Clinical Efficacy of Sodium Butyrate in Managing Pediatric Inflammatory Bowel Disease
Abstract
1. Introduction
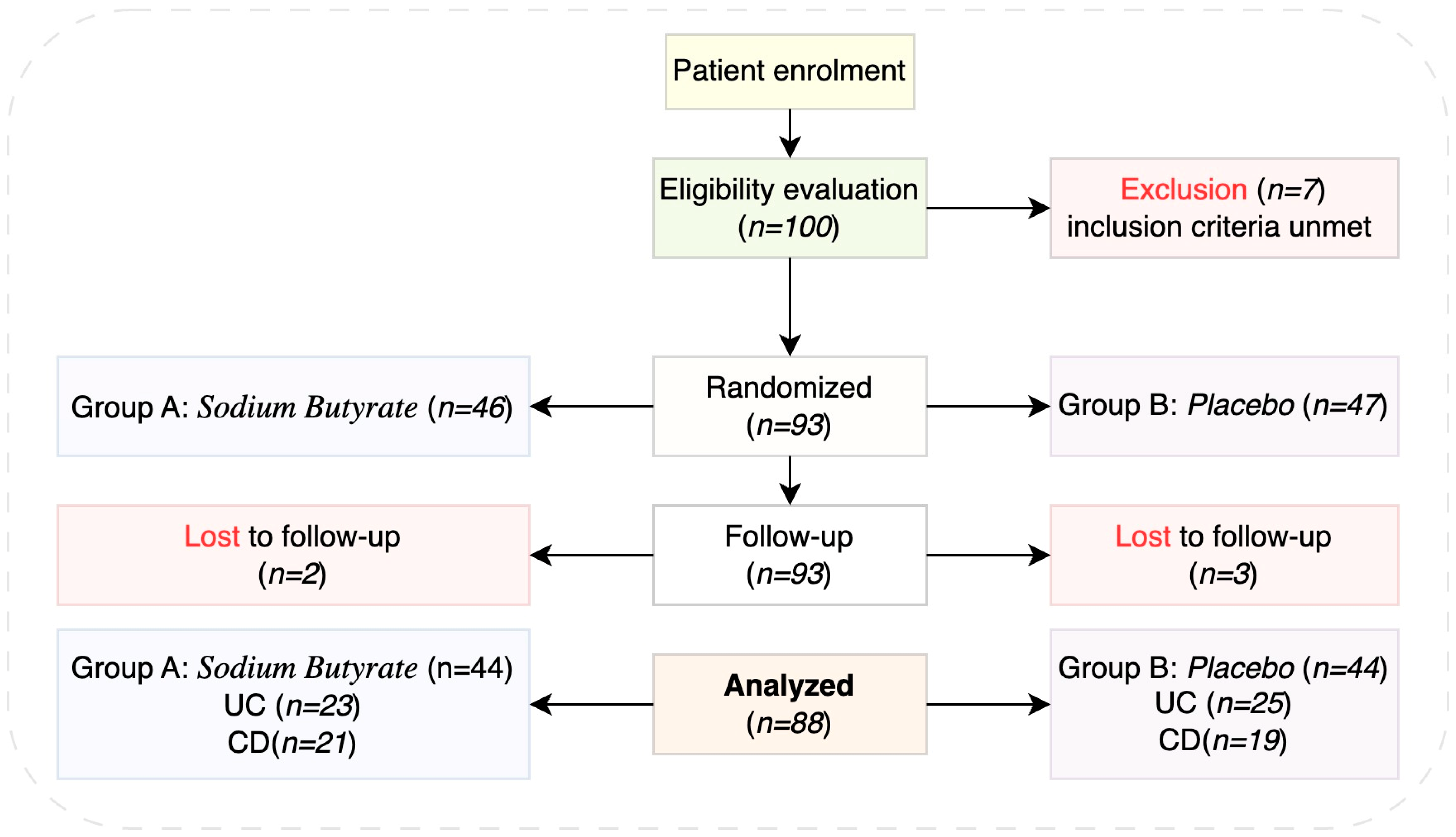
2. Materials and Methods
2.1. Study Design and Population
2.2. Inclusion Criteria
- Age 7–18 years
- Newly diagnosed inflammatory bowel disease
- Signed informed consent
- No probiotics or other dietary supplements were taken in the last 2 weeks before enrolment in the study.
2.3. Endpoints
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Maurício, J.; Domingues, I. Distinguishing between Crohn’s Disease and Ulcerative Colitis Using Deep Learning Models with Interpretability. Pattern Anal. Applic. 2024, 27, 1. [Google Scholar] [CrossRef]
- Peyrin-Biroulet, L.; Loftus, E.V.; Colombel, J.-F.; Sandborn, W.J. Long-Term Complications, Extraintestinal Manifestations, and Mortality in Adult Crohn’s Disease in Population-Based Cohorts. Inflamm. Bowel Dis. 2011, 17, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Georgescu, D.; Ancusa, O.-E.; Azoulay, D.; Lascu, A.; Ionita, I.; Calamar-Popovici, D.; Ionita, M.; Rosca, C.I.; Brează, G.-M.; Reisz, D.; et al. Portal Vein Thrombosis in Patients with Liver Cirrhosis: What Went Wrong? Int. J. Gen. Med. 2023, 16, 3889–3906. [Google Scholar] [CrossRef] [PubMed]
- Manser, C.N.; Borovicka, J.; Seibold, F.; Vavricka, S.R.; Lakatos, P.L.; Fried, M.; Rogler, G. Investigators of the Swiss Inflammatory Bowel Disease Cohort Study Risk Factors for Complications in Patients with Ulcerative Colitis. UEG J. 2016, 4, 281–287. [Google Scholar] [CrossRef]
- Georgescu, D.; Iurciuc, M.S.; Petre, I.; Georgescu, L.A.; Szasz, F.; Ionita, I.; Ancusa, O.E.; Ionita, M.; Lighezan, D. Chronic Pelvic Pain and Irritable Bowel Syndrome: Is Subclinical Inflammation Bridging the Gap? Rev. Chim. 2019, 70, 3634–3637. [Google Scholar] [CrossRef]
- Parragi, L.; Fournier, N.; Zeitz, J.; Scharl, M.; Greuter, T.; Schreiner, P.; Misselwitz, B.; Safroneeva, E.; Schoepfer, A.M.; Vavricka, S.R.; et al. Colectomy Rates in Ulcerative Colitis Are Low and Decreasing: 10-Year Follow-up Data from the Swiss IBD Cohort Study. J. Crohn’s Colitis 2018, 12, 811–818. [Google Scholar] [CrossRef] [PubMed]
- Benchimol, E.I.; Fortinsky, K.J.; Gozdyra, P.; Van den Heuvel, M.; Van Limbergen, J.; Griffiths, A.M. Epidemiology of Pediatric Inflammatory Bowel Disease: A Systematic Review of International Trends. Inflamm. Bowel Dis. 2011, 17, 423–439. [Google Scholar] [CrossRef]
- Kuenzig, M.E.; Fung, S.G.; Marderfeld, L.; Mak, J.W.Y.; Kaplan, G.G.; Ng, S.C.; Wilson, D.C.; Cameron, F.; Henderson, P.; Kotze, P.G.; et al. Twenty-First Century Trends in the Global Epidemiology of Pediatric-Onset Inflammatory Bowel Disease: Systematic Review. Gastroenterology 2022, 162, 1147–1159.e4. [Google Scholar] [CrossRef]
- Clark, J.G.; Srinath, A.I.; Youk, A.O.; Kirshner, M.A.; McCarthy, F.N.; Keljo, D.J.; Bousvaros, A.; DeMaso, D.R.; Szigethy, E.M. Predictors of Depression in Youth with Crohn Disease. J. Pediatr. Gastroenterol. Nutr. 2014, 58, 569–573. [Google Scholar] [CrossRef]
- Däbritz, J.; Gerner, P.; Enninger, A.; Claßen, M.; Radke, M. Inflammatory Bowel Disease in Childhood and Adolescence. Dtsch. Arztebl. Int. 2017, 114, 331–338. [Google Scholar] [CrossRef]
- Levine, A.; Griffiths, A.; Markowitz, J.; Wilson, D.C.; Turner, D.; Russell, R.K.; Fell, J.; Ruemmele, F.M.; Walters, T.; Sherlock, M.; et al. Pediatric Modification of the Montreal Classification for Inflammatory Bowel Disease: The Paris Classification. Inflamm. Bowel Dis. 2011, 17, 1314–1321. [Google Scholar] [CrossRef] [PubMed]
- Diefenbach, K.A. Pediatric Inflammatory Bowel Disease. World J. Gastroenterol. 2006, 12, 3204. [Google Scholar] [CrossRef] [PubMed]
- Sonavane, A.D.; Sonawane, P.; Amarapurkar, D.N. Inflammatory Bowel Disease Across the Age Continuum: Similarity and Disparity. Indian J. Pediatr. 2018, 85, 989–994. [Google Scholar] [CrossRef] [PubMed]
- Granot, M.; Kopylov, U.; Loberman-Nachum, N.; Krauthammer, A.; Abitbol, C.M.; Ben-Horin, S.; Weiss, B.; Haberman, Y. Differences in Disease Characteristics and Treatment Exposures between Paediatric and Adult-onset Inflammatory Bowel Disease Using a Registry-based Cohort. Aliment. Pharmacol. Ther. 2024, 60, 1435–1446. [Google Scholar] [CrossRef] [PubMed]
- Bouhuys, M.; Lexmond, W.S.; Van Rheenen, P.F. Pediatric Inflammatory Bowel Disease. Pediatrics 2023, 151, e2022058037. [Google Scholar] [CrossRef]
- Kim, J.; Ye, B.D. Successful Transition from Pediatric to Adult Care in Inflammatory Bowel Disease: What Is the Key? Intest. Res. 2019, 17, 24–35. [Google Scholar] [CrossRef]
- Kahn, S.A. The Transition from Pediatric to Adult Inflammatory Bowel Disease Care. Gastroenterol. Hepatol. 2016, 12, 403–406. [Google Scholar]
- Halfvarson, J.; Brislawn, C.J.; Lamendella, R.; Vázquez-Baeza, Y.; Walters, W.A.; Bramer, L.M.; D’Amato, M.; Bonfiglio, F.; McDonald, D.; Gonzalez, A.; et al. Dynamics of the Human Gut Microbiome in Inflammatory Bowel Disease. Nat. Microbiol. 2017, 2, 17004. [Google Scholar] [CrossRef]
- Santana, P.T.; Rosas, S.L.B.; Ribeiro, B.E.; Marinho, Y.; De Souza, H.S.P. Dysbiosis in Inflammatory Bowel Disease: Pathogenic Role and Potential Therapeutic Targets. Int. J. Mol. Sci. 2022, 23, 3464. [Google Scholar] [CrossRef]
- Bernstein, C.N.; Forbes, J.D. Gut Microbiome in Inflammatory Bowel Disease and Other Chronic Immune-Mediated Inflammatory Diseases. Inflamm. Intest. Dis. 2017, 2, 116–123. [Google Scholar] [CrossRef]
- Singh, V.; Lee, G.; Son, H.; Koh, H.; Kim, E.S.; Unno, T.; Shin, J.-H. Butyrate Producers, “The Sentinel of Gut”: Their Intestinal Significance with and beyond Butyrate, and Prospective Use as Microbial Therapeutics. Front. Microbiol. 2023, 13, 1103836. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, J.; He, T.; Becker, S.; Zhang, G.; Li, D.; Ma, X. Butyrate: A Double-Edged Sword for Health? Adv. Nutr. 2018, 9, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, M.T.; Cresci, G.A. The Immunomodulatory Functions of Butyrate. J. Inflamm. Res. 2021, 14, 6025–6041. [Google Scholar] [CrossRef]
- Lee, C.; Kim, B.G.; Kim, J.H.; Chun, J.; Im, J.P.; Kim, J.S. Sodium Butyrate Inhibits the NF-Kappa B Signaling Pathway and Histone Deacetylation, and Attenuates Experimental Colitis in an IL-10 Independent Manner. Int. Immunopharmacol. 2017, 51, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Kedia, S. Eosinophilic Esophagitis. Trop. Gastroenterol. 2015, 36, 156–167. [Google Scholar] [CrossRef]
- Hyams, J.; Markowitz, J.; Otley, A.; Rosh, J.; Mack, D.; Bousvaros, A.; Kugathasan, S.; Pfefferkorn, M.; Tolia, V.; Evans, J.; et al. Evaluation of the Pediatric Crohn Disease Activity Index: A Prospective Multicenter Experience. J. Pediatr. Gastroenterol. Nutr. 2005, 41, 416–421. [Google Scholar] [CrossRef]
- Vuijk, S.A.; Camman, A.E.; De Ridder, L. Considerations in Paediatric and Adolescent Inflammatory Bowel Disease. J. Crohn’s Colitis 2024, 18, ii31–ii45. [Google Scholar] [CrossRef]
- Markowitz, J. Early Inflammatory Bowel Disease: Different Treatment Response to Specific or All Medications? Dig. Dis. 2009, 27, 358–365. [Google Scholar] [CrossRef]
- Ota, S.; Sakuraba, H. Uptake and Advanced Therapy of Butyrate in Inflammatory Bowel Disease. Immuno 2022, 2, 692–702. [Google Scholar] [CrossRef]
- Gasaly, N.; Hermoso, M.A.; Gotteland, M. Butyrate and the Fine-Tuning of Colonic Homeostasis: Implication for Inflammatory Bowel Diseases. Int. J. Mol. Sci. 2021, 22, 3061. [Google Scholar] [CrossRef]
- Pietrzak, A.; Banasiuk, M.; Szczepanik, M.; Borys-Iwanicka, A.; Pytrus, T.; Walkowiak, J.; Banaszkiewicz, A. Sodium Butyrate Effectiveness in Children and Adolescents with Newly Diagnosed Inflammatory Bowel Diseases—Randomized Placebo-Controlled Multicenter Trial. Nutrients 2022, 14, 3283. [Google Scholar] [CrossRef] [PubMed]
- Ventura, I.; Chomon-García, M.; Tomás-Aguirre, F.; Palau-Ferré, A.; Legidos-García, M.E.; Murillo-Llorente, M.T.; Pérez-Bermejo, M. Therapeutic and Immunologic Effects of Short-Chain Fatty Acids in Inflammatory Bowel Disease: A Systematic Review. Int. J. Mol. Sci. 2024, 25, 10879. [Google Scholar] [CrossRef] [PubMed]
- Haskey, N.; Estaki, M.; Ye, J.; Shim, R.K.; Singh, S.; Dieleman, L.A.; Jacobson, K.; Gibson, D.L. A Mediterranean Diet Pattern Improves Intestinal Inflammation Concomitant with Reshaping of the Bacteriome in Ulcerative Colitis: A Randomised Controlled Trial. J. Crohn’s Colitis 2023, 17, 1569–1578. [Google Scholar] [CrossRef]
- Facchin, S.; Vitulo, N.; Calgaro, M.; Buda, A.; Romualdi, C.; Pohl, D.; Perini, B.; Lorenzon, G.; Marinelli, C.; D’Incà, R.; et al. Microbiota Changes Induced by Microencapsulated Sodium Butyrate in Patients with Inflammatory Bowel Disease. Neurogastroenterol. Motil. 2020, 32, e13914. [Google Scholar] [CrossRef]
- Vernia, P.; Monteleone, G.; Grandinetti, G.; Villotti, G.; Di Giulio, E.; Frieri, G.; Marcheggiano, A.; Pallone, F.; Caprilli, R.; Torsoli, A. Combined Oral Sodium Butyrate and Mesalazine Treatment Compared to Oral Mesalazine Alone in Ulcerative Colitis. Dig. Dis. Sci. 2000, 45, 976–981. [Google Scholar] [CrossRef]
- Sabatino, A.D.; Morera, R.; Ciccocioppo, R.; Cazzola, P.; Gotti, S.; Tinozzi, F.P.; Tinozzi, S.; Corazza, G.R. Oral Butyrate for Mildly to Moderately Active Crohn’s Disease. Aliment. Pharmacol. Ther. 2005, 22, 789–794. [Google Scholar] [CrossRef]
- Lin, J.; Nafday, S.M.; Chauvin, S.N.; Magid, M.S.; Pabbatireddy, S.; Holzman, I.R.; Babyatsky, M.W. Variable Effects of Short Chain Fatty Acids and Lactic Acid in Inducing Intestinal Mucosal Injury in Newborn Rats. J. Pediatr. Gastroenterol. Nutr. 2002, 35, 545–550. [Google Scholar] [CrossRef]
- Hamer, H.M.H. Short Chain Fatty Acids and Colonic Health. Ph.D. Thesis, Maastricht University, Maastricht, The Netherlands, 2009. [Google Scholar]
- Pietrzak, A.; Banasiewicz, T. Applicability of Sodium Butyrate Preparations from a Surgeon’s and Gastroenterologist’s Perspective. Pol. Przegl. Chir. 2024, 96, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Ran, X.; Li, B.; Li, Y.; He, D.; Huang, B.; Fu, S.; Liu, J.; Wang, W. Sodium Butyrate Inhibits Inflammation and Maintains Epithelium Barrier Integrity in a TNBS-Induced Inflammatory Bowel Disease Mice Model. eBioMedicine 2018, 30, 317–325. [Google Scholar] [CrossRef]
- Dou, X.; Gao, N.; Yan, D.; Shan, A. Sodium Butyrate Alleviates Mouse Colitis by Regulating Gut Microbiota Dysbiosis. Animals 2020, 10, 1154. [Google Scholar] [CrossRef]
- Simeoli, R.; Mattace Raso, G.; Pirozzi, C.; Lama, A.; Santoro, A.; Russo, R.; Montero-Melendez, T.; Berni Canani, R.; Calignano, A.; Perretti, M.; et al. An Orally Administered Butyrate-releasing Derivative Reduces Neutrophil Recruitment and Inflammation in Dextran Sulphate Sodium-induced Murine Colitis. Br. J. Pharmacol. 2017, 174, 1484–1496. [Google Scholar] [CrossRef] [PubMed]
- Recharla, N.; Geesala, R.; Shi, X.-Z. Gut Microbial Metabolite Butyrate and Its Therapeutic Role in Inflammatory Bowel Disease: A Literature Review. Nutrients 2023, 15, 2275. [Google Scholar] [CrossRef] [PubMed]
- Di Meglio, L.; Grimaldi, G.; Esposito, F.; Gelzo, M.; Esposito, M.V.; Castaldo, G.; Canani, R.B. Step-Up Approach for Sodium Butyrate Treatment in Children With Congenital Chloride Diarrhea. Front. Pediatr. 2022, 9, 810765. [Google Scholar] [CrossRef]
- Hodgkinson, K.; El Abbar, F.; Dobranowski, P.; Manoogian, J.; Butcher, J.; Figeys, D.; Mack, D.; Stintzi, A. Butyrate’s Role in Human Health and the Current Progress towards Its Clinical Application to Treat Gastrointestinal Disease. Clin. Nutr. 2023, 42, 61–75. [Google Scholar] [CrossRef]
- Gibbs, B.; Brown, B. Butyrate Therapy for Treatment-Resistant Ulcerative Colitis: A Case Study. Nutr. Med. J. 2021, 1, 60–67. [Google Scholar]
- Abdulkhakov, S.; Markelova, M.; Safina, D.; Siniagina, M.; Khusnutdinova, D.; Abdulkhakov, R.; Grigoryeva, T. Butyric Acid Supplementation Reduces Changes in the Taxonomic and Functional Composition of Gut Microbiota Caused by H. Pylori Eradication Therapy. Microorganisms 2024, 12, 319. [Google Scholar] [CrossRef] [PubMed]
- Pituch, A.; Walkowiak, J.; Banaszkiewicz, A. Butyric Acid in Functional Constipation. Prz. Gastroenterol. 2013, 5, 295–298. [Google Scholar] [CrossRef]
- Kaźmierczak-Siedlecka, K.; Marano, L.; Merola, E.; Roviello, F.; Połom, K. Sodium Butyrate in Both Prevention and Supportive Treatment of Colorectal Cancer. Front. Cell. Infect. Microbiol. 2022, 12, 1023806. [Google Scholar] [CrossRef]
- Canani, R.B. Potential Beneficial Effects of Butyrate in Intestinal and Extraintestinal Diseases. World J. Gastroenterol. 2011, 17, 1519. [Google Scholar] [CrossRef]
- Coppola, S.; Nocerino, R.; Paparo, L.; Bedogni, G.; Calignano, A.; Di Scala, C.; De Giovanni Di Santa Severina, A.F.; De Filippis, F.; Ercolini, D.; Berni Canani, R. Therapeutic Effects of Butyrate on Pediatric Obesity: A Randomized Clinical Trial. JAMA Netw. Open 2022, 5, e2244912. [Google Scholar] [CrossRef]
- Andreev, D.N.; Kucheryavyy, Y.A.; Maev, I.V. Efficacy of Butyric Acid Inclusion in Eradication Regimens for Helicobacter Pylori Infection: A Meta-Analysis of Controlled Trials. Ter. Arkhiv 2021, 93, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Lewandowski, K.; Kaniewska, M.; Karłowicz, K.; Rosołowski, M.; Rydzewska, G. The Effectiveness of Microencapsulated Sodium Butyrate at Reducing Symptoms in Patients with Irritable Bowel Syndrome. Prz. Gastroenterol. 2022, 17, 28–34. [Google Scholar] [CrossRef]
- Gerunova, L.K.; Gerunov, T.V.; P’yanova, L.G.; Lavrenov, A.V.; Sedanova, A.V.; Delyagina, M.S.; Fedorov, Y.N.; Kornienko, N.V.; Kryuchek, Y.O.; Tarasenko, A.A. Butyric Acid and Prospects for Creation of New Medicines Based on Its Derivatives: A Literature Review. J. Vet. Sci. 2024, 25, e23. [Google Scholar] [CrossRef]
- Ferrer-Picón, E.; Dotti, I.; Corraliza, A.M.; Mayorgas, A.; Esteller, M.; Perales, J.C.; Ricart, E.; Masamunt, M.C.; Carrasco, A.; Tristán, E.; et al. Intestinal Inflammation Modulates the Epithelial Response to Butyrate in Patients With Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2020, 26, 43–55. [Google Scholar] [CrossRef]


| Score | Severity | Status | |
|---|---|---|---|
| PUCAI * | 0–10 | Remission | Inactive |
| 10–34 | Mild | Active | |
| 35–64 | Moderate | Active | |
| 65–85 | Severe | Active | |
| PCDAI * | 6.8 ± 6.6 | Remission | Inactive |
| 18.7 ± 7.3 | Mild | Active | |
| 38.5 ± 12.9 | Moderate | Active | |
| 54.2 ± 14.0 | Severe | Active |
| Score | Severity | |
|---|---|---|
| CRP * (mg/L) | <5.0 | Normal, no significant inflammation |
| 5–10 | Mild inflammation, may suggest mild IBD activity | |
| >10 | Moderate to severe inflammation, commonly seen in active IBD | |
| >50 | Severe inflammation, may indicate a flare or complication | |
| Fecal calprotectin * (µg/g) | <50 | Normal, no significant inflammation |
| ≤200 | Borderline, mild inflammation | |
| ≤500 | Moderate inflammation, suggestive of active IBD | |
| >500 | High inflammation, indicative of active IBD or flare |
| Characteristics | Group A Sodium Butyrate (N = 44) | Group B Placebo (N = 44) | p Value |
|---|---|---|---|
| Age (a) | 11.6 ± 3.5 | 11.9 ± 3.5 | 0.630 |
| Gender (b) | |||
| F | 22 (50%) | 23 (52.27%) | 0.831 |
| M | 22 (50%) | 21 (47.73%) | 0.831 |
| Disease (b) | |||
| Crohn disease | 21 (47.73%) | 19 (43.18%) | 0.669 |
| Ulcerative Colitis | 23 (52.27%) | 25 (56.82%) | 0.669 |
| Score (b) | |||
| PCDAI | 21 (47.73%) | 19 (43.18%) | 0.669 |
| PUCAI | 23 (52.27%) | 25 (56.82%) | 0.669 |
| Paris classification for disease extension (b) | |||
| E1 | 5 (11.36%) | 4 (9.09%) | 0.904 |
| E2 | 4 (9.09%) | 6 (13.64%) | 0.904 |
| E3 | 7 (15.91%) | 5 (11.36%) | 0.904 |
| E4 | 7 (15.91%) | 10 (22.73%) | 0.904 |
| L2 | 10 (22.73%) | 10 (22.73%) | 0.904 |
| L3 | 11 (25.00%) | 9 (20.45%) | 0.904 |
| Regimens used (b) | |||
| AS | 4 (9.09%) | 3 (6.82%) | 0.954 |
| AS, S | 6 (13.64%) | 5 (11.36%) | 0.954 |
| AS, S, ATB | 2 (4.55%) | 4 (9.09%) | 0.954 |
| AS, S, T | 5 (11.36%) | 6 (13.64%) | 0.954 |
| AS, S, T, ATB | 4 (9.09%) | 2 (4.54%) | 0.954 |
| S, T | 11 (25.00%) | 11 (25.00%) | 0.954 |
| S, T, ATB | 6 (13.64%) | 4 (9.09%) | 0.954 |
| T, anti-TNF | 4 (9.09%) | 6 (13.64%) | 0.954 |
| T, anti-TNF, ATB | 2 (4.54%) | 3 (6.82%) | 0.954 |
| Disease severity (b) | |||
| Remission | 36 (81.82%) | 21 (47.73%) | <0.001 |
| Mild | 1 (2.27%) | 6 (13.64%) | <0.001 |
| Moderate | 3 (6.82%) | 16 (36.36%) | <0.001 |
| Severe | 4 (9.09%) | 1 (2.27%) | <0.001 |
| Group | Severity | Crohn’s Disease | Ulcerative Colitis | Total |
|---|---|---|---|---|
| 21 | 23 | 44 | ||
| Group A | Remission | 16 | 20 | 36 |
| Mild | 1 | 0 | 1 | |
| Moderate | 2 | 1 | 3 | |
| Severe | 2 | 2 | 4 | |
| 19 | 25 | 44 | ||
| Group B | Remission | 6 | 15 | 21 |
| Mild | 4 | 2 | 6 | |
| Moderate | 8 | 8 | 16 | |
| Severe | 1 | 0 | 1 | |
| Total | 40 | 48 | 88 |
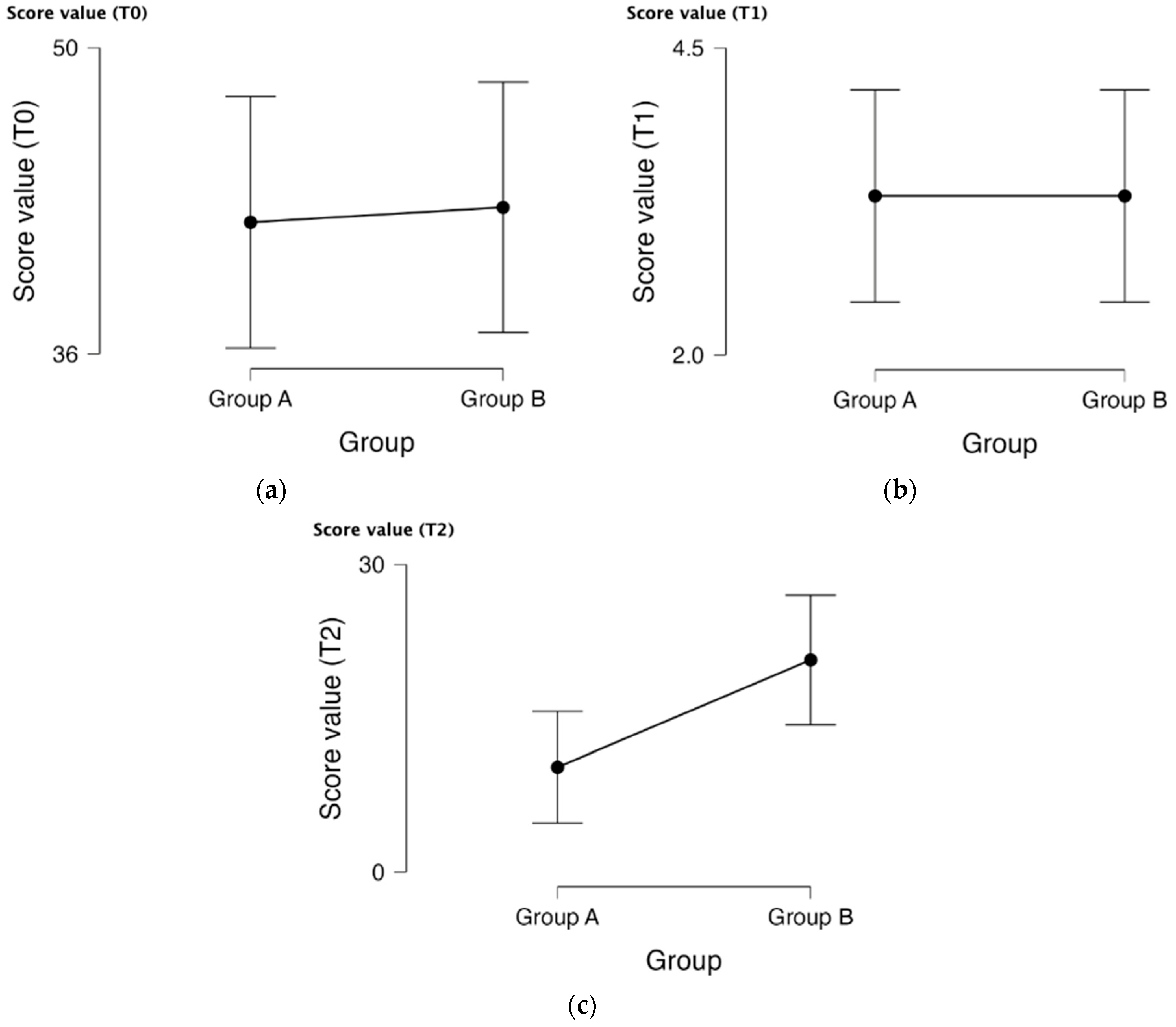
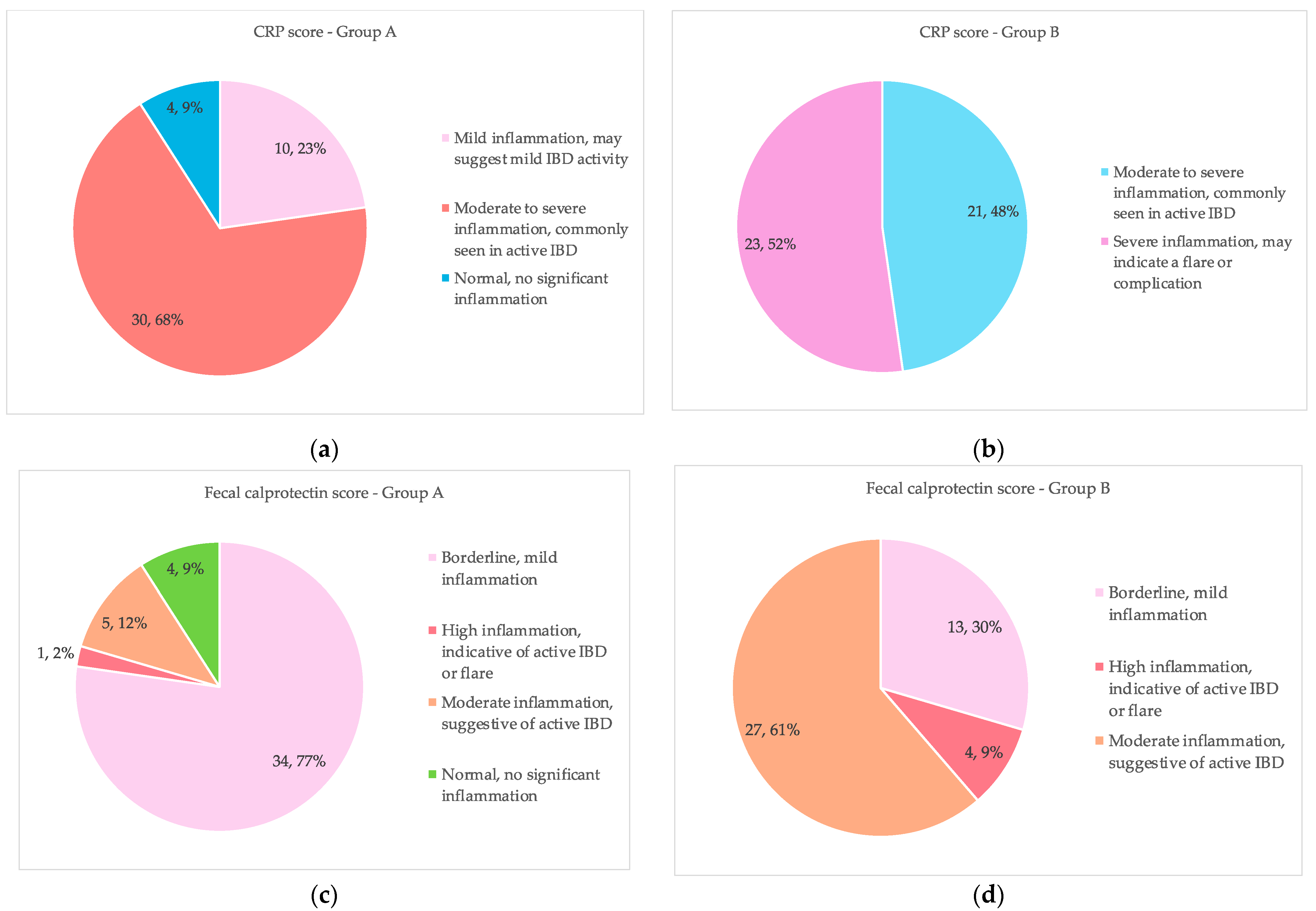
| Inflammatory Marker | Group A (N = 44) | Group B (N = 44) | p-Value | |
|---|---|---|---|---|
| CRP (a) | Baseline (T0) | 88.66 ± 37.43 | 83.93 ± 40.32 | 0.570 |
| After 12 weeks (T2) | 18.14 ± 11.19 | 57.00 ± 33.28 | <0.001 | |
| Fecal calprotectin (b) | Baseline (T0) | 937.27 ± 713.86 | 845.25 ± 608.458 | 0.517 |
| After 12 weeks (T2) | 125.80 ± 164.07 | 311.73 ± 248.69 | <0.001 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goldiș, A.; Dragomir, R.; Mercioni, M.A.; Sirca, D.; Goldiș, C.; Enatescu, I.; Olariu, L.; Belei, O. Clinical Efficacy of Sodium Butyrate in Managing Pediatric Inflammatory Bowel Disease. Life 2025, 15, 902. https://doi.org/10.3390/life15060902
Goldiș A, Dragomir R, Mercioni MA, Sirca D, Goldiș C, Enatescu I, Olariu L, Belei O. Clinical Efficacy of Sodium Butyrate in Managing Pediatric Inflammatory Bowel Disease. Life. 2025; 15(6):902. https://doi.org/10.3390/life15060902
Chicago/Turabian StyleGoldiș, Adrian, Radu Dragomir, Marina Adriana Mercioni, Diana Sirca, Christian Goldiș, Ileana Enatescu, Laura Olariu, and Oana Belei. 2025. "Clinical Efficacy of Sodium Butyrate in Managing Pediatric Inflammatory Bowel Disease" Life 15, no. 6: 902. https://doi.org/10.3390/life15060902
APA StyleGoldiș, A., Dragomir, R., Mercioni, M. A., Sirca, D., Goldiș, C., Enatescu, I., Olariu, L., & Belei, O. (2025). Clinical Efficacy of Sodium Butyrate in Managing Pediatric Inflammatory Bowel Disease. Life, 15(6), 902. https://doi.org/10.3390/life15060902






