Microsurgical Lymphatic Vessel Transplantation for Chronic Lymphedema: Long-Term Evaluation of Volume Reduction and Lymphatic Transport Kinetics
Abstract
1. Introduction
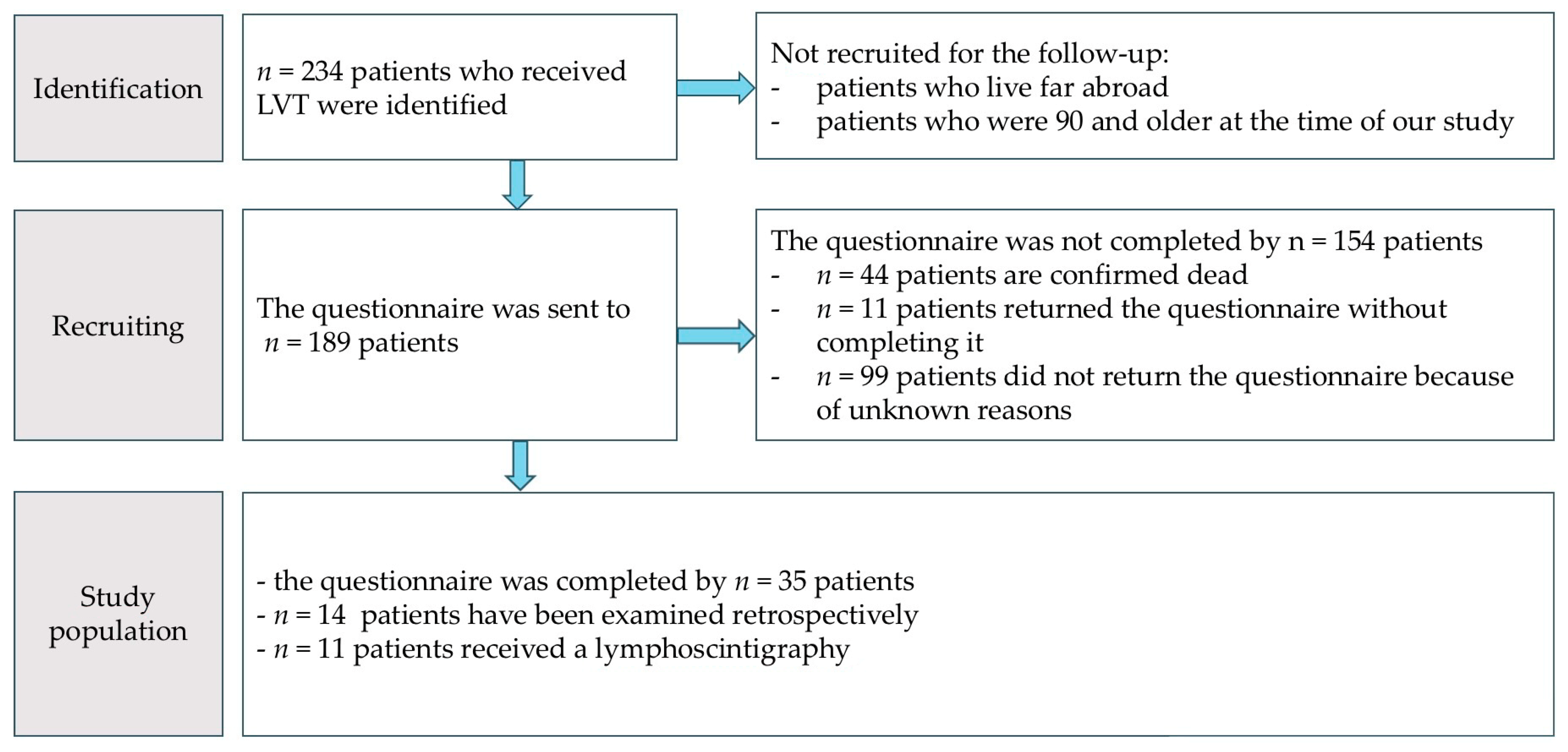
2. Materials and Methods
2.1. Assessment Instruments
2.1.1. Manual Volumetry
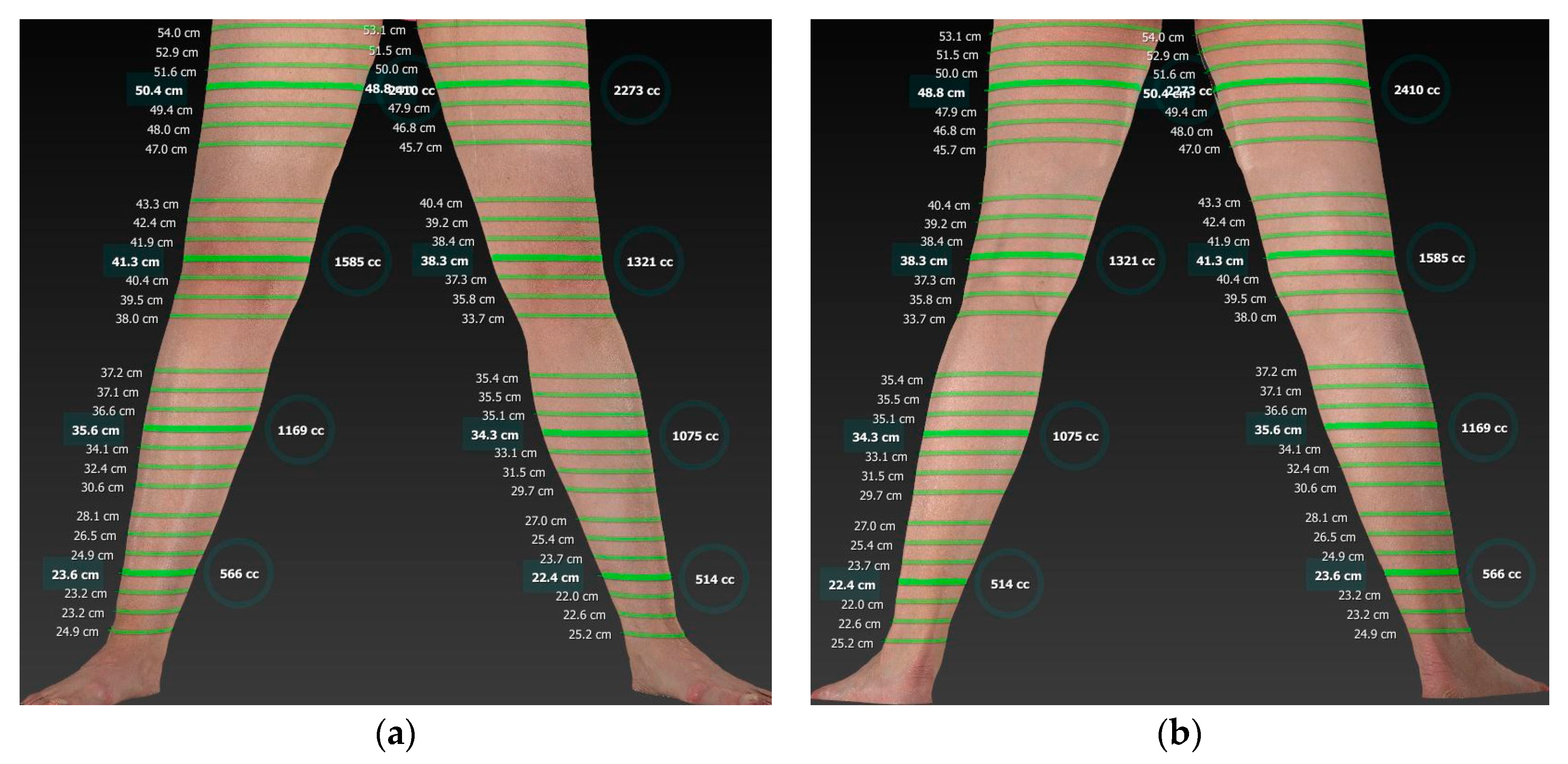
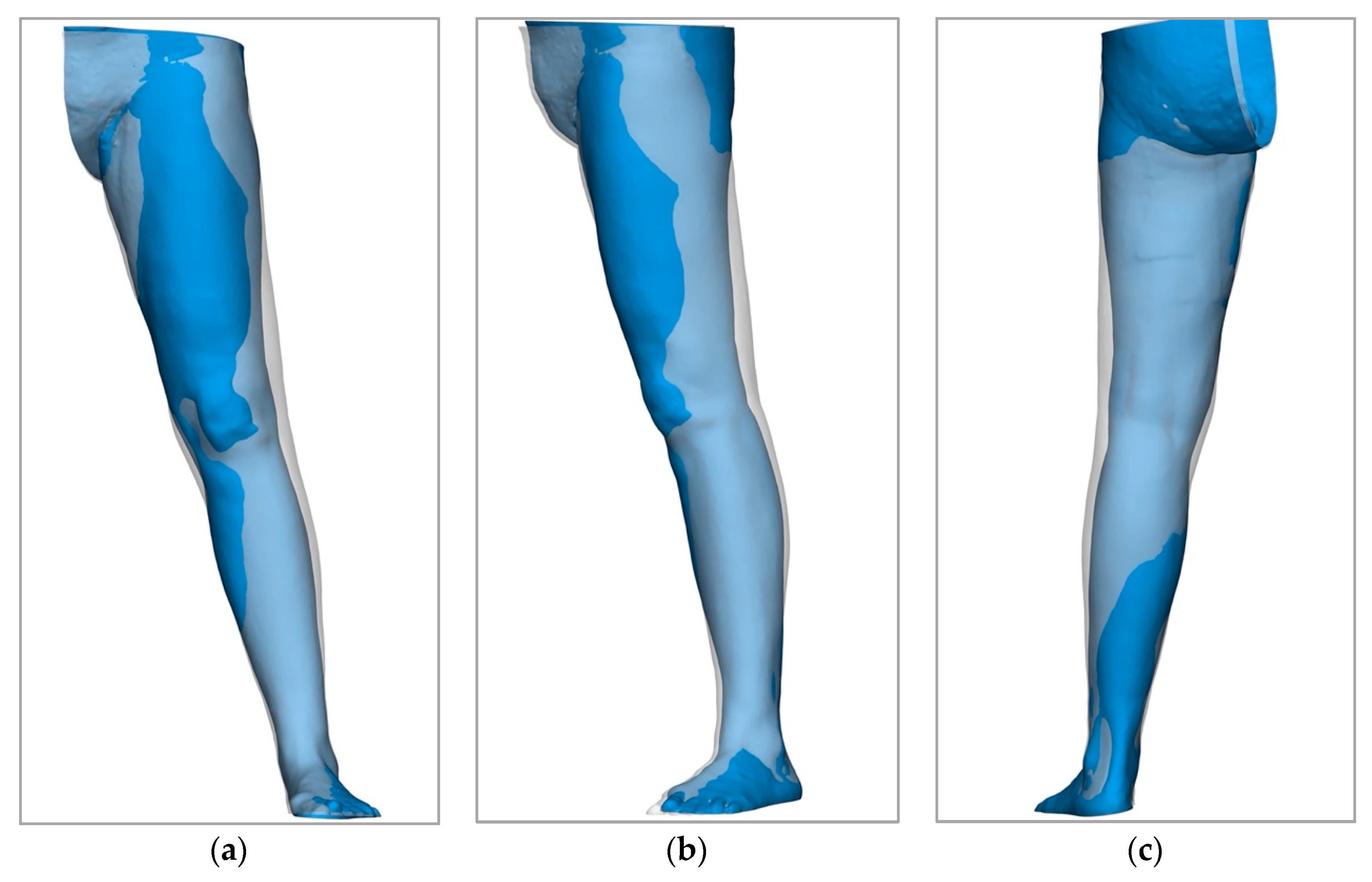
2.1.2. Digital 3D Volumetry
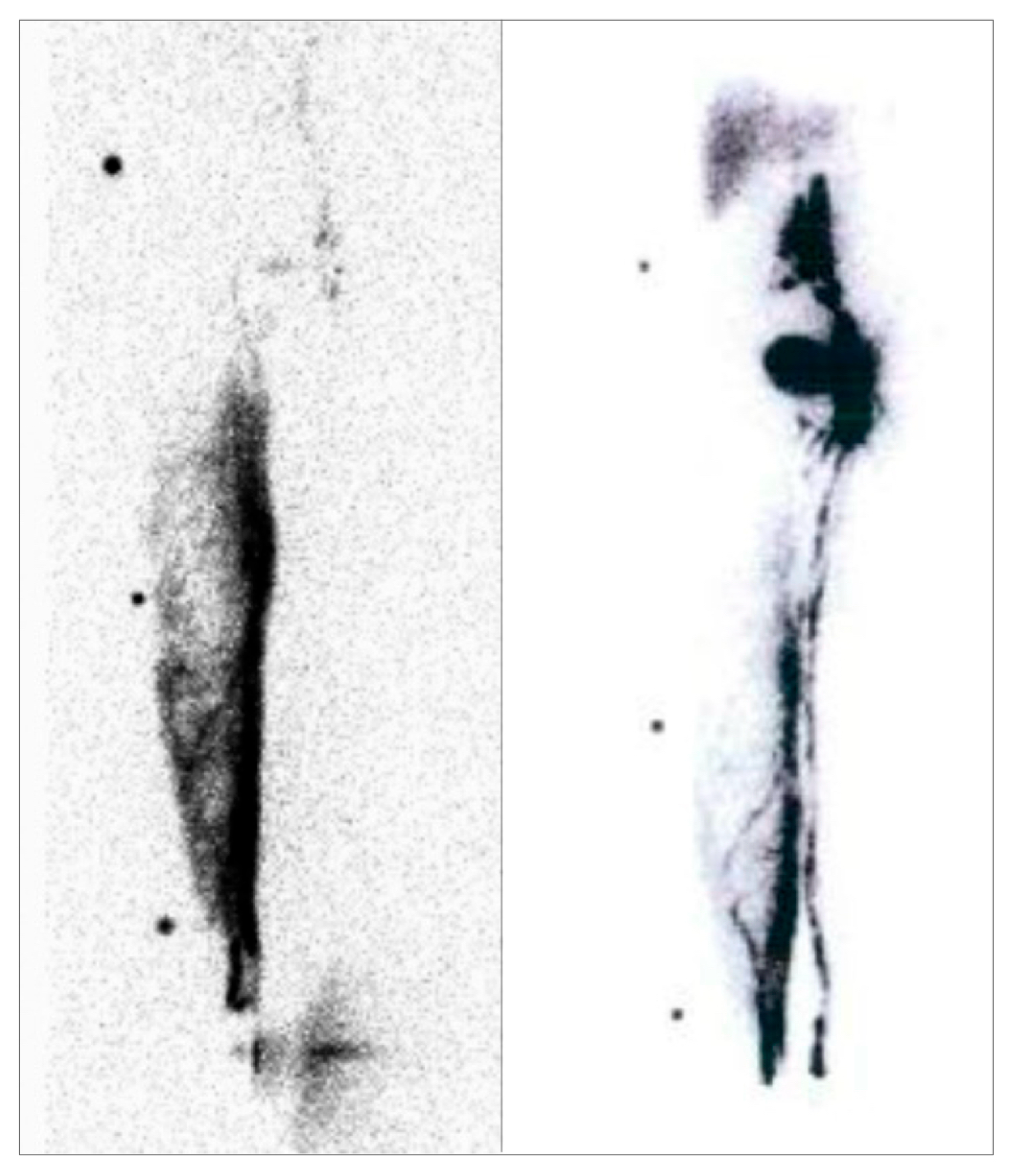
2.1.3. Lymphoscintigraphy
3. Results
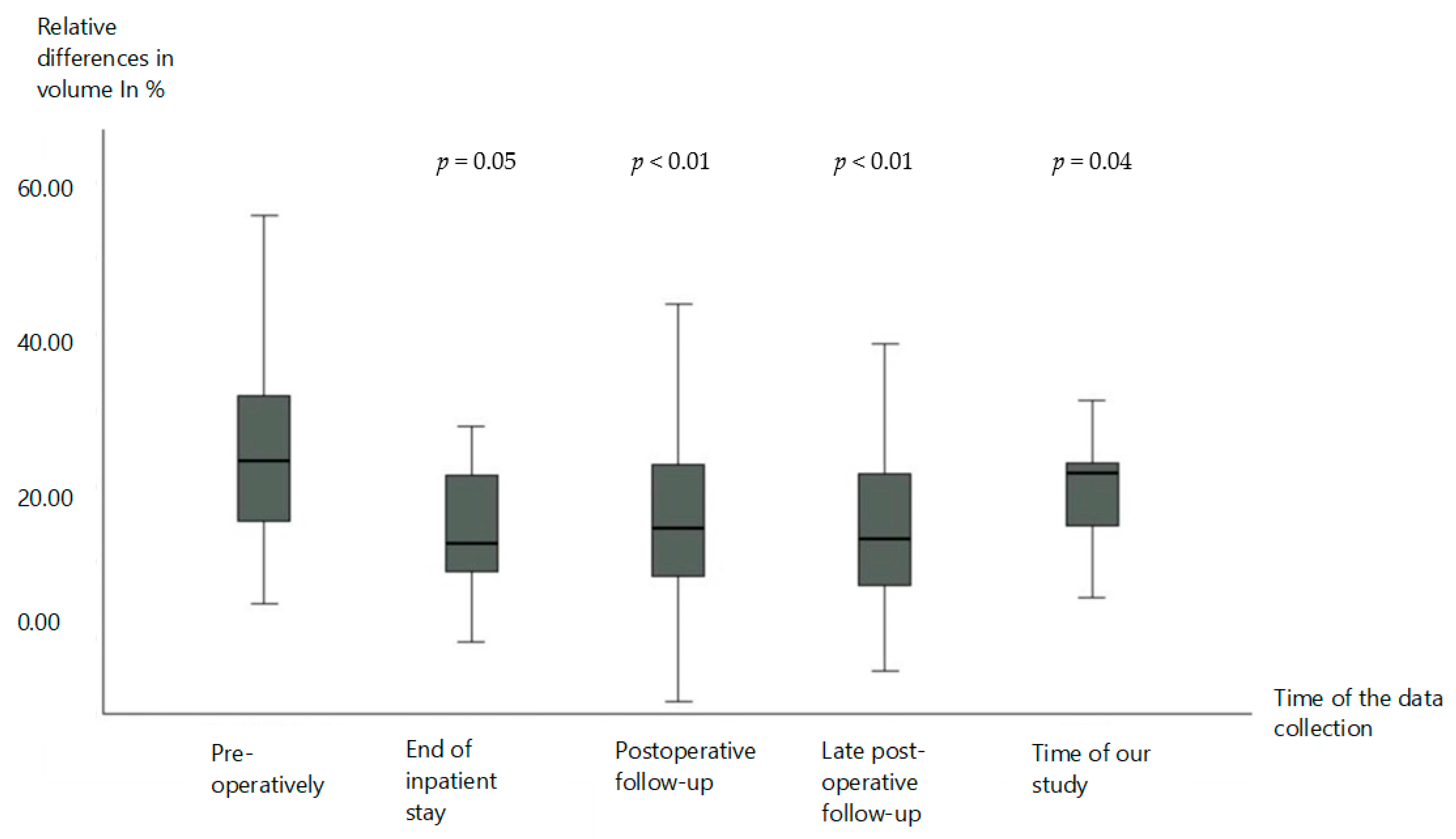
3.1. Volume Reduction
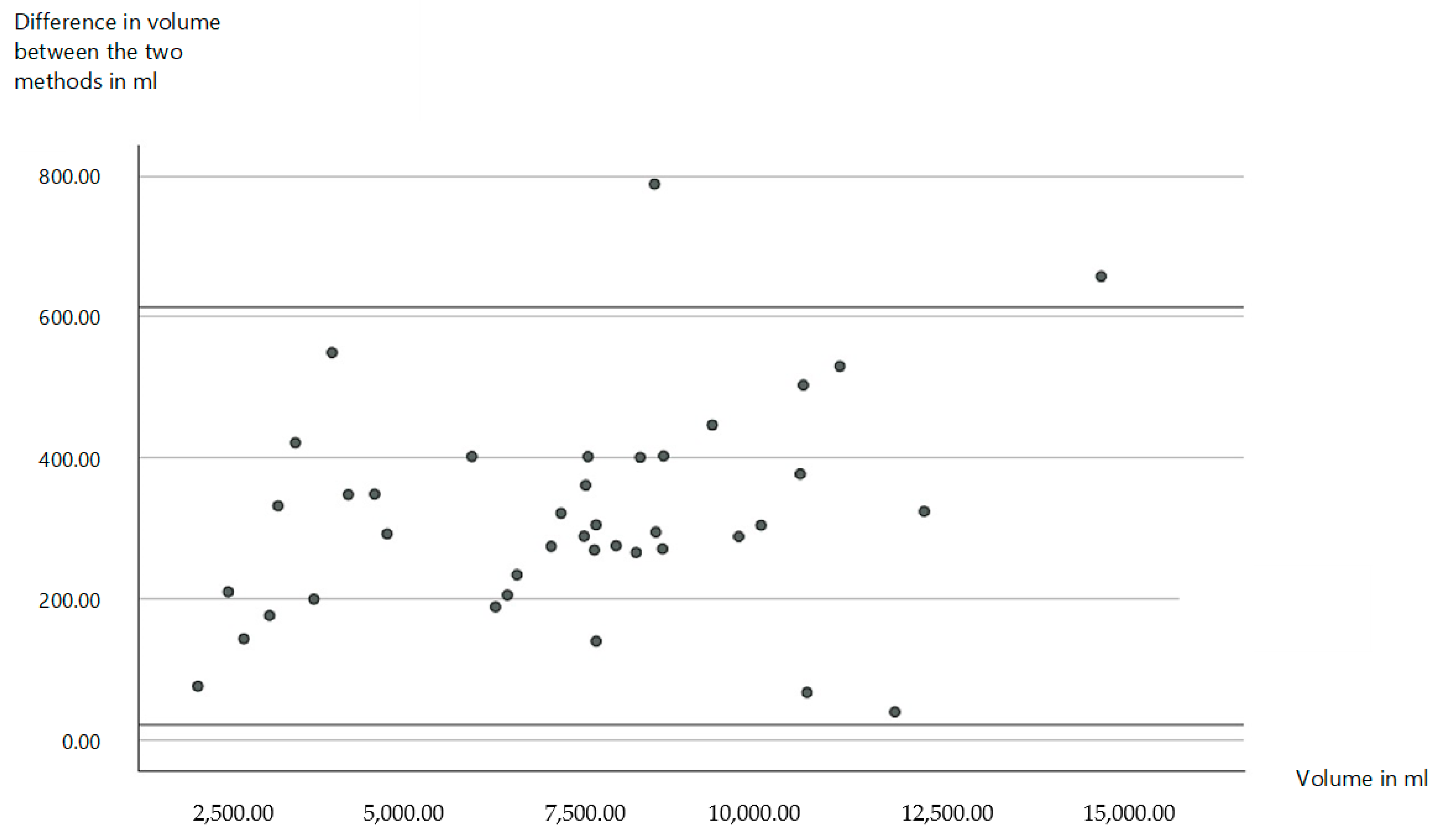
3.2. New Diagnostics for the Measurement of Volumes
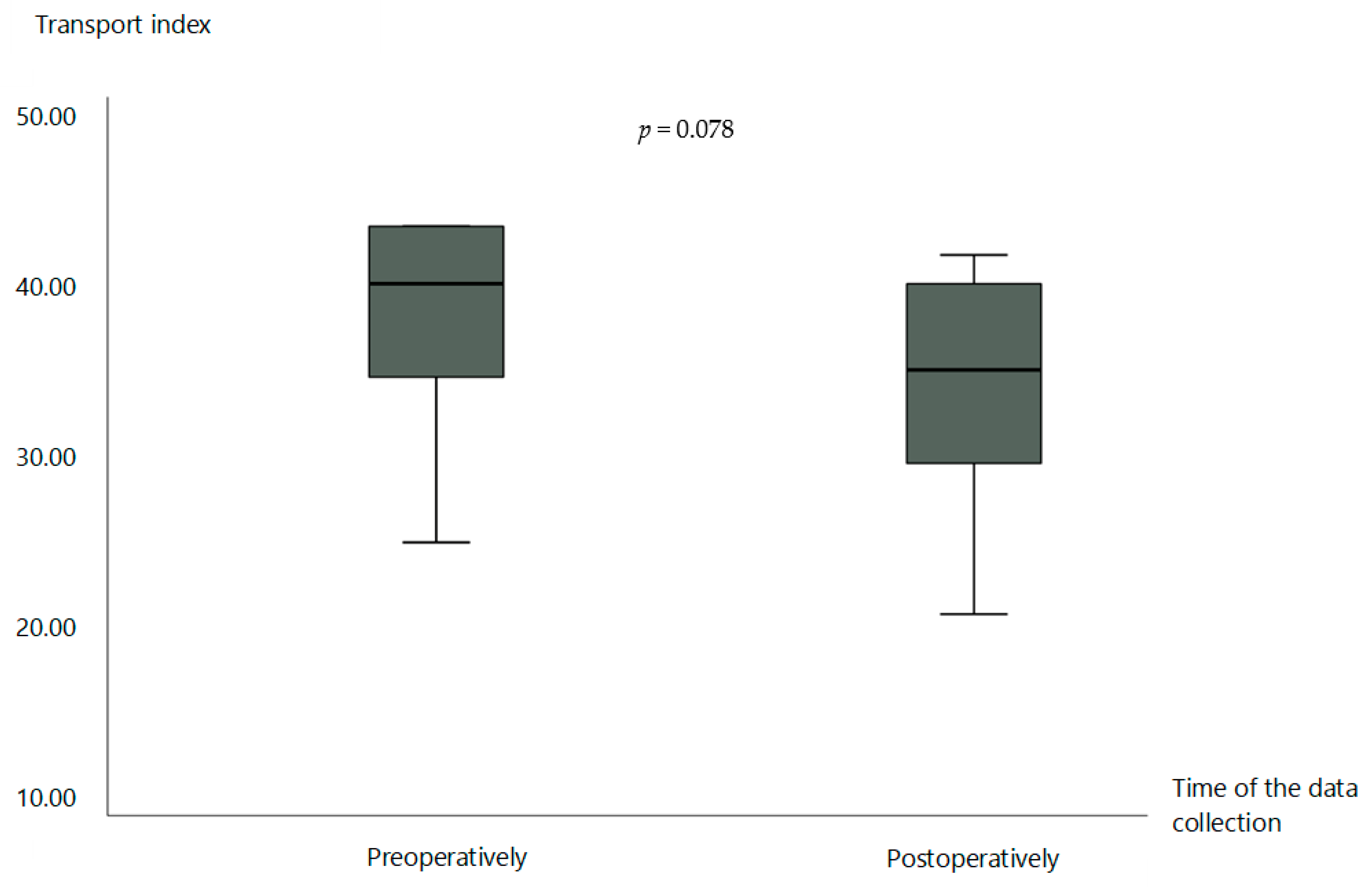
3.3. Lymphoscintigraphy
4. Discussion
5. Conclusions and Implications for Further Research
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Null, M.; Arbor, T.C.; Agarwal, M. Anatomy, Lymphatic System. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Ellis, S. Structure and function of the lymphatic system: An overview. Br. J. Community Nurs. 2006, 11, S4–S6. [Google Scholar] [CrossRef] [PubMed]
- Ozdowski, L.; Gupta, V. Physiology, Lymphatic System. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Mehrara, B.J.; Radtke, A.J.; Randolph, G.J.; Wachter, B.T.; Greenwel, P.; Rovira, I.I.; Galis, Z.S.; Muratoglu, S.C. The emerging importance of lymphatics in health and disease: An NIH workshop report. J. Clin. Investig. 2023, 133, e171582. [Google Scholar] [CrossRef] [PubMed]
- Casley-Smith, J.R.; Földi, M. Lymphangiology; Schattauer Verlag: Stuttgart, Germany, 1987. [Google Scholar]
- Sung, C.; Wang, S.; Hsu, J.; Yu, R.; Wong, A.K. Current Understanding of Pathological Mechanisms of Lymphedema. Adv. Wound Care 2022, 11, 361–373. [Google Scholar] [CrossRef] [PubMed]
- Baumeister, R.G.H. Management of Lymphedema. Dtsch. Med. Wochenschr. 2017, 142, 1790–1794. [Google Scholar] [CrossRef]
- Brix, B.; Sery, O.; Onorato, A.; Ure, C.; Roessler, A.; Goswami, N. Biology of Lymphedema. Biology 2021, 10, 261. [Google Scholar] [CrossRef]
- Flores, T.; Bergmeister, K.D.; Staudenherz, A.; Pieber, K.; Schrögendorfer, K.F. Diagnosis, prevention and therapy of lymphedema. Wien. Klin. Wochenschr. 2021, 133, 855–868. [Google Scholar] [CrossRef]
- Sleigh, B.C.; Manna, B. Lymphedema. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Zvonik, M.; Földi, E.; Felmerer, G. The effects of reduction operation with genital lymphedema on the frequency of erysipelas and the quality of life. Lymphology 2011, 44, 121–130. [Google Scholar]
- Földi, E.; Baumeister, R.G.H.; Bräutigam, P.; Tiedjen, K.U. Zur Diagnostik und Therapie des Lymphödems. Dtsch. Arztebl. International 1998, 95, 740. [Google Scholar]
- Földi, E.; Földi, M.; Kubik, S. (Eds.) Földi’s Textbook of Lymphology; Urban & Fischer Verlag/Elsevier GmbH: Munich, Germany, 2010. [Google Scholar]
- Jäger, G.; Döller, W.; Roth, R. Quality-of-life and body image impairments in patients with lymphedema. Lymphology 2006, 39, 193–200. [Google Scholar]
- Moffatt, C.J.; Franks, P.J.; Doherty, D.C.; Williams, A.F.; Badger, C.; Jeffs, E.; Bosanquet, N.; Mortimer, P.S. Lymphoedema: An underestimated health problem. QJM 2003, 96, 731–738. [Google Scholar] [CrossRef]
- Mercier, G.; Pastor, J.; Moffatt, C.; Franks, P.; Quéré, I. LIMPRINT: Health-Related Quality of Life in Adult Patients with Chronic Edema. Lymphat. Res. Biol. 2019, 17, 163–167. [Google Scholar] [CrossRef] [PubMed]
- Ridner, S.H. The psycho-social impact of lymphedema. Lymphat. Res. Biol. 2009, 7, 109–112. [Google Scholar] [CrossRef] [PubMed]
- Ton, T.G.; Mackenzie, C.; Molyneux, D.H. The burden of mental health in lymphatic filariasis. Infect. Dis. Poverty 2015, 4, 34. [Google Scholar] [CrossRef] [PubMed]
- Son, A.; O’Donnell, T.F.; Izhakoff, J.; Gaebler, J.A.; Niecko, T.; Iafrati, M.A. Lymphedema-associated comorbidities and treatment gap. J. Vasc. Surg. Venous Lymphat. Disord. 2019, 7, 724–730. [Google Scholar] [CrossRef]
- Gallagher, K.; Marulanda, K.; Gray, S. Surgical Intervention for Lymphedema. Surg. Oncol. Clin. N. Am. 2018, 27, 195–215. [Google Scholar] [CrossRef]
- Rabe, E.; Pannier-Fischer, F.; Bromen, K.; Schuldt, K.; Stang, A.; Poncar, C.; Wittenhorst, M.; Bock, E.; Weber, S.; Jockel, K.H.; et al. Bonn Vein Study by the German Society of Phlebology. Phlebologie 2003, 32, 1–14. [Google Scholar]
- Anuszkiewicz, K.; Jankau, J.; Kur, M. What do we know about treating breast-cancer-related lymphedema? Review of the current knowledge about therapeutic options. Breast Cancer 2023, 30, 187–199. [Google Scholar] [CrossRef]
- Baumeister, R.G.H. Reconstructive Lymph Vascular Surgery; Springer International Publishing: Munich, Germany, 2016. [Google Scholar]
- Garza, R.; Skoracki, R.; Hock, K.; Povoski, S.P. A comprehensive overview on the surgical management of secondary lymphedema of the upper and lower extremities related to prior oncologic therapies. BMC Cancer 2017, 17, 468. [Google Scholar] [CrossRef]
- Schaverien, M.V.; Coroneos, C.J. Surgical Treatment of Lymphedema. Plast. Reconstr. Surg. 2019, 144, 738–758. [Google Scholar] [CrossRef]
- Sanka, S.A.; Chryssofos, S.; Anolik, R.A.; Sacks, J.M. Advances in surgical management of chronic lymphedema: Current strategies and future directions. Med. Oncol. 2025, 42, 44. [Google Scholar] [CrossRef]
- Baumeister, R.G.; Frick, A. The microsurgical lymph vessel transplantation. Handchir. Mikrochir. Plast. Chir. 2003, 35, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Baumeister, R.G.; Mayo, W.; Notohamiprodjo, M.; Wallmichrath, J.; Springer, S.; Frick, A. Microsurgical Lymphatic Vessel Transplantation. J. Reconstr. Microsurg. 2016, 32, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Felmerer, G.; Sattler, T.; Lohrmann, C.; Tobbia, D. Treatment of various secondary lymphedemas by microsurgical lymph vessel transplantation. Microsurgery 2012, 32, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Baumeister, R.G.; Seifert, J.; Hahn, D. Autotransplantation of lymphatic vessels. Lancet 1981, 1, 147. [Google Scholar] [CrossRef]
- Baumeister, R.G.H.; Seifert, J.; Wiebecke, B. Transplantation of lymph vessels on rats as well as a first therapeutic application on the experimental lymphedema of the dog. In Proceedings of the 97th Congress of the German Society of Surgery, Munich, Germany, 14–19 May 1980; Heberer, G., Feifel, K., Meßmer, G., Eds.; FRG: Munich, Germany, 1980. [Google Scholar]
- Hackethal, A.; Hirschburger, M.; Eicker, S.O.; Mucke, T.; Lindner, C.; Buchweitz, O. Role of Indocyanine Green in Fluorescence Imaging with Near-Infrared Light to Identify Sentinel Lymph Nodes, Lymphatic Vessels and Pathways Prior to Surgery—A Critical Evaluation of Options. Geburtshilfe Frauenheilkd. 2018, 78, 54–62. [Google Scholar] [CrossRef]
- Springer, S.; Koller, M.; Baumeister, R.G.; Frick, A. Changes in quality of life of patients with lymphedema after lymphatic vessel transplantation. Lymphology 2011, 44, 65–71. [Google Scholar]
- Baumeister, R.G.; Siuda, S. Treatment of lymphedemas by microsurgical lymphatic grafting: What is proved? Plast. Reconstr. Surg. 1990, 85, 64–74; discussion 5–6. [Google Scholar] [CrossRef]
- Hock, L.A.; Nurnberger, T.; Koban, K.C.; Wiggenhauser, P.S.; Giunta, R.; Demmer, W. Quality of Life in Lymphedema Patients Treated by Microsurgical Lymphatic Vessel Transplantation-A Long-Term Follow-Up. Life 2024, 14, 957. [Google Scholar] [CrossRef]
- Farina, G.; Galli, M.; Borsari, L.; Aliverti, A.; Paraskevopoulos, I.T.; LoMauro, A. Limb Volume Measurements: A Comparison of Circumferential Techniques and Optoelectronic Systems against Water Displacement. Bioengineering 2024, 11, 382. [Google Scholar] [CrossRef]
- Armer, J.M.; Ballman, K.V.; McCall, L.; Armer, N.C.; Sun, Y.; Udmuangpia, T.; Hunt, K.K.; Mittendorf, E.A.; Byrd, D.R.; Julian, T.B.; et al. Lymphedema symptoms and limb measurement changes in breast cancer survivors treated with neoadjuvant chemotherapy and axillary dissection: Results of American College of Surgeons Oncology Group (ACOSOG) Z1071 (Alliance) substudy. Support. Care Cancer 2019, 27, 495–503. [Google Scholar] [CrossRef]
- De Stefani, A.; Barone, M.; Hatami Alamdari, S.; Barjami, A.; Baciliero, U.; Apolloni, F.; Gracco, A.; Bruno, G. Validation of Vectra 3D Imaging Systems: A Review. Int. J. Environ. Res. Public Health 2022, 19, 8820. [Google Scholar] [CrossRef] [PubMed]
- Hameeteman, M.; Verhulst, A.C.; Vreeken, R.D.; Maal, T.J.; Ulrich, D.J. 3D stereophotogrammetry in upper-extremity lymphedema: An accurate diagnostic method. J. Plast. Reconstr. Aesthetic Surg. 2016, 69, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Erends, M.; van der Aa, T.; de Grzymala, A.P.; van der Hulst, R. Validity and reliability of three-dimensional imaging for measuring the volume of the arm. Lymphat. Res. Biol. 2014, 12, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Hassanein, A.H.; Maclellan, R.A.; Grant, F.D.; Greene, A.K. Diagnostic Accuracy of Lymphoscintigraphy for Lymphedema and Analysis of False-Negative Tests. Plast. Reconstr. Surg. Glob. Open 2017, 5, e1396. [Google Scholar] [CrossRef]
- Weissleder, H.; Weissleder, R. Lymphedema: Evaluation of qualitative and quantitative lymphoscintigraphy in 238 patients. Radiology 1988, 167, 729–735. [Google Scholar] [CrossRef]
- Weiss, M.; Baumeister, R.G.; Tatsch, K.; Hahn, K. Lymphoscintigraphy for non-invasive long term follow-up of functional outcome in patients with autologous lymph vessel transplantation. Nuklearmedizin 1996, 35, 236–242. [Google Scholar]
- Weiss, M.; Baumeister, R.G.; Frick, A.; Wallmichrath, J.; Bartenstein, P.; Rominger, A. Lymphedema of the upper limb: Evaluation of the functional outcome by dynamic imaging of lymph kinetics after autologous lymph vessel transplantation. Clin. Nucl. Med. 2015, 40, e117–e123. [Google Scholar] [CrossRef]
- Pappalardo, M.; Cheng, M.H. Lymphoscintigraphy for the diagnosis of extremity lymphedema: Current controversies regarding protocol, interpretation, and clinical application. J. Surg. Oncol. 2020, 121, 37–47. [Google Scholar] [CrossRef]
- Kleinhans, E.; Baumeister, R.G.; Hahn, D.; Siuda, S.; Bull, U.; Moser, E. Evaluation of transport kinetics in lymphoscintigraphy: Follow-up study in patients with transplanted lymphatic vessels. Eur. J. Nucl. Med. 1985, 10, 349–352. [Google Scholar] [CrossRef]
- Thompson, B.; Gaitatzis, K.; Janse de Jonge, X.; Blackwell, R.; Koelmeyer, L.A. Manual lymphatic drainage treatment for lymphedema: A systematic review of the literature. J. Cancer Surviv. 2021, 15, 244–258. [Google Scholar] [CrossRef]
- Ramadan, F. Manual lymphatic drainage: The evidence behind the efficacy. Br. J. Community Nurs. 2024, 29, 83–84. [Google Scholar] [CrossRef] [PubMed]
- Zolla, V.; Nizamutdinova, I.T.; Scharf, B.; Clement, C.C.; Maejima, D.; Akl, T.; Nagai, T.; Luciani, P.; Leroux, J.C.; Halin, C.; et al. Aging-related anatomical and biochemical changes in lymphatic collectors impair lymph transport, fluid homeostasis, and pathogen clearance. Aging Cell 2015, 14, 582–594. [Google Scholar] [CrossRef] [PubMed]
- Weiss, M.; Baumeister, R.G.; Hahn, K. Dynamic lymph flow imaging in patients with oedema of the lower limb for evaluation of the functional outcome after autologous lymph vessel transplantation: An 8-year follow-up study. Eur. J. Nucl. Med. Mol. Imaging 2003, 30, 202–206. [Google Scholar] [CrossRef] [PubMed]
- Weiss, M.; Baumeister, R.G.; Hahn, K. Post-therapeutic lymphedema: Scintigraphy before and after autologous lymph vessel transplantation: 8 years of long-term follow-up. Clin. Nucl. Med. 2002, 27, 788–792. [Google Scholar] [CrossRef]
- Stucker, M.; Protz, K.; Eder, S.; Lauchli, S.; Traber, J.; Dissemond, J. Diagnosis of leg edema. Dermatologie 2023, 74, 182–189. [Google Scholar] [CrossRef]
- Koban, K.C.; Titze, V.; Etzel, L.; Frank, K.; Schenck, T.; Giunta, R. Quantitative volumetric analysis of the lower extremity: Validation against established tape measurement and water displacement. Handchir. Mikrochir. Plast. Chir. 2018, 50, 393–399. [Google Scholar] [CrossRef]
- Schiltz, D.; Diesch, S.T.; Kiermeier, N.; Eibl, D.; Felmerer, G.; Schreml, S.; Biermann, N.; Prantl, L.; Taeger, C.D. Digital Volumetric Measurements Based on 3D Scans of the Lower Limb: A Valid and Reproducible Method for Evaluation in Lymphedema Therapy. Ann. Vasc. Surg. 2024, 105, 209–217. [Google Scholar] [CrossRef]
- Etzel, L.; Schenck, T.L.; Giunta, R.E.; Li, Z.; Xu, Y.; Koban, K.C. Digital Leg Volume Quantification: Precision Assessment of a Novel Workflow Based on Single Capture Three-dimensional Whole-Body Surface Imaging. J. Digit. Imaging 2021, 34, 1171–1182. [Google Scholar] [CrossRef]
- Verhulst, A.C.; Wesselius, T.S.; Glas, H.H.; Vreeken, R.D.; Ulrich, D.J.O.; Maal, T.J.J. Accuracy and reproducibility of a newly developed tool for volume measurements of the arm using 3D stereophotogrammetry. J. Plast. Reconstr. Aesthetic Surg. 2017, 70, 1753–1759. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Demmer, W.; Hock, L.A.; Koban, K.C.; Wiggenhauser, P.S.; Brendel, M.; Giunta, R.; Nürnberger, T. Microsurgical Lymphatic Vessel Transplantation for Chronic Lymphedema: Long-Term Evaluation of Volume Reduction and Lymphatic Transport Kinetics. Life 2025, 15, 914. https://doi.org/10.3390/life15060914
Demmer W, Hock LA, Koban KC, Wiggenhauser PS, Brendel M, Giunta R, Nürnberger T. Microsurgical Lymphatic Vessel Transplantation for Chronic Lymphedema: Long-Term Evaluation of Volume Reduction and Lymphatic Transport Kinetics. Life. 2025; 15(6):914. https://doi.org/10.3390/life15060914
Chicago/Turabian StyleDemmer, Wolfram, Louisa Antonie Hock, Konstantin Christoph Koban, Paul Severin Wiggenhauser, Matthias Brendel, Riccardo Giunta, and Tim Nürnberger. 2025. "Microsurgical Lymphatic Vessel Transplantation for Chronic Lymphedema: Long-Term Evaluation of Volume Reduction and Lymphatic Transport Kinetics" Life 15, no. 6: 914. https://doi.org/10.3390/life15060914
APA StyleDemmer, W., Hock, L. A., Koban, K. C., Wiggenhauser, P. S., Brendel, M., Giunta, R., & Nürnberger, T. (2025). Microsurgical Lymphatic Vessel Transplantation for Chronic Lymphedema: Long-Term Evaluation of Volume Reduction and Lymphatic Transport Kinetics. Life, 15(6), 914. https://doi.org/10.3390/life15060914




