The Emerging Clinical Relevance of Artificial Intelligence, Data Science, and Wearable Devices in Headache: A Narrative Review
Abstract
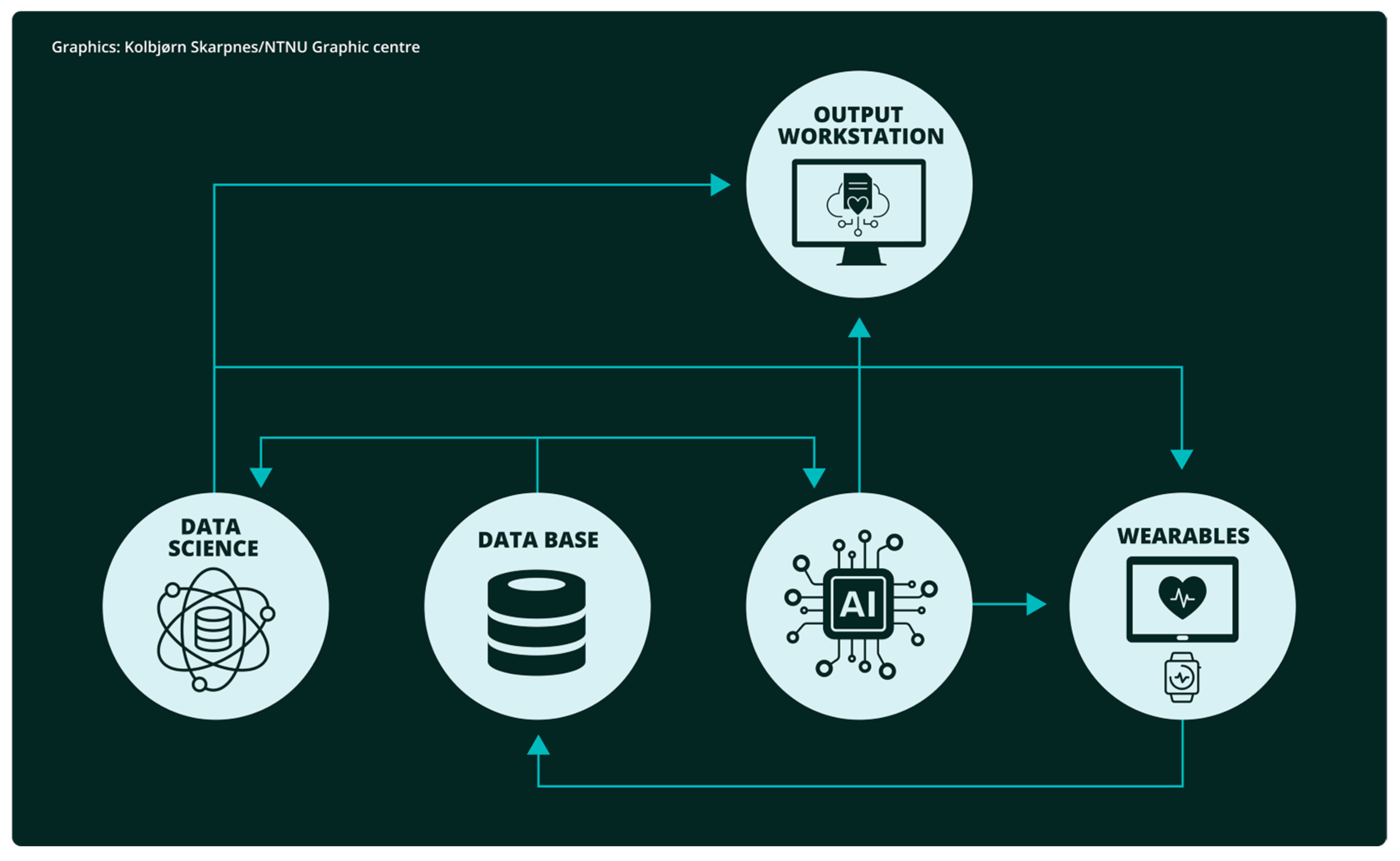
1. Introduction
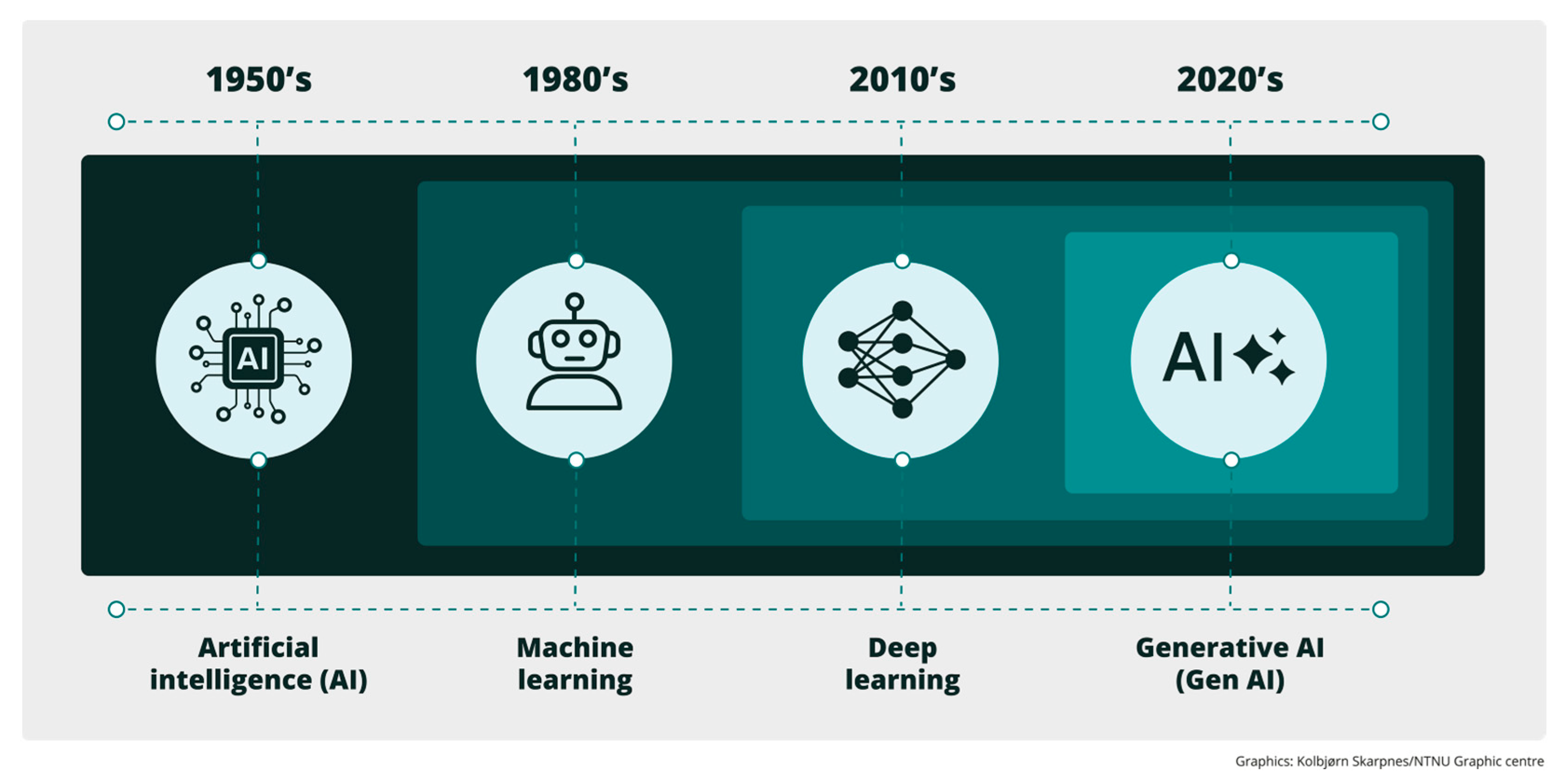
1.1. Artificial Intelligence
1.2. Data Science
1.3. Wearable Devices
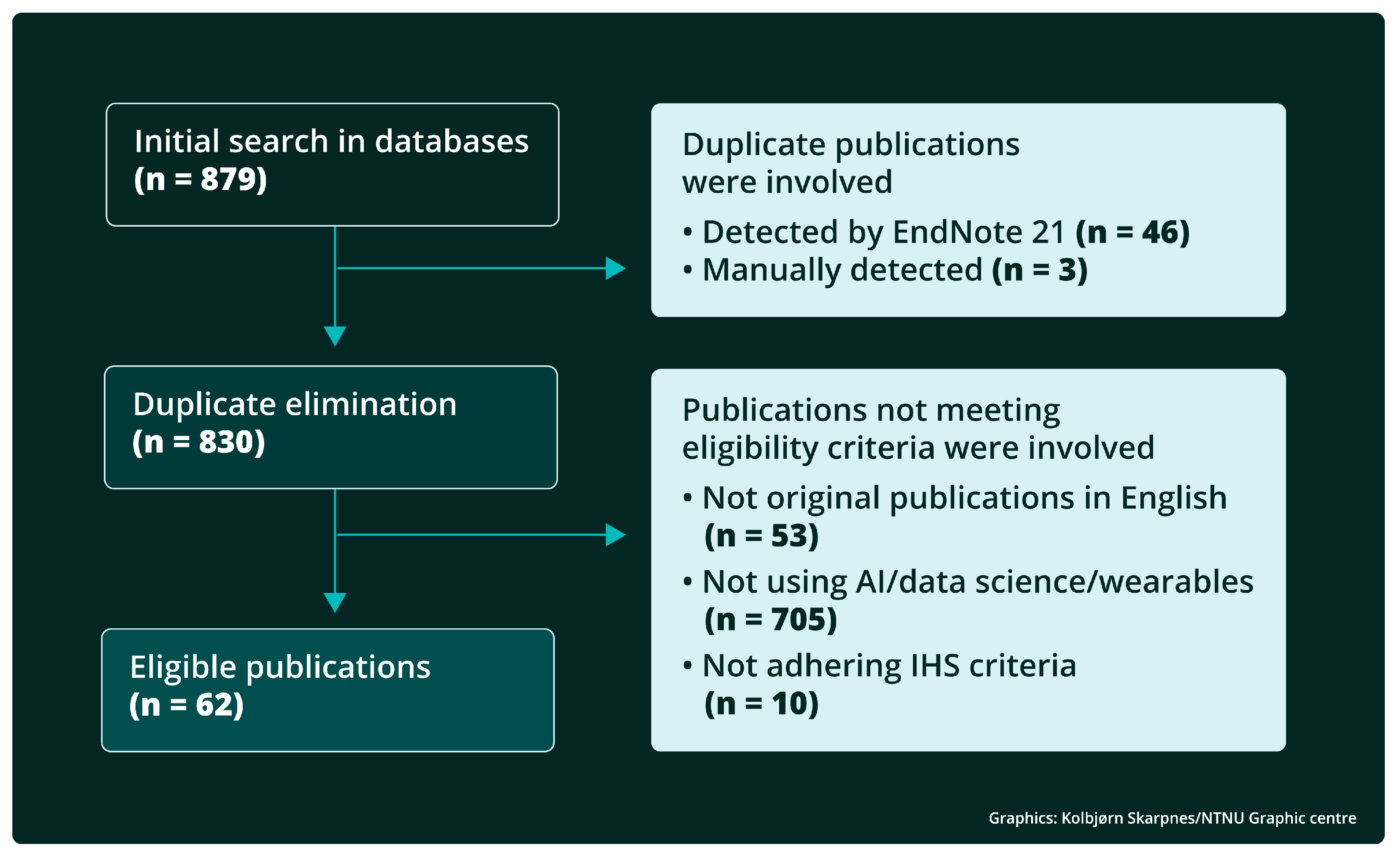
2. Methods
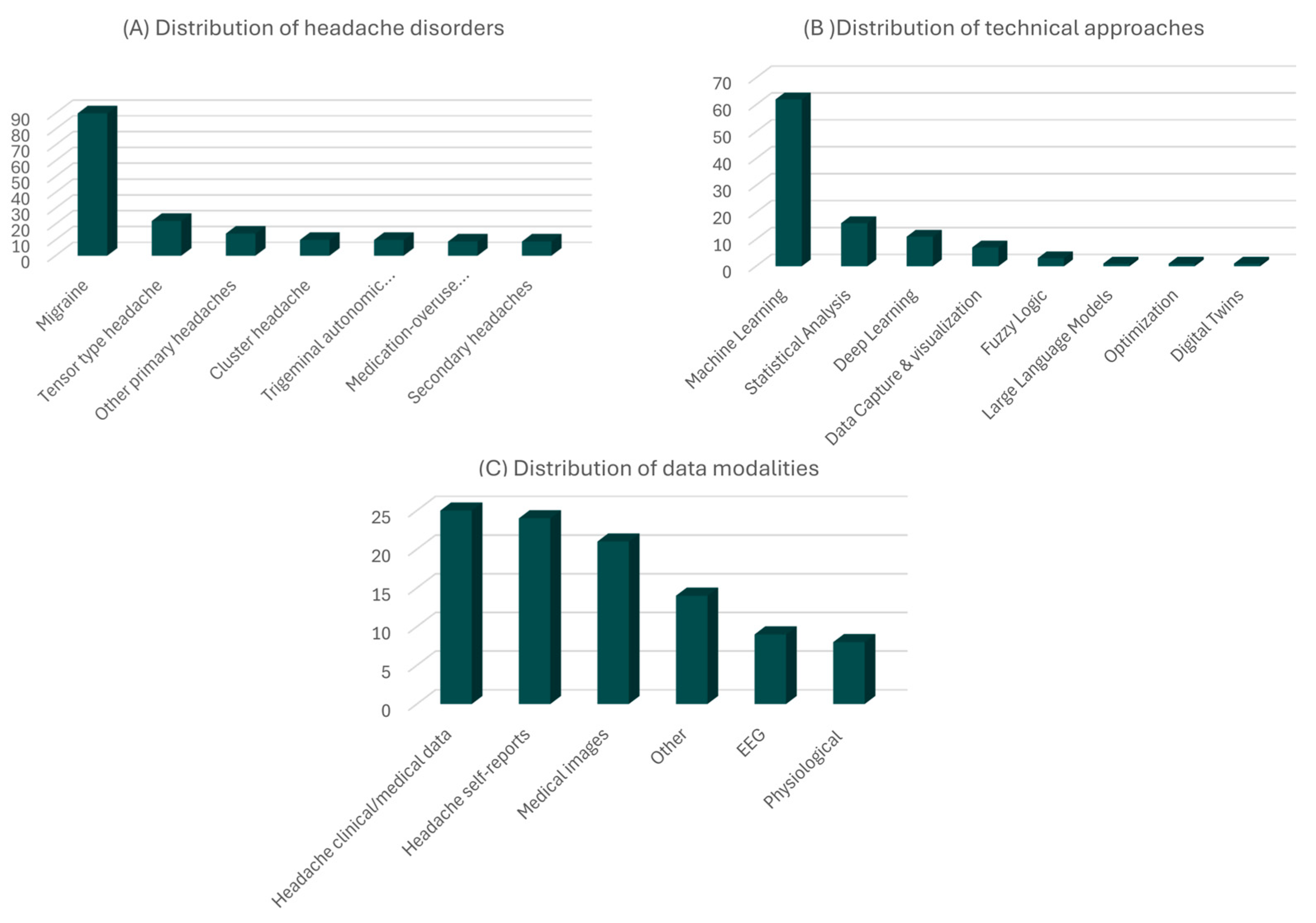
3. Results
3.1. Artificial Intelligence
3.2. Data Science
3.3. Wearable Devices
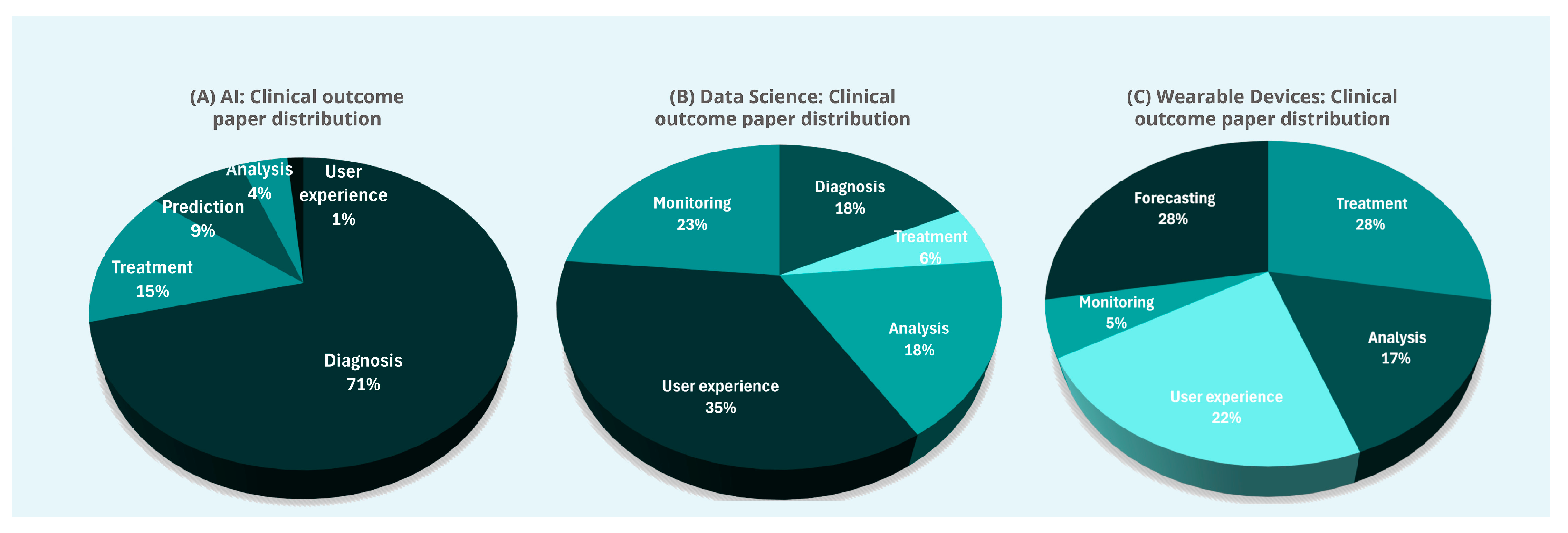
3.4. Statistical Summary
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AI | artificial intelligence |
| ML | machine learning |
| DL | deep learning |
| GenAI | generative artificial intelligence |
| LLM | large language models |
| EEG | electroencephalography |
| CGRP | calcitonin gene-related peptide |
| NSAID | non-steroidal anti-inflammatory drug |
| BoNT-A | botulinum toxin type A |
| AUC | area under the receiver operating characteristic curve |
| CBDA | comprehensive big data analytics |
| ICC | intra-class correlation |
| SD | standard deviation |
| MAE | mean absolute error |
| NDPH | new daily persistent headache |
| US | United States |
| ANOVA | ANalysis Of VAriance |
| SEP | somatosensory evoked potentials |
| sEMG-BF | surface electromyographic biofeedback |
| GDPR | general data protection regulation |
| XAI | explainable artificial intelligence |
References
- Russell, S.J.; Norvig, P. Artificial Intelligence: A Modern Approach, 3rd ed.; Pearson: London, UK, 2016. [Google Scholar]
- Jordan, M.I.; Mitchell, T.M. Machine learning: Trends, perspectives, and prospects. Science 2015, 349, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Goodfellow, I.; Bengio, Y.; Courville, A.; Bengio, Y. Deep Learning; The MIT Press: Cambridge, MA, USA, 2016. [Google Scholar]
- Feuerriegel, S.; Hartmann, J.; Janiesch, C.; Zschech, P. Generative AI. Bus. Inf. Syst. Eng. 2024, 66, 111–126. [Google Scholar] [CrossRef]
- Brown, T.; Mann, B.; Ryder, N.; Subbiah, M.; Kaplan, J.D.; Dhariwal, P.; Neelakantan, A.; Shyam, P.; Sastry, G.; Askell, A.; et al. Language models are few-shot learners. Adv. Neural Inf. Process. Syst. 2020, 33, 1877–1901. [Google Scholar]
- Stubberud, A.; Langseth, H.; Nachev, P.; Matharu, M.S.; Tronvik, E. Artificial intelligence and headache. Cephalalgia 2024, 44, 03331024241268290. [Google Scholar] [CrossRef]
- Aljaaf, A.J.; Al-Jumeily, D.; Hussain, A.J.; Fergus, P.; Al-Jumaily, M.; Abdel-Aziz, K. Toward an optimal use of artificial intelligence techniques within a clinical decision support system. In Proceedings of the Science and Information Conference (SAI), London, UK, 28–30 July 2015; IEEE: Piscataway NJ, USA; pp. 548–554. [Google Scholar]
- Li, L.; Li, P.; Wang, K.; Zhang, L.; Ji, H.; Zhao, H. Benchmarking state-of-the-art large language models for migraine patient education: Performance comparison of responses to common queries. J. Med. Internet Res. 2024, 26, e55927. [Google Scholar] [CrossRef] [PubMed]
- Wickham, H.; Çetinkaya-Rundel, M.; Grolemund, G. R for Data Science; O’Reilly Media Inc.: Sebastopol, CA, USA, 2023. [Google Scholar]
- Moreira, M.W.; Rodrigues, J.J.; Korotaev, V.; Al-Muhtadi, J.; Kumar, N. A comprehensive review on smart decision support systems for health care. IEEE Syst. J. 2019, 13, 3536–3545. [Google Scholar] [CrossRef]
- Patel, S.; Park, H.; Bonato, P.; Chan, L.; Rodgers, M. A review of wearable sensors and systems with application in rehabilitation. J. Neuroeng. Rehabil. 2012, 9, 21. [Google Scholar] [CrossRef]
- Petrušić, I.; Chiang, C.C.; Garcia-Azorin, D.; Ha, W.S.; Ornello, R.; Pellesi, L.; Rubio-Beltrán, E.; Ruscheweyh, R.; Waliszewska-Prosół, M.; Wells-Gatnik, W. Influence of next-generation artificial intelligence on headache research, diagnosis and treatment: The junior editorial board members’ vision–part 2. J. Headache Pain 2025, 26, 2. [Google Scholar] [CrossRef]
- Headache Classification Subcommittee of the International Headache Society. The international classification of headache disorders: 3rd edition. Cephalalgia 2018, 38, 1–211. [Google Scholar] [CrossRef]
- Headache classification subcommittee of the international headache society. The international classification of headache disorders: 2nd edition. Cephalalgia 2004, 24 (Suppl. 1), 9–160. [Google Scholar]
- Bie, B.; Ghosn, S.; Sheikh, S.R.; Araujo, M.L.D.; Mehra, R.; Mays, M.; Saab, C.Y. Electroencephalographic signatures of migraine in small prospective and large retrospective cohorts. Sci. Rep. 2024, 14, 28673. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xie, S.; Zhang, L.; Li, D.; Su, H.; Wang, R.; Ao, R.; Lin, X.; Liu, Y.; Zhang, S.; et al. Attentional network deficits in patients with migraine: Behavioral and electrophysiological evidence. J. Headache Pain 2024, 25, 195. [Google Scholar] [CrossRef] [PubMed]
- Ullah, M.E.; Haider, M.A.; Chowdhury, A.; Chowdhury, M.H. A Comparative Analysis of Stimuli Response among People with Migraine Classification: A Machine Learning Approach. In Proceedings of the International Conference on Advancement in Electrical and Electronic Engineering (ICAEEE), Gazipur, Bangladesh, 25–27 April 2024; IEEE: Piscataway NJ, USA; pp. 1–5. [Google Scholar]
- Hsiao, F.J.; Chen, W.T.; Liu, H.Y.; Wu, Y.T.; Wang, Y.F.; Pan, L.L.H.; Lai, K.L.; Chen, S.P.; Coppola, G.; Wang, S.J. Altered brainstem–cortex activation and interaction in migraine patients: Somatosensory evoked EEG responses with machine learning. J. Headache Pain 2024, 25, 185. [Google Scholar] [CrossRef]
- Li, X.; Wei, B.; Wu, H.; Li, X.; Cong, J. MwoA auxiliary diagnosis using 3D convolutional neural network. In Proceedings of the International Conference on Awareness Science and Technology (iCAST), Qingdao, China, 7–9 December 2020; IEEE: Piscataway NJ, USA; pp. 1–6. [Google Scholar]
- Chen, W.; Zhao, H.; Feng, Q.; Xiong, X.; Ke, J.; Dai, L.; Hu, C. Disrupted gray matter connectome in vestibular migraine: A combined machine learning and individual-level morphological brain network analysis. J. Headache Pain 2024, 25, 177. [Google Scholar] [CrossRef] [PubMed]
- Fernandes Jr, O.; Ramos, L.R.; Acchar, M.C.; Sanchez, T.A. Migraine aura discrimination using machine learning: An fMRI study during ictal and interictal periods. Med. Biol. Eng. Comput. 2024, 62, 2545–2556. [Google Scholar] [CrossRef]
- Jia, X.; Li, M.; Zhang, S.; Antwi, C.O.; Zhan, L.; Zhao, M.; Wen, J.; Hu, S.; Hao, Z.; Ren, J. Frequency-Specific Alternations in the Amplitude of Fluctuations in Tension-Type Headache: A Machine Learning Study. J. Neurosci. Res. 2024, 102, e25398. [Google Scholar] [CrossRef]
- Noh, E.; Namgung, J.Y.; Park, Y.; Jang, Y.; Lee, M.J.; Park, B.Y. Shifts in structural connectome organization in the limbic and sensory systems of patients with episodic migraine. J. Headache Pain 2024, 25, 99. [Google Scholar] [CrossRef]
- Li, X.; Wei, B.; Li, T.; Zhang, N. MwoA auxiliary diagnosis via RSN-based 3D deep multiple instance learning with spatial attention mechanism. In Proceedings of the International Conference on Awareness Science and Technology (iCAST), Qingdao, China, 7–9 December 2020; IEEE: Piscataway, NJ, USA; pp. 1–6. [Google Scholar]
- Wibowo, A.; Nugroho, F.A.; Sarwoko, E.A.; Setiawan, I.M.A. Classification of headache disorder using random Forest algorithm. In Proceedings of the International Conference on Informatics and Computational Sciences (ICICoS), 10–11 November 2020; IEEE: Piscataway NJ, USA; pp. 1–5. [Google Scholar]
- Pérez-Benito, F.J.; Conejero, J.A.; Sáez, C.; García-Gómez, J.M.; Navarro-Pardo, E.; Florencio, L.L.; Fernández-de-las-Peñas, C. Subgrouping Factors Influencing Migraine Intensity in Women: A Semi-automatic Methodology Based on Machine Learning and Information Geometry. Pain Pract. 2020, 20, 297–309. [Google Scholar] [CrossRef]
- Gulati, S.; Guleria, K.; Goyal, N. Classification of migraine disease using supervised machine learning. In Proceedings of the International Conference on Reliability, Infocom Technologies and Optimization (Trends and Future Directions) (ICRITO), Noida, India, 13–14 October 2022; pp. 1–7. [Google Scholar]
- Jalannavar, A.; Kanakaraddi, S.G.; Handur, V.S. Migraine prediction using deep learning model. In Proceedings of the International Conference on Emerging Research in Electronics, Computer Science and Technology (ICERECT), Mandya, India, 26–27 December 2022; IEEE: Piscataway NJ, USA; pp. 1–8. [Google Scholar]
- Romould, R.V.; Singh, V.; Gourisaria, M.K.; Das, H.; Dash, B.B. Deciphering Migraine Types: A Machine Learning Odyssey for Precision Prediction. In Proceedings of the International Conference on Intelligent Data Communication Technologies and Internet of Things (IDCIoT), Bengaluru, India, 4–6 January 2024; IEEE: Piscataway NJ, USA; pp. 1610–1616. [Google Scholar]
- Latıfoğlu, F.; Bulucu, F.O. Effect of Demographic Characteristics on Tension Type Headache and Migraine: A Machine Learning Based Analysis. In Proceedings of the Innovations in Intelligent Systems and Applications Conference (ASYU), Ankara, Turkey, 16–18 October 2024; IEEE: Piscataway NJ, USA; pp. 1–6. [Google Scholar]
- Mudassir, S.N.; Ravichandran, M. Enhancing Migraine Diagnosis and Classification with TabNet: A Data-Driven Approach. In Proceedings of the International Conference on Cloud Computing, Data Science & Engineering (Confluence), Noida, India, 18–19 January 2024; IEEE: Piscataway NJ, USA; pp. 679–684. [Google Scholar]
- Rehman, A.; Rehman, A.U.; Javaid, S.; Ali, T.M.; Mir, A.; Nirsanametla, Y. The Sophisticated Prognostication of Migraine Aura Using Machine Learning. In Proceedings of the International Conference on Emerging Trends in Networks and Computer Communications (ETNCC), Windhoek, Namibia, 23–25 July 2024; IEEE: Piscataway NJ, USA; pp. 1–7. [Google Scholar]
- Katsuki, M.; Shimazu, T.; Kikui, S.; Danno, D.; Miyahara, J.; Takeshima, R.; Takeshima, E.; Shimazu, Y.; Nakashima, T.; Matsuo, M.; et al. Developing an artificial intelligence-based headache diagnostic model and its utility for non-specialists’ diagnostic accuracy. Cephalalgia 2023, 43, 03331024231156925. [Google Scholar] [CrossRef]
- Esfahan, S.S.; Haratian, A.; Haratian, A.; Shayegh, F.; Kiani, S. Automatic classification of migraine and tension-type headaches using machine learning methods. In Proceedings of the International Iranian Conference on Biomedical Engineering (ICBME), Tehran, Iran, 30 November–1 December 2023; IEEE: Piscataway NJ, USA; pp. 220–225. [Google Scholar]
- Okada, M.; Katsuki, M.; Shimazu, T.; Takeshima, T.; Mitsufuji, T.; Ito, Y.; Ohbayashi, K.; Imai, N.; Miyahara, J.; Matsumori, Y.; et al. Preliminary External Validation Results of the Artificial Intelligence-Based Headache Diagnostic Model: A Multicenter Prospective Observational Study. Life 2024, 14, 744. [Google Scholar] [CrossRef]
- Simić, S.; Banković, Z.; Villar, J.R.; Simić, D.; Simić, S.D. A hybrid fuzzy clustering approach for diagnosing primary headache disorder. Log. J. IGPL 2021, 29, 220–235. [Google Scholar] [CrossRef]
- Danelakis, A.; Kumelj, T.; Winsvold, B.; Bjørk, M.; Nachev, P.; Matharu, M.; Giles, D. International Headache Genetic Consortium; Tronvik, E.; Langseth, H.; Stubberud, A. Diagnosing migraine from genome-wide genotype data: A machine learning analysis. Brain, 2025; in press. [Google Scholar] [CrossRef] [PubMed]
- Neumeier, M.S.; Stattmann, M.; Wegener, S.; Gantenbein, A.R.; Pohl, H. Interrater agreement in headache diagnoses. Cephalalgia Rep. 2022, 5, 25158163221115391. [Google Scholar] [CrossRef]
- Gonzalez-Martinez, A.; Pagan, J.; Sanz, A.; Garcia-Azorin, D.; Rodriguez Vico, J.; Jaimes Sanchez, A.; Gomez Garcia, A.; Diaz de Teran, J.; Gonzalez Garcia, N.; Quintas, S.; et al. Machine-learning based approach to predict CGRP response in patients with migraine: Multicenter Spanish study. Eur. J. Neurol. 2022, 29, 736. [Google Scholar] [CrossRef]
- Lu, Z.X.; Dong, B.Q.; Wei, H.L.; Chen, L. Prediction and associated factors of non-steroidal anti-inflammatory drugs efficacy in migraine treatment. Front. Pharmacol. 2022, 13, 1002080. [Google Scholar] [CrossRef]
- Tso, A.R.; Brudfors, M.; Danno, D.; Grangeon, L.; Cheema, S.; Matharu, M.; Nachev, P. Machine phenotyping of cluster headache and its response to verapamil. Brain 2021, 144, 655–664. [Google Scholar] [CrossRef]
- Stubberud, A.; Gray, R.; Tronvik, E.; Matharu, M.; Nachev, P. Machine prescription for chronic migraine. Brain Commun. 2022, 4, fcac059. [Google Scholar] [CrossRef]
- Parrales-Bravo, F.; Gomez-Rodriguez, V.; Cevallos-Torres, L. Multidimensional Bayesian Classifier for Predicting the Multi-stage Patient’s Response to the BoNT-A Treatment for Migraine. In Proceedings of the International Conference on Electrical, Computer and Energy Technologies (ICECET), Sydney, Australia, 25–27 July 2024; IEEE: Piscataway NJ, USA; pp. 1–6. [Google Scholar]
- Scalable AutoML in H2O-3 Open Source. Available online: https://h2o.ai/platform/h2o-automl/ (accessed on 31 March 2025).
- Chiang, C.C.; Schwedt, T.J.; Dumkrieger, G.; Wang, L.; Chao, C.J.; Ouellette, H.A.; Banerjee, I.; Chen, Y.C.; Jones, B.M.; Burke, K.M.; et al. Advancing toward precision migraine treatment: Predicting responses to preventive medications with machine learning models based on patient and migraine features. Headache J. Head Face Pain 2024, 64, 1094–1108. [Google Scholar] [CrossRef]
- Romozzi, M.; Lokhandwala, A.; Vollono, C.; Vigani, G.; Burgalassi, A.; García-Azorín, D.; Calabresi, P.; Chiarugi, A.; Geppetti, P.; Iannone, L.F. An evolving machine-learning-based algorithm to early predict response to anti-CGRP monoclonal antibodies in patients with migraine. Cephalalgia 2024, 44, 03331024241262751. [Google Scholar] [CrossRef]
- Wei, H.L.; Yu, Y.S.; Wang, M.Y.; Zhou, G.P.; Li, J.; Zhang, H.; Zhou, Z. Exploring potential neuroimaging biomarkers for the response to non-steroidal anti-inflammatory drugs in episodic migraine. J. Headache Pain 2024, 25, 104. [Google Scholar] [CrossRef]
- Ferroni, P.; Zanzotto, F.M.; Scarpato, N.; Spila, A.; Fofi, L.; Egeo, G.; Rullo, A.; Palmirotta, R.; Barbanti, P.; Guadagni, F. Machine learning approach to predict medication overuse in migraine patients. Comput. Struct. Biotechnol. J. 2020, 18, 1487–1496. [Google Scholar] [CrossRef] [PubMed]
- Faisal, F.; Danelakis, A.; Bjørk, M.H.; Winsvold, B.; Matharu, M.; Nachev, P.; Hagen, K.; International Headache Genetics Consortium; Tronvik, E.; Stubberud, A. Prediction of new-onset migraine using clinical-genotypic data from the HUNT Study: A machine learning analysis. J. Headache Pain 2025, 26, 1–13. [Google Scholar] [CrossRef] [PubMed]
- An, X.; Zhao, S.; Fang, J.; Li, Q.; Yue, C.; Jing, C.; Zhang, Y.; Zhang, J.; Zhou, J.; Chen, C.; et al. Identification of genetic susceptibility for Chinese migraine with depression using machine learning. Front. Neurol. 2024, 15, 1418529. [Google Scholar] [CrossRef]
- Ashina, S.; Muenzel, E.J.; Nicholson, R.A.; Zagar, A.J.; Buse, D.C.; Reed, M.L.; Shapiro, R.E.; Hutchinson, S.; Pearlman, E.M.; Lipton, R.B. Machine learning identifies factors most associated with seeking medical care for migraine: Results of the OVERCOME (US) study. Headache: J. Head Face Pain 2024, 64, 1027–1039. [Google Scholar] [CrossRef]
- Cui, S.W.; Pei, P.; Yang, W.M. Application value of a machine learning model in predicting mild depression associated with migraine without aura. Br. J. Hosp. Med. 2024, 85, 1–12. [Google Scholar] [CrossRef]
- Khayamnia, M.; Yazdchi, M.; Vahidiankamyad, A.; Foroughipour, M. The recognition of migraine headache by designation of fuzzy expert system and usage of LFE learning algorithm. In Proceedings of the Iranian Joint Congress on Fuzzy and Intelligent Systems (CFIS), Qazvin, Iran, 7–9 March 2017; IEEE: Piscataway NJ, USA; pp. 50–53. [Google Scholar]
- Marino, S.; Jassar, H.; Kim, D.J.; Lim, M.; Nascimento, T.D.; Dinov, I.D.; Koeppe, R.A.; DaSilva, A.F. Classifying migraine using PET compressive big data analytics of brain’s μ-opioid and D2/D3 dopamine neurotransmission. Front. Pharmacol. 2023, 14, 1–13. [Google Scholar] [CrossRef]
- Yin, Z.; Li, H.; Han, X.; Ran, Y.; Wang, Z.; Dong, Z. Clinical decision support system using hierarchical fuzzy diagnosis model for migraine and tension-type headache based on International Classification of Headache Disorders. Front. Neurol. 2024, 15, 1–12. [Google Scholar] [CrossRef]
- Filipi, J.M.; Khairat, S. Tracking and visualizing headache trends on a mobile or desktop website. In Proceedings of the World Congress on Medical and Health Informatics (MEDINFO), Copenhagen, Denmark, 20–23 August 2013; IOS Press: Amsterdam, The Netherlands; p. 1199. [Google Scholar]
- Tassorelli, C.; Jensen, R.; Allena, M.; De Icco, R.; Katsarava, Z.; Miguel Lainez, J.; Leston, J.A.; Fadic, R.; Spadafora, S.; Pagani, M.; et al. The added value of an electronic monitoring and alerting system in the management of medication-overuse headache: A controlled multicentre study. Cephalalgia 2017, 37, 1115–1125. [Google Scholar] [CrossRef] [PubMed]
- Raffaelli, B.; Mecklenburg, J.; Overeem, L.H.; Scholler, S.; Dahlem, M.A.; Kurth, T.; Oliveira Gonçalves, A.S.; Reuter, U.; Neeb, L. Determining the evolution of headache among regular users of a daily electronic diary via a smartphone app: Observational study. JMIR mHealth uHealth 2021, 9, e26401. [Google Scholar] [CrossRef]
- Aljaaf, A.J.; Al-Jumeily, D.; Hussain, A.J.; Baker, T.; Alloghani, M.; Mustafina, J. H-diary: Mobile application for headache diary and remote patient monitoring. In Proceedings of the International Conference on Developments in eSystems Engineering (DeSE), Cambridge, UK, 2–5 September 2018; IEEE: Piscataway, NJ, USA; pp. 18–22. [Google Scholar]
- DrugBank. Available online: https://docs.drugbank.com/v1/ (accessed on 31 March 2025).
- Kaytser, V.; Zhang, P. Non-interacting, non-opioid, and non-barbiturate containing acute medication combinations in headache: A pilot combinatorics approach based on DrugBank database. Front. Neurol. 2021, 12, 632830. [Google Scholar] [CrossRef]
- Lee, C.H.; Park, H.; Lee, M.J.; Park, B.Y. Whole-brain functional gradients reveal cortical and subcortical alterations in patients with episodic migraine. Hum. Brain Mapp. 2023, 44, 2224–2233. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.H.; Yang, F.Y.; Oyang, Y.J. Application of density estimation algorithms in analyzing co-morbidities of migraine. Netw. Model. Anal. Health Inform. Bioinform. 2013, 2, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Zong, F.; Mei, Y.; Zhao, K.; Qiu, D.; Xiong, Z.; Li, X.; Tang, H.; Zhang, P.; Zhang, M.; et al. Morphological similarity and white matter structural mapping of new daily persistent headache: A structural connectivity and tract-specific study. J. Headache Pain 2024, 25, 191. [Google Scholar] [CrossRef]
- Deniz-Garcia, A.; Fabelo, H.; Rodriguez-Almeida, A.J.; Zamora-Zamorano, G.; Castro-Fernandez, M.; Alberiche Ruano, M.D.P.; Solvoll, T.; Granja, C.; Schopf, T.R.; Callico, G.M.; et al. Quality, usability, and effectiveness of mHealth apps and the role of artificial intelligence: Current scenario and challenges. J. Med. Internet Res. 2023, 25, e44030. [Google Scholar] [CrossRef] [PubMed]
- Minen, M.T.; Jaran, J.; Boyers, T.; Corner, S. Understanding what people with migraine consider to be important features of migraine tracking: An analysis of the utilization of smartphone-based migraine tracking with a free-text feature. Headache J. Head Face Pain 2020, 60, 1402–1414. [Google Scholar] [CrossRef]
- Young, N.P.; Ridgeway, J.L.; Haddad, T.C.; Harper, S.B.; Philpot, L.M.; Christopherson, L.A.; McColley, S.M.; Phillips, S.A.; Brown, J.K.; Zimmerman, K.S.; et al. Feasibility and Usability of a Mobile App–Based Interactive Care Plan for Migraine in a Community Neurology Practice: Development and Pilot Implementation Study. JMIR Form. Res. 2023, 7, e48372. [Google Scholar] [CrossRef]
- Noser, A.E.; Robbertz, A.S.; Peugh, J.; Kabbouche, M.; Kacperski, J.; Powers, S.W.; Hershey, A.D.; Hommel, K.A. Single arm feasibility trial of a mobile application for adolescent migraine management. Pain Med. 2024, 26, pnae111. [Google Scholar] [CrossRef]
- Ulrich, S.; Gantenbein, A.R.; Zuber, V.; Von Wyl, A.; Kowatsch, T.; Künzli, H. Development and evaluation of a smartphone-based chatbot coach to facilitate a balanced lifestyle in individuals with headaches (BalanceUP App): Randomized controlled trial. J. Med. Internet Res. 2024, 26, e50132. [Google Scholar] [CrossRef]
- Van der Arend, B.W.; Holwerda, L.J.; Verhagen, I.E.; Van Casteren, D.S.; Timmers, T.; Terwindt, G.M. Practical Experience with the Use of Electronic Headache Diaries and Video Consultations in Migraine Care from a Longitudinal Cohort Study. Telemed. e-Health 2024, 30, 2696–2703. [Google Scholar] [CrossRef]
- Shapiro, R.E.; Muenzel, E.J.; Nicholson, R.A.; Zagar, A.J.; Reed, L.M.; Buse, D.C.; Hutchinson, S.; Ashina, S.; Pearlman, E.M.; Lipton, R.B. Factors and Reasons Associated with Hesitating to Seek Care for Migraine: Results of the OVERCOME (US) Study. Neurol. Ther. 2025, 14, 135–155. [Google Scholar] [CrossRef]
- Ashina, S.; Muenzel, J.; Nicholson, R.; Zagar, A.J.; Buse, D.; Reed, L.M.; Hutchinson, S.; Shapiro, R.; Pearlman, E.; Lipton, R. Factors Associated with Seeking Care for Migraine: Results of the OVERCOME (US) Study (P6-2.002). Neurology 2022, 98 (Suppl. 18), 3114. [Google Scholar] [CrossRef]
- Gazerani, P. Intelligent digital twins for personalized migraine care. J. Pers. Med. 2023, 13, 1255. [Google Scholar] [CrossRef]
- Martins, I.P.; Westerfield, M.; Lopes, M.; Maruta, C.; Gil-da-Costa, R. Brain state monitoring for the future prediction of migraine attacks. Cephalalgia 2020, 40, 255–265. [Google Scholar] [CrossRef]
- Cerrada, C.J.; Min, J.S.; Constantin, L.; Hitier, S.; Igracki Turudic, I.; Amand-Bourdon, C.; Stewart, A.; Ebel-Bitoun, C.; Goadsby, P.J. A prospective real-world study exploring associations between passively collected tracker data and headache burden among individuals with tension-type headache and migraine. Pain Ther. 2022, 11, 153–170. [Google Scholar] [CrossRef]
- Vandenbussche, N.; Van Der Donckt, J.; De Brouwer, M.; Steenwinckel, B.; Stojchevska, M.; Ongenae, F.; Van Hoecke, S.; Paemeleire, K. Patients with chronic cluster headache may show reduced activity energy expenditure on ambulatory wrist actigraphy recordings during daytime attacks. Brain Behav. 2024, 14, e3360. [Google Scholar] [CrossRef]
- Taufique, Z.; Zhu, B.; Coppola, G.; Shoaran, M.; Altaf, M.A.B. A low power multi-class migraine detection processor based on somatosensory evoked potentials. IEEE Trans. Circuits Syst. II Express Briefs 2021, 68, 1720–1724. [Google Scholar] [CrossRef]
- Taufique, Z.; Zhu, B.; Coppola, G.; Shoaran, M.; Saadeh, W.; Altaf, M.A.B. An 8.7 μJ/class. FFT accelerator and DNN-based configurable SoC for Multi-Class Chronic Neurological Disorder Detection. In Proceedings of the Asian Solid-State Circuits Conference (A-SSCC), Busan, Republic of Korea, 7–10 November 2021; IEEE: Piscataway NJ, USA. [Google Scholar]
- Siirtola, P.; Koskimäki, H.; Mönttinen, H.; Röning, J. Using sleep time data from wearable sensors for early detection of migraine attacks. Sensors 2018, 18, 1374. [Google Scholar] [CrossRef]
- Stubberud, A.; Ingvaldsen, S.H.; Brenner, E.; Winnberg, I.; Olsen, A.; Gravdahl, G.B.; Matharu, M.S.; Nachev, P.; Tronvik, E. Forecasting migraine with machine learning based on mobile phone diary and wearable data. Cephalalgia 2023, 43, 03331024231169244. [Google Scholar] [CrossRef] [PubMed]
- Kapustynska, V.; Abromavičius, V.; Serackis, A.; Paulikas, Š.; Ryliškienė, K.; Andruškevičius, S. Machine learning and wearable technology: Monitoring changes in biomedical signal patterns during pre-migraine nights. Healthcare 2024, 12, 1701. [Google Scholar] [CrossRef]
- Minen, M.T.; Corner, S.; Berk, T.; Levitan, V.; Friedman, S.; Adhikari, S.; Seng, E.B. Heartrate variability biofeedback for migraine using a smartphone application and sensor: A randomized controlled trial. Gen. Hosp. Psychiatry 2021, 69, 41–49. [Google Scholar] [CrossRef]
- Lazaridou, A.; Paschali, M.; Bernstein, C.; Curiel, M.; Moore, S.; Edwards, R.R. sEMG Biofeedback for Episodic Migraines: A Pilot Randomized Clinical Trial. Appl. Psychophysiol. Biofeedback 2024, 49, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Stubberud, A.; Tronvik, E.; Olsen, A.; Gravdahl, G.; Linde, M. Biofeedback treatment app for pediatric migraine: Development and usability study. Headache J. Head Face Pain 2020, 60, 889–901. [Google Scholar] [CrossRef] [PubMed]
- Connelly, M.A.; Boorigie, M.E. Feasibility of using “SMARTER” methodology for monitoring precipitating conditions of pediatric migraine episodes. Headache J. Head Face Pain 2021, 61, 500–510. [Google Scholar] [CrossRef] [PubMed]
- Ingvaldsen, S.H.; Tronvik, E.; Brenner, E.; Winnberg, I.; Olsen, A.; Gravdahl, G.B.; Stubberud, A. A biofeedback app for migraine: Development and usability study. JMIR Form. Res. 2021, 5, e23229. [Google Scholar] [CrossRef]
- Jogo Health. Available online: https://www.jogohealth.com/ (accessed on 31 March 2025).
- Steiner, T.J.; Stovner, L.J.; Katsarava, Z.; Lainez, J.M.; Lampl, C.; Lantéri-Minet, M.; Rastenyte, D.; Ruiz de la Torre, E.; Tassorelli, C.; Barré, J.; et al. The impact of headache in Europe: Principal results of the Eurolight project. J. Headache Pain 2014, 15, 1–11. [Google Scholar] [CrossRef]
- Stubberud, A.; Linde, M. Digital technology and mobile health in behavioral migraine therapy: A narrative review. Curr. Pain Headache Rep. 2017, 22, 1–6. [Google Scholar] [CrossRef]
- General Data Protection Regulation. Available online: https://gdpr-info.eu/ (accessed on 31 March 2025).
- EU. EC Regul (EC) No 178/2002 and Regulation (EC) No 1223/2009 and Repealing Council Directives 90/385/EEC and 93/42/EEC (Text with EEA Relevance) 2001/83; 745 of the European Parliament and of the Council of 5 April 2017 on Medical Devices, Amending Directive: 2017. Available online: https://eur-lex.europa.eu/eli/reg/2017/745/oj/eng (accessed on 2 May 2025).
- US Congress. 21st century Cures Act, Pub. L. No. 114–255. Stat 2016; 130: 1033. Available online: https://www.congress.gov/114/plaws/publ255/PLAW-114publ255.pdf (accessed on 2 May 2025).
- US Congress. Federal Food, Drug, and Cosmetic Act, 21 U.S.C. § 301 et Seq. 1938. Available online: https://www.wipo.int/wipolex/en/legislation/details/5473 (accessed on 2 May 2025).
- Böttcher, S.; Vieluf, S.; Bruno, E.; Joseph, B.; Epitashvili, N.; Biondi, A.; Zabler, N.; Glasstetter, M.; Dümpelmann, M.; Van Laerhoven, K.; et al. Data quality evaluation in wearable monitoring. Sci. Rep. 2022, 12, 21412. [Google Scholar] [CrossRef]
- Doshi-Velez, F.; Kim, B. Towards a rigorous science of interpretable machine learning. arXiv 2017, arXiv:1702.08608. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Danelakis, A.; Stubberud, A.; Tronvik, E.; Matharu, M. The Emerging Clinical Relevance of Artificial Intelligence, Data Science, and Wearable Devices in Headache: A Narrative Review. Life 2025, 15, 909. https://doi.org/10.3390/life15060909
Danelakis A, Stubberud A, Tronvik E, Matharu M. The Emerging Clinical Relevance of Artificial Intelligence, Data Science, and Wearable Devices in Headache: A Narrative Review. Life. 2025; 15(6):909. https://doi.org/10.3390/life15060909
Chicago/Turabian StyleDanelakis, Antonios, Anker Stubberud, Erling Tronvik, and Manjit Matharu. 2025. "The Emerging Clinical Relevance of Artificial Intelligence, Data Science, and Wearable Devices in Headache: A Narrative Review" Life 15, no. 6: 909. https://doi.org/10.3390/life15060909
APA StyleDanelakis, A., Stubberud, A., Tronvik, E., & Matharu, M. (2025). The Emerging Clinical Relevance of Artificial Intelligence, Data Science, and Wearable Devices in Headache: A Narrative Review. Life, 15(6), 909. https://doi.org/10.3390/life15060909







