Prognostic Value of Matrix Metalloproteinase 9 (MMP9) in Patients Following Off-Pump Coronary Artery Bypass Grafting
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Population
2.2. Perioperative Management
2.3. Measurement of MMP9 Concentration
2.4. Statistical Analysis
3. Results
3.1. Baseline Clinical Characteristics
3.2. MMP9 Levels in the Control Group
3.3. Association Between Preoperative Plasma MMP9 Concentrations and Baseline Characteristics
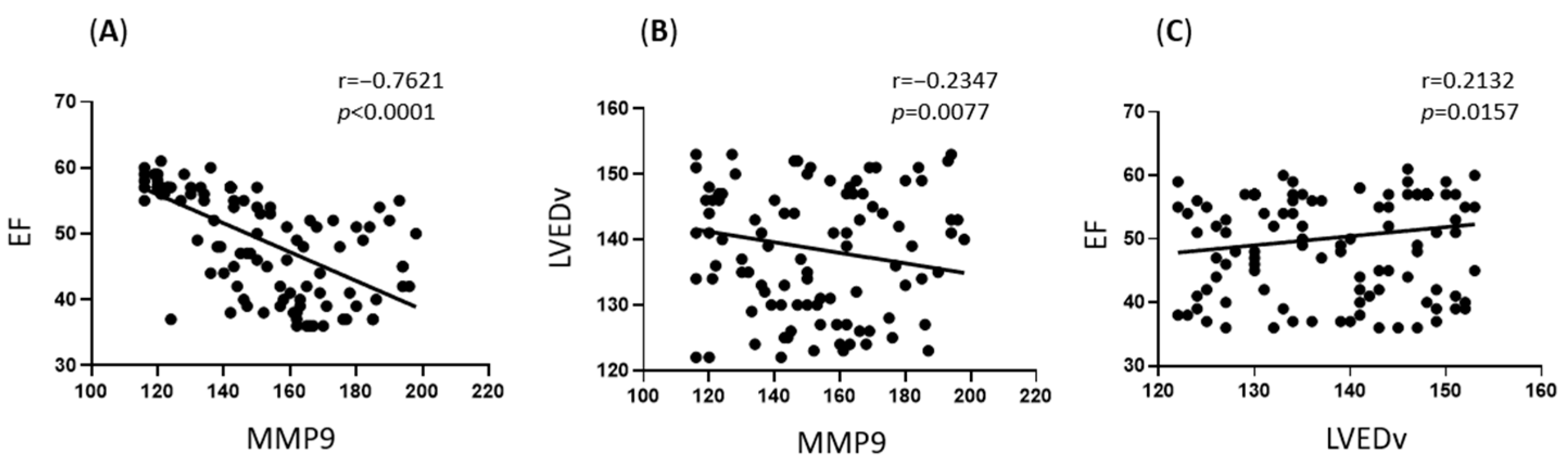
3.4. Association Between Postoperative MMP9 Levels and Echocardiographic Parameters
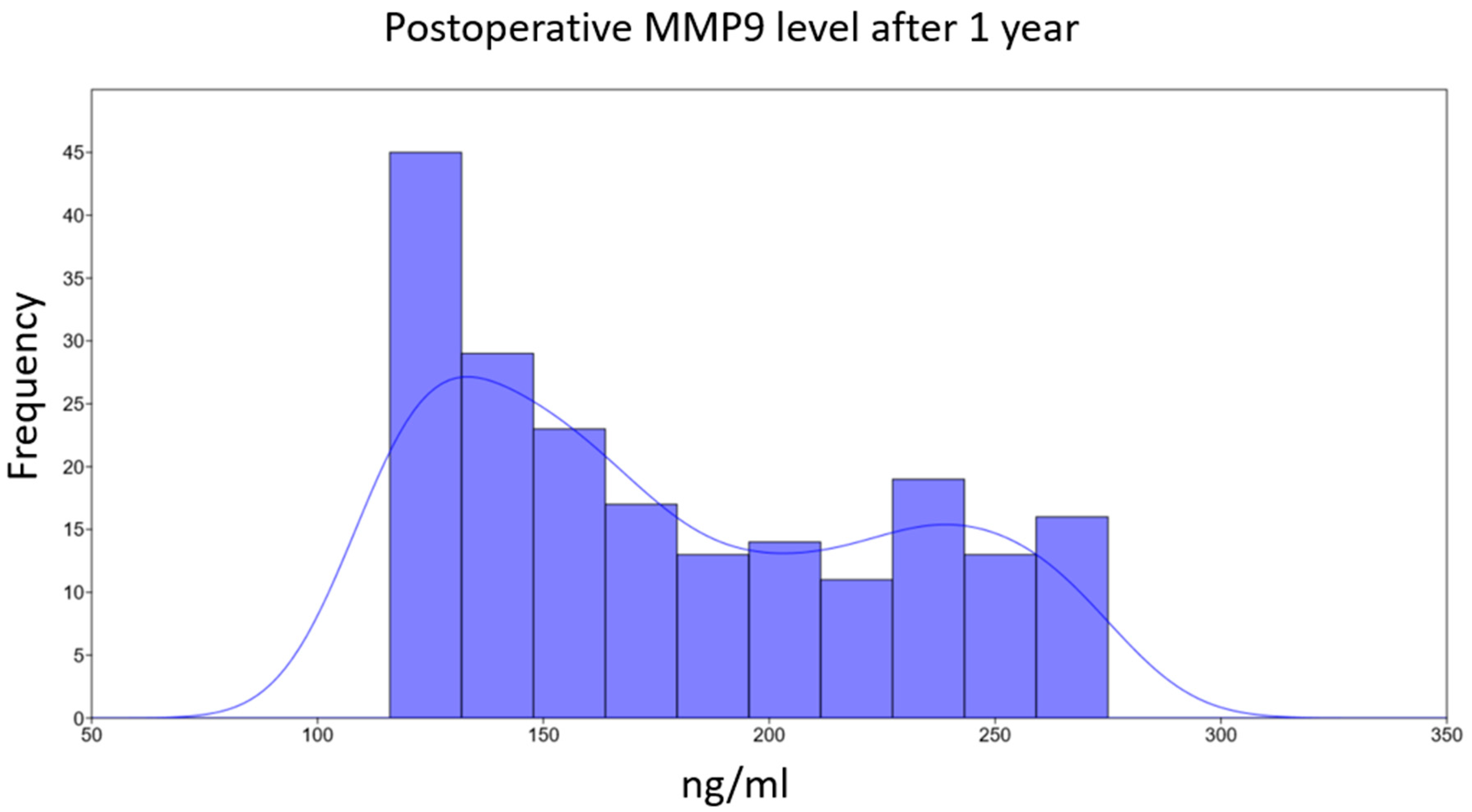
3.5. Association Between MMP9 Levels and Echocardiographic Parameters at Long-Term Follow-Up (1 Year)
4. Discussion
5. Study Strengths and Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, P.K.; Zhao, D.; Okamura, T.; Chang Kim, H.; Wong, N.D.; Yang, E. Atherosclerotic Cardiovascular Disease Risk Prediction Models in China, Japan, and Korea: Implications for East Asians? JACC Asia 2025, 5, 333–349. [Google Scholar] [CrossRef] [PubMed]
- Shumakov, D.V.; Shekhyan, G.G.; Zybin, D.I.; Yalymov, A.A.; Vedenikin, T.Y.U.; Popov, M.A. In-Stent Restenosis: Symptoms, Hemodynamic Signs, Pathogenesis and Treatment. Kardio. Vestn. 2021, 16, 20. [Google Scholar] [CrossRef]
- SCORE2 Asia-Pacific Writing Group; Abdullah, N.; Abdul Jalal, M.I.; Barr, E.L.M.; Chamnan, P.; Chong, C.L.; Cuenza, L.; Gao, P.; Graham, I.; Hilal, S.; et al. Risk Prediction of Cardiovascular Disease in the Asia-Pacific Region: The SCORE2 Asia-Pacific Model. Eur. Heart J. 2025, 46, 702–715. [Google Scholar] [CrossRef]
- Popov, M.A.; Shumakov, D.V.; Zybin, D.I.; Gurevich, L.E.; Ashevskaya, V.E.; Babokin, V.E.; Pronina, V.P. Role of Type IV Collagen and Matrix Metalloproteinase-9 in Remodeling of the Left Ventricular in Coronary Artery Disease. Russ. J. Cardiol. 2019, 8, 83–87. [Google Scholar] [CrossRef]
- Hueb, W.; Gersh, B.J.; Alves da Costa, L.M.; Costa Oikawa, F.T.; Vieira de Melo, R.M.; Rezende, P.C.; Garzillo, C.L.; Lima, E.G.; Nomura, C.H.; Villa, A.V.; et al. Accuracy of Myocardial Biomarkers in the Diagnosis of Myocardial Infarction After Revascularization as Assessed by Cardiac Resonance: The Medicine, Angioplasty, Surgery Study V (MASS-V) Trial. Ann. Thorac. Surg. 2016, 101, 2202–2208. [Google Scholar] [CrossRef]
- Johnson, C.; Sung, H.-J.; Lessner, S.M.; Fini, M.E.; Galis, Z.S. Matrix Metalloproteinase-9 Is Required for Adequate Angiogenic Revascularization of Ischemic Tissues: Potential Role in Capillary Branching. Circ. Res. 2004, 94, 262–268. [Google Scholar] [CrossRef]
- Halade, G.V.; Jin, Y.-F.; Lindsey, M.L. Matrix Metalloproteinase (MMP)-9: A Proximal Biomarker for Cardiac Remodeling and a Distal Biomarker for Inflammation. Pharmacol. Ther. 2013, 139, 32–40. [Google Scholar] [CrossRef]
- Ferroni, P.; Basili, S.; Martini, F.; Cardarello, C.M.; Ceci, F.; Franco, M.D.; Bertazzoni, G.; Gazzaniga, P.P.; Alessandri, C. Serum Metalloproteinase 9 Levels in Patients with Coronary Artery Disease: A Novel Marker of Inflammation. J. Investig. Med. 2003, 51, 295–300. [Google Scholar] [CrossRef]
- Packard, R.R.S.; Libby, P. Inflammation in Atherosclerosis: From Vascular Biology to Biomarker Discovery and Risk Prediction. Clin. Chem. 2008, 54, 24–38. [Google Scholar] [CrossRef]
- Apple, F.S.; Smith, S.W.; Pearce, L.A.; Murakami, M.M. Assessment of the Multiple-Biomarker Approach for Diagnosis of Myocardial Infarction in Patients Presenting with Symptoms Suggestive of Acute Coronary Syndrome. Clin. Chem. 2009, 55, 93–100. [Google Scholar] [CrossRef]
- Wagner, D.R.; Delagardelle, C.; Ernens, I.; Rouy, D.; Vaillant, M.; Beissel, J. Matrix Metalloproteinase-9 Is a Marker of Heart Failure After Acute Myocardial Infarction. J. Card. Fail. 2006, 12, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Shumakov, D.V.; Vishnyakova, M.V.; Pronina, V.P.; Zybin, D.I.; Abramenko, A.S.; Shekhyan, G.G.; Popov, M.A. Immediate and Long-Term Results of Left Ventricular Aneurysm Repair. Kardiol. Serdechno-sosud. khir. 2019, 12, 494. [Google Scholar] [CrossRef]
- Squiccimarro, E.; Labriola, C.; Malvindi, P.G.; Margari, V.; Guida, P.; Visicchio, G.; Kounakis, G.; Favale, A.; Dambruoso, P.; Mastrototaro, G.; et al. Prevalence and Clinical Impact of Systemic Inflammatory Reaction After Cardiac Surgery. J. Cardiothorac. Vasc. Anesth. 2019, 33, 1682–1690. [Google Scholar] [CrossRef] [PubMed]
- Cabral-Pacheco, G.A.; Garza-Veloz, I.; Castruita-De La Rosa, C.; Ramirez-Acuña, J.M.; Perez-Romero, B.A.; Guerrero-Rodriguez, J.F.; Martinez-Avila, N.; Martinez-Fierro, M.L. The Roles of Matrix Metalloproteinases and Their Inhibitors in Human Diseases. Int. J. Mol. Sci. 2020, 21, 9739. [Google Scholar] [CrossRef]
- DeLeon-Pennell, K.Y.; Meschiari, C.A.; Jung, M.; Lindsey, M.L. Matrix Metalloproteinases in Myocardial Infarction and Heart Failure. Prog. Mol. Biol. Transl. Sci. 2017, 147, 75–100. [Google Scholar] [CrossRef] [PubMed]
- Serraino, G.F.; Jiritano, F.; Costa, D.; Ielapi, N.; Battaglia, D.; Bracale, U.M.; Mastroroberto, P.; Andreucci, M.; Serra, R. Metalloproteinases in Cardiac Surgery: A Systematic Review. Biomolecules 2023, 13, 113. [Google Scholar] [CrossRef]
- Fang, L.; Yu, W.; Yu, G.; Ye, B.; Chen, G. Predictive Value of Matrix Metalloprotease 9 on Surgical Outcomes after Pericardiectomy. J. Cardiothorac. Surg. 2022, 17, 50. [Google Scholar] [CrossRef]
- Blankenberg, S.; Rupprecht, H.J.; Poirier, O.; Bickel, C.; Smieja, M.; Hafner, G.; Meyer, J.; Cambien, F.; Tiret, L. Plasma Concentrations and Genetic Variation of Matrix Metalloproteinase 9 and Prognosis of Patients with Cardiovascular Disease. Circulation 2003, 107, 1579–1585. [Google Scholar] [CrossRef]
- Squire, I.B.; Evans, J.; Ng, L.L.; Loftus, I.M.; Thompson, M.M. Plasma MMP-9 and MMP-2 Following Acute Myocardial Infarction in Man: Correlation with Echocardiographic and Neurohumoral Parameters of Left Ventricular Dysfunction. J. Card. Fail. 2004, 10, 328–333. [Google Scholar] [CrossRef]
- Inokubo, Y.; Hanada, H.; Ishizaka, H.; Fukushi, T.; Kamada, T.; Okumura, K. Plasma Levels of Matrix Metalloproteinase-9 and Tissue Inhibitor of Metalloproteinase-1 Are Increased in the Coronary Circulation in Patients with Acute Coronary Syndrome. Am. Heart J. 2001, 141, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Valente, F.M.; de Andrade, D.O.; Cosenso-Martin, L.N.; Cesarino, C.B.; Guimarães, S.M.; Guimarães, V.B.; Lacchini, R.; Tanus-Santos, J.E.; Yugar-Toledo, J.C.; Vilela-Martin, J.F. Plasma Levels of Matrix Metalloproteinase-9 Are Elevated in Individuals with Hypertensive Crisis. BMC Cardiovasc. Disord. 2020, 20, 132. [Google Scholar] [CrossRef] [PubMed]
- Olejarz, W.; Łacheta, D.; Kubiak-Tomaszewska, G. Matrix Metalloproteinases as Biomarkers of Atherosclerotic Plaque Instability. Int. J. Mol. Sci. 2020, 21, 3946. [Google Scholar] [CrossRef] [PubMed]
- Garvin, P.; Jonasson, L.; Nilsson, L.; Falk, M.; Kristenson, M. Plasma Matrix Metalloproteinase-9 Levels Predict First-Time Coronary Heart Disease: An 8-Year Follow-Up of a Community-Based Middle Aged Population. PLoS ONE 2015, 10, e0138290. [Google Scholar] [CrossRef]
- Popov, M.A.; Shumakov, D.V.; Gurevich, L.E.; Fedorov, D.N.; Zybin, D.I.; Ashevskaya, V.E.; Korosteleva, P.A.; Tyurina, V.M. The Evaluation of Hibernating Myocardium Function. Clin. Exp. Morphol. 2023, 12, 59–67. [Google Scholar] [CrossRef]
- Kelly, D.; Cockerill, G.; Ng, L.L.; Thompson, M.; Khan, S.; Samani, N.J.; Squire, I.B. Plasma Matrix Metalloproteinase-9 and Left Ventricular Remodelling after Acute Myocardial Infarction in Man: A Prospective Cohort Study. Eur. Heart J. 2007, 28, 711–718. [Google Scholar] [CrossRef]
- Kampourides, N.; Tziakas, D.; Chalikias, G.; Papazoglou, D.; Maltezos, E.; Symeonides, D.; Konstantinides, S. Usefulness of Matrix Metalloproteinase-9 Plasma Levels to Identify Patients with Preserved Left Ventricular Systolic Function After Acute Myocardial Infarction Who Could Benefit from Eplerenone. Am. J. Cardiol. 2012, 110, 1085–1091. [Google Scholar] [CrossRef]
- Ducharme, A.; Frantz, S.; Aikawa, M.; Rabkin, E.; Lindsey, M.; Rohde, L.E.; Schoen, F.J.; Kelly, R.A.; Werb, Z.; Libby, P.; et al. Targeted Deletion of Matrix Metalloproteinase-9 Attenuates Left Ventricular Enlargement and Collagen Accumulation after Experimental Myocardial Infarction. J. Clin. Investig. 2000, 106, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Sharma, C.; Dobson, G.P.; Davenport, L.M.; Morris, J.L.; Letson, H.L. The Role of Matrix Metalloproteinase-9 and Its Inhibitor TIMP-1 in Burn Injury: A Systematic Review. Int. J. Burn. Trauma. 2021, 11, 275–288. [Google Scholar]
- Baron, M.A.; Ferreira, L.R.P.; Teixeira, P.C.; Moretti, A.I.S.; Santos, R.H.B.; Frade, A.F.; Kuramoto, A.; Debbas, V.; Benvenuti, L.A.; Gaiotto, F.A.; et al. Matrix Metalloproteinase 2 and 9 Enzymatic Activities Are Selectively Increased in the Myocardium of Chronic Chagas Disease Cardiomyopathy Patients: Role of TIMPs. Front. Cell Infect. Microbiol. 2022, 12, 836242. [Google Scholar] [CrossRef]
- Cevik, C.; Otahbachi, M.; Nugent, K.; Warangkana, C.; Meyerrose, G. Effect of 3-Hydroxy-3-Methylglutaryl Coenzyme A Reductase Inhibition on Serum Matrix Metalloproteinase-13 and Tissue Inhibitor Matrix Metalloproteinase-1 Levels as a Sign of Plaque Stabilization. J. Cardiovasc. Med. 2008, 9, 1274–1278. [Google Scholar] [CrossRef] [PubMed]
- Luan, Z.; Chase, A.J.; Newby, A.C. Statins Inhibit Secretion of Metalloproteinases-1, -2, -3, and -9 From Vascular Smooth Muscle Cells and Macrophages. Arter. Thromb. Vasc. Biol. 2003, 23, 769–775. [Google Scholar] [CrossRef] [PubMed]
- Komukai, K.; Kubo, T.; Kitabata, H.; Matsuo, Y.; Ozaki, Y.; Takarada, S.; Okumoto, Y.; Shiono, Y.; Orii, M.; Shimamura, K.; et al. Effect of Atorvastatin Therapy on Fibrous Cap Thickness in Coronary Atherosclerotic Plaque as Assessed by Optical Coherence Tomography. J. Am. Coll. Cardiol. 2014, 64, 2207–2217. [Google Scholar] [CrossRef] [PubMed]
- Onal, I.K.; Altun, B.; Onal, E.D.; Kırkpantur, A.; Gul Oz, S.; Turgan, C. Serum Levels of MMP-9 and TIMP-1 in Primary Hypertension and Effect of Antihypertensive Treatment. Eur. J. Intern. Med. 2009, 20, 369–372. [Google Scholar] [CrossRef]
- Fontana, V.; Silva, P.S.; Belo, V.A.; Antonio, R.C.; Ceron, C.S.; Biagi, C.; Gerlach, R.F.; Tanus-Santos, J.E. Consistent Alterations of Circulating Matrix Metalloproteinases Levels in Untreated Hypertensives and in Spontaneously Hypertensive Rats: A Relevant Pharmacological Target. Basic. Clin. Pharmacol. Toxicol. 2011, 109, 130–137. [Google Scholar] [CrossRef]
- Martinez, M.L.; Lopes, L.F.; Coelho, E.B.; Nobre, F.; Rocha, J.B.T.; Gerlach, R.F.; Tanus-Santos, J.E. Lercanidipine Reduces Matrix Metalloproteinase-9 Activity in Patients with Hypertension. J. Cardiovasc. Pharmacol. 2006, 47, 117–122. [Google Scholar] [CrossRef]

| p | Patient Preoperative Data | Control Group Data | Parameter |
|---|---|---|---|
| 0.667 | 55 (50–57) [39–57] | 56 (48–58) [49–59] | Age, years |
| 0.001 | 100 | 78 | Sex (Male), % |
| 0.217 | 28 (27.2–28.6) [21.8–31] | 27 (26–28) [28–31] | BMI (kg/m2) |
| <0.001 | 139 (137–140) [130–156] | 137 (125–140) [111–150] | Systolic BP (mmHg) |
| 0.012 | 61 (53–66) [40–90] | 58 (54–65) [40–75] | Diastolic BP (mmHg) |
| 0.016 | 58 (54–64) [39–77] | 55 (52–58) [39–67] | Heart rate (bpm) |
| 0.008 | 94.5 | 58 | Smoking (current/last year), % |
| 0.011 | 4.9 (4.6–5.0) [4.0–5.8] | 4.8 (4.4–4.9) [4–5.8] | Glucose level (mmol/L) |
| 0.016 | 74 | 64 | Diabetes mellitus, % |
| 1 | 100 | 100 | Angina pectoris (NYHA Class I–II), % |
| 0.473 | 3.5 (1.7–4.0) [0.8–5.0] | 3.4 (1.7–3.50) [1.3–5] | Total Cholesterol (TC), mmol/L |
| 0.223 | 1.2 (1.0–1.4) [0.8–1.7] | 1.2 (1–1.3) [0.8–1.7] | HDL-C (mmol/L) |
| 0.007 | 2.4 (2.2–3.4) [1.6–3.9] | 2.2 (1.9–3.1) [1.2–3.9] | LDL-C (mmol/L) |
| 0.041 | 2.1 (1.7–2.7) [1.1–3.1] | 2 (1.5–2.5) [1.1–3.1] | Triglycerides (TG), mmol/L |
| 0.160 | 52 | 32 | Atherogenic Index, % |
| 0.008 | 25 | 34 | Prescribed diet, % |
| 0.031 | 86.5 | 62 | Lipid-lowering medication use, % |
| <0.001 | 70.5 | 62 | Antihypertensive medication use, % |
| 0.125 | 86.5 | 58 | Glucose-lowering medication use, % |
| <0.001 | 71.5 | 12 | Anticoagulant use, % |
| <0.001 | 71 | 62 | Antiplatelet use, % |
| 0.397 | 134 (128–138) [124–142] | 138 (134–139) [110–146] | LVEDV (Simpson’s biplane), mL |
| <0.001 | 43 (41–45) [39–47] | 64 (59–66) [54–77] | LVEF (Simpson’s biplane), % |
| <0.001 | 249 (222–275) [126–300] | 89 (78–95) [69–100] | MMP9, ng/mL |
| Patient Group—1-Year Follow-Up (n = 200) | Patient Group—Early Postoperative (48 h) (n = 200) | Control Group (n = 50) | Parameter |
|---|---|---|---|
| Echocardiographic Parameters | |||
| 139 (130–148) [122–153] p = 0.160 | 124 (121–127) [117–130] p < 0.001 | 138 (134–139) [110–146] | LVEDV (Simpson’s biplane), mL |
| 49 (41–57) [36–61] | 54 (54–55) [52–56] | 64 (59–66) [54–77] | LVEF (Simpson’s biplane), % |
| p < 0.001 | p < 0.001 | Biomarker | |
| 165 (136–224) [116–275] p < 0.001 | 126 (123–129) [108–131] p < 0.001 | 89 (78–95) [69–100] | Plasma MMP9, ng/mL |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popov, M.; Dabravolski, S.; Dontsov, V.; Vzvarov, S.; Agafonov, E.; Zybin, D.; Radchenkova, O.; Saveliev, D.; Pronina, V.; Kashirina, N.; et al. Prognostic Value of Matrix Metalloproteinase 9 (MMP9) in Patients Following Off-Pump Coronary Artery Bypass Grafting. Life 2025, 15, 908. https://doi.org/10.3390/life15060908
Popov M, Dabravolski S, Dontsov V, Vzvarov S, Agafonov E, Zybin D, Radchenkova O, Saveliev D, Pronina V, Kashirina N, et al. Prognostic Value of Matrix Metalloproteinase 9 (MMP9) in Patients Following Off-Pump Coronary Artery Bypass Grafting. Life. 2025; 15(6):908. https://doi.org/10.3390/life15060908
Chicago/Turabian StylePopov, Mikhail, Siarhei Dabravolski, Vladislav Dontsov, Sergei Vzvarov, Evgeniy Agafonov, Dmitriy Zybin, Olga Radchenkova, Dmitriy Saveliev, Victoria Pronina, Natalia Kashirina, and et al. 2025. "Prognostic Value of Matrix Metalloproteinase 9 (MMP9) in Patients Following Off-Pump Coronary Artery Bypass Grafting" Life 15, no. 6: 908. https://doi.org/10.3390/life15060908
APA StylePopov, M., Dabravolski, S., Dontsov, V., Vzvarov, S., Agafonov, E., Zybin, D., Radchenkova, O., Saveliev, D., Pronina, V., Kashirina, N., Lipatova, L., Peklo, M., Rutkevich, P., Yanushevskaya, E., Sokolovskaya, A., Metelkin, A., Verkhova, S., Nikiforov, N., & Shumakov, D. (2025). Prognostic Value of Matrix Metalloproteinase 9 (MMP9) in Patients Following Off-Pump Coronary Artery Bypass Grafting. Life, 15(6), 908. https://doi.org/10.3390/life15060908







