Early Real-World Outcomes of Switching to 8 mg Aflibercept for Neovascular Age-Related Macular Degeneration in the United Kingdom
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Collection
2.2. Treatment Protocol
2.3. OCT Imaging
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics
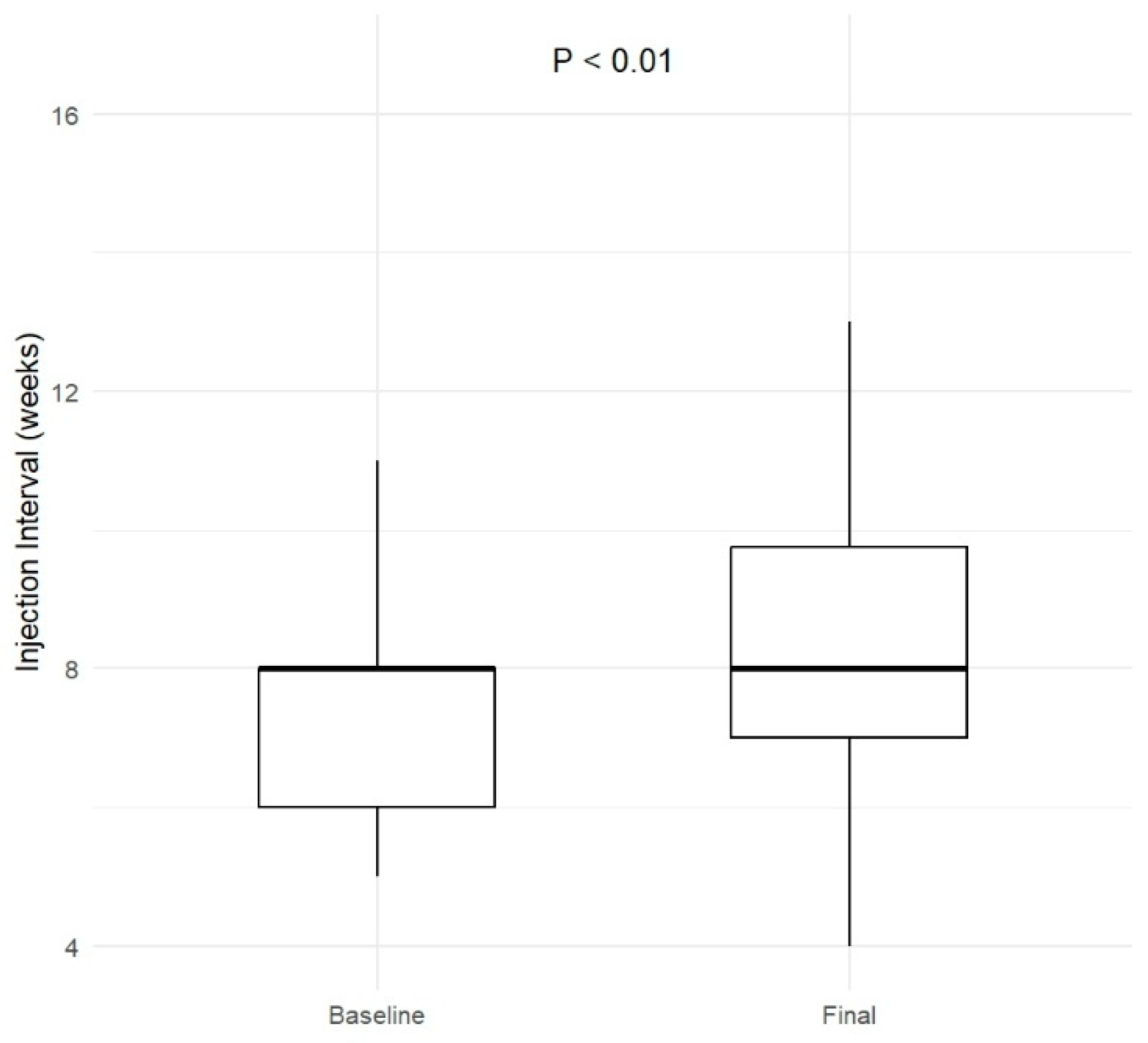
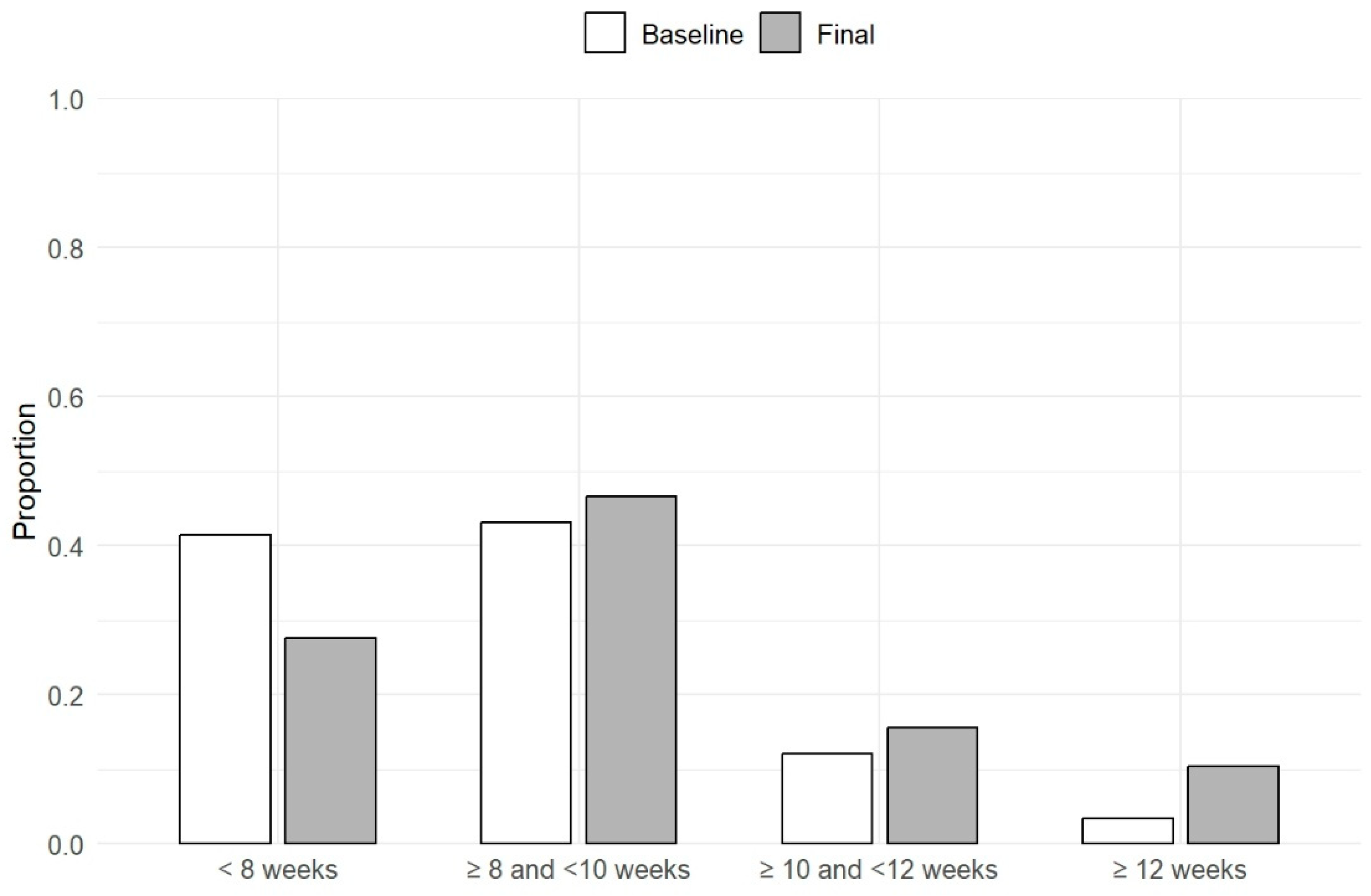
3.2. Treatment Summary
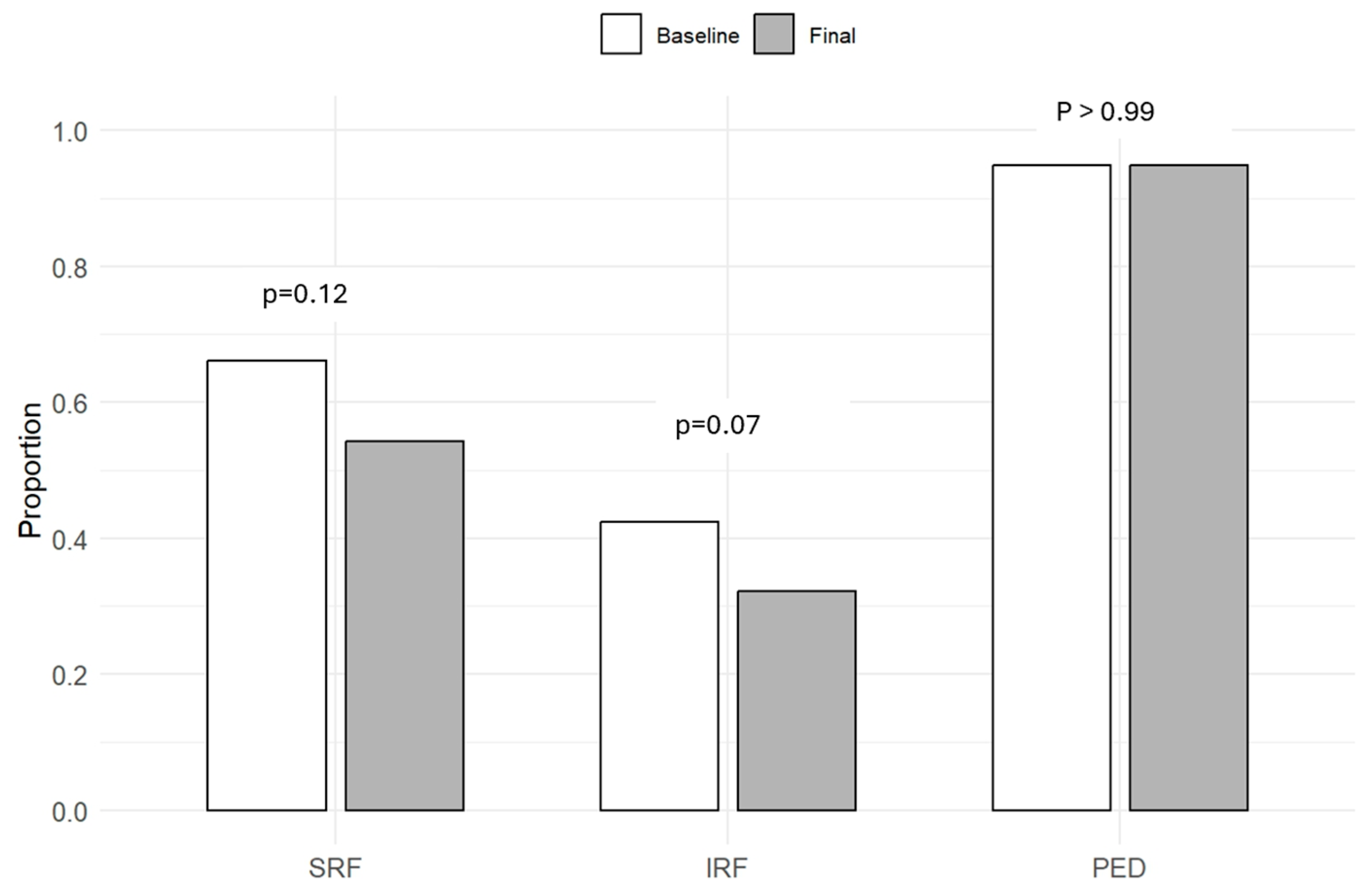
3.3. Functional and Anatomical Outcomes
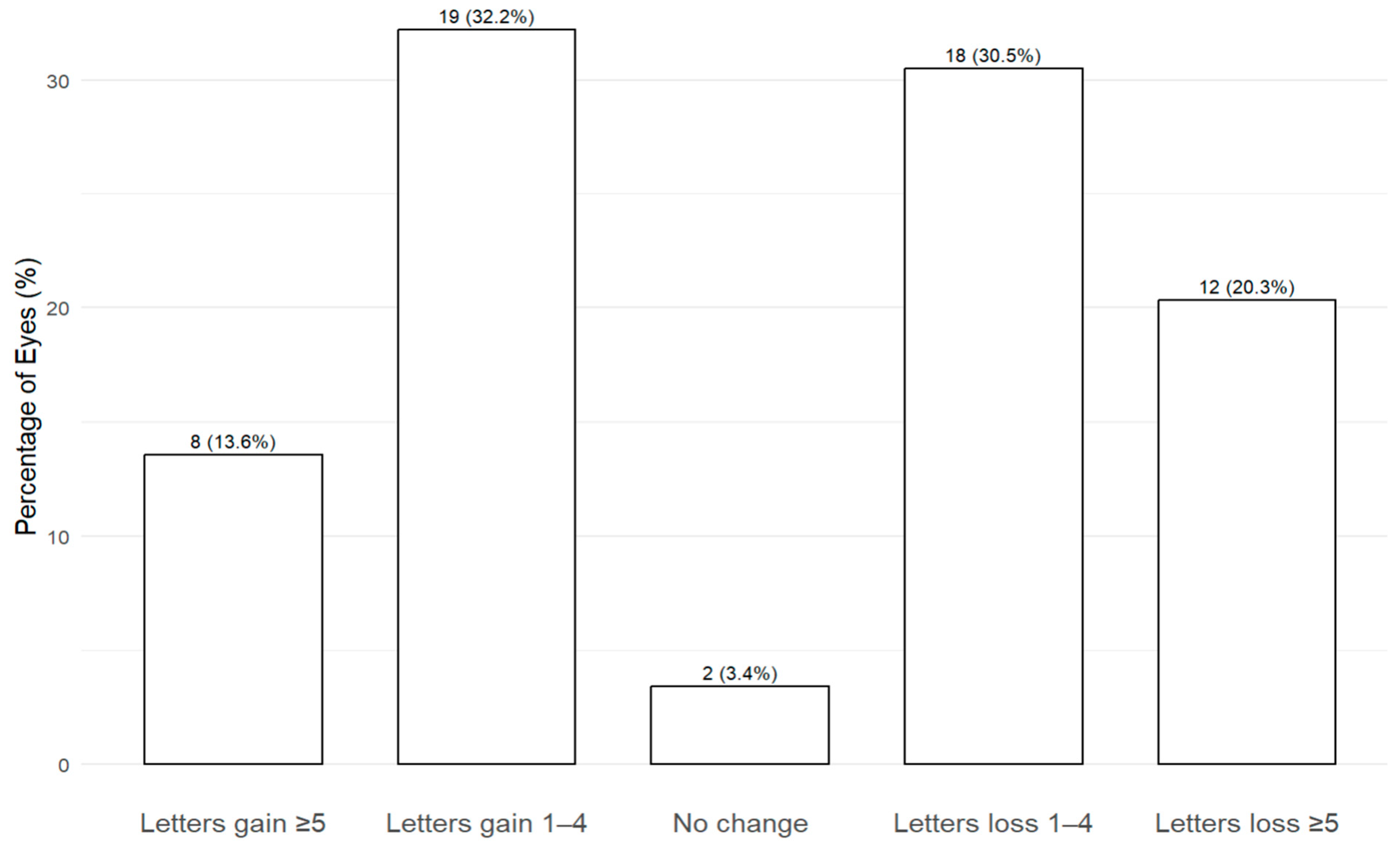
3.4. Visual Acuity Outcomes
3.5. Predictors of Visual Acuity Loss
3.6. Safety
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wong, W.L.; Su, X.; Li, X.; Cheung, C.M.G.; Klein, R.; Cheng, C.-Y.; Wong, T.Y. Global Prevalence of Age-Related Macular Degeneration and Disease Burden Projection for 2020 and 2040: A Systematic Review and Meta-Analysis. Lancet Glob. Health 2014, 2, e106–e116. [Google Scholar] [CrossRef] [PubMed]
- Fleckenstein, M.; Schmitz-Valckenberg, S.; Chakravarthy, U. Age-Related Macular Degeneration: A Review. JAMA 2024, 331, 147. [Google Scholar] [CrossRef] [PubMed]
- Lanzetta, P.; Loewenstein, A. Vision Academy Steering Committee Fundamental Principles of an Anti-VEGF Treatment Regimen: Optimal Application of Intravitreal Anti-Vascular Endothelial Growth Factor Therapy of Macular Diseases. Graefes Arch. Clin. Exp. Ophthalmol. 2017, 255, 1259–1273. [Google Scholar] [CrossRef] [PubMed]
- Chakravarthy, U.; Armendariz, B.G.; Fauser, S. 15 Years of Anti-VEGF Treatment for nAMD: Success or Failure or Something in Between? Eye 2022, 36, 2232–2233. [Google Scholar] [CrossRef] [PubMed]
- MHRA Approves Faricimab through International Work-Sharing Initiative 2022. Available online: https://www.gov.uk/government/news/mhra-approves-faricimab-through-international-work-sharing-initiative (accessed on 12 April 2025).
- Eylea 114.3 Mg/Ml Solution for Injection. Available online: https://www.medicines.org.uk/emc/product/15397/smpc#gref (accessed on 12 April 2025).
- Korobelnik, J.-F.; Lanzetta, P.; Wykoff, C.C.; Wong, T.Y.; Zhang, X.; Morgan-Warren, P.; Fitzpatrick, S.; Leal, S.; Brunck, L.; Hasanbasic, Z.; et al. Sustained Disease Control with Aflibercept 8 Mg: A New Benchmark in the Management of Retinal Neovascular Diseases. Eye 2024, 38, 3218–3221. [Google Scholar] [CrossRef] [PubMed]
- Lanzetta, P.; Korobelnik, J.-F.; Heier, J.S.; Leal, S.; Holz, F.G.; Clark, W.L.; Eichenbaum, D.; Iida, T.; Xiaodong, S.; Berliner, A.J.; et al. Intravitreal Aflibercept 8 Mg in Neovascular Age-Related Macular Degeneration (PULSAR): 48-Week Results from a Randomised, Double-Masked, Non-Inferiority, Phase 3 Trial. Lancet 2024, 403, 1141–1152. [Google Scholar] [CrossRef] [PubMed]
- Narendran, N.; Bailey, C.; Downey, L.; Gale, R.; Kotagiri, A.; Pearce, I.; Rennie, C.A.; Sivaprasad, S.; Talks, J.; Morgan-Warren, P.; et al. Expert Panel Consensus for Optimizing Outcomes in Neovascular Age-Related Macular Degeneration in the Context of Suboptimal Response to a Biosimilar: The Role of Aflibercept. Clin. Ophthalmol. 2024, 18, 3133–3142. [Google Scholar] [CrossRef] [PubMed]
- Bailey, C.; Cackett, P.; Kotagiri, A.; Mahmood, S.; Minos, E.; Narendran, N.; Patwardhan, A.; Sim, D.A.; Morgan-Warren, P.; O’Neil, C.; et al. Practical Implementation of a Q4–Q16 Aflibercept Treat-and-Extend Pathway for the Treatment of Neovascular Age-Related Macular Degeneration: Updated Guidance from a UK Expert Panel. Eye 2023, 37, 1916–1921. [Google Scholar] [CrossRef] [PubMed]
- NHS England National Health Service England. Operational Note: Updated Commissioning Recommendations for Medical Retinal Vascular Medicines Following the National Procurement for Ranibizumab Biosimilars; NHS England National Health Service England: London, UK, 2023. [Google Scholar]
- Borchert, G.A.; Kiire, C.A.; Stone, N.M.; Akil, H.; Gkika, T.; Fischer, M.D.; Xue, K.; Cehajic-Kapetanovic, J.; MacLaren, R.E.; Charbel Issa, P.; et al. Real-World Six-Month Outcomes in Patients Switched to Faricimab Following Partial Response to Anti-VEGF Therapy for Neovascular Age-Related Macular Degeneration and Diabetic Macular Oedema. Eye 2024, 38, 3569–3577. [Google Scholar] [CrossRef] [PubMed]
- Ng, B.; Kolli, H.; Ajith Kumar, N.; Azzopardi, M.; Logeswaran, A.; Buensalido, J.; Mushtaq, B.; Chavan, R.; Chong, Y.J. Real-World Data on Faricimab Switching in Treatment-Refractory Neovascular Age-Related Macular Degeneration. Life 2024, 14, 193. [Google Scholar] [CrossRef] [PubMed]
- Gale, R.P.; Pearce, I.; Eter, N.; Ghanchi, F.; Holz, F.G.; Schmitz-Valckenberg, S.; Balaskas, K.; Burton, B.J.L.; Downes, S.M.; Eleftheriadis, H.; et al. Anatomical and Functional Outcomes Following Switching from Aflibercept to Ranibizumab in Neovascular Age-Related Macular Degeneration in Europe: SAFARI Study. Br. J. Ophthalmol. 2020, 104, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Sambhara, D.; Vakharia, P.; Eichenbaum, D.A. Real-World Efficacy and Safety of 8 Mg Aflibercept in Neovascular AMD: A Case Series. BMJ Open Ophthalmol. 2025, 10, e002091. [Google Scholar] [CrossRef] [PubMed]
- Khanani, A.M.; Eichenbaum, D.; Schlottmann, P.G.; Tuomi, L.; Sarraf, D. Optimal management of pigment epithelial detachments in eyes with neovascular age-related macular degeneration. Retina 2018, 38, 2103–2117. [Google Scholar] [CrossRef] [PubMed]
- Aldhanhani, A.A.; Azzam, O.A.; AlAli, S.H.; Almasri, K.G.; Aljneibi, S.H.; Pichi, F. Switch to Faricimab after Initial Treatment with Aflibercept in Eyes with Neovascular Age-Related Macular Degeneration. Int. Ophthalmol. 2024, 44, 369. [Google Scholar] [CrossRef] [PubMed]
- Nasimi, N.; Nasimi, S.; Grauslund, J.; Vergmann, A.S.; Subhi, Y. Real-World Efficacy of Intravitreal Faricimab for Neovascular Age-Related Macular Degeneration: A Systematic Review. Int. J. Retin. Vitr. 2024, 10, 48. [Google Scholar] [CrossRef] [PubMed]
- Mehta, H.; Kim, L.N.; Mathis, T.; Zalmay, P.; Ghanchi, F.; Amoaku, W.M.; Kodjikian, L. Trends in Real-World Neovascular AMD Treatment Outcomes in the UK. Clin. Ophthalmol. 2020, 14, 3331–3342. [Google Scholar] [CrossRef] [PubMed]
- Lucentis 10 Mg/Ml Solution for Injection. Available online: https://www.medicines.org.uk/emc/product/5418/smpc#gref (accessed on 12 April 2025).
- Vabysmo 120 Mg/mL Solution for Injection. Available online: https://www.medicines.org.uk/emc/product/13741/smpc#gref (accessed on 12 April 2025).
- Eylea 40 Mg/Ml Solution for Injection in Pre-Filled Syringe. Available online: https://www.medicines.org.uk/emc/product/11273/smpc#gref (accessed on 12 April 2025).

| Patient level (n = 50) | ||
| Age, years | Mean (SD) | 80.2 (6.3) |
| Male sex | n (%) | 28 (56.0) |
| Ethnicity, white | n (%) | 50 (100) |
| Eye level (n = 59) | ||
| Pseudophakic | n (%) | 28 (46.7) |
| BCVA, letters | Mean (SD) | 66 (14.4) |
| ≥70 letters | n (%) | 33 (55.9) |
| Presence of SRF | n (%) | 39 (66.1%) |
| Presence of IRF | n (%) | 25 (42.4%) |
| Presence of PED | n (%) | 56 (94.9%) |
| Presence of SRF or IRF | n (%) | 53 (89.1%) |
| CST, µm | Mean (SD) | 367.2 (100.7) |
| Cmax, µm | Mean (SD) | 487.3 (146.6) |
| PED height, µm | Mean (SD) | 232.5 (162.4) |
| Previous anti-VEGF treatment | ||
| Number of prior injections | Mean (SD) | 26.9 (19.0) |
| Most recent interval, weeks | Mean (SD) | 7.7 (1.7) |
| Treatment before switch to aflibercept 8 mg | ||
| Faricimab | n (%) | 35 (59.3) |
| Aflibercept 2 mg | n (%) | 22 (37.3) |
| Brolucizumab | n (%) | 2 (3.4) |
| Reason for switching | ||
| Persistent disease activity | n (%) | 45 (76.3) |
| Interval extension | n (%) | 8 (13.6) |
| New disease activity | n (%) | 6 (10.2) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Musadiq, M.; Musadiq, M.; Latif, F.; Ng, B.; Azzopardi, M.; Gilead, N.; Needham, A.; Chong, Y.J. Early Real-World Outcomes of Switching to 8 mg Aflibercept for Neovascular Age-Related Macular Degeneration in the United Kingdom. Life 2025, 15, 903. https://doi.org/10.3390/life15060903
Musadiq M, Musadiq M, Latif F, Ng B, Azzopardi M, Gilead N, Needham A, Chong YJ. Early Real-World Outcomes of Switching to 8 mg Aflibercept for Neovascular Age-Related Macular Degeneration in the United Kingdom. Life. 2025; 15(6):903. https://doi.org/10.3390/life15060903
Chicago/Turabian StyleMusadiq, Muiz, Mohammed Musadiq, Fozia Latif, Benjamin Ng, Matthew Azzopardi, Noa Gilead, Andrew Needham, and Yu Jeat Chong. 2025. "Early Real-World Outcomes of Switching to 8 mg Aflibercept for Neovascular Age-Related Macular Degeneration in the United Kingdom" Life 15, no. 6: 903. https://doi.org/10.3390/life15060903
APA StyleMusadiq, M., Musadiq, M., Latif, F., Ng, B., Azzopardi, M., Gilead, N., Needham, A., & Chong, Y. J. (2025). Early Real-World Outcomes of Switching to 8 mg Aflibercept for Neovascular Age-Related Macular Degeneration in the United Kingdom. Life, 15(6), 903. https://doi.org/10.3390/life15060903





