 2011, 61, 250–281. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yan, W.; Wistuba, I.I.; Emmert-Buck, M.R.; Erickson, H.S. Squamous Cell Carcinoma—Similarities and Differences among Anatomical Sites. Am. J. Cancer Res. 2011, 1, 275–300. [Google Scholar] [PubMed] [PubMed Central]
- Santezi, C.; Reina, B.D.; Dovigo, L.N. Curcumin-mediated Photodynamic Therapy for the treatment of oral infections—A review. Photodiagnosis Photodyn. Ther. 2018, 21, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Ramqvist, T.; Dalianis, T. Oropharyngeal cancer epidemic and human papillomavirus. Emerg. Infect. Dis. 2010, 16, 1671–1677. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Benson, E.; Li, R.; Eisele, D.; Fakhry, C. The clinical impact of HPV tumor status upon head and neck squamous cell carcinomas. Oral Oncol. 2014, 50, 565–574. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dąbrowska, A.; Mastalerz, J.; Wilczyński, B.; Osiecka, B.; Choromańska, A. Determinants of Photodynamic Therapy Resistance in Cancer Cells. Int. J. Mol. Sci. 2024, 25, 12069. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- van Straten, D.; Mashayekhi, V.; De Bruijn, H.S.; Oliveira, S.; Robinson, D.J. Oncologic Photodynamic Therapy: Basic Principles, Current Clinical Status and Future Directions. Cancers 2017, 9, 19. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sarbadhikary, P.; George, B.P.; Abrahamse, H. Recent Advances in Photosensitizers as Multifunctional Theranostic Agents for Imaging-Guided Photodynamic Therapy of Cancer. Theranostics 2021, 11, 9054–9088. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Aebisher, D.; Czech, S.; Dynarowicz, K.; Misiołek, M.; Komosińska-Vassev, K.; Kawczyk-Krupka, A.; Bartusik-Aebisher, D. Photodynamic Therapy: Past, Current, and Future. Int. J. Mol. Sci. 2024, 25, 11325. [Google Scholar] [CrossRef] [PubMed]
- Komolibus, K.; Fisher, C.; Swartling, J.; Svanberg, S.; Svanberg, K.; Andersson-Engels, S. Perspectives on interstitial photodynamic therapy for malignant tumors. J. Biomed. Opt. 2021, 26, 070604. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sour, A.; Jenni, S.; Ortí-Suárez, A.; Schmitt, J.; Heitz, V.; Bolze, F.; de Sousa, P.L.; Po, C.; Bonnet, C.S.; Pallier, A.; et al. Four gadolinium(III) complexes appended to a porphyrin: A water-soluble molecular theranostic agent with remarkable relaxivity suited for MRI tracking of the photosensitizer. Inorg. Chem. 2016, 55, 4545–4554. [Google Scholar] [CrossRef]
- Rainey, N.; Motte, L.; Aggarwal, B.B.; Petit, P.X. Curcumin hormesis mediates a cross-talk between autophagy and cell death. Cell Death Dis. 2015, 6, e2003. [Google Scholar] [CrossRef]
- Anand, P.; Sundaram, C.; Jhurani, S.; Kunnumakkara, A.B.; Aggarwal, B.B. Curcumin and cancer: An “old-age” disease with an “age-old” solution. Cancer Lett. 2008, 267, 133–164. [Google Scholar] [CrossRef] [PubMed]
- Dembicka-Mączka, D.; Kępa, M.; Fiegler-Rudol, J.; Grzech-Leśniak, Z.; Matys, J.; Grzech-Leśniak, K.; Wiench, R. Evaluation of the Disinfection Efficacy of Er: YAG Laser Light on Single-Species Candida Biofilms—An In Vitro Study. Dent. J. 2025, 13, 88. [Google Scholar] [CrossRef] [PubMed]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of curcumin: Problems and promises. Mol. Pharm. 2007, 4, 807–818. [Google Scholar] [CrossRef] [PubMed]
- Jagetia, G.C.; Aggarwal, B.B. “Spicing Up” of the Immune System by Curcumin. J. Clin. Immunol. 2007, 27, 19–35. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B.; Deb, L.; Prasad, S. Curcumin Differs from Tetrahydrocurcumin for Molecular Targets, Signaling Pathways and Cellular Responses. Molecules 2015, 20, 185–205. [Google Scholar] [CrossRef]
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| In vitro studies using oral squamous cell carcinoma cells. Use of free curcumin or nanoformulated curcumin, specifically intended as a photosensitizer (not solely as a chemotherapeutic agent). The method of eliminating cancer cells used in in vitro studies was curcumin-mediated photodynamic therapy. Original, peer-reviewed in vitro research articles. Published in English. Studies published between 1 January 2009 and 31 December 2024. | Editorial correspondence or commentary (e.g., letters to the editor) Non-contemporary literature or historical analyses Secondary research articles, including literature and systematic reviews Non-peer-reviewed sources such as books, reports, or policy documents Redundant articles or those sharing identical ethical clearance identifiers Articles not published in the English language Studies where photodynamic therapy was not implemented as a treatment modality Investigations in which curcumin was not applied as the active photosensitizer Research employing a photosensitizer other than curcumin Use of light therapy in the absence of any photosensitizing agent Studies not involving oral malignancies as a target condition |
| Reason for Exclusion | Reference Number |
|---|---|
| review of the structure and pharmacokinetics of curcumin | [15,18,24] |
| chemo-photothermal therapy; human lung and liver cancer cells | [25,26,27] |
| oral mucositis in patients with cancer | [28,29,30,31,32] |
| breast cancer | [33] |
| no photodynamic therapy | [34,35,36,37,38,39] |
| ALA-PDT | [40] |
| review; PDT of OSCC | [41,42] |
| osteosarcoma | [43] |
| pharmacokinetics of two-photon active nucleus-targeting carbon dots | [44] |
| review; therapeutic role in cancer | [45] |
| no access | [46] |
| ovarian adenocarcinoma | [47] |
| no OSCC | [48] |
| Reference | [PS] | Origin of PS | Incubation Time | Light Source Parameters | Powermeter | Clinical Cancer Cell Lines | Negative Control Group | Numerical Results Available (Statistics) | No Missing Outcome Data | Total Score |
|---|---|---|---|---|---|---|---|---|---|---|
| [2] | yes | no | yes | yes | yes | yes | yes | yes | yes | 8 |
| [4] | yes | yes | yes | no | no | no | yes | yes | no | 5 |
| [39] | yes | yes | yes | yes | no | yes | yes | yes | yes | 8 |
| [49] | yes | yes | yes | yes | no | yes | yes | yes | yes | 7 |
| [50] | yes | yes | yes | yes | no | no | yes | yes | yes | 7 |
| [51] | yes | yes | yes | yes | no | no | yes | yes | yes | 7 |
| [52] | yes | no | yes | yes | yes | yes | yes | yes | no | 7 |
| [53] | yes | yes | yes | no | no | no | yes | yes | no | 5 |
| [54] | yes | no | yes | no | no | no | yes | yes | no | 4 |
| [55] | no | no | yes | no | no | no | yes | yes | no | 3 |
| Author/Year | Reference Number/Country/Year of Publication | Study Design | Cell Culture | Study Group | Outcomes |
|---|---|---|---|---|---|
| Ravera et al. (2024) [2] | [2] Italy South Africa 2024 | In vitro study | To eliminate potential bias associated with the FANC-A gene mutation, the OHSU-974 cell line (originally obtained from Cincinnati Children’s Hospital Medical Center, Cincinnati, OH, USA) was genetically corrected using the S11FAIN retroviral vector. | control untreated, laser, curcumin, curcumin + laser, lasered curcumin n = 3 | Curcumin shows an ability to reduce the aerobic energy metabolism function of HNSCC at concentrations of 1 and 10 μM. The anti-tumor activity of curcumin was further enhanced by a 60 s exposure to 15 J/cm2 of 450 nm laser light. |
| Beyer et al. (2017) [4] | [4] Germany Spain 2017 | In vitro study on 96-well microtiter plates with a density of approximately 2 × 104 cells/0.33 cm2 | The study utilized three human cell lines: the oral squamous cell carcinoma line HN ACC 417 (DSMZ, Leipzig, Germany), the spontaneously immortalized human keratinocyte line HaCaT 26, and the human epidermoid carcinoma line A431 (ATCC CRL-1555™). | control unirradiated 0 h, control unirradiated 16 h, control unirradiated 1 J/cm2 UVA 16 h, control unirradiated 5 min VIS 16 h; CUR 0.01–1 µg/mL | In cultures treated with curcumin concentrations of 0.8 µg/mL and 1 µg/mL in combination with UVA as well as in cultures treated with 0.6 µg/mL and 0.8 µg/mL curcumin in combination with VIS cell retraction and dynamic plasma membrane blebbing were evident. Lactate dehydrogenase activity in supernatants increased after UVA irradiation when applying 0.4 µg/mL–1 µg/mL curcumin and 0.4 µg/mL curcumin after VIS irradiation. DNA fragmentation of cell cultures treated with curcumin and light was significantly increased. |
| Ambreen et al. (2020) [56] | [56] Germany Pakistan 2020 | In vitro study on 96-well transparent microtiter plate at ~1 × 104 (HeLa and VX2) or ~6 × 104 (UD-SCC-2) cells per well (0.35 cm2) | The HeLa cell line, derived from cervical cancer, and the UD-SCC-2 cell line, originating from head and neck squamous cell carcinoma (HNSCC) at the University of Düsseldorf, Germany, were used. Additionally, fresh VX2 cells were obtained from a VX2 carcinoma in a New Zealand White (NZW) rabbit. | Control (Dark); 1/3/5 J/cm2; Curcumin Liposomes/DMSO 0–100 umol/L n = 3 | Curcumin liposomes were capable of generating a PDT-triggered response in three papilloma virus-associated tumor cell lines, leading to major cell death. |
| Wu et al. (2023) [49] | [49] China 2023 | In vitro study in 96-well plates at a density of 1 × 104 cell | The CAL-27 tongue cancer cell line was donated to the laboratory of Shanghai Ninth People’s Hospital. | CMCC+L, CMCC-L, CC+L, CMC+L, MCC+L; Ce6: 5, 2.5, 1.25 µg/mL; Cur: 2, 1, 0.5 µg/mL (CMCC—cancer cell membrane coated mesoporous silica nanoparticles loaded with Ce6/Cur, MCC—mesoporous silica nanoparticles loaded with Ce6 and Cur, CMC—cell membrane coated mesoporous silica nanoparticles loaded with Ce6, CC—Ce6 + Cur, Ce6—chlorin e6) | The biomimetic nanoplatform apparently enhanced the therapeutic effect of PDT by curcumin disturbing the ROS-defense system. |
| Pavarina et al. (2012) [50] | [50] Brazil 2012 | In vitro study on 24-well plates | The immortalized Hela cell line, purchased from Adolfo Lutz Institute (São Paulo, SP, Brazil) | C+L+, C+L-, C-L+, C-L- n = 5 | The association of CUR and light achieved a significant reduction in cell metabolism of 87.3%. |
| Roschenko et al. (2023) [51] | [51] Germany 2023 | In vitro study on 96-well plates 15,000 cells/0.35 cm2 (per well) | The cell lines UM-SCC-47, UPCI-SCC-154, and UM-SCC-104 represent the HPV-positive subgroup, while UM-SCC-3, UM-SCC-27, and UT-SCC-26A belong to the HPV-negative subgroup. These lines were sourced from the University of Michigan (USA), the University of Pennsylvania (USA), and the University of Turku (Finland). | CUR-LCNPs+L+, Free CUR+L+, CUR-LCNPs+L-, Free CUR+L- n = 3 | The photodynamic efficacy of CUR-LCNPs was evident in inhibiting cell viability across a total of six HNSCC cell lines, both HPVpos and HPVneg, with only low dark toxicity. |
| Singh et al. (2014) [52] | [52] India 2014 | In vitro study on 96-well plate 5 × 104 4451 cells | The human squamous cell carcinoma (4451) cell line, derived from an oral cavity carcinoma, was established at the Institute of Nuclear Medicine and Allied Sciences (INMAS) in Delhi, India. | control, Cur, Cur + 12 J/cm2, Cur + 20 J/cm2 (1 h/4 h) (Cru free/Cur-SiNp) | Curcumin–SiNp formulation enhances uptake and cytotoxic effects of curcumin in oral cancer cells. Photodynamic activity of curcumin in nanoformulation is enhanced as indicated by an increase in cell killing and by inhibition of NF-κB activity |
| Dujic et al. (2009) [53] | [53] Germany 2009 | In vitro study on microwell plates at a density of 2 × 104 cells per 0.33 cm2 | The A431 cell line, a human epidermoid carcinoma model, was obtained from the American Type Culture Collection (ATCC). | control/light-protected/UVA/VIS, 0/0.25/0.5/1/2 µg/mL n = 8 | A combination of light and curcumin amplifies the anti-growth and proapoptotic effects of curcumin in a tumor model. The dose of visible light showed stronger effects than 1 J/cm2 UVA when applied with equal amounts of curcumin. |
| Author/Year | Light Source | Wavelength (nm) | Energy Density (Fluence) (J/cm2) | Power Output (mW) | Irradiation Time (s) |
|---|---|---|---|---|---|
| Ravera et al. (2024) [2] | Diode laser (Garda Laser snc, Verona, Italy) | 450 | 15 | 250 | 60 |
| Beyer et al. (2017) [4] | UVA, Waldmann, Villingen-Schwenningen, Germany; VIS, 5500 lx, Philips GmbH, Hamburg, Germany | 315–400 (UVA) 400–550 (VIS) | 1 (UVA) 1.65 (VIS) | 300 | |
| Ambreen et al. (2020) [56] | A low-power LED device (Lumundus GmbH, Eisenach, Germany) was equipped with two different LEDs of 457 nm (blue) and 652 nm (red) wavelengths. | 457 | 1, 3, 5 | 220.2 W/m2 | 45, 136, 227 |
| Wu et al. (2023) [49] | Laser | 650 | 20 mW/cm2 | 300 | |
| Pavarina et al. (2012) [50] | A light emitting diode (LED) based device, composed of eight royal blue LEDs (LXHL-PR09, Luxeon® III Emitter, Lumileds Lighting, San Jose, CA, USA) | 455 | 5.28 | 22 mW/cm2 | 240 |
| Roschenko et al. (2023) [51] | A prototype low-power light-emitting diode (LED) device designed to fit multiwell plates (Lumundus GmbH, Eisenach, Germany) | 457 | 8.6 | 100 mA | 390 |
| Singh et al. (2014) [52] | Halogen lamp equipped with a multimode fiber (Applied Optical Technologies, Plot No. 147, Rd Number 24, Wagle Industrial Estate, Thane West, Thane, Maharashtra 400604, India) | 400–700 | 12, 20 | 200 | |
| Dujic et al. (2009) [53] | UV therapy system UV 3003 K, Waldmann, Villingen-Schwenningen, Germany; 5500 lx visible light (10x 40 W lamps, distance 45 cm, Philips GmbH, Hamburg, Germany) | 315–400 (UVA) 400–550 (VIS) | 1 (UVA) 1.65 (VIS) | 300 |
| Author/Year | Incubation Time (in Minutes) | The Way of Presentation of Curcumin | Concentration/s of PS Used |
|---|---|---|---|
| Ravera et al. (2024) [2] | 60 | Curcumin dissolved in DMSO | 0.1, 1, 10 µM |
| Beyer et al. (2017) [4] | 60 | Curcumin (Sigma-Aldrich Taufkirchen, Eschenstrasse 5, 82024 Taufkirchen, Germany.) | 0.01 μg/mL to 1 μg/mL |
| Ambreen et al. (2020) [56] | 240 | Curcumin (Sigma-Aldrich Taufkirchen, Germany) Curcumin liposomes | IC50 at 3 J·cm−2: 9.52 µmol/L for HeLa, 7.88 µmol/L for UD-SCC-2, and 20.70 µmol/L for VX2 cells |
| Wu et al. (2023) [49] | 240 | Curcumin (Sigma-Aldrich, Shanghai) was dissolved in DMSO to 20 mg/mL solution. Synthesis of mesoporous silica nanoparticles loaded with curcumin | 0.5, 1, 2 µg/mL |
| Pavarina et al. (2012) [50] | 20 | Curcumin (Sigma Aldrich, St. Louis, MO, USA); A stock solution of CUR (200 µM) was prepared in 10% DMSO and then diluted in saline solution to obtain the concentrations to be tested | 5, 10, 20 µM |
| Roschenko et al. (2023) [51] | 240 | Curcumin (Alfa Aesar, Kandel, Germany); CUR-LCNP | 0, 1, 5, 10, 20, 30, 40, 50 µM/L |
| Singh et al. (2014) [52] | 1, 2, 4 and 20 | Curcumin dissolved in DMSO and curcumin-SiNp complex | 10, 25 µM |
| Dujic et al. (2009) [53] | 60 | 30 mg curcumin (Sigma, Deisenhofen, Germany) was dissolved in 1 mL DMSO | 0.25 to 2 µg/mL |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kubizna, M.; Fiegler-Rudol, J.; Niemczyk, W.; Wiench, R. The Efficacy of Curcumin-Mediated Photodynamic Therapy in the Treatment of Oral Squamous Cell Carcinoma: A Systematic Review of In Vitro Studies. Life 2025, 15, 924. https://doi.org/10.3390/life15060924
Kubizna M, Fiegler-Rudol J, Niemczyk W, Wiench R. The Efficacy of Curcumin-Mediated Photodynamic Therapy in the Treatment of Oral Squamous Cell Carcinoma: A Systematic Review of In Vitro Studies. Life. 2025; 15(6):924. https://doi.org/10.3390/life15060924
Chicago/Turabian StyleKubizna, Magdalena, Jakub Fiegler-Rudol, Wojciech Niemczyk, and Rafał Wiench. 2025. "The Efficacy of Curcumin-Mediated Photodynamic Therapy in the Treatment of Oral Squamous Cell Carcinoma: A Systematic Review of In Vitro Studies" Life 15, no. 6: 924. https://doi.org/10.3390/life15060924
APA StyleKubizna, M., Fiegler-Rudol, J., Niemczyk, W., & Wiench, R. (2025). The Efficacy of Curcumin-Mediated Photodynamic Therapy in the Treatment of Oral Squamous Cell Carcinoma: A Systematic Review of In Vitro Studies. Life, 15(6), 924. https://doi.org/10.3390/life15060924






